Abstract
The rapid and increasing use of the nanomaterials (NMs), nanostructured materials (NSMs), metal nanoclusters (MNCs) or nanocomposites (NCs) in the development of electrochemiluminescence (ECL) nanobiosensors is a significant area of study for its massive potential in the practical application of nanobiosensor fabrication. Recently, NMs or NSMs (such as AuNPs, AgNPs, Fe3O4, CdS QDs, OMCs, graphene, CNTs and fullerenes) or MNCs (such as Au, Ag, and Pt) or NCs of both metallic and non-metallic origin are being employed for various purposes in the construction of biosensors. In this review, we have selected recently published articles (from 2014–2017) on the current development and prospects of label-free or direct ECL nanobiosensors that incorporate NCs, NMs, NSMs or MNCs.
Keywords: electrochemiluminescence, label-free, nanostructure materials, nanomaterials, nanocomposites, nanobiosensors, metal nanoclusters, ordered mesoporous carbon, signal amplification
1. Introduction
1.1. Biosensors
A biosensor is a powerful and innovative analytical tool, which integrates a biological recognition element with a physio-chemical transducer to detect biological substances. The International Union of Pure and Applied Chemistry (IUPAC) defines a biosensor as “a device that uses biochemical reactions mediated by isolated enzymes, organelles or whole cells to detect the effects of chemical compounds by electrical, thermal or optical signals” [1,2]. The world’s first biosensor was invented in 1962 by Clark and Lyons to electrochemically measure glucose in biological samples [3]. The sensor utilized the glucose oxidase enzyme as a recognition element in order to detect reduction in oxygen levels or production of hydrogen peroxide. These levels would act as a read-out for glucose level in the body due to their correlation to each other [4,5].
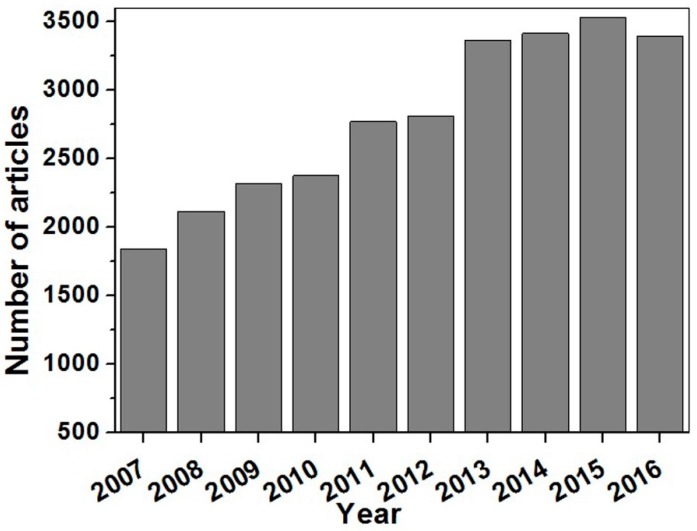
Since, their discovery Clark and Lyons, biosensors have gained a prominence that is evident by their extensive use in various fields. Figure 1 specifically shows the number of research articles that have been published on electrochemiluminescence (ECL) biosensors in the last 10 years. Some of the important applications of biosensors include medical diagnosis [6,7,8,9,10,11], the food industry [12,13,14,15,16,17], biomedicine [18], forensic science [19,20,21] environmental monitoring [16,22], water characteristic testing [23], defense and the military [24], as well as security [25]. In real-world settings, biosensors have replaced many tedious, expensive and complex conventional analytical techniques. Perhaps another reason for their popularity amongst researchers across the globe is that they create a way to unite different fields of biology, chemistry, physics, material sciences, nanosciences, electronics and optics [6,7,19] to allow for a multidisciplinary cooperation.
Figure 1.
Number of articles published on ECL biosensors in the last 10 years with the keyword search of “electrochemiluminescence biosensor”, either in the title or in the abstract [26].
1.2. Biosensor Components
A biosensor is a device that consists of two fundamental components: a bioreceptor and a transducer [2]. A bioreceptor is a biological factor that recognizes and consolidates biomolecules or the target analytes that is in intimate contact with the transducer [27]. Bioreceptors may contain nucleic acids (DNA or RNA), antibodies, enzymes, cell receptors, organelles, synthetic receptors, microorganism, tissues, and organs [28,29].
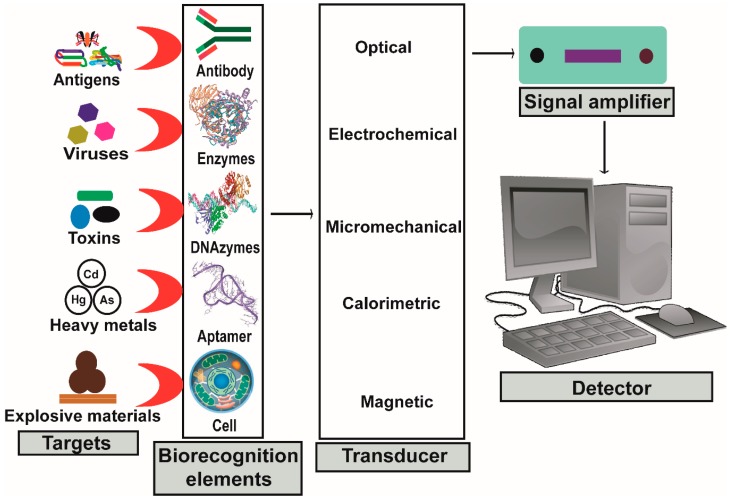
The other important component of biosensor is a transducer, a physicochemical detector that measures the physical change that occurs between the analyte and the bioreceptor and then transforms that energy into a measurable electrical signal equivalent to a single analyte. The transducer is connected to the detector system through a connector whereby signals become amplified, analyzed and converted to concentration units. These data are then displayed by the device or stored within the device for later extraction [30]. All fundamental components of the biosensor are illustrated in Figure 2.
Figure 2.
Illustrated scheme of a biosensor and its fundamental components.
1.3. Construction of Biosensors
1.3.1. Immobilization of Biomolecules
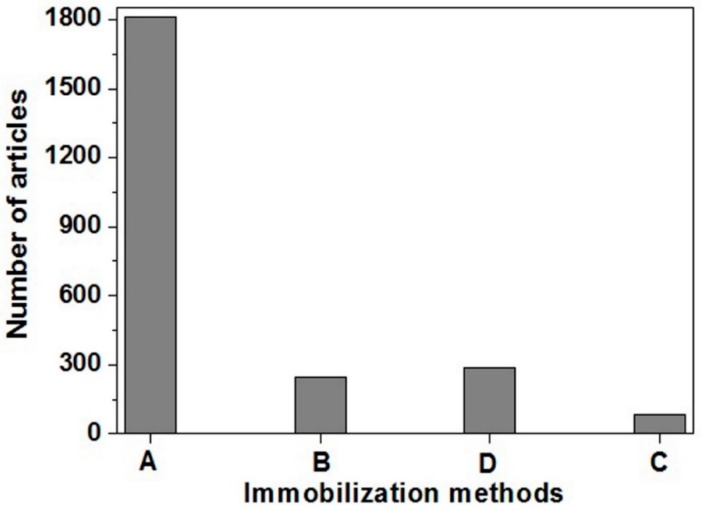
The first and foremost step in the construction of a biosensor is the adherence of biomolecules onto the transducer, a step referred to as immobilization. The two most common methods of immobilization are physical methods (such as adsorption and encapsulation) [6,7,31,32,33] and chemical methods (such as crosslinking and covalent bonding) [34,35,36,37]. Each technique has its own advantages and limitations. The most frequently used technique for immobilization of bioreceptor is adsorption. Figure 3 illustrates the number of articles which utilized adsorption techniques in comparison to encapsulation, cross-linking and covalent bonding [6,7,31,38]. Therefore, in this review we have focused on the adsorption-based ECL nanobiosensors. The adsorption method of immobilization is a simple and straightforward technique. The process is quick, without requiring any complex reactions. Moreover, no hazardous chemicals are used, ensuring that the functionality of biomolecules is not changed [6,7,31]. An illustration depicting immobilized biomolecules on transducer “without nanocomposite modification” and “with nanocomposites modification” via adsorption are shown in Figure 4A,B, respectively. Covalent bonding and cross-linking techniques via chemical immobilization method of biomolecules are respectively shown in Figure 4C,D. During encapsulation, biomolecules are first enclosed in a porous polymer matrix followed by immobilization on the transducer surface, as shown in Figure 4E.
Figure 3.
Research articles published in the last 10 years with the following keyword search: (A) “biosensor using adsorption,” (B) “biosensor using encapsulation,” (C) “biosensor using crosslinking” and (D) “biosensor using covalent bonding”, either in the title or in the abstract [26].
Figure 4.
Illustrated scheme of event when physically adsorbing biomolecules on the surface of (A) transducer; (B) nanocomposites-modified transducer; (C) covalently attaching biorecognition elements on the transducer; (D) crosslinking between biorecognition elements and the transducer; and (E) biorecognition elements entrapment in polymer matrix.
1.3.2. Transducer
The selection of the suitable biomolecule immobilization method is usually followed by deciding on the transducer for an effective bio/immunosensor. A transducer is a component which converts chemical [6,31], optical [7,15], mass [39] or temperature [40] signal into an electrical signal (see Table 1). Henceforth, depending on the types of transducer or the signal transduced, biosensors are mainly divided into electrochemical, optical, thermal, and piezoelectric devices. Essentially, an electrochemical biosensor generates an electrical signal when analytes interact with biomolecules to produce chemical changes on the chemically active area of the electrodes in the presence of an electrochemical probe. An optical biosensor, on the other hand, sense the change in the properties of electromagnetic wave when analytes communicate with biomolecules. Some examples of optical biosensors include those that are: surface plasmon resonance-based, fluorescence-based, chemiluminescence-based, and ECL-based. Thermal biosensors convert temperature change into electrical signal when analytes and biomolecules interact, while piezoelectric biosensors detect the difference in mass due to formation of a complex between analytes and biomolecules.
Table 1.
Types of biosensor based on transducers/signals transduced.
It is clear that the immobilization of biomolecules and the accompanying transduction method are the fundamental components of biosensor fabrication. Hence, researchers are continuously introducing new combinations of biomolecule immobilization methods coupled with quick and sensitive transduction methods to fabricate biosensors with advanced analytical properties. Some important analytical parameters of biosensors that are progressively being improved include limit of detection, speed of response, signal amplification, sensitivity and specificity, detection range, selectivity, resistance to interference, stability, capacity for regeneration and cost-effectiveness. Henceforth, this review will cover the more recent research articles (from 2014–2017) whereby nanostructure materials (NSMs)- or nanomaterials (NMs)- or metal nanoclusters (MNCs)-based nanocomposites (NCs) have been utilized in the fabrication of ECL nanobiosensors.
