Abstract
The present study aimed to investigate the role of microRNA-96 (miR-96) in the proliferation, invasion and apoptosis of bladder cancer cell lines, and the associated mechanisms. The expression of miR-96 and human ether-à-go-go-related (HERG1) potassium channel in the normal uroepithelium SV-HUC-1 cell line, and bladder cancer T24 and 5637 cell lines were examined using reverse transcription-polymerase chain reaction or/and western blotting. Transfection with miR-96 inhibitor or scrambled control (SC) was used to study the biological activities of miR-96 in bladder cancer cell lines. MTT, flow cytometric and Transwell assays were applied to detect cell viability, apoptosis and invasion, respectively. A dual-luciferase reporter assay was applied to determine the association between miR-96 and HERG1 expression. As demonstrated, miR-96 was highly expressed in the two bladder cancer cell lines, particularly in T24 cells. Following transfection with miR-96 inhibitor, miR-96 expression was significantly reduced in the T24 cell line, compared with SC. The miR-96 inhibitor suppressed cell proliferation and invasion, promoted apoptosis and arrested the cell cycle at the G1 phase. Consistently, HERG1 was also highly expressed in the two bladder cancer cell lines at the mRNA and protein level, but not in the normal uroepithelium cell line. The miR-96 inhibitor also significantly decreased HERG1 expression compared with SC. The results of the dual-luciferase reporter assay indicated that miR-96 directly targeted wild-type HERG1. In conclusion, miR-96 inhibitor exhibited anticancer effects on bladder cancer cells by inhibiting proliferation and invasion of cells, and promoting their apoptosis. HERG1 was an important target of miR-96. These results provided experimental evidence supporting miR-96 as a therapeutic target for patients with bladder cancer.
Keywords: microRNA-96, human ether-à-go-go-related gene, bladder cancer cell lines, proliferation, apoptosis
Introduction
Bladder cancer is one of the most common types of malignant tumors of the urinary and reproductive system, including bladder urothelial cell carcinoma, squamous cell carcinoma, adenocarcinoma and rare small cell carcinoma worldwide. Of these subtypes, bladder urothelial cell carcinoma accounts for 90% of cases. A series of pathogenic factors affect the occurrence of bladder cancer, including aging, smoking and environmental pollution (1). The mortality rate of bladder cancer is increasing, even in childhood (1,2). The pathogenesis of bladder cancer is complex, and involves the activation of oncogenes and the inactivation of tumor suppressor genes. The imbalanced activation of oncogenes and tumor suppressor genes impairs the normal growth mechanism of cells, leading to unrestricted cell proliferation, and increased invasiveness (3,4).
MicroRNAs (miRNAs/miRs) are a family of endogenous non-coding small RNA molecules with a length of 21–25 nucleotides. miRNAs function primarily through the targeted degradation of mRNAs and inhibition of translation into proteins. Therefore, miRNAs have numerous biological activities (5,6). Notably, miRNAs perform critical functions in the occurrence and development of lung, liver, gastric, cervical, breast, and colon cancer (7,8). Direct evidence has also documented the abnormal expression of miRNAs in bladder cancer tissue. miR-96 is a conserved miRNA that may be involved in the occurrence and development of bladder cancer (9,10). Further research also demonstrated that miR-96 was highly expressed in bladder cancer tissue and the blood plasma obtained from patients with bladder cancer (11–13). Yamada et al (14) confirmed that miR-96 expression in urine samples from bladder urothelial carcinoma was significantly increased compared with that from healthy subjects. Therefore, miR-96 is considered to be an important tumor diagnostic marker for bladder cancer. However, to the best of our knowledge, the influence of miR-96 on tumor cell biology and its mechanisms have not been reported previously. In the present study, miR-96 was selected as the research target; the aim was to investigate the effects of miR-96 on bladder cancer cell proliferation, apoptosis, invasion and metastasis and underlying mechanisms.
