Abstract
The present study was designed to evaluate the effect of one rare sugar, D-allose, on normal human cells and cutaneous tissue, and to investigate the radiosensitizing and chemosensitizing potential of D-allose in an in vivo model of head and neck cancer. Results indicated that D-allose did not inhibit the growth of normal human fibroblasts TIG-1 cells, and no apoptotic changes were observed after D-allose and D-glucose treatment. The mRNA expression levels of thioredoxin interacting protein (TXNIP) in TIG-1 cells after D-allose treatment increased by 2-fold (50.4 to 106.5). Conversely, the mRNA expression levels of TXNIP in HSC3 cancer cells increased by 74-fold (1.5 to 110.6), and the thioredoxin (TRX)/TXNIP ratio was markedly reduced from 61.7 to 1.4 following D-allose treatment. Combined multiple treatments with docetaxel, radiation and D-allose resulted in the greatest antitumor response in the in vivo model. Hyperkeratosis, epidermal thickening and tumor necrosis factor-α immunostaining were observed following irradiation treatment, but these pathophysiological reactions were reduced following D-allose administration. Thus, the present findings suggest that D-allose may enhance the antitumor effects of chemoradiotherapy whilst sparing normal tissues.
Keywords: D-allose, docetaxel, radiation, head and neck cancer, thioredoxin interacting protein
Introduction
Patients with loco-regionally advanced head and neck squamous cell carcinoma (HNSCC) are usually treated with surgery and postoperative adjuvant radiotherapy. However, the prognosis for loco-regionally advanced HNSCC patients remains poor. To improve loco-regional control and survival, various chemotherapy drugs that have shown antitumor activity in HNSCC as single agents have also been tested in combination (1–6). Docetaxel, an analog of taxane, is an inhibitor of microtubule depolymerisation that causes cell cycle arrest at the G2/M transition. As a single agent, docetaxel shows significant antitumor activity in HNSCC when used as a neoadjuvant therapy. It also exhibits a potent radiosensitizing effect (7), and has therefore been used as induction chemotherapy or concomitantly with radiotherapy.
In order to reduce the general toxicities associated with this treatment, weekly docetaxel and concomitant radiotherapy were tried as an alternative (8,9). Even low-dose docetaxel showed a strong antitumor effect in combination with radiation, with a high survival rate amongst patients who showed a complete response (8,10), although there were grade 3 or 4 adverse events consisting of stomatitis, dermatitis, and anorexia.
Thioredoxin (TRX), a small redox-active multifunctional protein, acts as a potent antioxidant and redox regulator in signal transduction (11). TRX expression is elevated in various types of cancer (12–14), and its over expression is associated with a poor prognosis (15,16). TRX is negatively regulated by thioredoxin interacting protein (TXNIP) (17), and reduced levels of active TRX lead to an accumulation of reactive oxygen species (18). TXNIP mediates the inhibition of cell proliferation and the induction of apoptosis through activation of apoptosis signal regulating kinase 1 (17). TXNIP has also been reported to act as a transcriptional repressor (19). These findings suggest that TXNIP could be a tumor suppressor gene, and furthermore, that the regulation of the redox state might be an important strategy in cancer treatment.
D-allose is a rare sugar that is found at only very low levels in nature. A number of studies have recently characterized the biological functions of D-allose, and we recently showed that it induces TXNIP expression and suppresses the growth of several types of cancer cells (20,21) by increasing the level of intracellular reactive oxygen species (ROS) and radiation induced apoptosis (22).
In this study, we investigated the effect of D-allose on normal human fibroblasts in order to establish its safety. The combined effect of D-allose and low-dose docetaxel plus radiation was also investigated using a mouse model of human head and neck cancer.
Materials and methods
Cell culture
The human head and neck carcinoma cell line HSC3 (tongue carcinoma) was obtained from the Health Science Research Resources Bank, Osaka, Japan. HSC3 cells were cultured in Eagle's minimal essential medium (EMEM). Medium contained 10% heat-inactivated fetal bovine calf serum and 1% penicillin-streptomycin. The human fibroblast cell line TIG-1 was kindly supplied by the Laboratory of Physiological Chemistry, Faculty of Pharmaceutical Sciences at Kagawa, Tokushima Bunri University, Sanuki, Japan. TIG-1 cells were cultured in DMEM with 10% FBS. Cells were incubated in a humidified 5% CO2 atmosphere at 37°C.
