Abstract
Asthma is a complex disease. The heterogeneity of airway inflammation during asthma indicates there are different mechanisms involved. In order to further study the mechanisms of asthma, different mouse models were established to mimic corresponding subtypes of asthma in clinic. Eosinophilic asthma was established by intraperitoneal injections of ovalbumin (OVA) on day 0 and day 7, followed by inhalation of aerosolized OVA on days 14–17. Neutrophilic asthma was established by transtracheal administration of a high dose of lipopolysaccharide (LPS; 10 µg) on days 15 and 17 in combination with OVA sensitization and challenge as described previously. Mix-granulocytic asthma was established by transtracheal administration of a low dose of LPS (1 µg) on day 15, in combination with OVA sensitization and challenge as described previously. Compared with healthy controls, increased numbers of eosinophils, elevated levels of T helper (Th)2 cytokines in bronchoalveolar lavage fluid (BALF), and moderated inflammation of lung tissues was observed in eosinophilic asthma. Increased numbers of neutrophils, elevated levels of Th1 and Th17 cytokines in BALF and severe inflammation of lung tissues was observed in neutrophilic asthma. Increased numbers of both eosinophils and neutrophils, elevated levels of Th1, Th2 and Th17 cytokines in BALF and severe inflammation of lung tissues was observed in mix-granulocytic asthma. Airway hyperresponsiveness, increased bronchial mucus secretion, and elevated serum levels of immunoglobin (Ig)E and OVA-IgE were detected in all three asthma models. Dexamethasone reduced the pathogenic symptoms of the mice in eosinophilic asthma, however had no effect on neutrophilic asthma or mix-granulocytic asthma. Each model of asthma established in the present study represents corresponding subtypes of asthma in clinic.
Keywords: eosinophilic asthma, neutrophilic asthma, mix-granulocytic asthma, dexamethasone
Introduction
Asthma is a complex disorder characterized by chronic inflammation of the airways, airway hyperresponsiveness (AHR), airflow obstruction, and airway wall remodeling (1). Eosinophilic inflammation is generally considered to be the main feature of asthmatic airways and is assumed to be crucial in the pathogenesis of allergic asthma (2). However, recent studies have profoundly altered this simplistic view of eosinophils and their function. A group of asthmatic patients characterized by the high levels of sputum neutrophils were found in clinic. Moreover, unlike conventional eosinophilia asthmatics, the patients with neutrophilia have poor response to corticosteroids which are the cornerstone of maintenance therapy for asthma (3). This strongly suggests that there are different underlying pathophysiological mechanisms in asthma. For this, researchers divided asthma into four subtypes according to the cell characteristics in the sputum in clinic: Eosinophilic asthma, neutrophilic asthma, mixed-granulocytic asthma and paucigranulocytic asthma (4). It is estimated that approximately 40% asthma cases are accompanied by characteristics of neutrophilia, in which half were accompanied by eosinophilia simultaneously. These patients are at great risk of dying, thus they require amounts of healthcare time and costs, due to the unclear pathomechanism (5).
As a result of ethical and moral issues preventing patients from mechanistic research, the development and characterization of animal models are needed to further understanding of asthma. Appropriate animal models which represent different subtypes of asthma are of great significance to the research and are beneficial to the targeted treatment of individial asthma patients. Immunization with ovalbumin (OVA) is a classic approach to induce eosinophilic asthma (6). However, using merely one animal model is not sufficient to reflect all the characteristics of asthma patients, with in-depth understanding of asthma, and considering the classification of clinical asthma in pathological features. Many studies have reported that asthma associated with neutrophilia was related to bacterial endotoxin, ozone and particulate air pullution (7–9). It has been demonstrated that domestic endotoxin exposure could trigger exacerbation of pre-existing asthma in children and adults (10). These patients are often accompanied by infection in airways, suggesting that the bacteria and virus may be the inducer of neutrophilia in acute attack of asthma.