Materials and methods
Cell culture
Human bladder cancer cell lines, T24 and 5637 (American Type Culture Collection, Bethesda, MD, USA) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 100 U/ml penicillin-streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in 5% CO2 at 37°C. SV-HUC-1, a normal uroepithelium cell line, was also obtained from the American Type Culture Collection, and was used as the control. Cells at 60% confluence were used in the following experiments.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted according to the instruction of the TRIzol kit (Takara Biotechnology Co., Ltd., Dalian, China) and the purity of RNA was confirmed by optical density (OD) at 280/260 nm. Following this, RNA was amplified using a one-step RT-PCR kit (Dalian Baosheng Biological Engineering Co., Ltd., Dalian, China) and PCR products were detected using 2% agarose gel electrophoresis. The primers were added into a 25-µl PCR reaction system following a protocol of 94°C denaturation for 45 sec, 59°C annealing for 45 sec and 72°C extension 60 sec for 35 cycles. The primers used are as follows: miR-96 forward, 5′-TTTGGCACTAGCACATT-3′ and reverse, 5′-TTTGGCACTAGCACATT-3′; human ether-à-go-go-related (HERG1) potassium channel forward, 5′-TCCAGCGGCTGTACTCGGGC-3′ and reverse, 5′-TGGACCAGAAGTGGTCGGAGAACTC-3′; and GAPDH forward, 5′-AGCCACATCGCTCAGACA-3′ and reverse 5′-TGGACTCCACGACGTACT-3′.
Cell transfection
When the confluence of T24 cells reached 50–70%, Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.) was applied to assist the transfection of scrambled control (SC) and miR-96 inhibitor. Then, 6 h later, the medium was changed to DMEM. The miR-96 inhibitor (5′-AGCAAAAAUGUGCUAGUGCCAAA-3′) (1 µl) and SC (5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized using Shanghai GenePharma Co., Ltd. (Shanghai, China). Cells were then cultured in DMEM containing 10% FBS for another 48 h. RT-PCR was applied to detect miR-96 expression.
MTT assay
T24 cells were seeded in 96-well plates. When the cell confluence reached 50–70%, Lipofectamine 2000 was applied to transfect cells with SC and miR-96 inhibitor, as aforementioned. At 6 h later, the medium was changed into DMEM. The cells were then cultured in DMEM containing 10% FBS for another 24, 48 and 72 h. An MTT assay was utilized to detect cell proliferation, as previously described (15). In total, 150 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to each well in order to dissolve formazan. The optical density (OD) was determined using a microplate reader at 570 nm.
Flow cytometry
Following transfection with miR-96 inhibitor and SC for 48 h, the T24 cells were collected for Annexin V-fluorescein isothiocyanate/propidium iodide (PI) staining using the Annexin V-FITC Apoptosis Detection kit (cat. no. C1062; Beyotime Institute of Biotechnology, Ningbo, China) and the proportion of apoptotic cells was detected within 1 h using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed using CellQuest Pro (version 5.1; BD Biosciences, Franklin Lakes, NJ, USA). Following transfection for 48 h, the cells were collected for PI staining and the cell cycle distribution was assessed by FACSCalibur (BD Biosciences) within 1 h of staining.
Transwell assay
Following transfection with miR-96 inhibitor and SC for 48 h, T24 cells (3×103) were digested and seeded into the upper chamber of Transwell with one-day starvation of FBS (Hyclone; GE Healthcare Life Sciences). The lower chamber contained DMEM with 10% FBS. the cells were seeded in the upper chamber. The lower chamber contained only DMEM. At 48 h after seeding, the cells in lower chamber were fixed in 4% paraformaldehyde at room temperature for 30 min and stained with 1% crystal violet for 5 min at room temperature. Images were captured using a light microscope (magnification, ×200). At least five fields in each image were counted.
