Abstract
Brucea javanica oil emulsion (BJOE) has been used clinically to treat esophageal cancer combined with radiotherapy for numerous years in China. However, the detailed mechanism remains unclear. Thus, the effects of BJOE on the radiosensitivity of esophageal squamous cell carcinoma (ESCC) were evaluated in vitro and in vivo. The growth inhibitory effects of different BJOE concentrations were determined through an MTT assay. Radiosensitivity was evaluated through focal formation measurements and clone formation assays. The effects of BJOE on radiation-induced apoptosis were examined through flow cytometric analysis. The effects of BJOE on hypoxia-inducible factor 1α (HIF-1α) protein levels in vitro and in vivo were respectively analyzed through western blot analyses and enzyme-linked immunosorbent assays. BJOE significantly inhibited ECA109 cell proliferation in a dose- and time-dependent manner. Pretreatment with 2.5 mg/ml BJOE increased ECA109 radiosensitivity. BJOE in combination with radiation increased the DNA double-strand breaks. Compared with radiation alone, BJOE and radiation significantly increased the apoptotic rate of ECA109 cells. BJOE also decreased the HIF-1α protein levels in vitro and in vivo. The results from the present study demonstrated that BJOE enhanced the radiosensitivity of human ESCC. This finding was associated with the inhibition of HIF-1α expression. Therefore, BJOE may be a potential radiotherapy sensitization drug due to its significant anti-hypoxic activity.
Keywords: Brucea javanica oil emulsion, esophageal cancer, hypoxia, radiosensitivity, hypoxia-inducible factor-1α
Introduction
Esophageal cancer is a highly lethal disease with a rapidly increasing incidence. In China, esophageal cancer is the fourth most common cause of mortality, resulting in 16.77 mortalities per 100,000 of the population in 2009 (1). In China, esophageal squamous cell carcinoma (ESCC) is a major histopathological subtype of esophageal cancer. Locally advanced ESCC is conventionally treated through radiotherapy; however, a large proportion of ESCC tumors develop resistance to radiation. This phenomenon emphasizes the importance of enhancing tumor radiosensitization in ESCC.
Hypoxia is a common phenomenon observed in solid tumors. Hypoxia-inducible factor (HIF)-1 is an important regulator of adaptive responses to hypoxia (2). HIF-1 plays an important role in tumor growth, proliferation, metastasis and therapeutic resistance. HIF-1 is a heterodimer protein consisting of HIF-1α and HIF-1β. HIF-1α is primarily involved in HIF-1 protein stabilization and transactivation (3). Therefore, HIF-1α expression should be suppressed to enhance the sensitivity of tumor cells to radiotherapy.
Laboratory and clinical studies have demonstrated that Brucea javanica oil emulsion (BJOE) provides an antitumor potential. However, BJOE, in combination with radiotherapy has yet to be used to treat esophageal cancer. The present study was designed to explore the radiosensitizing effect of BJOE on human esophageal cancer in vitro and in vivo.
Materials and methods
Cell culture and treatment
Human esophageal carcinoma cell line ECA109 was provided by the Central Experimental Laboratory, Nanjing Medical University (Nanjing, China). The cells were cultured in RPMI-1640 medium (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) containing 10% Sijiqing newborn bovine serum (Zhejiang Tianhang Biotech Co., Ltd., Huzhou, China) in a 5% CO2 thermostatic incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C. The cells were also incubated at 37°C in a sealed tank filled with complex air consisting of 1% O2, 94% N2, and 5% CO2 to stimulate growth under hypoxic conditions for 24 h.
The cells were irradiated with a single dose at a dose rate of 200 cGy/min using a 6 MV linear accelerator (Precise Treatment System™; Elekta AB, Stockholm, Sweden). The source-cell distance was 100 cm, and the radiation field was 20×20 cm.
Patients and treatment
A total of 20 patients with histological evidence of invasive thoracic ESCC were enrolled between January 2013 and December 2014. They underwent radiotherapy at the Center of Radiation Oncology in the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). Patients with tumors in clinical stage T1N1 or T2-3N0-1 and M0, in accordance with the Union for International Cancer Control Tumor Node Metastasis classification (Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer) (4), were chosen. The enrolled patients (12 males, 8 females) were between 40–70 years old, and were characterized by an Eastern Cooperative Oncology Group performance status score of ≤2. Adequate hematologic, renal, hepatic and pulmonary functions, absence of other cancer types, or previous radiotherapy or chemotherapy were required. The patients signed an informed consent prior to treatment administration. The present study was approved by the Ethics Committee of Huai'an First People's Hospital in Huai'an (Jiangsu, China).
