Abstract
It has been shown that head and neck squamous cell carcinoma (HNSCC) is infiltrated by plasmacytoid dendritic cells (pDCs). The HNSCC TH2 biased microenvironment leads to strong alterations of the cellular functions of pDC and thus impairs the initiation and function of adequate immune responses. In this work we comprehensively analyzed the capacity of CpG-oligonucleotides to activate interferon (IFN)-α secretion of human pDC in the presence of HNSCC. IFN-α secretion was measured using the ELISA Technique. Class A CpG dinucleotide 2216 was used in different concentrations and time frames to stimulate the IFN-α production of human pDC from peripheral blood in the absence and presence of the HNSCC microenvironment. To elucidate single components that might induce the reduction of IFN-α secretion, pDC were exposed to different concentrations of HNSCC relevant cytokines such as IL-6, IL-8 and IL-10. In accordance to former experiments we found that HNSCC micro milieu severely depresses up to 75% of IFN-α secretion capacity of pDCs, if the stimulating Class A CpG 2216 is added to the culture. Preincubation of HNSCC supernatant leads to unrestorable reduction of IFN-α secretion in pDC and can not be restored by CpG 2216. Incubation of pDCs with single cytokines relevant for cancer progression within the HNSCC micro milieu show that IL-6 or IL-8 have no influence on the IFN-α secretion in pDCs, whereas IL-10 massively impairs the secretion in a dose dependent manner. This effect can be potentiated by synergistic incubation with IL-6 and can be abrogated by blocking antibodies to the IL-10 receptor. Interestingly, incubation with IL-10 is not the only factor that impairs the IFN-α secretion, as incubation with the whole HNSCC supernatant is even more effective in reducing the secretion, implying that additional factors play a role. We conclude that restoration of HNSCC induced TH2 bias could be improved by the inhibition of immune cell cytokine receptors in addition to immunostimulating approaches with CpG motifs.
Keywords: CpG ODN, immunomodulation, plasmacytoid dendritic cell, IL-10, HNSCC
Introduction
Head and neck cancer is an aggressive malignancy comprising approximately 6% of all newly diagnosed cancers (1). 95% of tumors arising in the head and neck region are squamous cell carcinomas. HPV negative head and neck squamous cell carcinoma (HNSCC) are most commonly associated with smoking and alcohol abuse. HNSCC are known to be infiltrated by various kinds of immune cells such as dendritic cells (DCs), but efficient immune responses are strongly impaired (2,3). DCs are bone marrow derived leukocytes with an antigen-presenting function, such as B-cells and monocytes and can be divided into different subgroups (4–6). Human plasmacytoid DCs (pDCs) were first identified within the T-cell areas of lymphoid organs (7,8), but also in peripheral blood (9) and they are the principal source of IFN-α producing cells (10). pDCs are able to recognize CpG motifs within microbial DNA, which are unmethylated CG dinucleotides in a certain sequence context and trigger the production of INF-α in pDCs (11). It has been shown that CpG DNA can be recognized by TLR9 and thus stimulates the polyclonal activation of B lymphocytes and the secretion of proinflammatory cytokines by pDCs and macrophages (12–14). Cytokines in the HNSCC microenvironment also have a strong influence on the immune response and thus play a critical role in tumor aggressiveness and its response to chemo- and radiation therapy (15,16). The leading cytokines identified in the HNSCC microenvironment are interleukin (IL)-4, IL-6, IL-8 and IL-10, granulocyte macrophage-colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), prostaglandin E2 and basic fibroblast growth factor (bFGF) (17–19).
pDCs are considered to be primarily responsible for the establishment of an adaptive TH1 immune response (20,21). Under the influence of IL-3 and CD40-ligand they also can adjust the immune response to a tolerance induction. This happens by polarising the secretion of cytokines which lead to a TH2 specific immune response, for example IL-4 and IL-10 (22). In this work we comprehensively analyzed the capacity of CpG-oligonucleotides to induce IFN-α secretion in human pDC under the influence of HNSCC.