Western blotting
Following transfection with miR-96 inhibitor or SC for 48 h, the cells were collected for biochemical experiments. Protein concentration was quantified using the BCA method. An equal amount of protein per lane (20 µg) separated using 10% SDS-PAGE. Following electrophoresis, proteins were transferred to a nitrocellulose membrane. Non-specific protein binding was blocked with 4% non-fat milk (2 h at room temperature). Membranes were incubated with anti-HERG1 (1:1,000; cat. no. ab196301; Epitomics; Abcam, Cambridge, MA, USA) and anti-GAPDH (1:1,000; cat. no. AG019; Beyotime Institute of Biotechnology, Haimen, China) primary antibodies. Subsequent to rinsing with 0.1% phosphate-buffered saline (0.1% Tween-20), the membranes were incubated with a horseradish peroxidase-labeled secondary antibody (1:100; cat. no. ab131368; Abcam) at room temperature for 2 h. The signal was detected using an Enhanced Chemiluminescence Detection kit (GE Healthcare, Chicago, IL, USA) and scanned using ChemiDoc™ XRS (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The densities of the blots were analyzed using Quantity One analysis (version 1.4.6; Bio-Rad Laboratories, Inc.).
Dual-luciferase reporter assay
T24 cells (3×105) were seeded into 24-well plates and transfected with miR-96 and/or plasmid HERG1 using Lipofectamine 2000 according to the manufacturer's protocol, as aforementioned. Plasmids (cat. no. 60847; Wuhan Miaoling Biotech Science Co., Ltd., Wuhan, China) encoding an effective sequence to overexpress wild-type or mutant HERG1 were constructed. Experiments were divided into four groups: miR-96 inhibitor + wild-type HERG1 [pGL-ENNRA 3′-untranslated region (3′UTR)-Wt], SC + wild-type HERG1, miR-96 inhibitor + mutated HERG1 (pGL-ENNRA 3′UTR-Mut) and SC + mutated HERG1 (Shanghai Jima Industrial Co., Ltd., Shanghai, China). A Dual-Luciferase® Reporter Assay system (cat. no. E1960; Promega Corporation, Madison, WI, USA) was used to detect luciferase activity. Following transfection for 24 h, the medium was removed, cells were washed with PBS and 100 µl passive lysis buffer (provided by the Dual-Luciferase® system) was added and incubated for 10 min. Following centrifugation (11,656 × g) for 10 min at 4°C and the supernatant was collected. Next, 20 µl supernatant and 100 µl luciferase assay reagent were mixed. Firefly luciferase activity was determined using a Glomax 20/20 fluorescence luminometer. Next, 100 µl Stop & Glo reagent (provided by the Dual-Luciferase system) was added to detect Renilla luciferase activity, to which the data were normalized.
Statistical analysis
The data are presented as the mean ± standard deviation. Statistical analyses of the data were performed using one-way analysis of variance followed by a Bonferroni post hoc test or unpaired Student's t-test (version 17; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-96 is highly expressed in bladder cancer cell lines
As presented in Fig. 1, miR-96 was highly expressed in T24 and 5,637 cells, particularly in T24 cells. As a control, miR-96 was rarely expressed in the uroepithelium SV-HUC-1 cell line. In the subsequent experiments, T24 was selected as the cell line to be used.
Figure 1.

miR-96 is highly expressed in bladder cancer cell lines. **P<0.01 vs. SV-HUC-1 cells. miR-96, microRNA-96.
Transfection with miR-96 inhibitor suppresses cell proliferation, induces apoptosis, arrests cell cycle and inhibits cell invasion
Following transfection with miR-96 inhibitor for 6 h, expression miR-96 was markedly reduced (Fig. 2A). The effects of miR-96 inhibitor transfection on cell viability were assessed. As demonstrated using an MTT assay, cell viability was significantly reduced in cells transfected with the miR-96 inhibitor 24, 48 and 72 h after transfection, compared with those transfected with SC (Fig. 2B). Following transfection with miR-96 inhibitor for 48 h, the proliferation inhibition was ~50%, and therefore, the 48-h time point was selected for the other experiments. The proportion of apoptosis was detected using Annexin V-FITC/PI staining. Compared with cells transfected with SC, miR-96 inhibitor significantly increased the apoptosis rate (Fig. 2C and Table I). PI staining revealed that transfection with the miR-96 inhibitor arrested the cell cycle at the G1 phase (Fig. 2D and Table I). In addition, the Transwell assay demonstrated that transfection with a miR-96 inhibitor markedly decreased the cell invasion rate (Fig. 2E).