Determination of TIG-1 cell survival
D-allose was supplied by the Department of Biochemistry and Food Science, Faculty of Agriculture, Kagawa University, Kagawa, Japan. Docetaxel was obtained from Sanofi S.A. (Paris, France) and stored in frozen aliquots. Before use, it was thawed and diluted to the desired concentrations in the cell culture medium or normal saline. The growth inhibitory effect of D-allose was compared with that of D-glucose or medium only. TIG-1 cells were seeded in 96-well plates at 1.0×103 cells/100 µl and cultured for 24 h. The medium was then removed, and fresh medium containing D-allose or D-glucose was added. The cells that were seeded in 5 separate wells in each group were incubated for an additional 24–72 h. To investigate the effects of radiation, cells were treated with 25 mM D-allose or D-glucose 6 h before irradiation with X-rays (0, 4, 8 Gy), and then incubated for a further 72 h. The cells were irradiated with a dose of 0.59 Gy/min using an X-ray irradiator (HITEX type HW 260, 200 kV, mA, Osaka, Japan). The net number of viable cells was then determined using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. The absorbance was measured by a microplate reader at 450 nm after 2 h incubation. Values are the mean of 3 independent experiments.
Measurement of apoptosis
A terminal deoxynucleotidyl transferase d-UTP nick-end labelling (TUNEL) assay was performed using the Apoptosis Detection System Fluorescein kit (Promega Corporation, Madison, WI, USA). Briefly, treated TIG-1 cells were spread on a poly-L-lysine slide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Cells were incubated in 50 µl of TdT incubation buffer (nucleotide mix [fluorescein-12-dUTP] and TdT enzyme prepared according to the manufacturer's protocol) for 60 min at 37°C in a humidified chamber. The reaction was terminated by washing the cells in 2X saline sodium citrate buffer followed by 2 washes in PBS. Cells were counterstained with 1 µg/ml propidium iodide and then washed in distilled water. Staining was observed under a fluorescence microscope. Green fluorescence indicated DNA fragmentation due to fluorescein-12-dUTP labeling.
Analysis of mRNA expression
To investigate the effects of D-allose on the expression of TXNIP and TRX, cells were cultured in 6 cm dishes with 25 mM D-allose for 24 h. To investigate the effects of radiation on the expression of TXNIP and TRX, D-allose treated or untreated cells were incubated at 37°C for 6 h, and then exposed to a single 8 Gy X-ray dose. The cells were then incubated for a further 24 h. Quantitative polymerase chain reaction (qPCR) was carried out using TaqMan gene expression assay primers and the ABI7700 Real-Time PCR system. Each reaction was performed in duplicate. The GAPDH gene was used to normalize expression across assays and runs, and a quantification value (Cq) for each sample was used to determine the expression level of the gene.
In vivo xenograft experiment
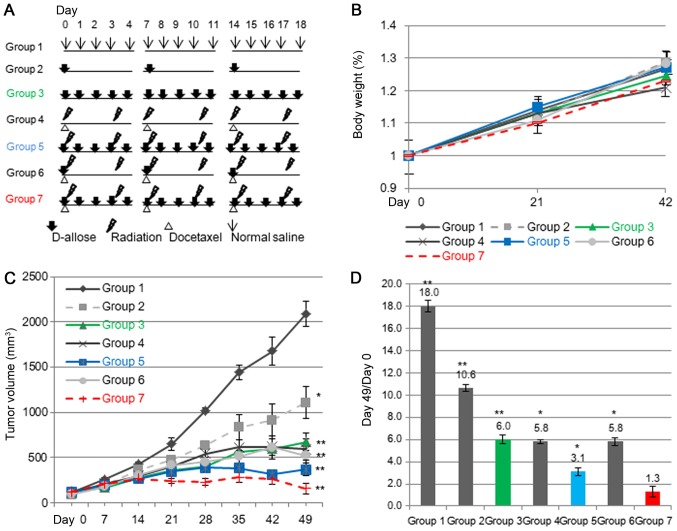
HSC3 cells were used in a xenograft model with female athymic nude mice (BALB/c nu/nu, 5–6 weeks old). A suspension of 1×106 cells in 0.1 ml EMEM was injected subcutaneously into both sides of the posterior flank using a 1-cc syringe with a 27G needle. Tumors were grown for 10 days until attaining an average size of 100 mm3 (day 0). A total of 42 nude mice were assigned to 7 treatment groups (including the control group), each consisting of 6 mice. The control group mice were injected with 0.1 ml normal saline at the same time points (group 1). For the two different D-allose treatment groups, 0.1 ml of 25 mM D-allose was injected into the tumor region once a week (group 2) or 5 times a week (group 3). For the low-dose weekly docetaxel and radiation treatment group which is established as clinical model, 3 mg/kg docetaxel (20% of the maximum tolerable dose) was injected intraperitoneally, and the mice were also irradiated on days 1 and 4 (Group 4). For the combined D-allose and radiation treatment, D-allose (with the same dosing regimen as Group 3) plus radiation with a 4 Gy dose on days 1 and 4 (Group 5). The docetaxel, radiation, and D-allose group was treated with the same regimen (group 5) and 0.1 ml of 25 mM D-allose was injected into tumor tissue on day 1 (group 6) or 5 times a week (group 7). These treatments were repeated for 3 weeks. This study was approved by the Animal Care and Use Committees of Kagawa University.