Materials and methods
Preparation and stimulation of pDCs
Buffy coats were provided by the blood bank of the University of Lübeck in an anonymized manner and were used to isolate human peripheral blood mononuclear cells (PBMCs). Blood donors were healthy, without medication or symptomatic allergies. PBMCs were obtained from buffy coats by Ficoll-Hypaque density gradient centrifugation as described previously (2). pDCs were isolated by magnetically activated cell sorting using the BDCA-4 DC isolation kit from Miltenyi Biotec (Bergisch-Gladbach, Germany). Cell numbers were calculated by light microscopy and cell viability was determined by trypan blue staining of dead cells as well as using flow cytometry.
5×104 pDCs were cultured and stimulated in 96-well round bottom plates in 100 µl of medium, comprising Dulbecco's modified Eagle's medium (Gibco Life Technologies, Carlsbad, CA, USA), 10% heat-inactivated FCS (Gibco Life Technologies), non-essential amino acids (Gibco Life Technologies), sodium pyruvate (Sigma-Aldrich, St. Louis, MO, USA) and X-Vivo (Cambrex Bioscience, Rockland, ME, USA). The cells were stimulated for 12, 24 and 48 h, respectively, with the following, in endotoxin-free water solved agents: 3 respective 6 µg/ml CpG ODN 2216 (Invivogen, Inc., San Diego, CA, USA; Metabion International AG, Martinsried, Germany) and HNSCC supernatant (preparation as described below) in a solution medium/supernatant of 1:1 or in cell suspension 1:4. Human recombinant IL-10, IL-8 and IL-6 were purchased from Biosource and added in a concentration of 1, 10 and 100 ng/ml (IL-10), resp. 100 pg/ml (IL-6), resp. 10 pg/ml, 100 pg/ml, 1 ng/ml. The IL-10 receptor antibody was purchased by R&D Systems, Inc. (Minneapolis, MN, USA) and used in a concentration of 2,5 µg/ml.
Preparation of HNSCC supernatants
Permanent HNSCC cell lines BHY (DSMZ, Braunschweig, Germany (23) and PCI-13 (Dr Theresa Whiteside, Hypopharyngeal cancer, Pittsburgh Cancer Institute, Pittsburgh, PA, USA) were used to generate HNSCC supernatants. HNSCC cells were cultured in DMEM-medium (Gibco Life Technologies) supplemented with 10% FCS, 1 mM glutamine and 0.1 mM sodium pyruvate. Cell-free supernatants were collected by centrifugation and filtration after 48 h of cell cultivation and frozen once (−20°C).
Detection of IFN-α
The IFN-α module set from Bender MedSystems (Vienna, Austria) was used to detect IFN-α in cell culture supernatants according to the instructions provided by the manufacturer. The photometric extinction was converted into pg/ml by inverse polynomic regression on the basis of the standard curve. The detection threshold is stated by 3.16 pg/ml in accordance to the manufacturer.
Statistical analysis
Data are expressed as mean with standard deviation from three independent experiments. Statistical significance was evaluated by paired Student's t-test.
Results
IFN-α production is reduced in response to soluble factors of the HNSCC microenvironment
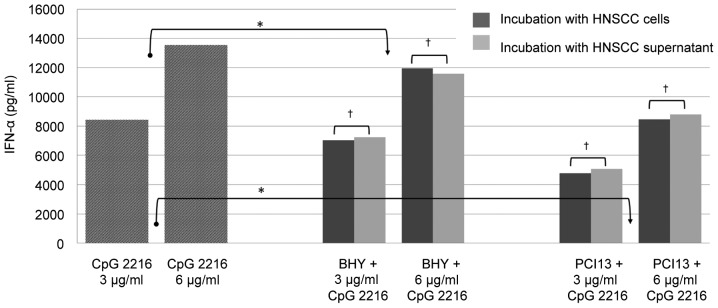
pDCs were simultaneously stimulated with 3 and 6 µg/ml CpG 2216 for 48 h in the presence and absence of HNSCC cells and supernatant, respectively. Therefore two different permanent HNSCC cells lines BHY and PCI13 were used. The IFN-α production was reduced approximately 15% by BHY and 40% by PCI 13 in the mean and was significant in both cell lines (P<0.05; Fig. 1).