Figure 2.
Effect of miR-96 inhibitor on cell biological activities in T24 cells. (A) miR-96 inhibitor decreased miR-96 expression. (B) miR-96 inhibitor suppressed cell proliferation. (C) miR-96 inhibitor induced apoptosis. (D) miR-96 inhibitor arrested cell cycle. (E) miR-96 inhibitor prohibited cell invasion (magnification, ×200). **P<0.01 vs. SC. miR-96, microRNA-96; SC, scrambled control; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Table I.
miR-96 inhibitor arrests the cell cycle at the G1 phase and induces apoptosis in T24 cells.
| Cell cycle, % | Apoptosis, % | ||||
|---|---|---|---|---|---|
| Group | G1 | S | G2 | Early | Late |
| SC | 68.38±6.69 | 20.13±2.01 | 11.49±1.15 | 3.35±0.32 | 8.29±1.83 |
| miRI-96 | 87.37±8.46a | 6.13±0.56a | 6.50±0.58a | 12.28±0.28a | 19.46±1.92a |
P<0.01, compared with SC. SC, scrambled control; miRI-96, microRNA-96 inhibitor.
HERG1 is highly expressed in bladder cancer cell lines
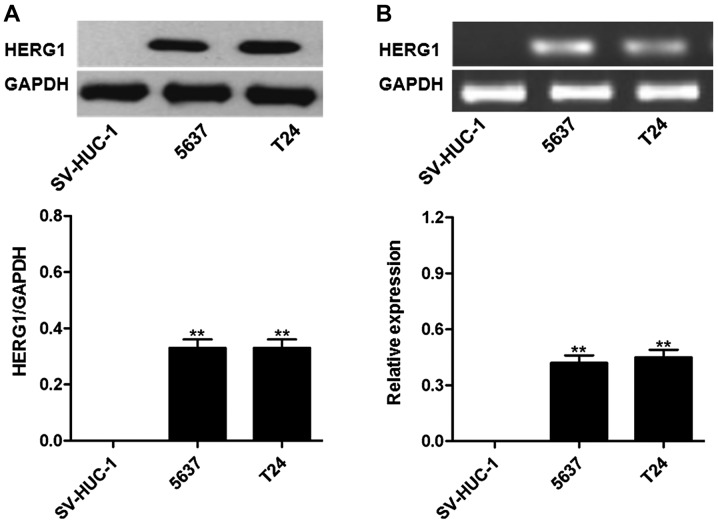
As presented in Fig. 3, HERG1 protein and mRNA was highly expressed in the bladder cancer T24 and 5,637 cell lines, but not in uroepithelium SV-HUC-1 cells.
Figure 3.
HERG1 is highly expressed in bladder cancer cell lines. (A) Protein level; (B) mRNA level. **P<0.01 vs. SV-HUC-1 cells. HERG1, human ether-à-go-go-related.
miR-96 inhibitor regulates HERG1 expression in T24 cells
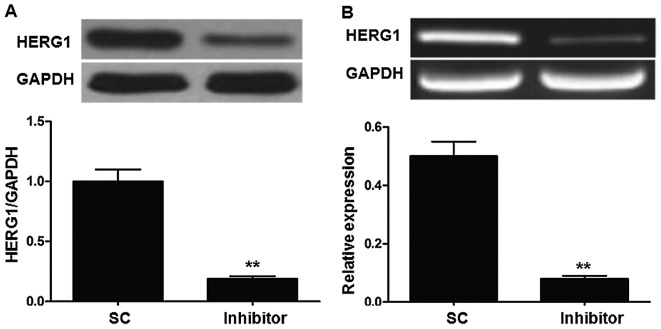
As miR-96 and HERG1 were highly expressed in bladder cancer cell lines, whether HERG1 was regulated by the expression of miR-96 was investigated. The T24 cell line was selected for use in these experiments. As demonstrated in Fig. 4, transfection with the miR-96 inhibitor significantly decreased HERG1 expression at the mRNA and protein level. To assess the direct regulation of HERG1 by miR-96, a dual-luciferase reporter assay was performed. As demonstrated in Fig. 5, transfection with the miR-96 inhibitor significantly decreased the relative fluorescence in the group transfected with the wild-type HERG1 3′UTR compared with the SC group.