The patients were randomly divided into two groups: Radiation group and radiation + BJOE group. They received a total radiation dose of 56–60 Gy in 28–30 fractions with 2 Gy/fraction, 5 days/week using three-dimensional conformal radiotherapy technique. In the radiation + BJOE group, 30 ml of BJOE dissolved in 250 ml normal saline was intravenously injected once daily.
Reagents and antibodies
BJOE was provided by Shenyang Yaoda Pharmaceutical Co., Ltd. (Liaoning, China). Anti-HIF-1α (cat, no. sc13515) and anti-β-actin monoclonal antibodies (cat. no. sc70319) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit antihuman phosphorylated H2A histone family member X (γH2AX) polyclonal antibody (cat. no. 309-005-003), horseradish peroxidase (HRP)-conjugated secondary antibody (cat. no. 111-035-003) and fluorescein (FITC)-conjugated secondary antibody (cat. no. 111-005-003) were obtained from Jackson ImmunoResearch Laboratories, Inc. (Westgrove, PA, USA).
MTT assay
ECA109 cells in the logarithmic growth phase were inoculated in 96-well plates at a density of 5×103 cells/ml. The cells were cultured in an incubator containing 5% CO2 at 37°C. The medium was removed and replaced with fresh medium supplemented with different BJOE concentrations (1.25, 2.5, 5, 10, 20, 40, 80, and 100 mg/ml), when the monolayers had reached confluency. An untreated cell culture medium was used as an experimental control. After treatments were administered for 24 and 48 h, medium was removed, and cells were washed with PBS (pH 7.4), once or twice. Following this, 20 µl MTT (5 mg/ml; Beyotime Institute of Biotechnology, Haimen, China) was added to each well. After the medium was inoculated for another 4 h, the supernatant was removed and 150 µl dimethyl sulfoxide (Nanjing KeyGen Biotech Co., Ltd.) was added to each well to dissolve formazan crystals. Cell viability was determined through MTT cell proliferation and cytotoxicity assay (Beyotime Institute of Biotechnology). Absorbance was obtained at a wavelength of 490 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Clone formation assay
ESCC cells in the logarithmic growth phase were trypsinized and diluted to single-cell suspensions. Following this, 200 cells/ml were seeded into 6-well plates and cultured overnight. Adherent cells were randomly divided into two groups: Radiation group and the BJOE (2.5 mg/ml) + radiation group. After the cells were cultured under hypoxic conditions, both groups were irradiated with different absorbance doses of 0, 2, 4, 6, and 8 Gy. The cells were then cultured in an incubator with 5% CO2 at 37°C for ~2 weeks until the colonies were visible. The colonies were fixed with methanol for 10 min at room temperature and stained with Giemsa for 15 min at room temperature. The number of colonies (>50 cells/colony) were counted. A cell survival curve was fitted on the basis of single-hit multiple-target model by using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) to determine the survival fraction=1-(1-e−D/D0)n, where D0 is the dose required when the curve index decreased by 63%. D37 is the required dose when the curve index decreased by 37%. The sensitization enhancement ratio (SER) was calculated as follows: D0 in the control group/D0 in the experimental group.
γH2AX immunofluorescent measurement
ECA109 cells were cultured on 6-well plates at a density of 5×104 cells/ml. The cells were treated with or without BJOE (2.5 mg/ml) and exposed to 6 Gy radiation. The control cells did not receive BJOE or radiation treatment. Each group was incubated under hypoxic conditions for 24 h. Thereafter, the cells were fixed with 4% paraformaldehyde for 15 min at 4°C, permeabilized with 0.3% Triton X-100 for 15 min at 4°C, and blocked with 1% bovine serum albumin for 2 h at room temperature (all from Nanjing KeyGen Biotech Co., Ltd.). The cells were incubated with an anti-γH2AX primary antibody (dilution, 1:250) at 4°C overnight and the following day with secondary antibody conjugated with FITC (dilution, 1:150) for 1 h at room temperature. The nuclei were counterstained with DAPI (Nanjing KeyGen Biotech Co., Ltd.) for 3 min. Focal formation was verified by using a laser scanning confocal microscope.