Figure 1.
Abrogation of IFN-α production after stimulation with CpG and under influence of HNSCC is caused by a soluble factor in the microenvironment of the tumor. *P<0.05, †not significant.
Our data demonstrates that the inhibitory effect of HNSCC does not depend on the presence of tumor cells. There is no significant difference between the incubation with HNSCC cells or supernatant, which underlines that the inhibition is caused by soluble factors and does not require a direct cell contact.
Pre-incubation with HNSCC has a negative effect on the IFN-α secretion
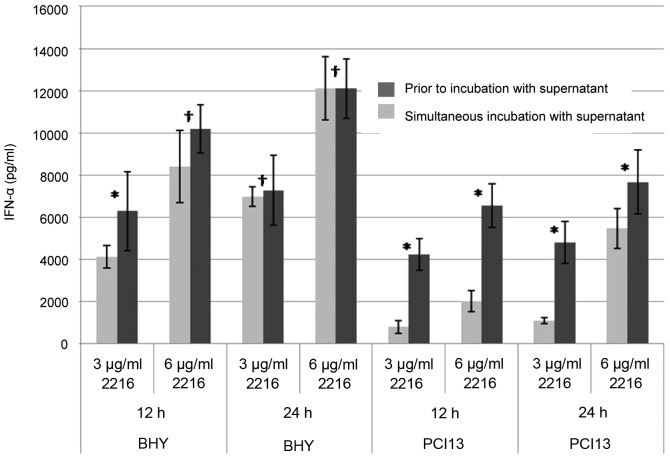
pDCs of healthy donors were pre-incubated with HNSCC supernatants for 24 or 36 h, respectively. Subsequently CpG ODN was added for another 12 and 24 h, so that the overall incubation time was 48 h. As positive control cells of the same donors were incubated with HNSCC supernatant and CpG simultaneously, unstimulated pDCs were used as a negative control (data not shown).
pDCs which were pre-incubated with HNSCC prior to CpG stimulation showed a stronger impairment in the IFN-α secretion than those cells incubated simultaneously (Fig. 2). Longer pre-incubation times led to a stronger impaired IFN-α secretion. After 24 h of incubation with PCI13 supernatant prior to CpG stimulation, the IFN-α is reduced by 25%; after 36 h incubation by 75% (Fig. 2B). The overall reduction by BHY was almost the same after 24 h regardless the pre-incubation period. Nevertheless, after 36 h of incubation with supernatant, the impairment is significant (Fig. 2A).
Figure 2.
Suppression of the IFN-α production is dependent on timeframe and point of stimulation. *P<0.05, †not significant.
IL-10 is the main inhibitor of pDC IFN-α secretion
To further elucidate which component in the supernatant of HNSCC might be causing the impairment of pDC function, we exposed native pDC to different concentrations of cytokines relevant in the micro milieu of HNSCC, such as IL-6, IL-8 and IL-10.
The IL-6 concentrations were defined by the average serum levels in HNSCC patients (19,5 pg/ml) and the maximum (312 pg/ml) as well as levels in supernatants of immortalized cell lines (up to 4,000 pg/ml). IL-8 was used in concentrations of 10 pg/ml, 100 pg/ml and 1 ng/ml. IL-10 was used in concentrations of 1, 10 and 100 ng/ml. The concentrations were also defined with respect to our previous data and in accordance to the literature (19,24–26).
There was no significant effect on the CpG induced IFN-α secretion in response to the addition of IL-6 and IL-8. Neither different concentrations nor different combinations of both cytokines were able to impair the pDC IFN-α secretion (data not shown).