Figure 4.
miR-96 inhibitor suppresses HERG1 expression in the T24 cell line. (A) Protein level; (B) mRNA level. **P<0.01 vs. SC. miR-96, microRNA96; HERG1, human ether-à-go-go-related; SC, scrambled control.
Figure 5.

Luciferase report assay reported the association between miR-96 and HERG1. **P<0.01 vs. Pgl-HERG1 3′UTR-Wt. miR-96, microRNA96; HERG1, human ether-à-go-go-related; 3′UTR, 3′-untranslated region; Wt, wild-type.
Discussion
The pathogenesis of cancer is a complex process; various aspects affect its genesis and development. A commonly held view is that the imbalanced expression of oncogenes and tumor suppressor genes contributes to tumor cell proliferation, and invasion. Genes promoting the tumor development are known as oncogenes and the opposite are considered tumor suppressor genes (16). miRNAs are a class of highly conserved small RNAs that bind the 3′-UTR region of its target gene and regulate the expression of target genes. The present study demonstrated that miR-96 was a potential pro-oncogenic factor in bladder cancer cells. Inhibition of miR-96 expression was able to suppress cellular proliferation and invasion, promote apoptosis, and arrest the cell cycle at the G1 phase.
Recent studies have indicated that miRNAs function either as oncogenes or tumor suppressor genes, regulating the proliferation, apoptosis, differentiation, migration and invasion of tumor cells (17,18). miR-96 belongs to the miR-183 family, the other two members of which are miR-182 and miR-183. These three miRNAs are found at similar locations in the genome. miR-96 is located at human chromosome locus 7q32.2, and the amplification of human chromosome 7 is important in lung cancer (19), hepatocellular carcinoma (20), prostate cancer (21), gastric cancer (22), esophageal cancer (23), breast cancer (24) and other types of tumor (9–13). miR-96 is highly expressed in those types of cancer and has been considered to be an oncogenic miRNA (25). Han et al (9) applied the gene expression microarray to validate that miR-96 expression was upregulated in bladder cancer tissue. Yoshino et al (13) also confirmed that miR-96 was highly expressed in bladder cancer tissue. Scheffer et al (12) confirmed that the expression of 22 miRNAs in bladder cancer tissues was upregulated, including miR-96. Kriebel et al (11) reported that the expression of plasma miR-96 in patients with bladder cancer was significantly increased. Eissa et al (26) demonstrated that miR-96 was highly expressed in bladder cancer tissues, but not in benign bladder tumors. An additional report stated that miR-96 levels in the urine samples of patients with bladder cancer were increased (14). Therefore, it appears that miR-96 may serve important roles in the development and progression of bladder cancer. The present study demonstrated that miR-96 expression was upregulated in bladder cancer cell lines. These cell lines served as in vitro models, allowing for the study of the biological activity of miR-96 in bladder cancer.