Immunohistochemical staining
For the histological studies, one mouse in each treatment group was euthanized 3 weeks after the initiation of treatment. The posterior flank skin specimens were fixed in phosphate-buffered paraformaldehyde (4%), embedded in paraffin, and cut into 4 µm thick sections. The immunohistochemistry was performed using the Vectastain ABC rabbit IgG kit (Vector Laboratories, Inc., Burlingame, CA, USA) following the manufacturer's instructions. The following primary antibodies were used: anti-tumor necrosis factor (TNF)-α (NBP1-19532) polyclonal (Novus Biologicals, LLC, Littleton, CO, USA), anti-TXNIP rabbit polyclonal (Sigma-Aldrich; Merck KGaA), and anti-TRX (C63C6) rabbit monoclonal (Cell Signaling Technology, Inc., Danvers, MA, USA).
Intensity of staining were divided into four groups-no staining, weak staining, moderate staining and strong staining. One pathologist evaluated all pathological sections without the information of experimental design.
Western blot analysis
Protein was extracted from untreated normal skin, normal skin treated with 25 mM D-allose for 2 weeks, untreated tumor tissue, and tumor tissue treated with 25 mM D-allose for 2 or 3 weeks. For the Western blot analyses, proteins were separated on 10% SDS-PAGE gels, transferred to nitrocellulose membranes, blocked with 5% (w/v) non-fat dried milk in PBS, and incubated with anti-TXNIP (MBL, Nagoya, Japan), anti-TRX (MBL), and anti-GAPDH (14c10) antibodies (Cell Signaling Technology, Inc., Tokyo, Japan). Membranes were probed with a horseradish peroxidase-conjugated anti-mouse IgG (Amersham, Tokyo, Japan), and signals were detected using an enhanced chemiluminescence system (Amersham).
Statistical analysis
Comparisons between groups for cell growth assay and mRNA expressions were compared using the Kruskal-Wallis test. Post-hoc test was carried out using the Tukey's test. Pretreatment mRNA expressions between TIG cell and HSC3 cell were compared using the Student's t-test. Significant difference between in vivo experimental groups was estimated using the Kruskal-Wallis test. Post-hoc test was carried out using the Mann-Whitney U test with Bonferrioni's correction. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of D-allose on the proliferation of TIG-1 cells
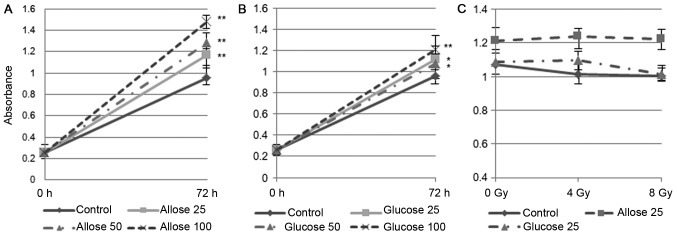
Compared to untreated cells, the growth of TIG-1 cells exposed to 25 mM D-allose or D-glucose increased significantly, by 116.8% (P<0.001) and 112.1% (P<0.01), respectively. The growth promoting effect of D-allose was dose dependent (Fig. 1A and B). No significant reduction in cell number was observed following irradiation with 4 Gy (94.6%, P=0.2) or 8 Gy (93.9%, P=0.2) in the control cells. D-glucose treated cells were also unaffected by 4 Gy irradiation (101%, P=0.84), although their growth was marginally suppressed after an 8 Gy irradiation (93%, P=0.051). D-allose treated cells were unaffected by either of the radiation doses (4 Gy: 102%, P=0.62; 8 Gy: 101%, P=0.784) (Fig. 1C).
Figure 1.
CCK-8 assay to analyze the effects of D-allose, D-glucose, and radiation on TIG-1 cells. Varying doses (25, 50 and 100 mM) of (A) D-allose and (B) D-glucose were added and cells were incubated for 72 h. (C) Cells were incubated with 25 mM D-allose or D-glucose for 6 h, irradiated, and incubated for 72 h (*P<0.01, **P<0.001). CCK-8, Cell Counting Kit-8.