In the present study, we established eosinophilic asthma through traditional OVA immunization and, on this basis, established neutrophilic asthma and mixed-granulocytic asthma by intratracheal administration of different doses of lipopolysaccharide (LPS). The cell characteristics in bronchoalveolar lavage fluid (BALF), pathological changes of lung tissue and disease related protein levels were analyzed to verify whether each model of asthma has been successfully established. Furthermore, the changes of the asthma symptoms in mice with different asthma models were identified following treatment of dexamethasone, a type of corticosteroid medication.
Materials and methods
Mice
Female BALB/c mice (6–8 weeks of age) were purchased from Centers for Disease Control (CDC; Hubei, China). It has been demonstrated that female mice develop a more pronounced type of allergic airway inflammation than male mice after OVA challenge (11). Mice were housed in a condition of SPF room, and allowed access to food and water ad libitum. All the experiments in this study were performed according to the Guidelines for Use and Experimentation as set forth by the Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The study was approved by the ethics committee of Department of Immunology, School of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology.
Asthma models
For the eosinophilic asthma group (Eos), mice were sensitized with an intraperitoneal (i.p.) injection of 10 µg OVA (Grade V; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) emulsified in aluminium hydroxide gel (InvivoGen, San Diego, CA, USA) on days 0 and 7. The animals were then challenged with 6% OVA diluted in phosphate-buffered saline (PBS) for 25 min on days 14–17. The aerosol exposure was performed in a chamber coupled to an ultrasonic nebulizer (Leyi Industry Co., Ltd., Shanghai, China). For the neutrophilic asthma group (Neu), mice were sensitized and challenged with OVA as described above, and performed transtracheal administration of 10 µg LPS (Escherichia coli serotype O55:B5; Sigma-Aldrich; Merck KGaA) diluted in PBS on days 15 and 17. For the mixed-granulocytic asthma group (Mix), mice were sensitized and challenged with OVA as described above, and performed transtracheal administration of 1 µg LPS diluted in PBS on day 15 (Fig. 1). For dexamethasone treatment (Con + DEX, Eos + DEX, Neu + DEX, Mix + DEX), mice were administered 5 µg/kg dexamethasone i.p. one day before the first challenge and lasted for five consecutive days until sacrificed. PBS aerosol was used as a negative control (Con). At 24 h after the last OVA challenge (day 18), all mice were sacrificed for assay.
Figure 1.
Asthma mouse model protocol. OVA, ovalbumin; LPS, lipopolysaccharide; i.p., intraperitoneal; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos, eosinophilic asthma.
Measurement of AHR
Mice were anesthetized with i.p. injections of pentobarbital sodium and a tracheostomy tube was placed. The internal jugular vein was cannulated and connected to a microsyringe for intravenous methacholine administration. Lung resistance (RI) in response to increasing concentrations of nebulized methacholine (3, 6, 12 and 24 mg/ml) were recorded using the FinePointe data acquisition and analysis software (Buxco, Wilmington, NC, USA). Results are expressed as a percentage of the respective basal values in response to PBS.
Bronchoalveolar lavage and cell count
Tracheae was cannulated and the left lung was lavaged slowly 2 times with 0.6 ml PBS following the right lung ligation. Each fluid was centrifuged and the supernatant was rapidly frozen at −80°C. The cells in BALF were resuspended in PBS and centrifuged in a cytocentrifuge. The cells were then stained with Wright-Giemsa (Quick Wright-Giemsa Stain; Jiancheng Bioengineering Institute, Nanjing, China) and identified as macrophages, eosinophils, neutrophils and lymphocytes based on cellular morphology and staining characteristics. At least 200 cells were counted under ×400 magnification.
Blood collection
Blood sample was obtained from the orbital venous plexus, and the serum was collected for immunoglobin (Ig)E and OVA specific IgE assay.
Lung histology
The right lung tissues were fixed with 4% paraformaldehyde, embedded, sectioned and stained with hematoxylin and eosin (H&E) for detection of inflammatory cells and periodic acid-schiff (PAS) for detection of mucin in goblet cells (mucus-secreting cells) by light microscopy.