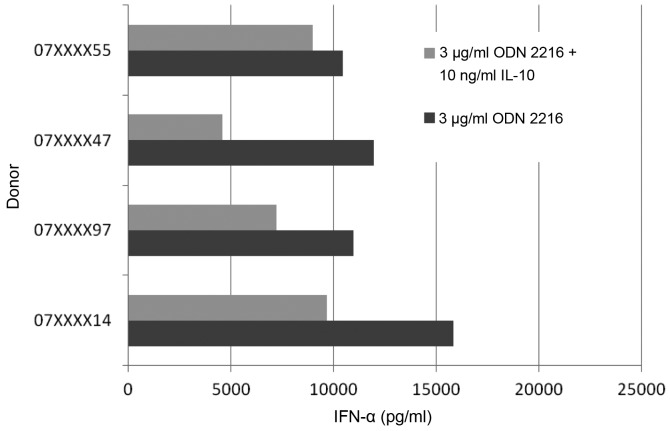
On the contrary, IL-10 showed a significant effect on the CpG induced IFN-α secretion. These findings correlate with data from Duramad et al (25) in 2003. We were able to reproduce these findings in our settings. Furthermore the CpG induced IFN-α secretion showed more or less a wide range of variation (Fig. 3). The four tested pDCs of healthy donors showed a fluctuation margin of approximately 45%. The decrease of the IFN-α secretion compared to positive control was 14, 62, 52 and 39%.
Figure 3.
IL-10 altered CpG induced IFN-α secretion in pDCs of healthy donors. The reduction differed between 14–62% depending on the specimen.
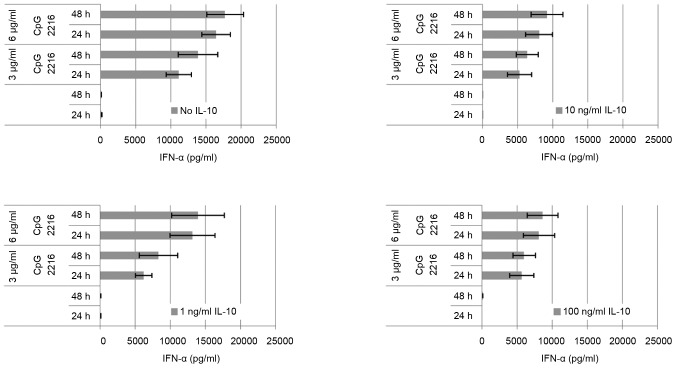
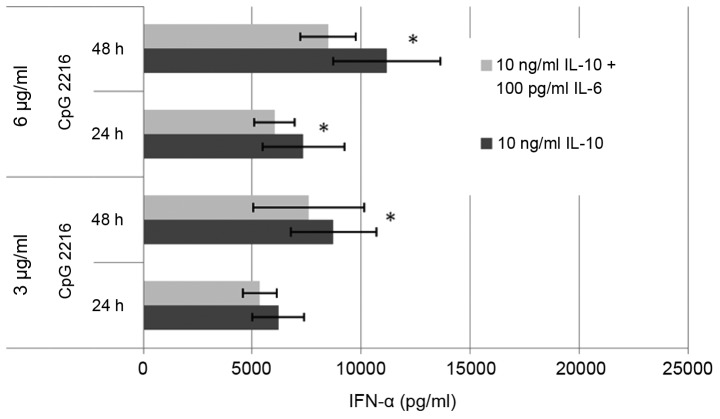
The IL-10 induced reduction on the CpG induced IFN-α secretion in pDC is dose-dependent (Fig. 4). The maximum is reached at 10 ng/ml. At a concentration of 1 ng/ml IL-10, the suppression of the IFN-α secretion can be outweighed by elongation of incubation and dose escalation. At a concentration of 10 ng/ml IL-10 this is no longer the case, and the difference between 3 and 6 µg/ml ODN 2216 is minor. The effect of 1 ng/ml IL-10 is already higher at 3 µg/ml ODN than at 6 µg/ml. The amount of secreted cytokine on stimulation by CpG changes only slightly at IL-10 concentration of 10 ng/ml. The dose escalation up to 100 ng/ml has no additional significant effect on the secreted amount of IFN-α.