In the present study, RT-PCR was used to detect miR-96 expression, which demonstrated high expression of miR-96 in normal bladder cells, particularly in bladder cancer cells. In the subsequent experiments, transfection with miR-96 inhibitor was found to suppress the cell proliferation and invasion, promote apoptosis and arrest the cell cycle at the G1 phase. Tumor cells must complete the cell cycle to replicate. Thus, cells that do not pass the G1 checkpoint ‘loop out’ of the cell cycle and into a resting state termed the G0 phase. Data from the present study were consistent with a previously published study, in which it was verified that transfection with miR-96 inhibitor arrested the Panc-1 cells at the G1 phase and inhibited cellular proliferation (27). Apoptosis is a process of programmed cell death that occurs in multicellular organisms. In tumor cells, apoptosis is impaired, leading to unrestricted cell proliferation (28). Apoptosis is a method of eliciting cell death of cancer cells during chemotherapy or radiotherapy (29). The present study also found that miR-96 inhibitor promoted apoptosis of bladder cancer cells. In addition, transfection with the miR-96 inhibitor also prohibited the migration of bladder cancer cells. Consistently, Yu et al (30) also verified that transfection with the miR-96 inhibitor suppressed the invasion of renal cancer cell lines Caki-1 and 786-O.
Previous studies indicated that potassium ion channels serve important roles in the process of cellular proliferation (31,32). In addition, calcium and chloride channels also have roles in the occurrence and development of tumors (33). The HERG1 channel is a potassium ion channel and a member of the fast delayed rectifier voltage-gated potassium ion family. HERG1 is located on chromosome 7q35-36 and was identified by Warmke and Ganetzky in 1994 (34) in Drosophila and in mammals. HERG1 consists of six transmembrane proteins, and is inward-rectifying and ion-selective. HERG1 regulates the entry of calcium into the cell, promotes calcium second messenger signaling transduction, regulates cytoskeletal changes, and alters cellular proliferation and migration (31,32). HERG1 was initially reported to serve important roles in the process of repolarizing cardiac membrane potential (32). HERG1 was identified to be highly expressed in various tumor tissues, and was closely associated with tumor cell proliferation, apoptosis, differentiation, migration and invasion (35–37).
Downregulation of HERG1 in tumor cells can inhibit the proliferation, migration and invasion of tumor cells, all of which are malignant biological activities (38). The present study screened the expression of HERG1 in bladder cancer T24 and 5,637 cell lines, and the normal uroepithelium SV-HUC-1 cell line. The results of the current study revealed that HERG1 expression was significantly increased in bladder cancer cell lines compared with that in SV-HUC-1 cells. Bioinformatic analysis has demonstrated that HERG1 may be a target gene of miR-96 (39). The present study also utilized a dual luciferase reporter assay to verify that HERG1 was a target gene of miR-96. In addition, results of western blot analysis and RT-PCR also revealed that miR-96 inhibitor significantly reduced HERG1 expression.
The present study demonstrated that miR-96 was highly expressed in bladder cancer cell lines. miR-96 inhibition resulted in a number of anticancer effects on bladder cancer cells, including inhibition of proliferation and invasion, and promotion of apoptosis. Notably, evidence revealed that HERG1 was a target of miR-96. These results provided experimental evidence that support the role of miR-96 as a therapeutic target for bladder cancer.