ELISA
Quantitation of cytokines IL-4, IL-5, IL-13, IL-17A, IL-33, IFN-γ in BALF and serum levels of IgE were measured by sandwich ELISA using paired Abs (BD Biosciences, San Jose, CA, USA). Serum levels of OVA-IgE were measured using a mouse OVA specific IgE ELISA kit (Cusabio Biotech, Wuhan, China). The sensitivity of detection were 4 pg/ml for IL-4, IL-5 IL-13, IL-17A, 15 pg/ml for IFN-γ, 25 pg/ml for IL-33 and 5 ng/ml for IgE.
Statistical analysis
Results were shown as the mean ± SEM. Statistical significance of differences was performed with the unpaired two-tailed Student's t-test (for comparison between two groups), repeated-measures ANOVA with the Dunnett posttest (for AHR dose-response curves) or one-way ANOVA with the Tukey post test (for comparison between three or more groups). The software package GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was used for data analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
AHR
AHR is a hallmark of bronchial asthma. The essential measurement to assess AHR in mice is airway RI, which is defined as the pressure driving respiration divided by flow (12). In comparison with control mice that were instilled with PBS, OVA challenged mice of all three groups showed significantly enhanced RI after treatment with methacholine. Dexamethasone dramatically reduced RI in mice of Eos, suggesting that immune-mediated airway pathology in vivo was modified. However, dexamethasone did not show any inhibitory effects on AHR in mice of Neu and Mix (Fig. 2).
Figure 2.

The AHR of OVA-sensitized/challenged BALB/c mice supplemented with LPS or not. Airway responsiveness of mechanically ventilated mice in response to aerosolized methacholine was measured 24 h after the last phosphate-buffered saline aerosol or OVA aerosol. AHR is expressed as percentage change from the baseline level of lung resistance (RI, n=6 mice). Data (mean ± SEM) are representative of three independent-experiments (n=6). *P<0.05, **P<0.01, vs. control group. #P<0.05, Eos vs. Eos + DEX. Con, control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated with dexamethasone; Neu + Dex, neutrophilic asthma treated with dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with dexamethasone; Dex, dexamethasone; OVA, ovalbumin; AHR, airway hyperresponsiveness.
Characteristics of cells in BALF in each model of asthma
Cell accumulation in the pulmonary airways is another hallmark feature of allergic asthma. Recent studies using sputum induction and/or BAL techniques to measure and characterise airways inflammation in asthmatic subjects have shown that potentially a substantial proportion of cases have an underlying pathology that is related to non-eosinophiic asthma (13). Analysis of BALF from mice in Eos showed that the influx of inflammatory cells was dominated by eosinophils (Fig. 3A, green arrow), which constituted ~50% of total BALF cells. In Neu, huge amounts of neutrophil, not eosinophils, was observed in BALF (Fig. 3A, red arrow), which constituted ~60% of total BALF cells. Increased percentagesof both eosinophils and neutrophils were shown in BALF in Mix (Fig. 3A), which constituted ~20 and ~50% of total BALF cells, repsectively. In addition, the total number of cells in the BALF in mice of three groups were all increased (Fig. 3B). These results showed that the cell characteristics in BALF in each model of asthma were consistent with that have obsevered in corresponding subtypes of asthma in clinic.
Figure 3.
Characteristics of cells in BALF of mice. (A) Wright-Giemsa staining shows the macrophage (black arrow), eosinophil (green arrow) and neutrophil (red arrow) under microscopy (magnification, ×400). (B) The statistical analysis of the numbers and proportions of the eosinophil, neutrophil, macrophage and lymphocyte in BALF of each model of asthma with or without dexamethasone treatment. Data (mean ± SEM) are representative of three independent-experiments (n=6). *P<0.05, **P<0.01, ***P<0.001, vs. control group. #P<0.05, ###P<0.01, vs. Eos. $P<0.05, $$$P<0.001, vs. Mix. Con, control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated with dexamethasone; Neu + Dex, neutrophilic asthma treated with dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with dexamethasone; Dex, dexamethasone; BALF, bronchoalveolar lavage fluid.