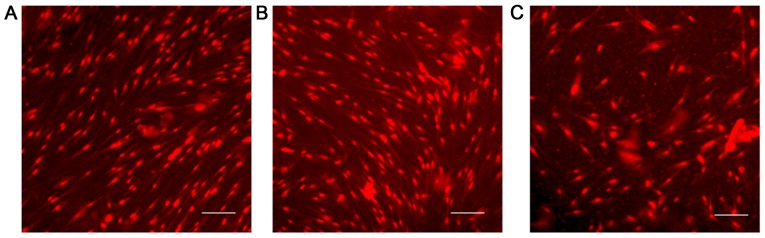
The TUNEL assay was carried out on the TIG-1 cell line exposed to each sugar at 25 mM for 48 h, but no apoptotic changes were observed (Fig. 2).
Figure 2.
Induction of apoptosis by D-allose and D-glucose treatment on TIG-1 cells. A TUNEL assay after treatment with each sugar for 72 h revealed no apoptosis. (A) Untreated cells; (B) 25 mM D-allose; (C) 25 mM D-glucose (scale bar: 30 µm).
D-allose alters TRX and TXNIP mRNA expression
To assess the effect of radiation on normal cells, 8 Gy was selected as the irradiation dose in this study. The mRNA expression of TRX and TXNIP is summarized in Table I. In untreated TIG-1 cells, the ratio of TRX and TXNIP (TRX/TXNIP) was 6.4. The mRNA expression of TXNIP after D-allose treatment increased approximately 2-fold, and as a result, TRX/TXNIP decreased to 2.2. No apparent changes were observed in either TXNIP or TRX mRNA expression after 8 Gy irradiation (ratio to control: 0.97 and 0.92, respectively), and the TRX/TXNIP ratio was only slightly lower (6.1). The effect of D-allose plus radiation was the same as that of D-allose treatment alone. Compared with TIG-1 cells, the mRNA expression level of TXNIP in HSC3 cells was relatively low (50.4 vs. 1.5) and TRX/TXNIP was very high (61.7). The mRNA expression of TXNIP after D-allose treatment had increased about 74-fold and TRX/TXNIP dramatically reduced to 1.4. The change of TXNIP mRNA expression after radiation treatment was no greater than 2.6-fold and TRX/TXNIP was 25.4. Combined D-allose and radiation treatment enhanced TXNIP mRNA expression (ratio to control: 135.6), and TRX/TXNIP was reduced to 1.1.
Table I.
Change of mRNA expression after the D-allose and radiation treatment.
| Cell line | Treatment | TXNIP | Ratio to control | TRX | Ratio to control | TRX/TXNIP |
|---|---|---|---|---|---|---|
| TIG-1 | Control | 50.4 | 323.2 | 6.4 | ||
| D-allose 25 mM | 106.5 | 2.1 | 237.5 | 0.74 | 2.2 | |
| 8 Gy irradiation | 48.7 | 0.97 | 295.9 | 0.92 | 6.1 | |
| D-allose 25 mM+8 Gy | 95.3 | 1.9 | 232.8 | 0.72 | 2.4 | |
| HSC3 | Control | 1.5 | 92.5 | 61.7 | ||
| D-allose 25 mM | 110.6 | 73.8 | 153.5 | 1.7 | 1.4 | |
| 8 Gy irradiation | 3.9 | 2.6 | 98.7 | 1.1 | 25.4 | |
| D-allose 25 mM+8 Gy | 203.3 | 135.6 | 220.7 | 2.4 | 1.1 |
TXNIP, thioredoxin interacting protein; TRX, thioredoxin.
D-allose inhibits tumor growth and enhances the efficacy of docetaxel and radiation in a mouse model of HNSCC
In order to determine the appropriate dose for tumor treatment, several different doses were tested, and we found that 25 mM D-allose had the same antitumor effect as 50 mM or even higher D-allose concentrations (data not shown).