References
- 1.Gan X, Lin X, He R, Lin X, Wang H, Yan L, Zhou H, Qin H, Chen G. Prognostic and clinicopathological significance of downregulated p16 expression in patients with bladder cancer: A systematic review and meta-analysis. Dis Markers. 2016;2016:5259602. doi: 10.1155/2016/5259602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, Kaag M, Fransen van de Putte EE, Horenblas S, Drabick JJ. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: A systematic review and two-step meta-analysis. Oncologist. 2016;21:708–715. doi: 10.1634/theoncologist.2015-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauberg Evelyne CC, de la Rosette JJ, de Reijke TM. Emerging optical techniques in advanced cystoscopy for bladder cancer diagnosis: A review of the current literature. Indian J Urol. 2011;27:245–251. doi: 10.4103/0970-1591.82845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahmy N, Lazo-Langner A, Iansavichene AE, Pautler SE. Effect of anticoagulants and antiplatelet agents on the efficacy of intravesical BCG treatment of bladder cancer: A systematic review. Can Urol Assoc J. 2013;7:E740–E749. doi: 10.5489/cuaj.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizuguchi Y, Takizawa T, Yoshida H, Uchida E. Dysregulated miRNA in progression of hepatocellular carcinoma: A systematic review. Hepatol Res. 2016;46:391–406. doi: 10.1111/hepr.12606. [DOI] [PubMed] [Google Scholar]
- 6.Wang QX, Zhu YQ, Zhang H, Xiao J. Altered MiRNA expression in gastric cancer: A systematic review and meta-analysis. Cell Physiol Biochem. 2015;35:933–944. doi: 10.1159/000369750. [DOI] [PubMed] [Google Scholar]
- 7.Gambari R, Brognara E, Spandidos DA, Fabbri E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Nuew trends in the development of miRNA therapeutic strategies in oncology (Review) Int J Oncol. 2016;49:5–32. doi: 10.3892/ijo.2016.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava K, Srivastava A. Comprehensive review of genetic association studies and meta-analyses on miRNA polymorphisms and cancer risk. PLoS One. 2012;7:e50966. doi: 10.1371/journal.pone.0050966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han Y, Chen J, Zhao X, Liang C, Wang Y, Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One. 2011;6:e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Liu K, Wang Y, Xu Z, Meng J, Gu S. Upregulation of microRNA-96 and its oncogenic functions by targeting CDKN1A in bladder cancer. Cancer Cell Int. 2015;15:107. doi: 10.1186/s12935-015-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriebel S, Schmidt D, Holdenrieder S, Goltz D, Kristiansen G, Moritz R, Fisang C, Müller SC, Ellinger J. Analysis of tissue and serum microRNA expression in patients with upper urinary tract urothelial cancer. PLoS One. 2015;10:e0117284. doi: 10.1371/journal.pone.0117284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffer AR, Holdenrieder S, Kristiansen G, von Ruecker A, Müller SC, Ellinger J. Circulating microRNAs in serum: Novel biomarkers for patients with bladder cancer? World J Urol. 2014;32:353–358. doi: 10.1007/s00345-012-1010-2. [DOI] [PubMed] [Google Scholar]
- 13.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10:396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 14.Yamada Y, Enokida H, Kojima S, Kawakami K, Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N, Nakagawa M. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: Correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 2011;102:522–529. doi: 10.1111/j.1349-7006.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu G, Wang X, Wu S, Li Q. Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int. 2012;60:400–408. doi: 10.1016/j.neuint.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Lv Q, Xue Y, Li G, Zou L, Zhang X, Ying M, Wang S, Guo L, Gao Y, Li G, et al. Beneficial effects of evodiamine on P2X(4)-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int Immunopharmacol. 2015;28:1044–1049. doi: 10.1016/j.intimp.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra S, Mulders PF, Schalken JA. Clinical use of novel urine and blood based prostate cancer biomarkers: A review. Clin Biochem. 2014;47:889–896. doi: 10.1016/j.clinbiochem.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Kaboli PJ, Rahmat A, Ismail P, Ling KH. MicroRNA-based therapy and breast cancer: A comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharmacol Res. 2015;97:104–121. doi: 10.1016/j.phrs.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Li Q, Li W, Zheng T, Zhao S, Liu Z. MiR-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells. Mol Cell Biochem. 2014;390:155–160. doi: 10.1007/s11010-014-1966-x. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Wang Y. MiR-96 targets SOX6 and promotes proliferation, migration and invasion of hepatocellular carcinoma. Biochem Cell Biol. 2017 Sep 11; doi: 10.1139/bcb-2017-0183. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Zhong J, Guo B, Zhu Q, Liang H, Wen N, Yun W, Zhang L. miR-96 promotes the growth of prostate carcinoma cells by suppressing MTSS1. Tumour Biol. 2016;37:12023–12032. doi: 10.1007/s13277-016-5058-2. [DOI] [PubMed] [Google Scholar]