2. Electrochemiluminescence
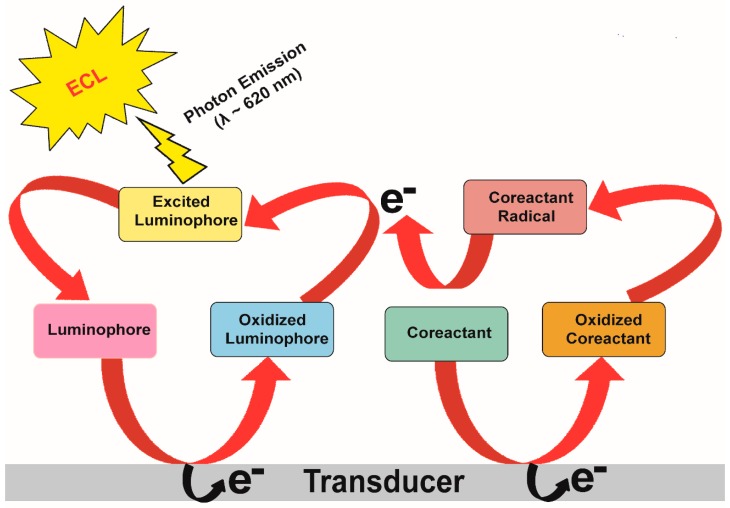
Electrochemiluminescence (ECL), alternatively known as electrogenerated chemiluminescence, is a technique whereby chemiluminescence is emitted upon electron-transfer reaction as depicted in Figure 5.
Figure 5.
Schematic illustration of ECL mechanism and its generation on electrode surface in presence of suitable luminophore and co-reactant.
ECL is highly versatile due to the ability to adjust these different parameters during the fabrication steps: potentials, currents, reaction time and electrode size. In terms of analytical ECL studies, co-reactant ECL predominates over its counterpart, annihilation ECL, due to the ease of ECL generation in a simple and environmentally safe aqueous solutions through a co-reactant pathway, as well as a wide range of ECL co-reactants to choose from. The method is, therefore, transferable to a wide range of assays for different purposes. ECL has diverse analytical applications, such as identifying co-reactants, ECL enhancers and ECL quenchers, or used in biomolecules detection, food and drug analysis, clinical diagnosis and environmental monitoring due to its high sensitivity, broad linear range and rapid analysis. ECL has also been coupled to other analytical techniques, such as flow injection analysis, high-performance liquid chromatography (HPLC), capillary electrophoresis, and micro total analysis (mTAS) [7,8,15,41,42,43]. The widespread use of ECL biosensors for biomolecule detection in various fields in the last decade has seen numerous adaptations incorporated in the fabrication of ECL biosensors, specifically to modify electrodes, ECL labels and ECL-emitting probes with different NSMs or NMs or MNCs and their composites. Although numerous review articles have been published on ECL and ECL biosensors [44,45,46,47,48,49,50,51,52,53,54], no recent review article includes the latest development on NSMs-, NMs- MNCs- and NCs-based ECL biosensors. Therefore, this review will specifically emphasize on the advancement in direct or label-free NSMs- or NMs- or MNCs nanocomposites-based ECL nanobiosensors from the year 2014 to 2017. NSMs- or NMs or MNCs-based nanocomposites are used for the fabrication of ECL immunosensors to enhance the following specifications: adsorption of recognition element on electrode, limit of detection, speed of response, signal amplification, sensitivity, detection range, selectivity, interference resistance, specificity, stability and cost-effectiveness. The main approach involves increasing the surface area and electron conductivity of the electrode surface. The selection of research articles cited in this review is arbitrary due to the large number of excellent and original research papers that have been published on this subject matter.
3. Mechanism of ECL Detection
ECL detection occurs by means of two well-known pathways [45,47,50,51,52,53,54]: (a) ion annihilation pathway and (b) co-reactant pathway.
(a) Ion annihilation pathway
Ion annihilation involves the generation of ECL by the formation of electrochemically generated and sufficiently stable intermediate cationic (R˙+) as well as anionic (R˙−) species by the emitter (R) at the electrode surface. These two oppositely charged radical ions annihilate one another to produce the excited state (R*) and the ground state (R). The excited state (R*) will emit light of wavelength hv to produce ECL and come to the ground state (R) as described in Scheme 1.
Scheme 1.
Ion annihilation pathways.
| R − e− |  |
R˙+ | (oxidation at the electrode surface) |
| R + e− |  |
R˙− | (reduction at the electrode surface) |
| R˙+ + R˙− |  |
R + R* | (annihilation process/formation of excited state) |
| R* |  |
R + hv | (emission of light) |
(b) Co-reactant pathway
The co-reactant pathway of ECL generation involves the presence of two species: emitter (R) and the co-reactant (C). Co-reactant ECL is generated by applying an anodic or cathodic potential in a solution containing the luminophore and the co-reactant. Therefore, both luminophore and co-reactant species can be oxidized or reduced at the electrode surface to form radical ions and intermediates species by changing the polarity of the applied potential. Finally, radical ions and intermediate species combined to form the excited state species, which emit light and can be detected by the ECL detector, such as a photomultiplier tube. Depending on the polarity of the applied potential, either highly reducing intermediate species are generated after electrochemical oxidation of co-reactant rendering the reaction as “oxidative-reduction”, or highly oxidizing intermediates are produced after electrochemical reduction rendering the reaction as “reductive-oxidation. The two possible mechanisms of the co-reactant ECL pathways are elucidated in Scheme 2 and Scheme 3 for (i) oxidative-reduction co-reactant ECL and (ii) reductive-oxidation co-reactant ECL, respectively.
-
(i)
Oxidative-reduction co-reactant ECL pathway
-
(ii)
Reductive-oxidation co-reactant ECL pathway
Scheme 2.
Oxidative-reduction co-reactant ECL pathways.
| R − e− |  |
R˙+ | (oxidation at the electrode surface) |
| C − e− |  |
C˙+ | (reduction at the electrode surface) |
| C + C |  |
R + C˙ | (homogenous chemical reactions) |
| C˙+ |  |
*Cr | (homogenous chemical reactions) |
| *Cr + R |  |
R˙− + P | (homogenous chemical reactions) |
| R˙− + R˙+ |  |
R + R* | Or R˙+ + *Cr
 R* + P (excited state formation species) R* + P (excited state formation species) |
| R* |  |
hv | (emission of light) |
Scheme 3.
Reductive-oxidation co-reactant ECL pathways. Here, *Cr and *Co are the co-reactant intermediates for the reducing and oxidizing agents, respectively, and P is the product associated with *Cr and *Co reactions, h is the Planck constant with a value of 6.634 × 10−34 J.s or 4.13 × 10−15 ev, v is the frequency (Hz) of the emitted light (v = c/λ, c = speed of light (3.00 × 108 ms−1), and λ is wavelength of the emitted light).
| R + e− |  |
R˙− | (reduction at the electrode surface) |
| C + e− |  |
C˙− | (reduction at the electrode surface) |
| R˙− + C |  |
R + C˙− | (homogenous chemical reactions) |
| C˙− |  |
*Co | (homogenous chemical reactions) |
| *Co + R |  |
R˙+ + P | (homogenous chemical reactions) |
| R˙− + R˙+ |  |
R + R* | Or R˙+ + *Co
 R* + P (excited state species formation) R* + P (excited state species formation) |
| R* |  |
hv | (emission of light) |
4. Nanostructure Materials, Nanomaterials, Metal Nanoclusters and Nanocomposite(s)
The current decade has seen tremendous development in nanosciences and nanotechnology in all areas of science and technology. Nanoscience or nanotechnology has gained particular importance in areas such as biomedical sciences [55], food industry [56], environment [57], catalysis [58], optics [59], and energy science [60]. To a large extent, this implicated the field of analytical chemistry, specifically in the fabrication of ECL nanobiosensors. The direct or indirect application of NSMs or NMs or MNCs as NCs mainly affect the limit of detection, sensitivity, range, selectivity, specificity and stability. Additionally, the use of NMs, NSMs and MNCs in NCs consequently results in the possibility of miniaturizing the devices with reduced volume of recognition element and sample needed, coupled with improved efficiency. NMs can enhance biosensor sensitivity by either enhancing the conductivity of the sensor platform or providing increased surface area for antigens or recognition elements due to their unique physical and chemical properties [7].
Figure 6 demonstrates the application of the NSMs or NMs or MNCs as nanocomposites being recently used in the fabrication of ECL nanobiosensors. The use of NSMs or NMs or MNCs have been previously reviewed in the context of ECL-based nanobiosensors fabrication [44,48,52,53,54]. However, in this particular review article, we have selected some of the more recent articles (from 2014 to 2017) that utilize metallic, magnetic (AuNPs, AgNPs and Fe3O4), quantum dots (CdS, CdSe, CdTe and graphene), carbon (ordered mesoporous carbons, graphene, carbon nano-onions, and carbon nanotubes) NSMs, NMs and MNCs (Au, Ag and Cu) composites in the fabrication of ECL nanobiosensors for point-of-care devices for diagnostic applications.
Figure 6.
Illustration of various nanostructure materials or nanomaterials or metal nanocluster or nanocomposite being used for the fabrication of ECL nanobiosensors (MNPs: Magnetic nanoparticles, CNOs: Carbon nano-onions, GR: Graphene, BNPs: Bimetallic nanoparticles, CNFs: Carbon nanofiber, CNTs: Carbon nanotubes, QDs: Quantum dots, NPs: Nanoparticles).
5. Metal- and Magnetic Nanocomposite-Based ECL Nanobiosensors
Due to the unique property of noble metals (gold and silver) and magnetic nanomaterials, they have been used as nanocomposites in the fabrication of ECL nanobiosensors [61,62,63]. Some of these unique physicochemical properties are increased surface area and mechanical strength, in addition to their substantial electronic and catalytic properties. These properties have been exploited in order to detect biomolecular recognition events through electrical signals or electrochemical transductions. This has driven the development of a new generation of electronic nanobiosensors devices, which rely on changes in the ohmic response of an electrical circuit or the flow of electrons arising from faradaic current generated by oxidation or reduction near the surface of an electrode in order to measure the presence or concentration of biological molecules [64]. Moreover, the large surface area and high mass transference of MNPs lead to their integration as transducer materials in the fabrication of nanobiosensors [65].