Flow cytometric analysis of apoptosis
Single-cell suspension of ESCC cells lines were seeded onto 6-well plates at a density of 5×106 cells/ml. The control group was cultured under hypoxic conditions for 24 h without any treatment. The two groups were treated with 6 Gy irradiation and cultured for 24 h under hypoxic conditions. They were further cultured for 48 h. The cells were routinely trypsinized, rinsed with cold PBS, resuspended in 1X Annexin-binding buffer and stained with the Annexin V-flourescein isothiocyanate FITC apoptosis kit (Nanjing KeyGen Biotech Co., Ltd.), according to the manufacturer's protocol. The samples were immediately analyzed by using a flow cytometer. Data was analyzed using FlowJo 7.6.2 (FlowJo LLC, Asland, OR, USA).
Western blot analysis
ESCC cells were treated with BJOE under hypoxic conditions for 24 h. The control cells were cultured without BJOE under normoxic, and hypoxic conditions for 12, 24, and 48 h. Subsequently, proteins were extracted from cells using RIPA lysis buffer (Beyotime Institute of Biotechnology), and protein concentration was determined through a BCA assay. Equal amounts of protein (40 µg) were loaded into each lane and separated through SDS-PAGE with 8% resolving gel and 5% stacking gel using an electrophoresis instrument (Bio-Rad Laboratories, Inc.) at 60 V for 15 min and at 120 V for 30 min. Proteins were transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA) using a wet electrophoretic transfer instrument (Bio-Rad Laboratories, Inc.) at 250 mA for 180 min. The membranes were blocked with 5% skim milk for 1 h at room temperature, and then incubated with anti-HIF-1α monoclonal antibodies (diluted 1:1,000) at 4°C overnight. The following day, membranes were incubated with goat anti-rabbit IgG (H+L) HRP-conjugated secondary antibody (diluted 1:5,000) for 1 h at room temperature. The immunostained membranes were visualized using an enhanced chemiluminescence detection kit (Nanjing KeyGen Biotech Co., Ltd.) with a Chemidoc XRS imaging system (Bio-Rad, Laboratories, Inc.).
Enzyme-linked immunosorbent assay (ELISA)
Venous blood samples were collected from the enrolled patients before and after radiotherapy was completed. Serum was isolated at (2,014 × g) and 4°C for 10 min. Serum HIF-1α levels were assayed using a human HIF-1α ELISA kit (Shanghai Bogoo Biological Technology Co., Ltd., Shanghai, China) in accordance with the manufacturer's protocol. Absorbance was determined at a wavelength of 450 nm on a microplate reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were statistically analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard deviation. All experiments were performed at least in triplicate, and differences between treatment groups were determined via one-way analysis of variance with post hoc contrasts by Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
BJOE enhances the radiosensitivity of hypoxic ESCC cell lines
The viability of ESCC cell lines was detected following treatment with different BJOE concentrations for 24 and 48 h. MTT assay revealed that BJOE inhibited the growth of ECA109 in a concentration- and time-dependent manner (Fig. 1). At 24 h, IC20 of ECA109 was 4.02 mg/ml. The treatment with 2.5 mg/ml BJOE for 24 h did not significantly affect the OD of ECA109 cells. Thus, 2.5 mg/ml BJOE was chosen for the radiosensitization experiments to avoid BJOE cytotoxicity.
Figure 1.