We then examined the growth inhibitory effect of D-allose with or without radiation or docetaxel in this model. The treatment schedules are shown in Fig. 3A. Although docetaxel plus radiation treated mice on average suffered a ~5% decrease in body weight compared with normal saline treated mice, the difference in weight was not statistically significant (Fig. 3B). Overall, drug treatment was well tolerated, with no apparent toxicity, and organ macroscopic examinations were normal at sacrifice. The tumor growth curves are shown in Fig. 3C. The mean tumor volumes in all of the treated groups were significantly lower than that of the control group at day 49 (P<0.0005). The greatest tumor inhibition was observed in group 7 and then group 5, with weaker inhibition in groups 3, 4 and 6. A moderate inhibition was achieved in group 2. The mean tumor volume in the group treated with multiple-doses of D-allose, weekly-docetaxel, and radiation (group 7) was significantly lower than in mice treated with weekly-docetaxel plus radiation (group 4) (P<0.001). The changes in tumor volume ratios are shown in Fig. 3D. The tumor volume had increased 18-fold in the saline treated group (group 1) at day 49, but only 10.6- and 6-fold in the mice treated with D-allose once or 5 times a week (group 2 and 3, respectively). The treatment effect in group 3 was the same as that achieved with docetaxel plus radiation (group 4, 5.8-fold). Treatment with D-allose 5 times a week and radiation (group 5) reduced the tumor volume significantly (3.1-fold). Compared with group 4, additional D-allose treatment once a week did not enhance the tumor inhibitory effect at day 49 (group 6: 5.8-fold). However, the growth inhibitory effect in group 6 persisted 11 weeks after the initiation of treatment, while the tumors in the group 4 and group 5 mice had re-grown. Half of the tumors disappeared in the group treated with multiple-doses of D-allose, weekly low-dose docetaxel, and radiation (group 7) (Fig. 4).
Figure 3.
Treatment scheme and effect of D-allose treatment in a xenofraft mouse model. (A) Treatment schedule; athymic nude mice were randomized to 7 different treatment groups. (B) Mice were weighed 6 weeks after the initiation of treatment. Effect of D-allose on tumor growth in vivo. (C) Tumor growth curves for the different treatment groups. Points, mean tumor volume; error bars, SE Statistically significant differences are indicated by asterisks: *P<0.01 vs. saline-treated mice. (D) The ratio of tumor volumes measured at day 0 and day 49. *P<0.05 and **P<0.01 vs. Group 1.
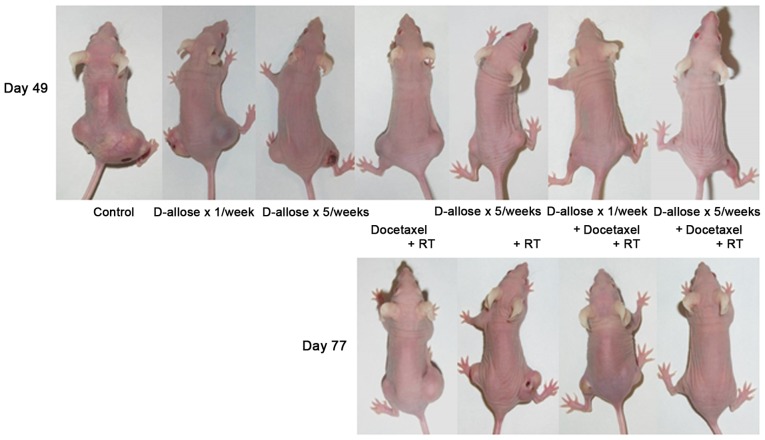
Figure 4.
The changes of tumor size after the treatment. Representative images 7 and 11 weeks after initial treatment. Due to tumor rupture, volume measurements could not be made for untreated and D-allose only-treated mice during the 11th week.
Radiation-induced skin inflammation
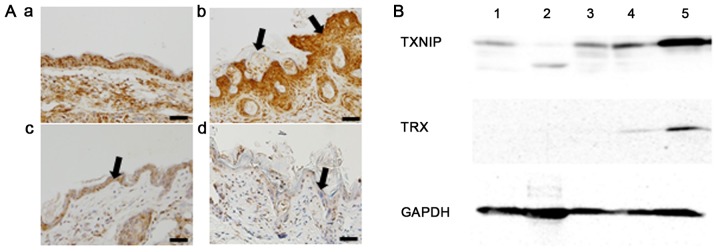
Histopathological findings of normal skin are shown in Fig. 5Aa. Weak to moderate increased TNF-α expression was observed in untreated epithelium. Radiation exposure resulted in an increase in epidermal thickening and hyperkeratosis (Fig. 5Ab). Strong increased TNF-α expression was also observed in the irradiated epithelium. Weak increased TNF-α expression was found in the D-allose treated epithelium (Fig. 5Ac). D-allose treatment suppressed TNF-α expression and epidermal thickening, whilst, hyperkeratosis followed the combined use of D-allose and radiation treatment (Fig. 5Ad).
Figure 5.