After the treatment of dexmathesone, 10-fold and 5-fold decrease in the proportion of eosinophils recovered in Eos + DEX and Mix + DEX were observed, respectively. However, the neutrophils was not decreased in Neu + DEX nor Mix + DEX, suggesting that dexamethasone has an inhibitory effect on eosinophils but not neutrophils (Fig. 3A and B).
Lung tissue inflammation and bronchial mucus secretionin each model of asthma
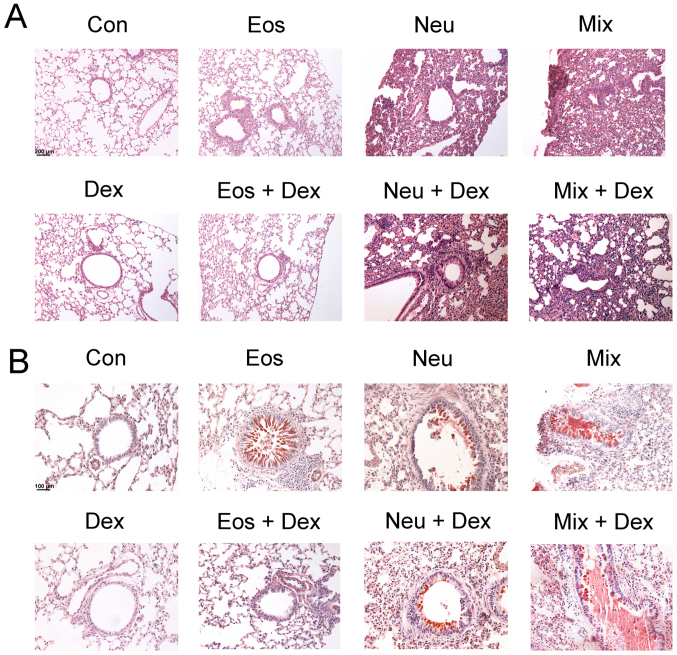
To examine the histological changes of lung tissues in each group of asthma model, H&E and PAS staining were performed to observe the inflammation and mucus secretion in the lung tissues. The inflammtory cells only infiltrated around the bronchi and vessels in Eos, which represent a moderate inflammation. The inflammatory cells infiltrated in nearly lungs in Neu and Mix, consistent with that asthma patients accompanied by neutrophilia have a more severe respiratory inflammation (Fig. 4A). On the other hand, OVA-challenged mice, but not saline-challenged mice, developed marked goblet cell hyperplasia and mucus hypersecretion within the bronchi in the lung (Fig. 4B).
Figure 4.
Degree of lung inflammation and mucus secretion in the bronchi of mice. The lung sections were stained with (A) H&E or (B) PAS and examined under microscopy (magnification, ×200). Con, control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated with dexamethasone; Neu + Dex, neutrophilic asthma treated with dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with dexamethasone; Dex, dexamethasone; H&E, hematoxylin and eosin; PAS, periodic acid-schiff.
The inhibitory effects of dexamethasone administration were evident in Eos + DEX, as lung tissue sections showed a reduction in inflammatory cells in airway and tissue mucus-secreting goblet cells in the airway epithelium. However, dexamethasone could not ameliorate the inflammation of lung tissues or bronchial mucus secretion in Neu + DEX and Mix + DEX (Fig. 4A and B).