5.1. Gold Nanoparticles (AuNPs)
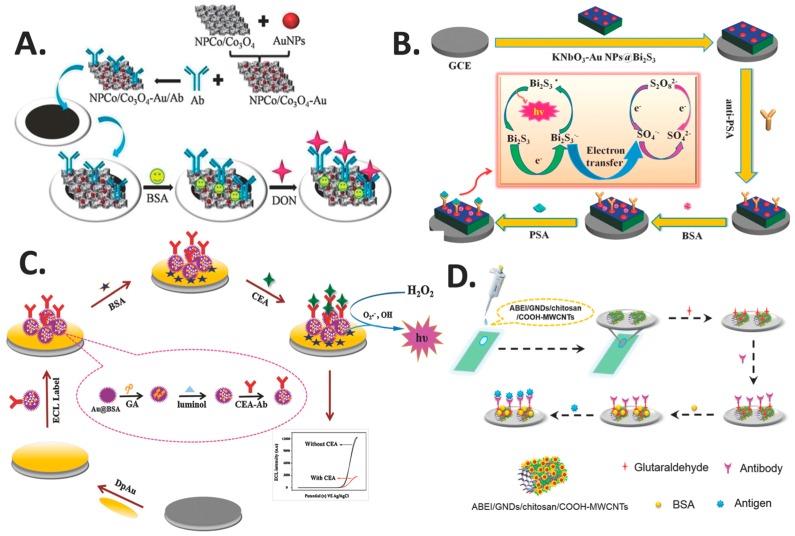
AuNPs is one of the most extensively studied NMs or NSMs in nanoscience and nanotechnology when compared to other metal-based nanomaterials. Monumental research and advanced applications of AuNPs have emerged only in the recent decade. Its highly favorable properties, which include a large surface area-to-volume ratio, unique optical and electronic properties, and easy surface modification, have contributed to the intensive focus on AuNPs from both academia and industry. Much effort has been devoted to tailoring the properties of AuNPs for specific biosensor applications [66]. AuNPs have been employed in ECL nanobiosensors as nanocomposites due their unique biocompatibility [67], high electrical conductivity, size-related electronic, magnetic and optical properties, catalysis and the ease by which they can self-assemble [68]. The unique properties of AuNPs-modified electrode interfaces lead to the development of novel ECL nanobiosensors with high sensitivity and good stability [7], which is mainly attributed to the increased surface area of the electrodes [69]. Table 2 summarizes the AuNPs-based NCs recently used in the fabrication of ECL-based direct or label-free nanobiosensors. Lv et al. fabricated an ECL nanobiosensor (Figure 7A) using AuNPs functionalized nonporous Co/Co3O4 (NPCo/Co3O4-Au) NCs to modify the electrodes for detecting mycotoxin deoxynivalenol (DON). NCs of NPCo/Co3O4 and AuNPs enhanced the conductivity of the electrodes and their catalytic properties for the luminescence of RuSi@Ru(bpy)32+, resulting in a highly sensitive ECL nanobiosensor. The ECL electrodes exhibited a wide linear range of activity for DON from 5 pg mL−1 to 100 ng mL−1, with the ability to detect very low concentration of DON at 1 pg mL−1 and displaying excellent stability and reproducibility [14]. In another study, Li et al. used AuNPs to prepare the NCs niobate-Au nanoparticles@bismuth sulphide (KNbO3-AuNPs@Bi2S3) to modify glassy carbon electrode (GCE) for the detection of prostate specific antigen (PSA) (Figure 7B). AuNPs were combined with Bi2S3 via the Au-S covalent bond and anti-PSA via Au-NH2 covalent bond without crosslinking. AuNPs elevated the electron transfer efficiency of the nanocomposite at the surface of the electrode, which in turn enhanced the sensitivity and improved both the ECL signal produced and the stability of the ECL nanobiosensor. The study reported that the ECL nanobiosensor exhibited high sensitivity, selectivity, good repeatability and long-term stability, with a wide detection range of 0.005 to 5 ng mL−1 and a low detection limit of 3 pg mL−1 [70].
Table 2.
Metals- and magnetic-NMs- or NSMs- or MNCs- or NCs-based ECL nanobiosensors.
| Electrode | NMs/NSMs | NCs | Function | Assay type | Bioanalyte | LOD | Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| GCE | AuNPs | MWCNTs-Cys-AuNPs | Signal amplification, reproducibility, high sensitivity, stability and cost-effective | Direct | Diphen-hydramine hydrochloride (DPH) | 6.7 × 10−9 M | 2 × 10−8–7.5 × 10−4 M | [72] |
| QDs-SPE | AuNPs | AuNPs@CNOs/Chitosan | High ECL, increased effective surface area, high anti-β2M binding, enhanced photons capture, high detection limit, wide linear range, long-term stability and good selectivity | Label-free | Beta-2 microglobulin (β2M) | 1 fg mL−1 | 1 fg mL−1–100 ng mL−1 | [7] |
| GCE | AuNPs | MWCNTs-Au | Signal amplification, sensitivity, selectivity, reproducibility, stability and low cost | Direct | Bisphenol A | 0.083 μM | 0.25–100 μM | [61] |
| GCE | AuNPs | Au@BSA nanoparticles | Large surface area, biocompatibility, specificity, stability, reproducibility, favorable selectivity, wide dynamic range and an ultralow detection limit | Label-free | CEACEA) | 0.0003 ng mL−1 | 0.001–200 ng mL−1 | [67] |
| GCE | AuNPs | AuNPs/ion liquid/hollowed TiO2 nano-shell | Specificity, repeatability, stability, storage, reproducibility, recovery for real sample test, ultralow detection limit, wide linear range, reliability, low-cost and on-site monitoring | Enzymatic | Cholesterol | 6.30 × 10−9 M | 8.33 × 10−9–4.17 × 10−7 M | [68] |
| GCE | AuNPs | NPCo/Co3O4–Au | Stability, reproducibility and immobilization of large amount of biomolecules | Label-free | DON | 1 pg mL−1 | 5 pg mL−1–100 ng mL−1 | [15] |
| GCE | AuNPs | MWCNTs-GO-Thi-Au | Enhanced ECL signal, high sensitivity, good selectivity and stability | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | AuNPs | KNbO3-AuNPs@Bi2S3 | Strong ECL signal, sensitivity, stability, long-term stability, acceptable selectivity, precision and accuracy | Label-free | PSA | 3 pg mL−1 | 0.0055 ng mL−1 | [70] |
| GCE | AuNPs | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, repeatability, selectivity, long-term stability, wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| GCE | AuNPs | AuNPs-CdSe QDs | Sensitivity, large surface area, rapid, acceptable precision, good stability, bioactivity and low-cost | Competitive | Phenylethanolamine A (PA) | 0.0047 ng mL−1 | 0.02 –50 ng mL−1 | [69] |
| GCE | AuNP | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity, good reproducibility, stability and repeatability | Direct | Dopamine (DA) | 0.067 µM | 0.20–70 µM | [75] |
| GCE | AuNPs | AuNPs-CdSe QDs | Rapid and ultrasensitive, good stability, specificity and fabrication reproducibility | Competitive | Clenbuterol (CLB) | 0.0084 ng mL−1 | 0.02–50 ng mL−1 | [76] |
| GCE | AuNPs | Fe3O4 (Au-FrGO) | Large ECL signal, surface area, biocompatibility, sensitive response, excellent stability, repeatability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | AuNPs | petal-like CdS/AuNPs | high sensitivity, stability, good selectivity and wide linear range | Label-free | Immunoglobulin E | 8.0 × 10−14 M | 5.0 × 10−13–1.0 × 10−9 M | [77] |
| ITO | Gold nanodots | ABEI/GNDs/chitosan/COOH-MWCNTs | Strong and stable ECL signal, selectivity, stable and reliable response, extremely high sensitivity, satisfactory recovery | Label-free | N-Terminal Pro-brain natriuretic peptide (NT-proBNP) | 3.86 fg mL−1 | 0.01–100 pg mL−1 | [71] |
| GCE | AgNPs | luminol-AgNPs@OMC | Fast, sensitive, specific, stable and reliable | Label-free | Aflatoxin B1 (AFB1) | 50 fg mL−1 | 0.1 pg mL−1–50 ng mL−1 | [62] |
| GCE | Au@Ag nanorods | Au and Ag Bimetallic | Catalytic, sensitive, stable, specific and reproducible | Label-free | CEA | 30 fg mL−1 | 0.1 pg mL−1–380 ng mL−1 | [78] |
| GCE | nanoFe3O4 | nanoFe3O4@GO | Good conductivity, magnetism, stability, reproducibility and regeneration | Label-free | Carbohydrate antigen 19-9 (CA19-9) | 0.0005 U mL−1 | 0.001–5 U mL−1 | [79] |
| GCE | Fe3O4 | Fe3O4 (Au-FrGO) | Large ECL signal, specific surface area, biocompatibility, sensitive response, excellent stability, repeatability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | Fe3O4 | (RGO)/Fe3O4/CdSe | Sensitivity, excellent reproducibility and stability, wide linear range and high selectivity | Label-free | Interleukin-6 (IL-6) | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | Fe3O4 | CdS-Fe3O4 | Sensitive response, wide linear range, low detection limit, rapid, specific, stable, reliable and ultrasensitive ECL | Label-free | Ochratoxin A (OTA) | 2 pg mL−1 | 0.01–100 ng mL−1 | [80] |
| GCE | Magnetic nanofibers/CNHs | Magnetic nanofibers-Fe3O4 | Excellent electrical conductivity, large surface areas, variable porosity, amplified ECL, acceptable precision, reproducibility, biocompatibility, sensitivity, low detection limit and stability | Label-free | AFB1 | 0.02 ng mL−1 | 0.05–200 ng mL−1 | [81] |
| Modified titanium ribbon | AuNCs | Au NCs/GR | High sensitivity, low detection limit, wide dynamic range, low toxicity, good regeneration and can be used with real samples | Direct | Pentachlorophenol (PCP) | 0.1 fM | 0.1 fM–0.1 nM | [82] |
| GCE | AuNCs | lum-AuNCs | 100-folds enhancement of ECL, high specificity, good stability and potential clinical application | Direct | Alkaline phosphatase (ALP) | 0.1 nM | 0.3–12 nM | [83] |
Figure 7.
AuNPs nanocomposite-based ECL nanobiosensors: (A) ECL nanobiosensor with gold functionalized nonporous CO/Co3O4. Reprinted from Ref. [14] with permission from Elsevier; (B) ECL nanobiosensor incorporating the nanocomposite of KNbO3-AuNPs@Bi2S3. Reprinted from Ref. [70] with permission from Elsevier; (C) ECL nanobiosensor utilizing the nanocomposite of Au@BSA. Reprinted from Ref. [67] with permission from Elsevier; and (D) ECL nanobiosensor with ABEI/GNDs/chitosan/COOH-MWCNTs. Reprinted from [71] with permission from the American Chemical Society.
In another report by Zhang et al., a novel ECL nanobiosensor that utilized multifunctionalized flower-like Au@BSA nanoparticles was created for the determination of tumor marker carcinoembryonic antigen (CEA). The Au@BSA nanoparticles provided a large surface area, good electrical conductivity and excellent biocompatibility as an ECL interface. Moreover, they are also ideal carriers for the immobilization of luminol molecules that act as signal probes, and CEA capture antibodies that act as molecule recognition probes (Figure 7C). The nanobiosensor was reported to achieve good stability, excellent reproducibility and favorable selectivity. A wide detection range of 0.001 to 200 ng mL−1 was exhibited by the nanobiosensor, with a low limit of detection of 0.0003 ng mL−1 [67]. In a separate report, another group fabricated an ECL nanobiosensor using N-(aminobutyl)-N-(ethylisoluminol) (ABEI)-functionalized gold nanodots/chitosan/multiwalled carbon nanotubes (ABEI/GNDs/chitosan/COOH-MWCNTs) nanocomposite-modified ITO electrodes. Grafting the nanocomposite onto the electrode was possible due to the film-forming property of the nanocomposite. This is followed by the conjugation of N-Terminal Pro-brain natriuretic peptide antibody (anti-NT-proBNP) onto the modified surface using glutaraldehyde (Figure 7D). Strong and stable ECL signal was produced by the nanobiosensor with a wide linear range of 0.01–100 pg mL−1 and a limit of detection of 3.86 fg mL−1, which is three orders lower than the electrochemistry method reported previously. The ECL nanobiosensor also demonstrated high sensitivity, high selectivity coupled to simple and fast analysis [71].