Figure 4.
The effect of IL-10 on the CpG induced IFN-α secretion depends on the concentration of IL-10 and also on the concentration of CpG. The maximum effect of IL-10 with 6 µg/ml ODN is reached by 10 ng/ml, whereas with 3 µg/ml CpG ODN the maximum effect is already reached at 1 ng/ml-10 ng/ml. At 1 ng/ml the alteration can be counteracted by a higher CpG dose and/or longer incubation duration of CpG. At 10 ng/ml the effect can not be counteracted like this anymore. An increase of the IL-10 dose to 100 ng/ml has no additional effect.
We also were able to show a synergistic effect of IL-10 and IL-6 (Fig. 5). The combination of IL-10 with IL-8 had no additional effect (data not shown). The combination of IL-10 and IL-6 raised the suppressive effect on the IFN-α secretion by 13% in average.
Figure 5.
There is a synergistic average effect of 13% of the combination IL-6 and IL-10 compared to the reduction of the CpG induced IFN-α by IL-10 alone. n=3. *P<0.05
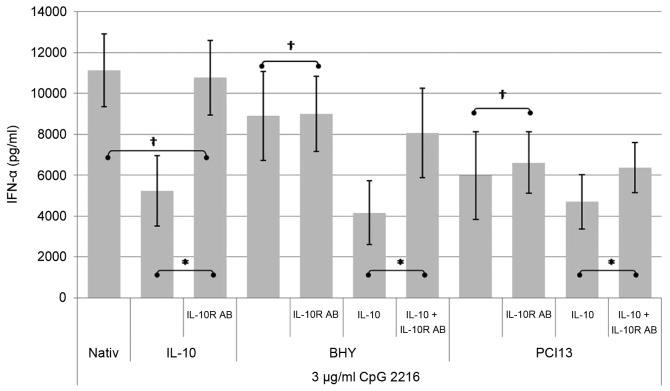
The inhibitory effect of HNSCC can be antagonized by antibodies to the IL-10 receptor
PDCs were incubated with HNSCC supernatant and/or IL-10 in the presence and absence of blocking antibodies to the IL-10-receptor. The IFN-α concentration was detected by ELISA after 24 h. The negative control were cells incubated with medium and IL-10 (data not shown). The positive control pDCs were incubated with CpG ODN 2216.
As shown in Fig. 6, the administration of a blocking antibody to the IL-10 receptor is not able to neutralize the suppressive effect of the complete HNSCC supernatant on the CpG induced IFN-α secretion completely. However pDCs incubated under the influence of IL-10 can be fully restored by adding the IL-10 receptor blocking antibody, which clearly documents that various other factors play a role in the IFN alpha suppressing mechanism.
Figure 6.
By adding the IL-10 receptor antibody, the IL-10 induced inhibition of the IFN-α secretion can be blocked. When added to the HNSCC supernatant the effect can not be blocked entirely. *P<0.05, †not significant.
The suppressive influence of HNSCC supernatant can be additionally boosted by adding IL-10. Antibodies to the IL-10 receptor can diminish this additional effect but are not able to restore the full functionality.
Discussion
The HNSCC micro milieu severely impairs the CpG ODN induced IFN-α secretion in pDCs in vitro up to 40%. The intensity of inhibition varies among different HNSCC cell lines according to incubation time and CpG dosage. BHY and PCI13 show different concentrations of soluble factors. BHY supernatant contains high amounts of IL-6 (3,750 pg/ml) and a lower concentration of IL-8 (820 pg/ml). The supernatant of PCI13 contains a low concentration of IL-6 (620 pg/ml) but nearly the same amount of IL-8 (780 pg/ml) like BHY. The cytokines IL-4 and IL-10 were measured in much lower concentrations in the supernatant of both cell lines. The immunosuppressive IL-10 and also IL-1 are not primarily secreted by the tumor itself. The micro milieu of the tumor induces secretion in mDC and other cells of its environment. IL-1 stimulates the increased secretion of the tumor stimulating and -relevant cytokines IL-4, IL-6 and GM-CSF. The different concentrations of the cytokines explain the varying influence of HNSCC on the CpG induced IFN-α secretion.