Cytokine levels in BALF in each model of asthma
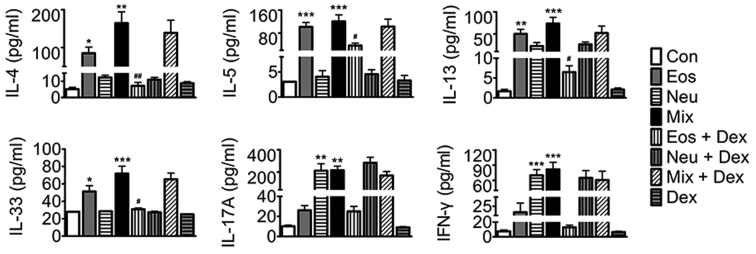
To probe the mechanisms of AHR and cell accumulation in lung tissues, we assayed key mediaors of disease in each asthma model. Eosinophilic asthma includes either allergic or nonallergic phenotypes underlying immune responses mediated by T helper (Th)2 cell-derived cytokines, whilst neutrophilic asthma is mostly dependent on Th1 and Th17 cell-induced mechanisms (14,15). In the present study, our results showed that elevated levels of Th2 cytokines (IL-4, IL-5, IL-13 and IL-33) were detected in BALF in Eos, and elevated levels of Th1 (IFN-γ) and Th17 (IL-17A) cytokines, but not Th2 cytokines in BALF were detected in Neu. Surprisingly, elevated levels of Th1, Th2 and Th17 cytokines were detected in BALF in Mix. Dexamethasone abated OVA-induced elevation of BALF Th2 cytokines in Eos + DEX, buthas no effect on Th1 and Th17 cytokine production in Neu nor Th1, Th2 and Th17 cytokines in Mix (Fig. 5).
Figure 5.
The levels of IL-4, IL-5, IL-13, IL-33, IFN-γ and IL-17A in BALF of mice. Data (mean ± SEM) are representative of three independent-experiments (n=6). *P<0.05, **P<0.01, ***P<0.001, vs. control group. #P<0.05, ##P<0.01, vs. Eos. Con, control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated with dexamethasone; Neu + Dex, neutrophilic asthma treated with dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with dexamethasone; Dex, dexamethasone; IL, interleukin; BALF, bronchoalveolar lavage fluid.
The levels of IgE and OVA-IgE in serum in each model of asthma
IgE is an important mediator of allergic reactions and has a central role in allergic asthma pathophysiology. Our results showed that IgE and OVA-IgE levels were elevated in the serum in all three models of asthma. Dexamethasone decreases the levels of IgE and OVA-IgE in Eos, whereas was ineffective in Neu or Mix, suggesting that dexamethasone exihibit a poor effect on asthma with neutrophilia (Fig. 6).
Figure 6.
The levels of IgE and OVA-IgE in serum of mice. Data (mean ± SEM) are representative of three independent-experiments (n=6). **P<0.01, ***P<0.001, vs. control group. ###P<0.01, vs. Eos. Con, control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated with dexamethasone; Neu + Dex, neutrophilic asthma treated with dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with dexamethasone; Dex, dexamethasone; IgE, immunoglobin E; OVA, ovalbumin.
Discussion
Asthma is now viewed as a heterogeneous inflammatory airways disorder which gives rise to several different clinical phenotypes. Eosinophilic inflammation is generally considered to be the main feature of asthmatic airways, however, recent reports suggest that many patients had sputum evidence of neutrophilic airway inflammation (16,17). Researchers have divided asthma into four subtypes according to the cell characteristics in the sputum: Eosinophilic, neutrophilic, paucigranulocytic or mixed cellularity (4). This classification, based on the underlying pattern of airway inflammation, has been demonstrated to be in agreement with the findings in bronchoalveolar lavage, airway biopsy and peripheral blood now (18,19). Moreover, sputum induction using nebulized hypertonic saline has been used as an alternative method to obtain lower airway lining fluid, with evidence of good repeatability and reproducibility.