5.2. Silver Nanoparticles (AgNPs)
Among the noble metal nanomaterials, AgNPs are capable of facilitating electron transfer from the reaction center to the electrode surface, which makes them capable of higher intensity electron transfer. AgNPs have few desirable properties such as their ease-of-functionalization, good biocompatibility, ease of immobilization of biomolecules and its ability to increase ECL intensity of luminophore [62]. These facts have led AgNPs to be incorporated in ECL nanobiosensor fabrication. Some of the currently fabricated ECL nanobiosensors using AgNPs are summarized in Table 2. Lv et al. used luminol functionalized with silver nanoparticles loaded onto ordered mesoporous carbon (luminol-AgNPs@OMC) electrode to conjugate AFB1 antibody (anti-AFB1) onto the sensing platform for the ECL-based detection of the AFB1 (Figure 8A). The AgNPs were used to improve ECL signal, absorption capacity of antibody, and catalysis of the luminescence of luminol. The ECL nanobiosensor demonstrated fast, sensitive, specific, stable and reliable results for the detection of AFB1 with a linear range of 0.1 pg mL−1 to 50 ng mL−1 and a low detection limit of 50 fg mL−1. Luminol-AgNPs@OMC proved to be an excellent sensing platform for the fabrication of simple and sensitive nanobioensors [62]. Zhang et al. constructed a label-free ECL nanobiosensor for the detection of CEA by employing NH4CoPO4/Au@Ag-luminol/chitosan matrix as antibody carriers. NH4CoPO4 was utilized for stabilizing the ECL system, while the Au@Ag nanorods act as a mimic enzyme for the catalysis within the luminol-H2O2 ECL system. The ECL nanobiosensor was fabricated by placing the materials down onto a GCE in the following order: chitosan film containing NH4CoPO4, Au@Ag and luminol, and immobilization of anti-CEA antibody (Figure 8B). In the presence of H2O2, ECL signal is generated when electrochemical reaction of luminol occurred on the surface of the Au@Ag-luminol film. The nanobiosensor was found to have a detection range of 0.1 pg mL−1 to 380 ng mL−1, with a low detection limit of 30 fg mL−1 [78].
Figure 8.
AgNPs nanocomposite-based ECL nanobiosensors: (A) ECL nanobiosensor with nanocomposite of luminol-AgNPs@OMC. Reprinted from Ref. [62] with permission from Elsevier; (B) ECL nanobiosensor with nanocomposite of NH4CoPO4/Au@Ag-luminol. Reprinted from Ref. [78] with permission from Springer; (C) ECL nanobiosensor with nanocomposite of nanoFe3O4@GO. Reprinted from Ref. [79] with permission from Elsevier; (D) ECL nanobiosensor with nanocomposite of Fe3O4-NFs. Reprinted from [81] with permission from Elsevier.
5.3. Magnetic Nanoparticles (MNPs)
Currently, MNPs have become an emerging focus in the development and fabrication of sensors and biosensors. MNPs can be integrated into the transducer material or be dispersed in the sample. Due to its ability to be manipulated by magnetic fields, applying an external magnetic field will attract them onto the biosensor’s active detection surface, which can be best accomplished when MNPs are at sizes of 10–20 nm due to super-magnetism. Furthermore, MNPs possess large surface areas and high mass transference which enhance the sensitivity and stability in the fabrication of biosensors and other detection systems in clinical, food and environmental applications. Some recent applications of the MNPs in the fabrication of ECL nanobiosensors are presented in Table 2.
MNPs made of Fe3O4 have the advantage of being easily separated and immobilized [65]. Sha et al. reported the construction of an effective ECL naobiosensor for the ultrasensitive determination of (CA19-9) that utilized multi-functionalized graphene oxide anti-CA19-9/ABEI-nanoFe3O4@GO (Figure 8C). The presence of nanoFe3O4@GO extended the outer Helmholtz plane (OHP), which enabled all N-(4-aminobutyl)-N-ethylisoluminol (ABEI) molecules to be immobilized onto GO and became electrochemically active. This improved the effective emission of ECL signals and the detection sensitivity due to increased conductivity. In this set-up, anti-CA19-9 antibodies acted as the recognition element for CA19-9 and ABEI acted as the electrochemiluminophore. The nanobiosensor could detect concentrations of CA19-9 in the range of 0.001 to 5 U mL−1 and a detection limit of 0.0005 U mL−1 with satisfactory specificity, stability, reproducibility and regeneration [79]. In a different study, Xu et al. fabricated an ultrasensitive label-free ECL nanobiosensor for the detection of AFB1 based on magnetic nanoarchitecture (Figure 8D). By using magnetic control technology, magnetic nanofibers (Fe3O4-NFs) could be immobilized tightly onto CNHs matrix with the ability to accommodate a large number of antibodies on the interface. This afforded the nanobiosensor a high sensitivity for AFB1, a wide detection linear range from 0.05 ng mL−1 to 200 ng mL−1 and a low detection limit of 0.02 ng mL−1. Moreover, the ECL nanobiosensor exhibited good reliability, fast detection and good reproducibility at a low cost [81].
5.4. Metal Nanoclusters (MNCs)
Recently, MNCs such as Au, Ag, Cu, Pt, Pd, and some alloys have become promising materials for ECL investigations and the construction of nanobiosensors due to their unique features, such as chemical inertness, high electrical conductivity, low toxicity, easy labeling and excellent biocompatibility as well as stability [82,84]. MNCs are clusters of few atoms to a maximum 100 atoms, and depending on the number of atoms that exist within the cluster, their sizes lies typically in the range of sub-nanometer (less than 2 nm) to several nanometers (small nanoparticles) [83]. This size of MNCs is comparable to the Fermi wavelength of electrons in metal, leading to the continuous density of states splitting into discrete energy levels, which provide MNCs with attractive characteristics, such as excellent optical, electrical, electrochemical, magnetic, and chemical properties, leading to their use in the fabrication of ECL based nanobiosensors [84]. Table 2 summarizes the MNCs-based NCs recently used in the fabrication of ECL-based nanobiosensors. In these new systems, MNCs participate in ECL reactions as luminophores, quenchers and catalysts. In-addition to that, MNCs have low toxicity, high stability, good water solubility, easy preparation and biocompatibility, which make MNCs promising materials for ECL investigation.
Report have shown that among the MNC composites, the composite of AuNCs endows them with distinct ECL behavior, rendering them popular in electroanalytical applications [84]. Due to the fascinating features of the AuNCs such as chemical inertness, low toxicity, easy labeling and excellent stability, Luo et al. constructed an ECL nanobiosensor based on Au nanoclusters/graphene (AuNCs/GR) hybrid for the sensitive detection of pentacholophenol (PCP) using S2O82− as co-reactant. AuNCs/GR hybrid based-ECL nanobiosensor demonstrated a wide linear range from 0.1 fM to 0.1 nM with a low detection limit of 10 fM. Additionally, the constructed ECL-nanobiosensor demonstrated easier, simpler and sensitive detection of PCP in real water samples [82]. Meanwhile, Nie et al. prepared luminol functional AuNCs (lum-AuNCs) for the fabrication of the nanobiosensor to detect alkaline phosphatase (ALP). Compared to individual luminol, AuNCs, and the mixture of luminol and AuNCs, the composite of lum-AuNCs had special ECL properties, which caused up to 100-folds enhancement in ECL intensity than the ECL intensity of the mixture. The fabricated nanobiosensor exhibited a dynamic detection range of ALP from 0.3 to 12.0 nM with a low detection limit of 0.1 nM. Furthermore, this nanobiosensor displayed high specificity, good stability and great potential in the detection of ALP in human serum samples [83].
6. Quantum Dots Nanocomposite-Based ECL Nanobiosensors
Quantum dots (QDs) are roughly spherical semiconductor nanoparticles or colloidal semiconductor nanocrystals that have unique optical, electronic and photophysical properties. They have been increasingly investigated in ECL mechanism study and has been applied in the labeling, imaging, and detection of biological material [85]. Some of the more extensively used QDs in ECL nanobiosensors are CdS, CdSe, CdTe and graphene quantum dots, as summarized in Table 3. Lv et al. designed an ECL nanobiosensor using the nanocomposite of CdS-Fe3O4 for the ultra-sensitive detection of ochratoxin A (OTA), as shown in Figure 9A. CdS QDs act as the ECL emitters and were immobilized onto mesoporous Fe3O4 along with anti-OTA antibodies. The ECL nanobiosensor exhibited high ECL signal intensity and sensitivity, with a detection limit of 2 pg mL−1 and a concentration range of 0.01 to 100 ng mL−1 [80]. In a separate study, Wu et al. constructed an ECL nanobiosensor for the detection of the PSA using nanocomposites of AuNPs, AgNPs and graphene quantum dots (Au/AgrGO) as sensing platform (Figure 9B). Aminated carboxyl Au/AgrGO was synthesized and loaded onto the electrode, which augmented the surface area for reaction and elevated electron conductivity in the presence of co-reactant K2S2O8. This was followed by the immobilization of anti-PSA through the adsorption of Au/Ag on proteins. The nanobiosensor demonstrated a detection range of 1 pg mL−1 to 10 ng mL−1, with a detection limit of 0.29 pg mL−1. The ECL nanobiosensor demonstrated superior stability, sensitivity, repeatability and selectivity [38].
Table 3.
Quantum dots-based ECL nanobiosensors.
| Electrode | NMs/NSMs | Nanocomposites | Function | Assay Type | Bioanalyte | LOD | Range | Ref |
|---|---|---|---|---|---|---|---|---|
| GCE | CdS QDs | CdS-Fe3O4 | Ultrasensitive ECL detection, wide linear range, low detection limit, rapid, specific, stable and reliable | Label-free | OTA | 2 pg mL−1 | 0.01–100 ng mL−1 | [80] |
| GCE | CdSe QDs | CdSe QDs/PICA-MWNT | Good biocompatibility, high ECL intensity, synergistic improvement of sensitivity, good selectivity and reproducibility | Label-free | Alpha-fetoprotein (AFP) | 0.4 pg mL−1 | 0.002–2000 ng mL−1 | [86] |
| GCE | CdSe QDs | HCNTs-Polyallylamine hydrochloride (PAH)-CdSe QDs | Signal amplification, increased surface area | Direct | DA | 0.2 × 10−9 M | 1.0 × 10−9–2.0 × 10−5 M | [87] |
| GCE | CdSe QDs | AuNPs-CdSe QDs | Electron transport accelerators, rapid, acceptable, precision, good stability, bioactivity, low-cost and lower detection limit | Competitive | PA | 0.0047 ng mL−1 | 0.02–50 ng mL−1 | [69] |
| GCE | CdSe QDs | AuNPs-CdSe QDs | Rapid, ultrasensitive, stability, specificity, fabrication, reproducibility and sensitivity | Competitive | CLB | 0.0084 ng mL−1 | 0.02–50 ng mL−1 | [76] |
| GCE | CdSe QDs | (RGO)/Fe3O4/CdSe | Sensitivity, stability, wide linear range, reproducibility and selectivity | Label-free | IL-6 | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | CdTe QDs | Graphene nanosheets (GNs)/CdTe QDs | Sensitivity, selectivity, reproducibility and ideal stability | Enzymatic | organophosphate pesticides (OPs) | 0.06 ng mL−1 | 0.2–10 ng mL−1 | [88] |
| Au/AgrGO | Graphene QDs | Graphene QDs | Ultrasensitive ECL, excellent electron conductivity, stability, sensitivity and repeatability | Label-free | PSA | 0.29 pg mL−1 | 1 pg mL−1–10 ng mL−1 | [38] |
Figure 9.