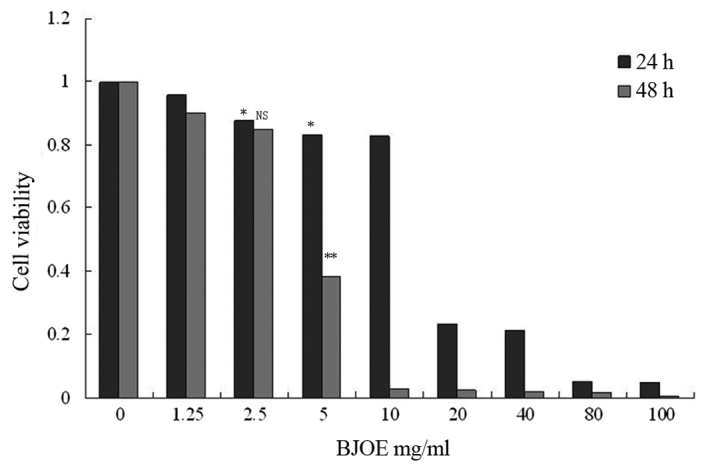
Effects of different concentrations of BJOE on cell viability of ECA109 cells determined by an MTT assay. ECA109 cells were inoculated in 96-well plates at a density of 5×103 cells/ml. The cells were cultured in an incubator containing 5% CO2 at 37°C. The medium was removed and replaced with fresh medium supplemented with different BJOE concentrations (1.25, 2.5, 5, 10, 20, 40, 80, and 100 mg/ml), when the monolayers had reached confluency. An untreated cell culture medium was used as an experimental control. After 24 and 48 h, cell viability was determined. BJOE inhibited the growth of ECA109 in a concentration- and time-dependent manner. The treatment with 2.5 mg/ml BJOE for 24 h did not significantly affect the optical density of ECA109 cells. Data are shown as mean ± standard deviation. *P<0.05 and **P<0.01, compared with the control; NS, no significance, compared with the control; BJOE, Brucea javanica oil emulsion.
Effects of radiation and BJOE on colony formation
ECA109 cells were exposed to different radiation doses after they were pretreated with 2.5 mg/ml BJOE for 24 h under hypoxic conditions to assess clone formation. The dose-survival curves (Fig. 2) were generated by using GraphPad Prism 6.0. The survival fraction data were fitted into the aforementioned single-hit multiple-target model. For ECA109 cells, the radiation group and the radiation + BJOE group yielded D0 of 1.98±0.04, and 1.17±0.03 Gy, respectively. The SERs of ECA109 was 1.66. The results from the present study revealed that BJOE significantly sensitized hypoxia cancer cells to radiation. ECA109 reached D37 of 5.21. Therefore, 6 Gy was the administered radiation dose for the focal formation experiment and apoptosis analysis.
Figure 2.
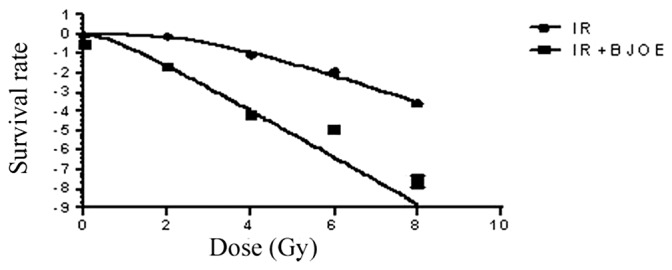
Effects of 2.5 mg/ml BJOE pretreatment on the radiosensitivity of ECA109 as determined by clonogenic survival assay. A concentration of 200 cells/ml ECA109 cells were seeded into 6-well plates and cultured overnight. Adherent cells were randomly divided into two groups: Radiation group; and the BJOE (2.5 mg/ml) and radiation group. After the cells were cultured under hypoxic conditions, both groups were irradiated with different absorbance doses of 0, 2, 4, 6, and 8 Gy. The cells were then cultured in an incubator with 5% CO2 at 37°C for 2 weeks until the colonies were visible. After fixed with methanol and stained with Giemsa, the number of colonies. The survival fraction data were fitted into single-hit multiple-target model. BJOE significantly sensitized hypoxia cancer cells to radiation. BJOE, Brucea javanica oil emulsion; IR, radiation.