Because of the ethical and moral issues preventing patients from mechanistic research, the development of animal models is of great significance to the research. Immunization with OVA is a classic approach to induce eosinophilic asthma (20). The features in Eos, including AHR to methacholine, inflammation of the airways (with infiltrates containing many eosinophils), airway remodeling (increases in mucus secretion of epithelial goblet cells), markedly increased lung expression of Th2 cytokines and serum expression of IgE and OVA-IgE, demonstrated that we have successfully established the eosinophilic asthma. Corticosteroid, which could effectively inhibit eosinophils activation and recruitment, and reduce their survival by inducing them apoptosis, is considered to be the most traditional approach for asthma (21,22). The results in the study showed that the dexamethasone, a drug belong to corticosteroid, inhibited hallmark features of eosinophilic asthma, including AHR, eosinophilic accumulation, airway remodeling, Th2 cytokine production and serum IgE synthesis.
There is increasing evidence that inflammatory mechanisms other than eosinophilic inflammation may be involved in producing the final common pathway of enhanced bronchial reactivity and reversible airflow obstruction that characterises asthma (23). It is supposed that a major proportion of asthma is based on neutrophilic airway inflammation, possibly triggered by environmental exposure to bacterial endotoxin, particulate air pollution, and ozone, as well as viral infections (24). As a powerful bacterial virulence factor, we hypothesize that LPS may participate in the pathogenesis of neutrophilic asthma. For this, we try to establish the mouse model of neutrophilic asthma using high dose of LPS (10 µg) in combination with OVA sensitization and chanllenge. Despite eosinophilic asthma is considered a Th2 cell-associated disorder, both the Th1-associated cytokine IFN-γ and Th17-associated cytokine IL-17 have been implicated in neutrophilic asthma. For example, transgenic mouse experiments clearly demonstrated that high levels of IFN-γ in airways induce neutrophilic lung inflammationand AHR (25). In addition, Th17 cytokines could recruit neutrophils to the airway by increasing secretion of epithelial-derived neutrophilic chemokines, and have pleotropic effects on airway smooth muscle resulting in airway narrowing (26). Asthma patients accomanied by neutrophilia frequently do not respond to corticosteroid, which attributed to that it decrease apoptosis in neutrophils and thus prolong their survival (27). Our results demonstrated that the asthma symptoms does not alleviated after the treatment of dexamethasone, and what is more, the numbers of neutrophilis increased. Together, all the results above demonstrate that we developed a model that exhibits several features of neutrophilic asthma in clinic, including airway neutrophilia, AHR, serum IgE and OVA-IgE synthesis, and what is more, resistent to the dexamethasone treatment.
Apart from neutrophilic asthma, some patients with severe disease is sustained by mixed patterns of inflammation including both eosinophils and neutrophils (28,29). In a recent study, Bafadhel et al reported that approximately 15% asthma patients who found in sputum with mixture of those two types of cells were resistant to corticosteroid (30). When establishing the mouse model of neutrophilic asthma, we found that the high dose of LPS have an inhibitory effect on eosinophilia. Thus, we try to establish the mouse model of mixed-granulocytic asthma using low dose of LPS (1 µg) in combination with OVA sensitization and chanllenge, which may partially suppress eosinophilia. The results showed that we successfully established the mixed-granulocytic asthma as demonstrated by AHR, inflammation of the airways (with infiltrates containing both eosinophils and neutrophils), airway remodeling (increases in mucus secretion of epithelial goblet cells) and serum IgE synthesis and, moreover, the infiltration of eosinophils and neutrophils as well as AHR has nochange after dexamethasone treatment. Interestingly, we found that the levels of Th1, Th2 and Th17 cytokines in BALF all elevated in this model. The explanation for this is not clear. Although many reports have demonstrated that both Th1 and Th17 cells are crucial for the development of neutrophilic inflammation in the airways, an increasing evidence in both humans and animal models suggest that a mixed Th2/Th17 response drives the development of more severe AHR (31,32). In addition, in an earlier study, Hansen et al found that in their mouse models, Th1 cells have been shown to function in a cooperative manner with Th2 cells to mediate severe airway inflammatory responses (33). Since there is no study on the mechanism of mixed-granulocytic asthma, our results may give a new hint about the pathogenesis of this heterogeneous disease.