Quantum dots nanocomposite based ECL nanobiosensors: (A) ECL nanobiosensor with nanocomposite of CdS-Fe3O4. Reprinted from Ref. [80] with permission from the Royal Society of Chemistry; (B) ECL nanobiosensor with nanocomposite of Au/AgrGO. Reprinted from Ref. [38] with permission from Nature.
7. Carbon Nanomaterials (CNMs)- or Carbon Nanostructured Materials (CNSMs)- Nanocomposite-Based ECL Nanobiosensors
Research in CNMs or CNSMs have advanced greatly in recent years, with a particular focus on graphene and carbon nanotubes (CNTs) for the development of carbon NCs-based biosensors. CNMs and CNSMs are attractive avenue as sensor components due to their exceptional electrical, thermal, chemical and mechanical properties, which render sensors more reliable, accurate and fast. Depending on the types of target molecules, different approaches can be taken in order to design a sensor device, making use of either zero-dimensional (carbon nano-onions) or one-dimensional (CNTs), or two-dimensional (graphene) or three-dimensional ordered mesoporous carbons (OMCs) or CNMs or CNSMs as NCs in order to fabricate a much improved analytical ECL-based nanobiosensors [89,90,91,92,93]. Presently, carbon nano-onions have emerged as the popular choice for the fabrication of sensitive and selective nanobiosensor due to their high surface area-to-volume ratio that provides a large surface area for biorecognition element binding, as well as improve electronic conductivity [90]. The latest examples of CNMs or CNSMs or OMCs NCs-based ECL nanobiosensors have been summarized in Table 4, with specific examples of each class of CNMs or CNSMs-based NCs.
Table 4.
CNMs-, CNSMs- and NCs-based nanobiosensors.
| Electrode | NMs/NSMs | NCs | Function | Assay type | Bioanalyte | LOD | Range | Ref |
|---|---|---|---|---|---|---|---|---|
| GCE | MWCNTs | MWCNTs-Cys-AuNPs | Signal amplification, reproducibility, stability, sensitivity and cost-effective | Direct | DPH | 6.7 × 10−9 M | 2 × 10−8–7.5 × 10−4 M | [72] |
| GCE | MWCNTs | MWCNT-Pt-luminol | ECL signal, sensitivity, fast analysis, stability and specificity | Label-free | CA19-9 | 0.00004 U mL−1 | 0.0001–10.0 U mL−1 | [63] |
| GCW | MWCNTs | MWCNTs-Au | Signal amplification, selectivity, stability and low-cost | Direct | Bisphenol A | 0.083 μM | 0.25–100 μM | [61] |
| GCE | HCNTs | HCNTs-Polyallylamine hydrochloride (PAH)-CdSe QDs | Signal amplification, increased surface area, stability, sensitivity and excellent specificity | Direct | DA | 0.2 × 10−9 M | 1.0 × 10−9–2.0 × 10−5 M | [87] |
| GCE | Magnetic nanofibers/CNHs | CNHs | Excellent electrical conductivity, biocompatibility, large surface areas, variable porosity, amplified ECL, large antibody, acceptable precision, low detection limit, reproducibility and stability | Label-free | AFB1 | 0.02 ng mL−1 | 0.05–200 ng mL−1 | [81] |
| GCE | MWCNTs | MWCNT-Pt-luminol | ECL signal, fast analysis, stability, reproducibility and specificity | Label-free | CA19-9 | 0.00004 U mL−1 | 0.0001–10.0 U mL−1 | [85] |
| GCE | MWCNTs | nanoCo-MWCNTs | Sensitivity, economical and practical applications | Direct | Glucose | 50 nM | 0.5–600 μM | [86] |
| GCE | MWCNTs | MWCNTs-GO-Thi-Au | Enhancing ECL, high sensitivity and good selectivity | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | MWCNTs | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, repeatability, accelerated electrons transfer, long-term stability and wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| ITO | MWCNTs | ABEI/GNDs/chitosan/COOH-MWCNTs | Strong and stable ECL signal, selectivity, stability and extremely high sensitivity | Label-free | NT-proBNP | 3.86 fg mL−1 | 0.01–100 pg mL−1 | [71] |
| GCE | MWCNTs | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity, good reproducibility and repeatability | Direct | DA | 0.067 µM | 0.20–70 µM | [75] |
| GCE | GO | MWCNTs-GO-Thi-Au | Enhanced ECL, high sensitivity and excellent stability | Enzymatic | Cholesterol | 50 nM | 0.15–828 µM | [73] |
| GCE | GO | GO/MWCNTs-COOH/Au@CeO2 | High sensitivity, accelerated electron transfer, selectivity and wide linear range | Label-free | CEA | 0.02 ng mL−1 | 0.05–100 ng mL−1 | [74] |
| GCE | GO | nanoFe3O4@GO | Good conductivity, magnetism, reproducibility and regeneration | Label-free | CA19-9 | 0.0005 U mL−1 | 0.001–5 U mL−1 | [79] |
| GCE | rGO | rGO/MWCNTs/AuNPs | Enhanced ECL signal intensity, high sensitivity and repeatability | Direct | DA | 0.067 µM | 0.20–70 µM | [75] |
| GCE | rGO | Fe3O4 (Au-FrGO) | Increased ECL, specific surface area, biocompatibility, sensitive response, excellent stability and selectivity | Label-free | CEA | 3.28 fg mL−1 | 0.01 pg mL−1–10 ng mL−1 | [9] |
| GCE | rGO | (RGO)/Fe3O4/CdSe | Sensitivity, stability, excellent reproducibility and selectivity | Label-free | IL-6 | 0.65 pg mL−1 | 0.002–20 ng mL−1 | [10] |
| GCE | GO | PtNFs/GO/GODx | High electrocatalytic activity, enhanced luminol ECL, reliability, high surface area, linear range and low detection limit | Direct | Glucose | 2.8 μM | 5–80 μM | [94] |
| QDs-SPE | CNOs | AuNPs@CNOs | High ECL, increased effective surface area for capturing and binding of antibodies, improved electron transmission rate, enhanced photon capture and highly sensitive | Label-free | β2M | 1 fg mL−1 | 1 fg mL−1–100 ng mL−1 | [7] |
| GCE | SWCNH | Aptamer/SWCNH | Quenching of ECL, sensitive, selective, simple, time-saving and cost-effective | Label-free | Adenosine triphosphate (ATP) | 1 nM | 5 nM–500 μM | [95] |
| GCE | OMCs | luminol-AgNPs@OMC | Fast, sensitive, specific, stable and reliable | Label-free | AFB1 | 50 fg mL−1 | 0.1 pg mL−1–50 ng mL−1 | [62] |
7.1. Ordered Mesoporous Carbons (OMCs)
OMCs constitute a subclass of 3D nanostructured porous CNMs or CNSMs. These materials have recently attracted great attention of researchers in electroanalytical applications for the fabrication of highly sensitive ECL nanobiosensors due to their good electronic conductivity, chemical inertness, great porosity (high specific surface area, large pore volume and size) and widely open ordered structure made of uniform and tunable pore sizes, which ensure fast mass transport rates [62,93].
7.2. Graphene
Graphene, a two-dimensional (2D) carbon material of one-atom thickness, is one of the most exciting CNMs or CNSMs being researched today for its potential applications in developing a new generation of nanobiosensors due to its unique and novel properties. Its sp2 hybridization drives the carbon atoms in graphene to be in a closely packed, honeycomb lattice structure, acting as a fundamental building block for carbon-based nanomaterials such as the zero-dimensional (0D) CNOs, one-dimensional (1D) CNTs, and three-dimensional (3D) graphite. Graphene and its derivatives possess a few exceptional properties such as high thermal conductivity, superior tensile strength, tunable optical property, remarkable elasticity, and perhaps most importantly, is their exceptional electrical conductivity due to factors such as its high quantum Hall effect and electron-hole symmetry [89,96]. Yang et al. constructed a reduced graphene oxide (RGO)/Fe3O4/PDDA/CdSe nanocomposite-based ECL nanobiosensor (Figure 10A) for the detection of human IL-6. The two-dimensional RGO can be modified to improve electrical performance, and overall the nanobiosensor exhibited good magnetism, ECL properties and biocompatibility, and low toxicity. Additionally, high analytical performance is demonstrated by the nanobiosensor in terms of linear range, stability, reproducibility, selectivity and sensitivity. The ECL intensity demonstrated by the nanobiosensor decreased linearly with IL-6 concentrations in the range of 0.002–20 ng mL−1, with a limit of detection of 0.65 pg mL−1 [10]. In another report, Yang et al. constructed a composite to be incorporated into an ECL nanobiosensor that consisted of macroporous gold nanoparticles (AuNPs) functionalized reduced graphene oxide (rGO) capped Fe3O4 (Au-FrGO) and CeO2@TiO2 composite (Figure 10B) for the label-free detection of CEA. The microporous structure of FrGO was assembled by layering rGO sheets capped cationic Fe3O4 nanoparticles, culminating in a larger surface area for reaction compared to rGO. On its own, Fe3O4 maintained a rather low magnetism, but when combined with AuNPs and FrGO, it imparted the nanocomposite with a more favorable biocompatibility and electrical conductivity, favoring further immobilization of CeO2@TiO2 and antibodies. The capacity of applying magnetic separation further simplified the preparation procedure. The ECL nanobiosensor exhibited excellent stability, repeatability and selectivity, with a linear range of 0.01 pg mL−1 to 10 ng mL−1 and a limit of detection of 3.288 fg mL−1 [9].
Figure 10.
Carbon nanocomposite-based ECL nanobiosensors: (A) ECL nanobiosensor with nanocomposite of Au-FrGO and CeO2@TiO2. Reprinted from Ref. [10] with permission from Elsevier; (B) ECL nanobiosensor with nanocomposite of (RGO)/Fe3O4/PDDA/CdSe. Reprinted from Ref. [9] with permission from Elsevier; (C) ECL nanobiosensor with nanocomposite of GO/MWCNTs-COOH/Au@CeO2. Reprinted from Ref. [68] with permission from the American Chemical Society; (D) ECL nanobiosensor with nanocomposite of AuNPs@CNOs-CS. Reprinted from Ref. [7] with permission from the Royal Society of Chemistry.