With HNSCC supernatant, pre-incubated pDCs prior to CpG stimulation show a more efficient impairment of the INF-α secretion than those cells simultaneously incubated with CpG and HNSCC (Fig. 2). Longer pre-incubation times lead to a stronger impairment of IFN-α secretion than in the case of PCI13 which can not be compensated by an extended incubation time with CpG or a higher dosage of CpG.
Furthermore our data revealed that the inhibitory effect of HNSCC depends on soluble factors and cell to cell contact is not required (Fig. 1).
Our study was able to identify IL-10 as one soluble factor in the HNSCC micro milieu that markedly reduces the IFN-α secretion of pDCs.
The presumption that this would be key to the explanation of the mechanism was deceptive. As shown in Fig. 6 IL-10R antibody can not abolish the effect of HNSCC on the CpG induced IFN-α secretion entirely. The ability of pDCs incubated with mere IL-10 can be restored almost entirely by adding IL-10R antibody. Combined with the HNSCC supernatant, the ability can only be retrieved by a smaller level. This leads to the assumption that there are other factors in the HNSCC supernatant besides IL-10 that lead to a reduction of the CpG induced IFN-α secretion. Waibler et al had stated IL-10 before as a negative regulator regarding the GpG induced IFN-α secretion, but we were able to show that it is not the only soluble factor in the HNSCC micro milieu.
In literature, a cytotoxic effect of IL-10 for CpG-activated pDCs is stated, nevertheless in our work we were not able to reproduce this statement by FACS Annexin and PI staining (data not shown) [Duramad et al (25)].
We were able to show a synergism of IL-6 and IL-10 (Fig. 5). Mere IL-6 has no influence on the CpG induced IFN-α secretion but in combination with IL-10 it alters the secretion.
It is therefore most likely that even more factors have an influence on this mechanism.
More factors which have been described in the HNSCC supernatant are for example the VEGF family as multifunctional factors in angiogenesis, tumor progression, immunosuppression and immunotolerance (27). A synergism of IL-6, IL-1 and GM-CSF has already been described in matters of down regulation of CD80 in tumor cells, which can be reversed by IFN-γ (28).
The important role of IL-6 was shown in clinical studies by Riedel et al (29) and Duffy et al (30), and discussed as a biomarker. The important role of IL-6 within the Stat3 signaling in tumor proliferation has also been shown. When Stat3 is missing in this cascade, the tumor growth is reduced (31).
IL-8 plays a role in terms of proliferation and cell survival in HNSCC. We were not able to show an influence on the CpG induced IFN-α secretion in our work.
That cytokines have a strong influence of the pathogenesis in cancer has been shown in several studies. Patients with advanced disease show a shifted immune profile toward TH2 compared to patients with less advanced disease.
Elevations of IL-10 have been detected in diseases like cancer and chronic infection. The strong TH1-priming ability of CpG is the basis for future clinical trials to revert this immune tolerating status in infectious disease, cancer, asthma and allergic rhinitis.
Acknowledgements
We thank Brigitte Wollmann for skilful support in some parts of this study. We are grateful to all members of the Department of Otorhinolaryngology for helpful discussions and a comfortable atmosphere. This study was supported by grants of the Mildred-Scheel-Stiftung (Deutsche Krebshilfe), the Werner and Klara Kreitz-Stiftung, the Monika-Kutzner-Stiftung and the Rudolf-Bartling-Stiftung.