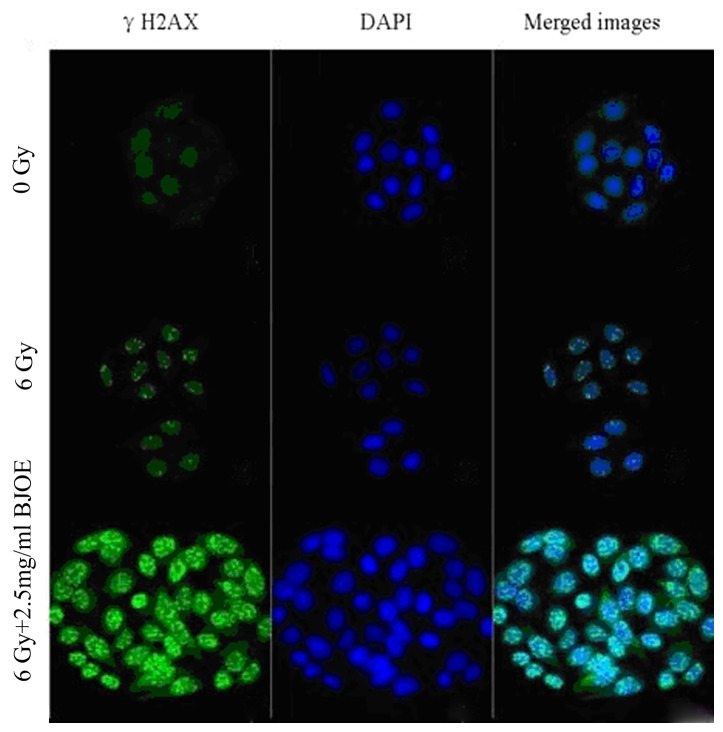
Immunofluorescence of γH2AX
The γH2AX focal formation levels were determined through immunofluorescent measurement to investigate the radiosensitization effect of BJOE on ESCC cells (Fig. 3). The control cells barely exhibited focal formation. However, following exposure to radiation, focal formation occurred primarily in the nuclei. BJOE + radiation treatment markedly promoted focal formation. This observation suggested that BJOE elicited a radiosensitizing effect.
Figure 3.
Effects of 2.5 mg/ml BJOE radiosensitization in ECA109 as using γH2AX immunofluorescent measurement. The control cells barely exhibited focal formation. However, following exposure to radiation, focal formation occurred primarily in the nuclei. BJOE and radiation treatment markedly promoted focal formation. BJOE elicited a radiosensitizing effect. BJOE, Brucea javanica oil emulsion; γH2AX, phosphorylated H2A histone family member X.
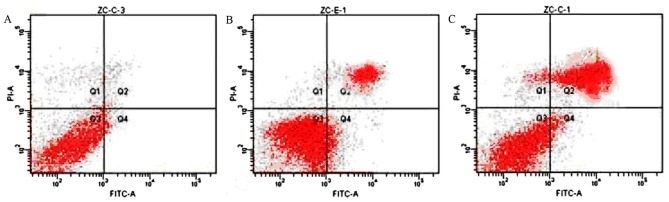
Radiation and BJOE renders ESCC cells to increased apoptosis
The effects of BJOE on the apoptosis of hypoxic ESCC cells treated with or without radiation were detected (Fig. 4). ECA109 in the control, radiation and radiation + BJOE groups yielded apoptotic rates of 5.65±0.80, 39.09±4.57, and 67.38±3.69%, respectively. Irradiation exposure significantly induced apoptotic events in ECA109 compared with the control cells (P<0.001). Compared with radiation alone, BJOE + radiation significantly increased apoptotic rates (P<0.001).
Figure 4.
The effects of BJOE plus radiation evaluated by flow cytometry. (A) ECA109 control cells. (B) ECA109 cells treated with radiation. (C) ECA109 cells treated with BJOE and radiation. Single-cell suspension of ECA109 cells were seeded onto 6-well plates at a density of 5×106 cells/ml. The control group was cultured under hypoxic conditions for 24 h without any treatment. The two groups were treated with 6 Gy irradiation and cultured for 24 h under hypoxic conditions. They were further cultured for 48 h. Then, the apoptosis rates were analyzed using a flow cytometer. Irradiation exposure significantly induced apoptotic events in ECA109, compared with the control cells (P<0.001). Compared with radiation alone, BJOE and radiation significantly increased apoptotic rates (P<0.001). BJOE, Brucea javanica oil emulsion.