7.3. Carbon Nanotubes and Carbon Nano-Onions
Since the significant discovery of carbon nanotubes (CNTs) in 1991, a flurry of research has focused on this specific allotrope of carbon. CNTs are well-ordered, high aspect ratio one-dimensional (1D) CNMs or CNSMs that can be rolled into seamless cylinders due to the sp2-hybridized carbon atoms in the graphene sheet. CNTs have diameters measuring in the nanometre range with lengths in the microns range. Two classes of CNTs have been identified, which are single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs). SWNTs are composed of only one rolled-up graphene layer (diameter 0.4–2 nm), whereas MWNTs are composed of multiple nested graphene layers of increasing diameter arranged in concentric tubes (2–100 nm) [89,96,97]. Due to their unique electronic, chemical, and mechanical properties, CNTs have been found to be useful in a wide range of applications. They have since become one of the more popular carbon nanomaterials for use in ECL biosensors as their conducting properties can accelerate electron transfer for electrochemical and ECL-based sensors and they can act as conducting pathways between luminophores and electrode. In addition to their excellent chemical and thermal stability, enhanced electrochemical reactivity, it has been reported that CNTs can accumulate important biomolecules, alleviate surface fouling effects, and their increased surface area and porosity result in faster diffusion of co-reactants [98,99,100].
Carbon nano-onions (CNOs) or onion-like graphitic particles or onion-like carbons (OLCs), also known as multi-shell fullerenes, are zero-dimensional (0D) carbon nanomaterials structured as concentric layers closed carbon shells, resembling an onion. Depending on the synthetic protocol implemented, a diameter of CNOs between 60 and 300 nm can be obtained, with a distance of 0.34 nm between each layer. The unique structure and properties of CNOs render them effective in a large number of applications, including incorporation into a biosensor. Like carbon nanotubes, CNOs generally exhibit poor solubility in both aqueous and organic solvents, hence they are mainly used in a composite form in nanobiosensor construction. In addition to possessing a high surface area-to-volume ratio and biocompatibility, CNOs can act as a linking layer between biomolecules and the surface of the sensor to amplify signals generated by the nanobiosensor [7]. Pang et al. constructed a highly sensitive ECL nanobiosensor in order to detect CEA as shown in Figure 10C. Nanocomposites of graphene oxide/carboxylated multiwall carbon nanotubes/gold/cerium oxide nanoparticles (GO/MWCNTs-COOH/Au@CeO2) were used to modify glassy GCE. The nanocomposite acted as anti-CEA antibody carriers and sensing platforms within the nanobiosensor, resulting in superior conductivity and large surface area for reaction. The GO/MWCNTs-COOH/Au@CeO2 nanocomposite-modified platform showed excellent cathodic ECL performance and sensitive response to CEA, with a limit of detection of 0.02 ng mL−1 and a wide linear range of 0.05–100 ng mL−1 [74]. Rizwan et al. designed a label-free ECL nanobiosensor for the detection of β2M based on AuNPs@CNOs-CS nanocomposite-modified QDs-SPE (Figure 10D). This nanocomposite greatly improved the electronic transmission rate and enhanced the capture of photons emitted from the luminophore tris(bipyridine)ruthenium(II) chloride ([Ru(bpy)3]2+Cl). Moreover, the biocompatible surface of the electrode possessed an increased effective surface area for capturing and binding of a large number of anti-β2M. These properties resulted in an ECL nanobiosensor that demonstrated 3 × 106 higher sensitivity when compared to existing nanobiosensor, good reproducibility, long-term stability and selectivity, with a linear detection range of 1 fg mL−1 to 100 ng mL−1 and a low detection limit of 1 fg mL−1 [7].
8. Conclusions and Prospects
Due to the increasing applications of ECL-based nanobiosensors in medical diagnostics, pharmaceuticals, food quality control, environmental safety and security, NMs, NSMs, MNCs and NCs are increasingly being incorporated into biosensors. These nanomaterials allow for miniaturization of biosensors, low production cost, ease of use, the possibility of mass production, use of small sample volumes and high analytical performance. In this review article, we have summarized various NMs, NSMs, MNCs and NCs that have recently been used in the fabrication, construction, design and development of direct or label-free ECL nanobiosensors. Special emphasis has been placed on nanocomposite-modified sensing platform that are used in the fabrication of ECL-based nanobiosensors for medical diagnosis. Applications of NMs, NSMs, MNCs and NCs lead to various advantageous properties such as increased surface area for capture and binding of a large number of antibodies, ECL signal amplification, increased sensitivity, increased long term stability and improved electronic transmission rate [73]. Furthermore, these nanocomposites exhibited good selectivity, excellent biocompatibility, specificity, reproducibility and selectivity, improved magnetism, cost-effective and potential for regeneration of ECL nanobiosensors [7,67,79]. Despite these favorable characteristics, incorporation of novel NMs, NSMs, MNCs or polymer-based nanocomposite in biosensors are still required in order to further improve the sensitivity and the long-term stability of ECL nanobiosensors [7]. Currently, only very few label-free ECL-based nanobiosensors exploited polymer-based nanocomposite (such as: polyethylene glycol, chitosan and nafion), whereas materials such as MNCs, buckyballs, OMCs, carbon nanofibers, CNOs or SWCNTs have not been heavily utilized in the preparation of nanocomposites to modify the sensing platform of ECL-based nanobiosensors, which could increase the ECL signal intensity [17,84,93]. Similarly, disposable screen-printed electrodes and paper-based electrodes have not been employed as frequently in the preparation of nanocomposites in ECL nanobiosensors for point-of-care devices. Development of specialized ECL nanobiosensors, including enzymatic ECL nanobiosensors, ECL nano-immunosensors and ECL aptasensors, has great potential for practical applications, especially in the field of clinical diagnostics for the purpose of personalized medical monitoring. We predict that in the future, the demand for ECL-based nanobiosensors will be high due to their potential in a diverse range of applications. We anticipate the elimination of lengthy laboratory assays on bodily fluids (urine, blood, saliva and tears) as ECL-based nanobiosensors became a more sensible alternative approach in diagnosing various pathologies and diseases.
Acknowledgments
The authors would like to acknowledge partly the financial support for the project given by Brunei Research Council of Negara Brunei Darussalam through Grant# BRC-10. Mohammad Rizwan wishes to thank UBD’s Graduate Research Scholarship (GRS) for his PhD fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fetz V., Knauer S.K., Bier C., Von Kries J.P., Stauber R.H. Translocation biosensors cellular system integrators to dissect CRM1-dependent nuclear export by chemicogenomics. Sensors. 2009;9:5423–5445. doi: 10.3390/s90705423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim S.A., Ahmed M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016;6:24995–25014. doi: 10.1039/C6RA00333H. [DOI] [Google Scholar]
- 3.Clark L.C., Lyons C. Electrode systems for continuous monitoring cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 4.Fracchiolla N.S., Artuso S., Cortelezzi A. Biosensors in clinical practice: Focus on oncohematology. Sensors. 2013;13:6423–6447. doi: 10.3390/s130506423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013;42:3184–3196. doi: 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- 6.Rizwan M., Koh D., Booth M.A., Ahmed M.U. Combining a gold nanoparticle-polyethylene glycol nanocomposite and carbon nanofiber electrodes to develop a highly sensitive salivary secretory immunoglobulin A immunosensor. Sens. Actuators B Chem. 2018;255:557–563. doi: 10.1016/j.snb.2017.08.079. [DOI] [Google Scholar]
- 7.Rizwan M., Mohd-Naim N.F., Keasberry N.A., Ahmed M.U. A highly sensitive and label-free electrochemiluminescence immunosensor for beta 2-microglobulin. Anal. Methods. 2017;9:2570–2577. doi: 10.1039/C7AY00263G. [DOI] [Google Scholar]
- 8.Inoue Y., Inoue M., Saito M., Yoshikawa H., Tamiya E. Sensitive detection of glycated albumin in human serum albumin using electrochemiluminescence. Anal. Chem. 2017;89:5909–5915. doi: 10.1021/acs.analchem.7b00280. [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Zhu W., Ren X., Khan M.S., Zhang Y., Du B., Wei Q. Macroporous graphene capped Fe3O4 for amplified electrochemiluminescence immunosensing of carcinoembryonic antigen detection based on CeO2@TiO2. Biosens. Bioelectron. 2017;15:842–848. doi: 10.1016/j.bios.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y., Liu Q., Liu X.-P., Liu P.-Z., Mao C.-J., Niu H.-L., Jin B.-K., Zhang S.-Y. Multifunctional reduced graphene oxide (RGO)/Fe3O4/CdSe nanocomposite for electrochemiluminescence immunosensor. Electrochim. Acta. 2016;190:948–955. doi: 10.1016/j.electacta.2016.01.014. [DOI] [Google Scholar]
- 11.Ahmed M.U., Hossain M.M., Safavieh M., Wong Y.L., Rahman I.A., Zourob M., Tamiya E. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2016;36:495–505. doi: 10.3109/07388551.2014.992387. [DOI] [PubMed] [Google Scholar]
- 12.Omanovic-Miklicanin E., Valzacchi S. Development of new chemiluminescence biosensors for determination of biogenic amines in meat. Food Chem. 2017;235:98–103. doi: 10.1016/j.foodchem.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Yang L., Zhang Y., Li R., Lin C., Guo L., Qiu B., Lin Z., Chen G. Electrochemiluminescence biosensor for ultrasensitive determination of ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens. Bioelectron. 2015;70:268–274. doi: 10.1016/j.bios.2015.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Lv X., Li Y., Yan T., Pang X., Cao W., Du B., Wu D., Wei Q. Electrochemiluminescence modified electrodes based on RuSi@Ru(bpy)32+ loaded with gold functioned nanoporous CO/Co3O4 for detection of mycotoxin deoxynivalenol. Biosens. Bioelectron. 2015;70:28–33. doi: 10.1016/j.bios.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Roy S., Wei S.X., Ying Z.L.J., Safavieh M., Ahmed M.U. A novel, sensitive and label-free loop-mediated isothermal amplification detection method for nucleic acids using luminophore dyes. Biosens. Bioelectron. 2016;86:346–352. doi: 10.1016/j.bios.2016.06.065. [DOI] [PubMed] [Google Scholar]
- 16.Miao S.S., Sheng M., Ma L.Y., He X.J., Yang H. Electrochemiluminescence biosensor for determination of organophosphorous pesticides based on bimetallic Pt-Au/multi-walled carbon nanotubes modified electrode. Talanta. 2016;158:142–151. doi: 10.1016/j.talanta.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 17.Kitte S.A., Gao W., Zholudov Y.T., Qi L., Nsabimana A., Liu Z., Xu G. Stainless steel electrode for sensitive luminol electrochemiluminescence detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017;89:9864–9869. doi: 10.1021/acs.analchem.7b01939. [DOI] [PubMed] [Google Scholar]
- 18.Fu X., Tan X., Yuan R., Chen S. A dual-potential electrochemiluminescence ratiometric sensor for sensitive detection of dopamine based on graphene-CdTe quantum dots and self-enhanced Ru(II) complex. Biosens. Bioelectron. 2017;90:61–68. doi: 10.1016/j.bios.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Gao W., Wang C., Muzyka K., Kitte S.A., Li J., Zhang W., Xu G. Artemisinin-luminol chemiluminescence for forensic bloodstain detection using a smart phone as a detector. Anal. Chem. 2017;89:6160–6165. doi: 10.1021/acs.analchem.7b01000. [DOI] [PubMed] [Google Scholar]
- 20.Stewart A.J., Hendry J., Dennany L. Whole blood electrochemiluminescent detection of dopamine. Anal. Chem. 2015;87:11847–11853. doi: 10.1021/acs.analchem.5b03345. [DOI] [PubMed] [Google Scholar]
- 21.McGeehan J., Dennany L. Electrochemiluminescent detection of methamphetamine and amphetamine. Forensic Sci. Int. 2016;264:1–6. doi: 10.1016/j.forsciint.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Yu B., Zhang X., You T. A novel electrochemiluminescence sensor based on [Ru(bpy)2]3+ doped carbon nanodots system for the detection of bisphenol A. Anal. Chim. Acta. 2015;895:104–111. doi: 10.1016/j.aca.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J.-J., Kang T.-F., Hao Y.-C., Lu L.-P., Cheng S.-Y. Electrochemiluminescent immunosensor based on CdS quantum dots for ultrasensitive detection of microcystin-LR. Sens. Actuators B. 2015;214:117–123. doi: 10.1016/j.snb.2015.03.019. [DOI] [Google Scholar]
- 24.Li G., Yu X., Liu D., Liu X., Li F., Cui H. Label-free electrochemiluminescence aptasensor for 2,4,6-Trinitrotoluene based on bilayer structure of luminescence functionalized graphene hybrids. Anal. Chem. 2015;87:10976–10981. doi: 10.1021/acs.analchem.5b02913. [DOI] [PubMed] [Google Scholar]
- 25.Spehar-Délèze A.M., Gransee R., Martinez-Montequin S., Bejarano-Nosas D., Dulay S., Julich S., Tomaso H., O’Sullivan C.K. Electrochemiluminescence DNA sensor array for multiplex detection of biowarfare agents. Anal. Bioanal. Chem. 2015;407:6657–6667. doi: 10.1007/s00216-015-8831-y. [DOI] [PubMed] [Google Scholar]
- 26.Scopus Elsevier’s abstract and citation database. [(accessed on 13 September 2017)]; Available online: https://www.scopus.com/search/form.uri?display=basic.