TNF-α, TXNIP and TRX expression after the treatment. Histological features of the skin region. Three weeks after the initial treatment, skin specimens were obtained and fixed with 4% PFA. To observe inflammatory changes, TNF-α antibody was used. (Aa) Normal, saline-treated skin was included as a control (bar, 50 µm). (Ab) Hyperkeratosis and epidermal thickening (arrows) were observed after radiotherapy, with strong TNF-α staining. (Ac) Weak TNF-α staining was observed with D-allose treatment (arrow). (Ad) Radiation-induced epidermal thickening and TNF-α staining were reduced with additional D-allose treatment (arrow). (B) Western blot analysis of TXNIP and TRX expression. Proteins were obtained from: 1, normal skin with saline treatment; 2, normal skin with D-allose treatment for 2 weeks; 3, tumor tissue with saline treatment; 4, tumor tissue with D-allose treatment for 2 weeks; and 5, tumor tissue with D-allose treatment for 3 weeks. TXNIP, thioredoxin interacting protein; TRX, thioredoxin; TNF-α, tumor necrosis factor-α.
Additive effect of D-allose
Western blot analyses revealed that no apparent change was observed about the expression of TXNIP in normal skin by D-allose treatment. The expression of TXNIP in transplanted tumor tissue after 3 weeks of D-allose treatment was markedly increased in comparison to tumors treated with D-allose for only 2 weeks (Fig. 5B). TRX expression increased slightly after 3 weeks of D-allose treatment.
Discussion
Oxidant stress induced by irradiation or anticancer drugs produces a variety of highly reactive free radicals that damage cells, initiate signal transduction pathways, and alter gene expression. Therefore, regulation of the redox state is one of the key mechanisms that can be used to control cancer cell growth.
In the present study, the mRNA expression of TXNIP in HSC3 cancer cells increased about 74 times (1.5 to 110.6), and the TRX/TXNIP ratio was reduced from 61.7 to 1.4 after D-allose treatment. We previously reported that induction of TXNIP by D-allose can enhance the radiation effects by increasing the intracellular ROS level and radiation-induced apoptosis (22). Combined use of D-allose and docetaxel also enhanced antitumor effect following upregulation of TXNIP expression and control of the intracellular ROS level (23).
In addition, D-allose inhibited the growth of head and neck cancer cells by inducing of cell cycle arrest, apoptosis and competition with glucose uptake (24). On the other hand, TXNIP expression in normal cells (TIG-1) was high (50.4) and the TRX/TXNIP ratio was much lower than in HSC3 cancer cells (6.4 vs. 61.7).
The in vivo experiment revealed that the tumor inhibitory effect of D-allose was greater when it was administered 5 times a week rather than once a week. Western blot analysis also showed that the expression of TXNIP was greater after D-allose treatment for 3 weeks compared to only 2 weeks. These results suggest that the tumor inhibitory effect of D-allose depends on the frequency or period of administration rather than just the total dose. D-allose combined with weekly-docetaxel and radiation markedly suppressed tumor growth, and 5 of the 10 transplanted tumors disappeared when treated with additional, multiple doses of D-allose together with docetaxel and radiation. None of the remaining 5 residual tumors showed any sign of re-growth in the observation period. Furthermore, D-allose had no growth inhibitory effect on human fibroblast TIG-1 cells, although the mRNA expression of TXNIP was slightly increased following D-allose administration. There was also no apoptotic change in these cells after D-allose treatment. TIG-1 cell line was established from human embryonic lung fibroblast and widely used as a standard normal cell with limited life span (25). These findings suggest that D-allose may not induce the local damage to normal tissue. In the mouse model, D-allose treatment seemed to suppress radiation toxicities such as epidermal thickening and inflammation. TNF-α is one of the important mediators of inflammation, a key event in the cutaneous radiation reaction (26). Radiation induced TNF-α expression was reduced with combined use of D-allose. Taken together, these findings suggest that D-allose might specifically radiosensitize cancer cells and thus could potentially reduce treatment-related toxicity in the clinical setting.
Several agents have been shown to act as chemosensitizers and radiosensitizers, including nimorazole (27), flavoperidol (28), and curcumin (29). Although each agent showed efficacy in preclinical tests, this has not been supported by the findings of clinical trials. Suberoylanilide hydroxamic acid, which is a strong histone deacetylase inhibitor (HDACi), arrests cancer cell growth by up-regulating TXNIP and down-regulating TRX expression (30). The modulation of DNA damage signaling and repair by HDACi may be one underlying mechanism by which they radiosensitize cancer cells (31–33). Several clinical trials have been carried out using combined HDACi and radiation (34–36). Although HDACi was more effective as a single agent in hematological malignancies rather than in solid tumors, its ability to radiosensitize cells remains unclear.