References
- 1.Liu Z, Yang L, Cui Y, Wang X, Guo C, Huang Z, Kan Q, Liu Z, Liu Y. Il-21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1133–1144. doi: 10.1002/ibd.20923. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 3.Reichert TE, Rabinowich H, Johnson JT, Whiteside TL. Mechanisms responsible for signaling and functional defects. J Immunother. 1998;21:295–306. doi: 10.1097/00002371-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 5.Hart DN. Dendritic cells: Unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 6.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/S0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 7.Müller-Hermelink HK, Stein H, Steinmann G, Lennert K. Malignant lymphoma of plasmacytoid T-cells. Morphologic and immunologic studies characterizing a special type of T-cell. Am J Surg Pathol. 1983;7:849–862. doi: 10.1097/00000478-198307080-00013. [DOI] [PubMed] [Google Scholar]
- 8.Vollenweider R, Lennert K. Plasmacytoid T-cell clusters in non-specific lymphadenitis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44:1–14. doi: 10.1007/BF02890155. [DOI] [PubMed] [Google Scholar]
- 9.Galy A, Christopherson I, Ferlazzo G, Liu G, Spits H, Georgopoulos K. Distinct signals control the hematopoiesis of lymphoid-related dendritic cells. Blood. 2000;95:128–137. [PubMed] [Google Scholar]
- 10.Kirkwood J. Cancer immunotherapy: The interferon-alpha experience. Semin Oncol. 2002;29(3 Suppl 7):S18–S26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 11.Krug A, Rothenfusser S, Hornung V, Jahrsdörfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::AID-IMMU2154>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Bauer S, Wagner H. Bacterial CpG-DNA licenses TLR9. Curr Top Microbiol Immunol. 2002;270:145–154. doi: 10.1007/978-3-642-59430-4_9. [DOI] [PubMed] [Google Scholar]
- 13.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 14.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 15.Chin D, Boyle GM, Theile DR, Parsons PG, Coman WB. Molecular introduction to head and neck cancer (HNSCC) carcinogenesis. Br J Plast Surg. 2004;57:595–602. doi: 10.1016/j.bjps.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Whiteside TL. Immune suppression in cancer: Effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev. 2006;17:141–146. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Mann EA, Spiro JD, Chen LL, Kreutzer DL. Cytokine expression by head and neck squamous cell carcinomas. Am J Surg. 1992;164:567–573. doi: 10.1016/S0002-9610(05)80708-1. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–1379. [PubMed] [Google Scholar]
- 20.Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 21.Cella MF, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 22.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 23.Kawamata H, Nakashiro K, Uchida D, Harada K, Yoshida H, Sato M. Possible contribution of active MMP2 to lymph-node metastasis and secreted cathepsin L to bone invasion of newly established human oral-squamous-cancer cell lines. Int J Cancer. 1997;70:120–127. doi: 10.1002/(SICI)1097-0215(19970106)70:1<120::AID-IJC18>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–7269. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 25.Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487–4492. doi: 10.1182/blood-2003-07-2465. [DOI] [PubMed] [Google Scholar]
- 26.Pries R, Thiel A, Brocks C, Wollenberg B. Secretion of tumor-promoting and immune suppressive cytokines by cell lines of head and neck squamous cell carcinoma. In Vivo. 2006;20:45–48. [PubMed] [Google Scholar]
- 27.Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): Possible link between angiogenesis and immune tolerance. Med Sci Monit. 2005;11:BR280–BR292. [PubMed] [Google Scholar]
- 28.Thomas GR, Chen Z, Leukinova E, Van Waes C, Wen J. Cytokines IL-1 alpha, IL-6, and GM-CSF constitutively secreted by oral squamous carcinoma induce down-regulation of CD80 costimulatory molecule expression: Restoration by interferon gamma. Cancer Immunol Immunother. 2004;53:33–40. doi: 10.1007/s00262-003-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedel F, Zaiss I, Herzog D, Götte K, Naim R, Hörmann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25:2761–2765. [PubMed] [Google Scholar]
- 30.Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 31.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]