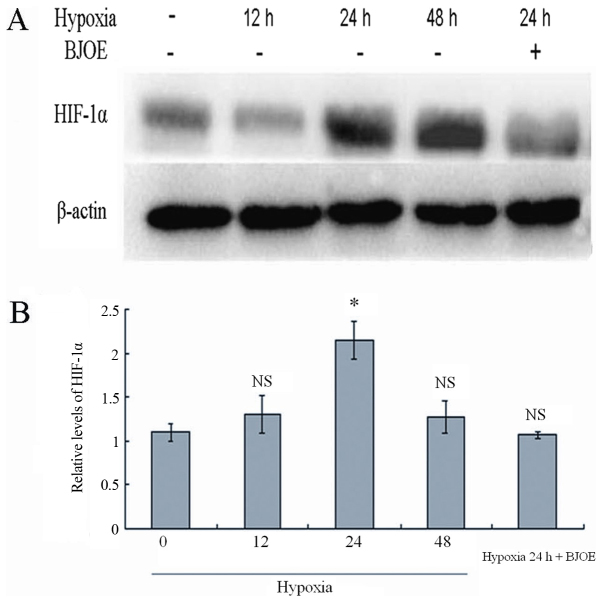
BJOE inhibits HIF-1α in ESCC cells under hypoxic conditions
The HIF-1α expression in ESCC cells was analyzed by western blotting at different time points under hypoxic conditions (Fig. 5). Comparable to normal protein levels were detected after 12 h under hypoxic conditions. However, HIF-1α levels increased to the maximum in ECA109 cells after 24 h under hypoxic conditions. In response to treatment with 5 mg/ml BJOE for 24 h under hypoxic conditions, the HIF-1α protein levels in ECA109 cells were markedly inhibited by BJOE.
Figure 5.
(A) Western blot analysis for HIF-1α in ECA109. (B) Semi-quantitative levels of HIF-1α in ECA109. ECA109 cells were treated with BJOE under hypoxic conditions for 24 h. The control cells were cultured without BJOE under normoxic, and hypoxic conditions for 12, 24, and 48 h. Subsequently, proteins were extracted from cells. A total of 40 µg protein were loaded. The experiments were performed according to the procedures aforementioned in the materials and methods section. Comparable to normal protein levels were detected after 12 h under hypoxic conditions. However, HIF-1α levels increased to the maximum in ECA109 cells after 24 h under hypoxic conditions. In response to treatment with 5 mg/ml BJOE for 24 h under hypoxic conditions, the HIF-1α protein levels in ECA109 cells were notably inhibited by BJOE. Data are shown as mean ± standard deviation. *P<0.05, compared with the control; NS, no significance, compared with the control. HIF-1α, hypoxia-inducible factor 1α; BJOE, Brucea javanica oil emulsion.
BJOE increases the radiation sensitivity of ESCC in vivo
HIF-1α levels did not significantly differ between the radiation group and the radiation + BJOE group before the treatment was administered. Conversely, the HIF-1α level in the radiation + BJOE group was significantly higher compared with that in the radiation group following treatment (Table I). In terms of demographic parameters, including age and gender, HIF-1α levels did not significantly differ between the two groups.
Table I.
Comparison of HIF-1α levels between pre-radiotherapy and post-radiotherapy.
| HIF-1α (ng/ml) | |||
|---|---|---|---|
| Group | n | Pre-treatment | Post-treatment |
| Radiotherapy | 10 | 35.66±7.26 | 29.15±4.77 |
| BJOE+Radiotherapy | 10 | 38.79±10.64 | 17.76±3.66 |
| P-valuea | 0.45 | <0.001a | |
P<0.05, HIF-1α, hypoxia-inducible factor 1α; BJOE, Brucea javanica oil emulsion.
Discussion
Tumor hypoxia is a well-recognized characteristic associated with resistance to radiotherapy and chemotherapy. Tumor cells adapt to hypoxic microenvironments by activating associated signaling pathways (5,6). HIF-1α plays a crucial role in tumor growth and metastasis. The following mechanisms are involved in these phenomena: i) HIF-1α promotes tumor angiogenesis by upregulating the expression of vascular endothelial growth factor (VEGF), angiopoietin-1, angiopoietin-2 and other factors (7). The correlation between HIF-1α and VEGF has been clarified (8). However, blood and nutrient supply of tumors become insufficient as they grow in mass. HIF-1α overexpression promotes VEGF expression as a result of hypoxia, and VEGF further stimulates VEGF-R1 and VEGF-R2 expression. This phenomenon increases the number of tumor blood vessels (8). ii) HIF-1α activates target genes encoding glucose transporters, namely, glucose transporter (GLUT)1 and GLUT3, and hexokinase, the first enzyme of the glycolytic pathway. This mechanism produces a rapid and effective pathway for tumor energy metabolism (9). iii) HIF-1α overexpression is able to inhibit P53 gene expression. Nevertheless, P53 is an important tumor suppressor. P53 induces apoptosis by upregulating Bcl-2 associated-X protein (Bax) and downregulating B-cell lymphoma-2 (Bcl-2) (10). iv) HIF-1α upregulates the expression of c-Met and matrix metalloproteinases (MMPs), and these proteins are associated with tumor progression and metastasis. HIF-1α may also inhibit β-catenin and E-cadherin expression. These associated catenins are the major constituents of cell-cell adhesion. Therefore, the decreased expression of β-catenin and E-cadherin is correlated with tumor progression (11,12). HIF-1α increases the metastatic potential of tumor cells by increasing VEGF expression, as previously described.