Generally, head and neck cancers are present within the field of vision and are palpable. Therefore, local injection might be a more effective route than oral intake or intravenous injection. However, other delivery routes or systems are needed to deliver D-allose to other tumor types. In addition, further evaluation is needed of D-allose combined with other anticancer drugs.
In conclusion, our findings show that D-allose acts as an enhancer of radiotherapy and chemotherapy and may reduce the severity of radiation-induced reactions such as dermatitis and mucositis. These preclinical studies justify clinical trials to further evaluate the potential of D-allose for the treatment of HNSCC.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Project to accelerate development of Rare Sugar Research in 2017, Kagawa Prefectural Government, Japan.
References
- 1.Pignon JP, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet. 2000;355:949–955. doi: 10.1016/S0140-6736(00)90011-4. [DOI] [PubMed] [Google Scholar]
- 2.Bourhis J, Le Maître A, Baujat B, Audry H, Pignon J, Meta-Analysis of Chemotherapy in Head, Neck Cancer Collaborative Group. Meta-Analysis of Radiotherapy in Carcinoma of Head, Neck Collaborative Group. Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma Collaborative Group Individual patients' data meta-analyses in head and neck cancer. Curr Opin Oncol. 2007;19:188–194. doi: 10.1097/CCO.0b013e3280f01010. [DOI] [PubMed] [Google Scholar]
- 3.Browman GP, Hodson DI, Mackenzie RJ, Bestic N, Zuraw L, Cancer Care Ontario Practice Guideline Initiative Head and Neck Cancer Disease Site Group Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: A systematic review of the published literature with subgroup analysis. Head Neck. 2001;23:579–589. doi: 10.1002/hed.1081. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2209. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 5.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Calais G. Final results of the 94-01 french head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Wendt TG, Grabenbauer GG, Rödel CM, Thiel HJ, Aydin H, Rohloff R, Wustrow TP, Iro H, Popella C, Schalhorn A. Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: A randomized multicenter study. J Clin Oncol. 1998;16:1318–1324. doi: 10.1200/JCO.1998.16.4.1318. [DOI] [PubMed] [Google Scholar]
- 7.Nabell L, Spencer S. Docetaxel with concurrent radiotherapy in head and neck cancer. Semin Oncol. 2003;30(6 Suppl 18):S89–S93. doi: 10.1053/j.seminoncol.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Fujii M, Tsukuda M, Satake B, Kubota A, Kida A, Kohno N, Okami K, Inuyama Y, Japan Cooperative Head and Neck Oncology Group (JCHNOG) Phase I/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol. 2004;9:107–112. doi: 10.1007/s10147-003-0375-z. [DOI] [PubMed] [Google Scholar]
- 9.Furusaka T, Matsuda A, Saito T, Katsura Y, Ikeda M. Concurrent chemoradiation therapy with docetaxel (DOC) for laryngeal preservation in T2N0M0 glottic squamous cell carcinomas. Acta Otolaryngol. 2013;133:99–112. doi: 10.3109/00016489.2012.744144. [DOI] [PubMed] [Google Scholar]
- 10.Calais G, Bardet E, Sire C, Alfonsi M, Bourhis J, Rhein B, Tortochaux J, Man YT, Auvray H, Garaud P. Radiotherapy with concomitant weekly docetaxel for stages III/IV oropharynx carcinoma. Results of the 98-02 GORTEC phase II trial. Int J Radiat Oncol Biol Phys. 2004;58:161–166. doi: 10.1016/S0360-3016(03)01370-1. [DOI] [PubMed] [Google Scholar]
- 11.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki K, Noda N, Okada S, Hagiwara Y, Miyata M, Sakurabayashi I, Yamaguchi N, Sugimura T, Terada M, Wakasugi H. Elevated serum level of thioredoxin in patients with hepatocellular carcinoma. Biotherapy. 1998;11:277–288. doi: 10.1023/A:1008032703468. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura H, Bai J, Nishinaka Y, Ueda S, Sasada T, Ohshio G, Imamura M, Takabayashi A, Yamaoka Y, Yodoi J. Expression of thioredoxin and glutaredoxin, redox-regulating proteins, in pancreatic cancer. Cancer Detect Prev. 2000;24:53–60. [PubMed] [Google Scholar]
- 14.Grogan TM, Fenoglio-Prieser C, Zeheb R, Bellamy W, Frutiger Y, Vela E, Stemmerman G, Macdonald J, Richter L, Gallegos A, Powis G. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–481. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- 15.Kakolyris S, Giatromanolaki A, Koukourakis M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC, Harris AL. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res. 2001;7:3087–3091. [PubMed] [Google Scholar]