- 27.Newman J.D., Tigwell L.J., Warner P.J., Turner A.P.F. Biosensors: Boldly going into the new millennium. Sens. Rev. 2001;21:268–271. doi: 10.1108/EUM0000000005999. [DOI] [Google Scholar]
- 28.Shana A., Rogers K.R. Biosensors. Meas. Sci. Technol. 1994;5:461–472. [Google Scholar]
- 29.Yang L., Li Y. AFM and impedance spectroscopy characterization of the immobilization of antibodies on indium-tin oxide electrode through self-assembled monolayer of epoxysilane and their capture of Escherichia coli O157:H7. Biosens. Bioelectron. 2005;20:1407–1416. doi: 10.1016/j.bios.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Grieshaber D., MacKenzie1 R., Voros J., Reimhult E. Electrochemical biosensors-Sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s8031400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim S.A., Yoshikawa H., Tamiya E., Yasin H.M., Ahmed M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014;4:58460–58466. doi: 10.1039/C4RA11066H. [DOI] [Google Scholar]
- 32.Wang X., Chen L., Su X., Ai S. Electrochemical immunosensor with graphene quantum dots and apoferritin-encapsulated Cu nanoparticles double-assisted signal amplification for detection of avian leucosis virus sub group J. Biosens. Bioelectron. 2013;47:171–177. doi: 10.1016/j.bios.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Lina C.-C., Chub Y.-M., Chang H.-C. In situ encapsulation of antibody on TiO2 nanowire immunosensor via electro-polymerization of polypyrrole propylic acid. Sens. Actuators B. 2013;187:533–539. doi: 10.1016/j.snb.2013.03.045. [DOI] [Google Scholar]
- 34.Lim S.A., Ahmed M.U. A carbon nanofiber-based label-free immunosensor for high sensitive detection of recombinant bovine somatotropin. Biosens. Bioelectron. 2015;70:48–53. doi: 10.1016/j.bios.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Lim S.A., Ahmed M.U. A label free electrochemical immunosensor for sensitive detection of porcine serum albumin as a marker for pork adulteration in raw meat. Food Chem. 2016;206:197–203. doi: 10.1016/j.foodchem.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs M., Selvam A.P., Craven J.E., Prasad S. Antibody-conjugated gold nanoparticle-based immunosensor for ultra-sensitive detection of Troponin-T. J. Lab. Autom. 2014;19:546–554. doi: 10.1177/2211068214538971. [DOI] [PubMed] [Google Scholar]
- 37.Ma H., Zhou J., Li Y., Han T., Zhang Y., Hu L., Du B., Wei Q. A label-free electrochemiluminescence immunosensor based on EuPO4 nanowire for the ultrasensitive detection of prostate specific antigen. Biosens. Bioelectron. 2016;80:352–358. doi: 10.1016/j.bios.2016.01.069. [DOI] [PubMed] [Google Scholar]
- 38.Wu D., Liu Y., Wang Y., Hu L., Ma H., Wang G., Wei Q. Label-free electrochemiluminescent immunosensor for detection of prostate specific antigen based on aminated graphene quantum dots and carboxyl graphene quantum dots. Sci. Rep. 2016;4:20511. doi: 10.1038/srep20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaseva N., Ermolaeva T. A regenerable piezoelectric immunosensor on the basis of electropolymerized polypyrrole for highly selective detection of Staphylococcal Enterotoxin A in foodstuffs. Microchim. Acta. 2015;182:1329–1335. doi: 10.1007/s00604-015-1456-1. [DOI] [Google Scholar]
- 40.Caldeira J.M.L.P., Rodrigues J.J.P.C., Garcia J.F.R., Torre I.D.L. A new wireless biosensor for intra-vaginal temperature monitoring. Sensors. 2010;10:10314–10327. doi: 10.3390/s101110314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao J., Li L., Li P., Yang M. Quantum dots: From fluorescence, chemiluminescence, bioluminescence and electrochemiluminescence to electrochemistry. Nanoscale. 2017;9:13364–13383. doi: 10.1039/C7NR05233B. [DOI] [PubMed] [Google Scholar]
- 42.Cui H., Paolucci F., Sojic N., Xu G. Analytical electrochemiluminescence. Anal. Bioanal. Chem. 2016;408:7001–7002. doi: 10.1007/s00216-016-9837-9. [DOI] [PubMed] [Google Scholar]
- 43.Bist I., Bano K., Rusling J.F. Screening Genotoxicity Chemistry with Microfluidic Electrochemiluminescent Arrays. Sensors. 2017;17:1008. doi: 10.3390/s17051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Zhou S., Li L., Zhu J.-J. Nanomaterials-based sensitive electrochemiluminescence biosensing. Nano Today. 2017;12:98–115. doi: 10.1016/j.nantod.2016.12.013. [DOI] [Google Scholar]
- 45.Gao W., Saqib M., Qi L., Zhang W., Xu G. Recent advances in electrochemiluminescence devices for point-of-care testing. Curr. Opin. Electrochem. 2017;3:4–10. doi: 10.1016/j.coelec.2017.03.003. [DOI] [Google Scholar]
- 46.Liu Z., Qi W., Xu G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015;44:3117–3142. doi: 10.1039/C5CS00086F. [DOI] [PubMed] [Google Scholar]
- 47.Ding C., Zhang W., Wang W., Chen Y., Li X. Amplification strategies using electrochemiluminescence biosensors for the detection of DNA, bioactive molecules and cancer biomarkers. Trends Anal. Chem. 2015;65:137–150. doi: 10.1016/j.trac.2014.10.015. [DOI] [Google Scholar]
- 48.Bertoncello P., Stewart A.J., Dennany L. Analytical applications of nanomaterials in electrogenerated chemiluminescence. Anal. Bioanal. Chem. 2014;406:5573–5587. doi: 10.1007/s00216-014-7946-x. [DOI] [PubMed] [Google Scholar]
- 49.Muzyka K. Current trends in the development of the electrochemiluminescent immunosensors. Biosens. Bioelectron. 2014;54:393–407. doi: 10.1016/j.bios.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Richter M.M. Electrochemiluminescence (ECL) Chem. Rev. 2004;104:3003–3036. doi: 10.1021/cr020373d. [DOI] [PubMed] [Google Scholar]
- 51.Miao W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008;108:2506–2553. doi: 10.1021/cr068083a. [DOI] [PubMed] [Google Scholar]
- 52.Bertoncello P., Forster R.J. Nanostructured materials for electrochemiluminescence (ECL)-based detection methods: Recent advances and future perspectives. Biosens. Bioelectron. 2009;24:3191–3200. doi: 10.1016/j.bios.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Bertoncello P. Nanomaterials for biosensing with electrochemiluminescence (ECL) detection. Front. Biosci. 2011;16:1084–1108. doi: 10.2741/3737. [DOI] [PubMed] [Google Scholar]
- 54.Bertoncello P., Ugo P. Recent Advances in Electrochemiluminescence with Quantum Dots and Arrays of Nanoelectrodes. ChemElectroChem. 2017;4:1663–1676. doi: 10.1002/celc.201700201. [DOI] [Google Scholar]
- 55.Ramos A.P., Cruz M.A.E., Tovani C.B., Ciancaglini P. Biomedical applications of nanotechnology. Biophys. Rev. 2017;9:79–89. doi: 10.1007/s12551-016-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He X., Hwang H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016;24:671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim R.K., Hayyan M., AlSaadi M.A., Hayyan A., Ibrahim S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. Int. 2016;23:13754–13788. doi: 10.1007/s11356-016-6457-z. [DOI] [PubMed] [Google Scholar]
- 58.Sharma N., Ojha H., Bharadwaj A., Pathak D.P., Sharma R.K. Preparation and catalytic applications of nanomaterials: A review. RSC Adv. 2015;5:53381–53403. doi: 10.1039/C5RA06778B. [DOI] [Google Scholar]
- 59.Peng H.-S., Chiu D.T. Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 2015;44:4699–4722. doi: 10.1039/C4CS00294F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussein A.K. Applications of nanotechnology in renewable energies-A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015;42:460–476. doi: 10.1016/j.rser.2014.10.027. [DOI] [Google Scholar]
- 61.Guo W., Zhang A., Zhang X., Huang C., Yang D., Jia N. Multiwalled carbon nanotubes/gold nanocomposites-based electrochemiluminescent sensor for sensitive determination of bisphenol A. Anal. Bioanal. Chem. 2016;408:7173–7180. doi: 10.1007/s00216-016-9746-y. [DOI] [PubMed] [Google Scholar]
- 62.Lv X., Li Y., Cao W., Yan T., Li Y., Du B., Wei Q. A label-free electrochemiluminescence immunosensor based on silver nanoparticle hybridized mesoporous carbon for the detection of Aflatoxin B1. Sens. Actuators B. 2014;202:53–59. doi: 10.1016/j.snb.2014.05.012. [DOI] [Google Scholar]
- 63.Zhang X., Ke H., Wang Z., Guo W., Zhang A., Huang C., Jia N. An ultrasensitive multi-walled carbon nanotube-platinum-luminol nanocomposite-based electrochemiluminescence immunosensor. Analyst. 2017;142:2253–2260. doi: 10.1039/C7AN00417F. [DOI] [PubMed] [Google Scholar]
- 64.Doria G., Conde J., Veigas B., Giestas L., Almeida C., Assunção M., Rosa J., Baptista P.V. Noble metal nanoparticles for biosensing applications. Sensors. 2012;12:1657–1687. doi: 10.3390/s120201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rocha-Santos T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014;62:28–36. doi: 10.1016/j.trac.2014.06.016. [DOI] [Google Scholar]