BJOE is a traditional Chinese medicine, which is extracted from B. javanica. In China, BJOE has been used to clinically treat patients with lung cancer, brain metastases and gastrointestinal cancer for numerous years. Clinical studies have revealed that BJOE combined with radiotherapy or chemotherapy is able to increase therapeutic efficacy, and reduce the side effects of patients with lung cancer, brain metastases and gastrointestinal cancer (13–16). Furthermore, BJOE elicits cytotoxic effects on tumor cell lines, including A431 (human skin cancer cell line), HT1080 (human fibrosarcoma cell line), LNCaP (human prostate cancer cell line), MCF-7 (human breast carcinoma cell line), HTB-126 (human breast carcinoma cell line), HeLa (HPV 18 positive human cervical carcinoma cell line), Caski (HPV 16 positive human cervical carcinoma cell line), SiHa (HPV 16 positive human cervical carcinoma cell line), HCT116 (human colon carcinoma cell line), KB (human oral cancer cell line), and ORL-48 (human oral cancer cell line) (16–19). However, the potential effect of the combination of BJOE with irradiation on ESCC has yet to be investigated.
Therefore, in the present study, the effect and mechanism of the combination of BJOE with irradiation on ESCC were preliminarily investigated in vitro and in vivo. MTT assay demonstrated that BJOE inhibited the proliferation of esophageal cancer cells in a concentration- and time-dependent manner, and this finding is consistent with that of Zhang et al (17). The dose-survival curves fitted the single-hit multiple-target model and the SERs of ECA109 was 1.66. The shoulder region of the survival curve was also shortened and the D0 value decreased. These findings demonstrate that BJOE enhanced the radiosensitivity of hypoxic ECA109 cells. γH2AX is closely associated with DNA double-strand breaks (DSB) (20). DSB is also directly associated with radiosensitivity. Immunofluorescent measurement further demonstrated that BJOE efficiently increased the radiosensitivity of hypoxic esophageal cancer cells. Majid et al (18) demonstrated that BJOE exhibits apoptosis-inducing activity on human oral cancer cell lines KB and ORL-48. Zhang et al (17) also described the pro-apoptotic function of BJOE on human acute myeloid leukemia cells U937 and HL-60. In the present study, similar results were obtained in ECA109 cells. BJOE + irradiation induced a markedly higher apoptotic rate of ESCC cells compared with the effects of irradiation alone. BJOE also increased the radiosensitivity of ESCC cells by enhancing radiation-induced apoptosis. The signaling mechanism by which BJOE increased the radiosensitivity of ESCC cells was examined through western blot analysis. Hypoxia stimulated the HIF-1α expression in ESCC cells. Thus, hypoxia contributes to tumor radiotherapy resistance. However, BJOE markedly downregulated HIF-1α expression. Taken together, these data suggested that BJOE may increase the radiosensitivity of ESCC cells by suppressing the activation of HIF-1α.
In vivo data also revealed that BJOE potentially enhanced tumor radiosensitivity by decreasing HIF-1α expression. In conclusion, the present study provided in vitro and in vivo evidence supporting the hypothesis that BJOE enhances the radiosensitivity of ESCC cells. This phenomenon is associated with the downregulation of HIF-1α expression. Therefore, BJOE is a potential radiotherapy sensitization agent due to its anti-hypoxia activity.