- 16.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 18.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 19.Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, et al. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003;22:4035–4046. doi: 10.1038/sj.onc.1206610. [DOI] [PubMed] [Google Scholar]
- 20.Sui L, Dong Y, Watanabe Y, Yamaguchi F, Hatano N, Izumori K, Tokuda M. Growth inhibitory effect of D-allose on human ovarian carcinoma cells in vitro. Anticancer Res. 2005;25:2639–2644. [PubMed] [Google Scholar]
- 21.Yamaguchi F, Takata M, Kamitori K, Nonaka M, Dong Y, Sui L, Tokuda M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int J Oncol. 2008;32:377–385. [PubMed] [Google Scholar]
- 22.Hoshikawa H, Indo K, Mori T, Mori N. Enhancement of the radiation effects by D-allose in head and neck cancer cells. Cancer Lett. 2011;306:60–66. doi: 10.1016/j.canlet.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Indo K, Hoshikawa H, Kamitori K, Yamaguchi F, Mori T, Tokuda M, Mori N. Effects of D-allose in combination with docetaxel in human head and neck cancer cells. Int J Onclol. 2014;45:2044–2050. doi: 10.3892/ijo.2014.2590. [DOI] [PubMed] [Google Scholar]
- 24.Mitani T, Hoshikawa H, Mori T, Hosokawa T, Tsukamoto I, Yamaguchi F, Kamitori K, Tokuda M, Mori N. Growth inhibition of head and neck carcinomas by D-allose. Head Neck. 2009;31:1049–1055. doi: 10.1002/hed.21070. [DOI] [PubMed] [Google Scholar]
- 25.Kamada M, Kumazaki T, Matsuo T, Mitsui Y, Takahashi T. Establishment of ultra long-lived cell lines by transfection of TERT into normal human fibroblast TIG-1 and their characterization. Cell Biol Int. 2012;36:519–527. doi: 10.1042/CBI20110460. [DOI] [PubMed] [Google Scholar]
- 26.Müller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35(4 Suppl 1):S96–S104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Metwally MA, Frederiksen KD, Overgaard J. Compliance and toxicity of the hypoxic radiosensitizer nimorazole in the treatment of patients with head and neck squamous cell carcinoma (HNSCC) Acta Oncol. 2014;53:654–661. doi: 10.3109/0284186X.2013.864050. [DOI] [PubMed] [Google Scholar]
- 28.Zhai S, Senderowicz AM, Sausville EA, Figg WD. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in clinical development. Ann Pharmacother. 2002;36:905–911. doi: 10.1345/aph.1A162. [DOI] [PubMed] [Google Scholar]
- 29.Goel A, Aggarwal BB. Curcumin, the golden spice from indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62:919–930. doi: 10.1080/01635581.2010.509835. [DOI] [PubMed] [Google Scholar]
- 30.Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin; Proc Natl Acad Sci USA; 2002; pp. 11700–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nolan L, Johnson PW, Ganesan A, Packham G, Crabb SJ. Will histone deacetylase inhibitors require combination with other agents to fulfil their therapeutic potential? Br J Cancer. 2008;99:689–694. doi: 10.1038/sj.bjc.6604557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shabason JE, Tofilon PJ, Camphausen K. Grand rounds at the national institutes of health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med. 2011;15:2735–2744. doi: 10.1111/j.1582-4934.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel S, Milstien S, Grant S. Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene. 2012;31:537–551. doi: 10.1038/onc.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masoudi A, Elopre M, Amini E, Nagel ME, Ater JL, Gopalakrishnan V, Wolff JE. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008;28:2437–2442. [PubMed] [Google Scholar]
- 35.Ree AH, Dueland S, Folkvord S, Hole KH, Seierstad T, Johansen M, Abrahamsen TW, Flatmark K. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: The Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncol. 2010;11:459–464. doi: 10.1016/S1470-2045(10)70058-9. [DOI] [PubMed] [Google Scholar]
- 36.Candelaria M, Cetina L, Pérez-Cárdenas E, De La Cruz-Hernández E, González-Fierro A, Trejo-Becerril C, Taja-Chayeb L, Chanona J, Arias D, Dueñas-González A. Epigenetic therapy and cisplatin chemoradiation in FIGO stage IIIB cervical cancer. Eur J Gynaecol Oncol. 2010;31:386–391. [PubMed] [Google Scholar]