- 66.Zhang Y., Chu W., Foroushani A.D., Wang H., Li D., Liu J., Barrow C.J., Wang X., Yang W. New gold nanostructures for sensor applications. Materials. 2014;7:5169–5201. doi: 10.3390/ma7075169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang A., Huang C., Shi H., Guo W., Zhang X., Xiang H., Jia T., Miao F., Jia N. Electrochemiluminescence immunosensor for sensitive determination of tumor biomarker CEA based on multifunctionalized Flower-like Au@BSA nanoparticles. Sens. Actuators B. 2017;238:24–31. doi: 10.1016/j.snb.2016.07.009. [DOI] [Google Scholar]
- 68.Tang S., Zhao Q., Tua Y. A sensitive electrochemiluminescent cholesterol biosensor based on Au/hollowed-TiO2 nanocomposite pre-functionalized electrode. Sens. Actuators B. 2016;237:416–422. doi: 10.1016/j.snb.2016.06.110. [DOI] [Google Scholar]
- 69.Yan P., Zhang J., Tang Q., Deng A., Li J. A quantum dot based electrochemiluminescent immunosensor for the detection of pg level phenylethanolamine A using gold nanoparticles as substrates and electron transfer accelerators. Analyst. 2014;139:4365–4372. doi: 10.1039/C4AN00378K. [DOI] [PubMed] [Google Scholar]
- 70.Li J., Ma H., Wu D., Li X., Zhao Y., Zhang Y., Du B., Wei Q. A label-free electrochemiluminescence immunosensor based on KNbO3-Au nanoparticles@Bi2S3 for the detection of prostate specific antigen. Biosens. Bioelectron. 2015;74:104–112. doi: 10.1016/j.bios.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H., Han Z., Wang X., Li F., Cui H., Yang D., Bian Z. Sensitive immunosensor for N-Terminal Pro-brain natriuretic peptide based on N-(Aminobutyl)-N-(ethylisoluminol)-functionalized gold nanodots/multiwalled carbon nanotube electrochemiluminescence nanointerface. ACS Appl. Mater. Interfaces. 2015;7:7599–7604. doi: 10.1021/am509094p. [DOI] [PubMed] [Google Scholar]
- 72.Miao C., Zhang A., Xu Y., Chen S., Ma F., Huang C., Jia N. An ultrasensitive electrochemiluminescence sensor for detecting diphenhydramine hydrochloride based on l-cysteine-functionalized multiwalled carbon nanotubes/gold nanoparticles nanocomposites. Sens. Actuators B. 2015;213:5–11. doi: 10.1016/j.snb.2015.02.069. [DOI] [Google Scholar]
- 73.Wu X., Chai Y., Yuan R., Zhong X., Zhang J. Synthesis of multiwall carbon nanotubes-graphene oxide-thionine-Au nanocomposites for electrochemiluminescence detection of cholesterol. Electrochim. Acta. 2014;129:441–449. doi: 10.1016/j.electacta.2014.02.103. [DOI] [Google Scholar]
- 74.Li J., Zhao Y., Wu D., Zhang Y., Du B., Ma H., Wei Q. Label-free electrochemiluminescent immunosensor for detection of carcinoembryonic antigen based on nanocomposites of GO/MWCNTs-COOH/Au@CeO2. ACS Appl. Mater. Interfaces. 2015;7:19260–19267. doi: 10.1021/acsami.5b05185. [DOI] [PubMed] [Google Scholar]
- 75.Yuan D., Chen S., Yuan R., Zhang J., Liu X. An ECL sensor for dopamine using reduced graphene oxide/multiwallcarbon nanotubes/gold nanoparticles. Sens. Actuators B. 2014;191:415–420. doi: 10.1016/j.snb.2013.10.013. [DOI] [Google Scholar]
- 76.Yana P., Tanga Q., Denga A., Li J. Ultrasensitive detection of clenbuterol by quantum dots based electrochemiluminescent immunosensor using gold nanoparticles as substrate and electron transport accelerator. Sens. Actuators B. 2014;191:508–515. doi: 10.1016/j.snb.2013.10.047. [DOI] [Google Scholar]
- 77.Cao J., Wang H., Liu Y. Petal-like CdS nanospheres-based electrochemiluminescence aptasensor for detection of IgE with gold nanoparticles amplification. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;151:274–279. doi: 10.1016/j.saa.2015.06.104. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y., Lu F., Yan Z., Wu D., Ma H., Du B., Wei Q. Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: Application to the determination of carcinoembryonic antigen. Microchim. Acta. 2015;182:1421–1429. doi: 10.1007/s00604-015-1473-0. [DOI] [Google Scholar]
- 79.Sha Y., Guo Z., Chen B., Wang S., Ge G., Qiu B., Jiang X. A one-step electrochemiluminescence immunosensor preparation for ultrasensitive detection of carbohydrate antigen 19-9 based on multifunctionalized graphene oxide. Biosens. Bioelectron. 2015;66:468–473. doi: 10.1016/j.bios.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Lv X., Li Y., Yan T., Pang X., Hu L., Du B., Wei Q. An electrochemiluminescent immunosensor based on CdS–Fe O nanocomposite electrodes for the detection of Ochratoxin A. New J. Chem. 2015;39:4259–4264. doi: 10.1039/C5NJ00320B. [DOI] [Google Scholar]
- 81.Xu G., Zhang S., Zhang Q., Gong L., Daia H., Lin Y. Magnetic functionalized electrospun nanofibers for magnetically controlled ultrasensitive label-free electrochemiluminescent immunedetection of aflatoxin B1. Sens. Actuators B. 2016;222:707–713. doi: 10.1016/j.snb.2015.08.129. [DOI] [Google Scholar]
- 82.Luo S., Xiao H., Yang S., Liu C., Liang J., Tang Y. Ultrasensitive detection of pentachlorophenol based on enhanced electrochemiluminescence of Au nanoclusters/graphene hybrids. Sens. Actuators B. 2014;194:325–331. doi: 10.1016/j.snb.2013.12.108. [DOI] [Google Scholar]
- 83.Nie F., Luo K., Zheng X., Zheng J., Song Z. Novel preparation and electrochemiluminescence application of luminol functional-Au nanoclusters for ALP determination. Sens. Actuators B. 2015;218:152–159. doi: 10.1016/j.snb.2015.04.112. [DOI] [Google Scholar]
- 84.Han S., Zhang Z., Li S., Qi L., Xu G. Chemiluminescence and electrochemiluminescence applications of metal nanoclusters. Sci. China Chem. 2016;59:794–801. doi: 10.1007/s11426-016-0043-3. [DOI] [Google Scholar]
- 85.Bard A.J. Electrogenerated Chemiluminesence. Marcel Dekker, Inc.; New York, NY, USA: 2004. [Google Scholar]
- 86.Nie G., Li C., Zhang L., Wang L. Fabrication of a simple and sensitive QDs-based electrochemiluminescence immunosensor using a nanostructured composite material for the detection of tumor markers alpha-fetoprotein. J. Mater. Chem. B. 2014;2:8321–8328. doi: 10.1039/C4TB01308E. [DOI] [PubMed] [Google Scholar]
- 87.Wang L., Mei L., Liu X., Shi J., Li Y., Gu N., Cui R. A nanocomposite prepared from helical carbon nanotubes, polyallylamine hydrochloride and CdSe quantum dots for electrochemiluminescent determination of dopamine. Microchim. Acta. 2015;182:1661–1668. doi: 10.1007/s00604-015-1490-z. [DOI] [Google Scholar]
- 88.Liang H., Song D., Gong J. Signal-on electrochemiluminescence of biofunctional CdTe quantum dots for biosensing of organophosphate pesticides. Biosens. Bioelectron. 2014;53:363–369. doi: 10.1016/j.bios.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Z. An overview of carbon nanotubes and graphene for biosensing applications. Nano-Micro Lett. 2017;9:1–24. doi: 10.1007/s40820-017-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jariwala D., Sangwan V.K., Lauhon L.J., Marks T.J., Hersam M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013;42:2824–2860. doi: 10.1039/C2CS35335K. [DOI] [PubMed] [Google Scholar]
- 91.Bartelmess J., Giordani S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014;5:1980–1998. doi: 10.3762/bjnano.5.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haghighi B., Tavakoli A., Bozorgzadeh S. Improved electrogenerated chemiluminescence of luminol by cobalt nanoparticles decorated multi-walled carbon nanotubes. J. Electroanal. Chem. 2016;762:80–86. doi: 10.1016/j.jelechem.2015.12.035. [DOI] [Google Scholar]
- 93.Walcarius A. Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon. Sensors. 2017;17:1863. doi: 10.3390/s17081863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian X., Lian S., Zhao L., Chen X., Huang Z., Chen X. A novel electrochemiluminescence glucose biosensor based on platinum nanoflowers/graphene oxide/glucose oxidase modified glassy carbon electrode. J. Solid State Electrochem. 2014;18:2375–2382. doi: 10.1007/s10008-014-2485-0. [DOI] [Google Scholar]
- 95.Liu Z., Zhang W., Qi W., Gao W., Hanif S., Saqib M., Xu G. Label-free signal-on ATP aptasensor based on the remarkable quenching of tris(2,2′-bipyridine)-ruthenium(II) electrochemiluminescence by single-walled carbon nanohorn. Chem. Commun. 2015;51:4256–4258. doi: 10.1039/c5cc00037h. [DOI] [PubMed] [Google Scholar]
- 96.Choudhary N., Hwang S., Choi W. Carbon Nanomaterials: A Review. In: Bhushan B., Luo D., Schricker S., Sigmund W., Zauscher S., editors. Handbook of Nanomaterials Properties. Springer; Berlin/Heidelberg, Germany: 2014. [Google Scholar]
- 97.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- 98.Guo Z., Dong S. Electrogenerated Chemiluminescence from Ru(bpy)32+ ion-exchange in carbon nanotube/perfluorosulfonated ionomer composite films. Anal. Chem. 2004;76:2683–2688. doi: 10.1021/ac035276e. [DOI] [PubMed] [Google Scholar]
- 99.Lin Z.Y., Chen J.H., Chen G.N. An ECL biosensor for glucose based on carbon-nanotube/Nafion film modified glass carbon electrode. Electrochim. Acta. 2008;53:2396–2401. doi: 10.1016/j.electacta.2007.09.063. [DOI] [Google Scholar]
- 100.Chen J.H., Lin Z.Y., Chen G.N. Enhancement of electrochemiluminesence of lucigenin by ascorbic acid at single-wall carbon nanotube film-modified glassy carbon electrode. Electrochim. Acta. 2007;52:4457–4462. doi: 10.1016/j.electacta.2006.12.030. [DOI] [Google Scholar]