- 22.Cao LL, Xie JW, Lin Y, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Huang CM. miR-183 inhibits invasion of gastric cancer by targeting Ezrin. Int J Clin Exp Pathol. 2014;7:5582–5594. [PMC free article] [PubMed] [Google Scholar]
- 23.Xia H, Chen S, Chen K, Huang H, Ma H. MiR-96 promotes proliferation and chemo- or radioresistance by down-regulating RECK in esophageal cancer. Biomed Pharmacother. 2014;68:951–958. doi: 10.1016/j.biopha.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Hong Y, Liang H, Uzair-Ur-Rehman, Wang Y, Zhang W, Zhou Y, Chen S, Yu M, Cui S, Liu M, et al. miR-96 promotes cell proliferation, migration and invasion by targeting PTPN9 in breast cancer. Sci Rep. 2016;6:37421. doi: 10.1038/srep37421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Liang AJ, Fan YP, Huang YR, Zhao XM, Sun Y, Chen XF. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget. 2016;7:42805–42825. doi: 10.18632/oncotarget.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eissa S, Matboli M, Essawy NO, Kotb YM. Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumour Biol. 2015;36:9545–9552. doi: 10.1007/s13277-015-3722-6. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Du X, Tai S, Zhong X, Wang Z, Hu Z, Zhang L, Kang P, Ji D, Jiang X, et al. GPC1 regulated by miR-96-5p, rather than miR-182-5p, in inhibition of pancreatic carcinoma cell proliferation. Int J Mol Sci. 2014;15:6314–6327. doi: 10.3390/ijms15046314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 29.Croce CM, Reed JC. Finally, an apoptosis-targeting therapeutic for cancer. Cancer Res. 2016;76:5914–5920. doi: 10.1158/0008-5472.CAN-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu N, Fu S, Liu Y, Xu Z, Liu Y, Hao J, Wang B, Zhang A. miR-96 suppresses renal cell carcinoma invasion via downregulation of Ezrin expression. J Exp Clin Cancer Res. 2015;34:107. doi: 10.1186/s13046-015-0224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He FZ, McLeod HL, Zhang W. Current pharmacogenomic studies on hERG potassium channels. Trends Mol Med. 2013;19:227–238. doi: 10.1016/j.molmed.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Sanguinetti MC. HERG1 channel agonists and cardiac arrhythmia. Curr Opin Pharmacol. 2014;15:22–27. doi: 10.1016/j.coph.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litan A, Langhans SA. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015;9:86. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals; Proc Natl Acad Sci USA; 1994; pp. 3438–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lastraioli E, Lottini T, Bencini L, Bernini M, Arcangeli A. hERG1 potassium channels: Novel biomarkers in human solid cancers. Biomed Res Int. 2015;2015:896432. doi: 10.1155/2015/896432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lastraioli E, Perrone G, Sette A, Fiore A, Crociani O, Manoli S, D'Amico M, Masselli M, Iorio J, Callea M, et al. hERG1 channels drive tumour malignancy and may serve as prognostic factor in pancreatic ductal adenocarcinoma. Br J Cancer. 2015;112:1076–1087. doi: 10.1038/bjc.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pillozzi S, Arcangeli A. Physical and functional interaction between integrins and hERG1 channels in cancer cells. Adv Exp Med Biol. 2010;674:55–67. doi: 10.1007/978-1-4419-6066-5_6. [DOI] [PubMed] [Google Scholar]
- 38.Zeng W, Liu Q, Chen Z, Wu X, Zhong Y, Wu J. Silencing of hERG1 gene inhibits proliferation and invasion, and induces apoptosis in human osteosarcoma cells by targeting the NF-κB pathway. J Cancer. 2016;7:746–757. doi: 10.7150/jca.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng J, Yu J, Pan X, Li Z, Chen Z, Zhang W, Wang B, Yang L, Xu H, Zhang G, Xu Z. HERG1 functions as an oncogene in pancreatic cancer and is downregulated by miR-96. Oncotarget. 2014;5:5832–5844. doi: 10.18632/oncotarget.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]