The evidence of epidemiology and clinic points to the fact that asthma has several distinct subgroups need to be treated differently. Since treatment and prevention strategies now are almost entirely focused on eosinophilic asthma, an improved asthma management which rely on clinically sustainable classification of disease phenotypes seems to be important (34,35). In this study, we developed three mouse models of allergic inflammation of the airways that exhibits several features of chronic asthma in clinic. We have checked the repeatability of these developed different experimental asthma mouse models, and the data are representative of three independent-experiments. These mouse models might therefore allow studying pathophysiological processes occurring in the subgroup of persistent asthmatics with non-eosinophilic asthma responding to inhaled steroids. Although we are aware that murine models cannot reflect all features of a complex disorder such as asthma, we believe that mimicking different types of inflammation and assessing the relationship to the development of AHR will allow to further dissect different asthma phenotypes.
Acknowledgements
The study was supported with funds from the Major State Basic Research Development Program of China (973 Program) (no. 2013CB530505).
References
- 1.Baarnes CB, Hansen AV, Ulrik CS. Enrolment in an asthma management program during pregnancy and adherence with inhaled corticosteroids: The ‘Management of Asthma during Pregnancy’ Program. Respiration. 2016;92:9–15. doi: 10.1159/000447244. [DOI] [PubMed] [Google Scholar]
- 2.Uhm TG, Kim BS, Chung IY. Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2012;4:68–79. doi: 10.4168/aair.2012.4.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson NC. Novel approaches to the management of noneosinophilic asthma. Ther Adv Respir Dis. 2016;10:211–234. doi: 10.1177/1753465816632638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 5.Schleich FN, Manise M, Sele J, Henket M, Seidel L, Louis R. Distribution of sputum cellular phenotype in a large asthma cohort: Predicting factors for eosinophilic vs. neutrophilic inflammation. BMC Pulm Med. 2013;13:11. doi: 10.1186/1471-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MY, Seo CS, Lee NH, Ha H, Lee JA, Lee H, Lee KY, Shin HK. Anti-asthmatic effect of schizandrin on OVA-induced airway inflammation in a murine asthma model. Int Immunopharmacol. 2010;10:1374–1379. doi: 10.1016/j.intimp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: Importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol. 2005;115:213–219; quiz 220. doi: 10.1016/j.jaci.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Chang HS, Lee TH, Jun JA, Baek AR, Park JS, Koo SM, Kim YK, Lee HS, Park CS. Neutrophilic inflammation in asthma: Mechanisms and therapeutic considerations. Expert Rev Respir Med. 2017;11:29–40. doi: 10.1080/17476348.2017.1268919. [DOI] [PubMed] [Google Scholar]
- 10.Kim KH, Jahan SA, Kabir E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ Int. 2013;59:41–52. doi: 10.1016/j.envint.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang HY, Mitzner W, Watson J. Variation in airway responsiveness of male C57BL/6 mice from 5 vendors. J Am Assoc Lab Anim Sci. 2012;51:401–406. [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman CM, Crudgington S, Stolberg VR, Brown JP, Sonstein J, Alexis NE, Doerschuk CM, Basta PV, Carretta EE, Couper DJ, et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) J Transl Med. 2015;13:19. doi: 10.1186/s12967-014-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima H, Hirose K. Role of IL-23 and Th17 cells in airway inflammation in asthma. Immune Netw. 2010;10:1–4. doi: 10.4110/in.2010.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, Maselli R. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015:879783. doi: 10.1155/2015/879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parameswaran K, Pizzichini E, Pizzichini MM, Hussack P, Efthimiadis A, Hargreave FE. Clinical judgement of airway inflammation versus sputum cell counts in patients with asthma. Eur Respir J. 2000;15:486–490. doi: 10.1034/j.1399-3003.2000.15.10.x. [DOI] [PubMed] [Google Scholar]