Glossary
Abbreviation
- BJOE
Brucea javanica oil emulsion
References
- 1.Chen W, He Y, Zheng R, Zhang S, Zeng H, Zou X, He J. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis. 2013;5:19–26. doi: 10.3978/j.issn.2072-1439.2013.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajduković J. HIF-1: A big chapter in the cancer tale. Exp Oncol. 2016;38:9–12. [PubMed] [Google Scholar]
- 3.Cavadas MA, Nguyen LK, Cheong A. Hypoxia-inducible factor (HIF) network: Insights from mathematical models. Cell Commun Signal. 2013;11:42. doi: 10.1186/1478-811X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th edition. Wiley-Blackwell; Oxford: 2010. [Google Scholar]
- 5.Tang CM, Yu J. Hypoxia-inducible factor-1 as a therapeutic target in cancer. J Gastroenterol Hepatol. 2013;28:401–405. doi: 10.1111/jgh.12038. [DOI] [PubMed] [Google Scholar]
- 6.Dhani N, Fyles A, Hedley D, Milosevic M. The clinical significance of hypoxia in human cancers. Semin Nucl Med. 2015;45:110–121. doi: 10.1053/j.semnuclmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic Reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like Vascular Endothelial Growth Factor (VEGF) J Biol Chem. 2014;289:3352–3364. doi: 10.1074/jbc.M113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Hernández-Lizoain JL, Baixauli J, Valentí V, Pardo F, et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem. 2011;22:634–641. doi: 10.1016/j.jnutbio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YR, Wei JL, Mo XF, Yuan ZW, Wang JL, Zhang C, Xie YY, You QD, Sun HP. Discovery and optimization of new benzofuran derivatives against p53-independent malignant cancer cells through inhibition of HIF-1 pathway. Bioorg Med Chem Lett. 2016;26:2713–2718. doi: 10.1016/j.bmcl.2016.03.112. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JC, Klausen C, Leung PC. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett. 2013;329:197–206. doi: 10.1016/j.canlet.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YY, Huang XE, Cao J, Xu X, Wu XY, Liu J, Xiang J, Xu L. Phase II study on Javanica oil emulsion injection (Yadanzi®) combined with chemotherapy in treating patients with advanced lung adenocarcinoma. Asian Pac J Cancer Prev. 2013;14:4791–4794. doi: 10.7314/APJCP.2013.14.8.4791. [DOI] [PubMed] [Google Scholar]
- 14.Nie YL, Liu KX, Mao XY, Li YL, Li J, Zhang MM. Effect of injection of Brucea javanica oil emulsion plus chemoradiotherapy for lung cancer: A review of clinical evidence. J Evid Based Med. 2012;5:216–225. doi: 10.1111/jebm.12001. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Huang XE, Tian GY, Cao J, Lu YY, Wu XY, Xiang J. Phase II study on safety and efficacy of Yadanzi® (Javanica oil emulsion injection) combined with chemotherapy for patients with gastric cancer. Asian Pac J Cancer Prev. 2013;14:2009–2012. doi: 10.7314/APJCP.2013.14.3.2009. [DOI] [PubMed] [Google Scholar]
- 16.Ji ZQ, Huang XE, Wu XY, Liu J, Wang L, Tang JH. Safety of Brucea javanica and cantharidin combined with chemotherapy for treatment of NSCLC patients. Asian Pac J Cancer Prev. 2014;15:8603–8605. doi: 10.7314/APJCP.2014.15.20.8603. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Yang JY, Zhou F, Wang LH, Zhang W, Sha S, Wu CF. Seed oil of Brucea javanica induces apoptotic death of acute myeloid leukemia cells via both the death receptors and the mitochondrial-related pathways. Evid Based Complement Alternat Med. 2011;2011:965016. doi: 10.1155/2011/965016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majid MZ, Zaini ZM, Razak FA. Apoptosis-inducing effect of three medicinal plants on oral cancer cells KB and ORL-48. ScientificWorldJournal. 2014;2014:125353. doi: 10.1155/2014/125353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Z, Zhang B, Huang Y, Qiu H, Chen P, Guo GF. Involvement of autophagy inhibition in Brucea javanica oil emulsion-induced colon cancer cell death. Oncol Let. 2015;9:1425–1431. doi: 10.3892/ol.2015.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res. 2013;753:24–40. doi: 10.1016/j.mrrev.2013.02.001. [DOI] [PubMed] [Google Scholar]