- 17.Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172:559–565. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 18.Gasiuniene E, Lavinskiene S, Sakalauskas R, Sitkauskiene B. Levels of IL-32 in serum, induced sputum supernatant, and bronchial lavage fluid of patients with chronic obstructive pulmonary disease. COPD. 2016;13:569–575. doi: 10.3109/15412555.2016.1145201. [DOI] [PubMed] [Google Scholar]
- 19.Emmanouil P, Loukides S, Kostikas K, Papatheodorou G, Papaporfyriou A, Hillas G, Vamvakaris I, Triggidou R, Katafigiotis P, Kokkini A, et al. Sputum and BAL Clara cell secretory protein and surfactant protein D levels in asthma. Allergy. 2015;70:711–714. doi: 10.1111/all.12603. [DOI] [PubMed] [Google Scholar]
- 20.Kerzel S, Wagner J, Rogosch T, Yildirim AO, Sikula L, Fehrenbach H, Garn H, Maier RF, Schroeder HW, Jr, Zemlin M. Composition of the immunoglobulin classic antigen-binding site regulates allergic airway inflammation in a murine model of experimental asthma. Clin Exp Allergy. 2009;39:591–601. doi: 10.1111/j.1365-2222.2008.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson NC, Spears M. Inhaled corticosteroids for asthma: On-demand or continuous use. Expert Rev Respir Med. 2013;7:687–699. doi: 10.1586/17476348.2013.836062. [DOI] [PubMed] [Google Scholar]
- 22.Raissy HH, Kelly HW, Harkins M, Szefler SJ. Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med. 2013;187:798–803. doi: 10.1164/rccm.201210-1853PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, Hodge S, AMAZES Study Research Group Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy. 2013;43:29–35. doi: 10.1111/j.1365-2222.2012.04075.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang SK, Zhang Q, Qiu Z, Chung KF. Mechanistic impact of outdoor air pollution on asthma and allergic diseases. J Thorac Dis. 2015;7:23–33. doi: 10.3978/j.issn.2072-1439.2014.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosca MA, Silvestri M, Morandi F, Prigione I, Pistorio A, Ciprandi G, Rossi GA. Impairment of lung function might be related to IL-10 and IFN-γ defective production in allergic children. Immunol Lett. 2011;140:104–106. doi: 10.1016/j.imlet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb DC, Peebles RS., Jr Th17-mediated inflammation in asthma. Curr Opin Immunol. 2013;25:755–760. doi: 10.1016/j.coi.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa T, Sakagami T, Koya T, Hasegawa T, Kawakami H, Kimura Y, Hoshino Y, Sakamoto H, Shima K, Tsukioka K, et al. Characteristics of eosinophilic and non-eosinophilic asthma during treatment with inhaled corticosteroids. J Asthma. 2015;52:417–422. doi: 10.3109/02770903.2014.975357. [DOI] [PubMed] [Google Scholar]
- 28.Manise M, Bakayoko B, Schleich F, Corhay JL, Louis R. IgE mediated sensitisation to aeroallergens in an asthmatic cohort: Relationship with inflammatory phenotypes and disease severity. Int J Clin Pract. 2016;70:596–605. doi: 10.1111/ijcp.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao P, Gibson PG, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, et al. Anti-inflammatory deficiencies in neutrophilic asthma: Reduced galectin-3 and IL-1RA/IL-1β. Respir Res. 2015;16:5. doi: 10.1186/s12931-014-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hering T. Update of the GINA-recommendations. MMW Fortschr Med. 2017;159:63–64. doi: 10.1007/s15006-017-9710-6. (In German) [DOI] [PubMed] [Google Scholar]
- 35.Isidoro-García M, Sánchez-Martín A, García-Sánchez A, Sanz C, García-Berrocal B, Dávila I. Pharmacogenetics and the treatment of asthma. Pharmacogenomics. 2017;18:1271–1280. doi: 10.2217/pgs-2017-0024. [DOI] [PubMed] [Google Scholar]