Abstract
Discoidin domain receptor 1 (DDR1) is a receptor tyrosine kinase activated by various types of collagens that performs a critical role in cell attachment, migration, survival and proliferation. The functions of DDR1 in various types of tumor have been studied extensively. However, in breast carcinoma, the roles of collagen-evoked DDR1 remain ill defined. Although a number of studies have reported that DDR1 promotes apoptosis and inhibits migration in breast carcinoma, it has also been reported to be associated with tumor cell survival, chemoresistance to genotoxic drugs and the facilitation of invasion. The present review summarizes current progress and the complex effects of DDR1 in the field of breast carcinoma, and presents DDR1 as a promising therapeutic target.
Keywords: discoidin domain receptor 1, breast carcinoma, collagen, epithelial-mesenchymal transition, migration
1. Introduction
Discoidin domain receptors (DDRs) are receptor tyrosine kinases (RTKs) characterized by an ~155 amino acid extracellular discoidin homology domain that binds to and is activated by collagens in their native triple-helical form. There are two types of DDR kinases: DDR1 and DDR2. DDR1 is activated by collagens type I–VI and VIII, whereas DDR2 is activated by fibrillar collagens type I and III (1–3). Alternative splicing generates five DDR1 isoforms: DDR1a, DDR1b, DDR1c, DDR1d and DDR1e. DDR1a, DDR1b and DDR1c are full-length functional receptors, whereas DDR1d and DDR1e are truncated and kinase-inactive receptors (4). DDR1 signaling is required for differentiation, immune response, normal skeletal development, mammary gland branching morphogenesis, migration and wound healing (5). The expression of DDR1 in several different types of human cancer, including breast cancer, renal clear cell carcinoma, non-small cell lung carcinoma, esophageal cancer, astrocytoma, prostate cancer, hepatocellular carcinoma and Hodgkin's lymphoma, suggests a function in tumor progression (6–14).
Breast carcinoma is the most common type of malignancy in women, with 1.7 million new cases diagnosed worldwide in 2012 (15). Collagens are a major component of the extracellular matrix (ECM); increasing evidence has demonstrated that collagen performs a critical role in the development and progression of breast carcinoma (13,14). In normal breast tissues, if fibrillar collagen deposition is increased, high mammographic density will be detected; it leads to a 2-fold increased risk of breast carcinoma development (16,17). Therefore, DDR1 may serve an important role in breast carcinoma. In renal clear cell carcinoma and a number of other types of cancer, DDR1 was significantly overexpressed in high-grade and advanced-stage tumors (6,12), suggesting that it may be suitable for use as a prognostic marker or therapeutic target. However, in breast carcinoma, the expression of DDR1 and the stage of the cancer do not appear to be associated (18). Different expression levels of DDR1 may be a reflection of its complex effects in breast carcinoma. This review summarizes the current knowledge regarding DDR1 and discusses its complex effects in breast carcinoma.
2. Collagen-induced DDR1 activation
DDRs are unique among RTKs as they are activated by collagen, an ECM protein. Collagens are major components of the ECM, accounting for ~30% of the total protein mass in the human body (19). Evidence has demonstrated that high mammographic density, which is partly due to increased fibrillar collagen deposition, is associated with a 2-fold increased risk of breast cancer development (16,17). The native triple-helical collagen serves as a ligand of DDR1; DDR1 cannot be activated by heat-denatured collagen (20,21). DDR1 is activated by specific types of collagens, as aforementioned. The DDR collagen-binding sites are entirely contained within the discoidin 1 domains (1–3). Leitinger et al (21) demonstrated that the isolated discoidin 1 domains of DDR1 and DDR2 bind directly to collagen with high affinity and that binding requires these domains to be dimerized. Initial mutagenesis experiments mapped the collagen-binding sites to three spatially adjacent surface-exposed loops that are highly conserved between the DDRs (21,22). DDR1 and DDR2 bind the GVMGVO (O, hydroxyproline) motif within fibrillar collagens I–III (23–25). Analysis revealed that the activation of DDR1 by collagen results in the binding of CD9 to Tyr513, SH2-domain containing protein to Tyr703, 796 and 740, and the p85 subunit of phosphoinositide 3-kinase to Tyr881 (20,26–28). These interactions were confirmed and additional binding proteins, including Ras GTPase activating protein, SH-2 domain-containing inositol 5′ polyphosphatase (SHIP)1, SHIP2, signal transducer and activator of transcription and SRC family kinases, were identified using proteomics approaches (29). Therefore, following binding to collagen, DDR1 becomes phosphorylated at tyrosine residues and can activate various downstream signaling pathways.
3. DDR1 expression level in breast carcinoma
Studies with large sets of clinical follow-up data and patients have been performed to verify the DDR1 expression profiles of different histological types of breast carcinoma (Table I). Invasive ductal and lobular carcinomas are the most common histological types of breast carcinoma (30,31). DDR1 was identified to be differentially expressed between lobular and ductal carcinomas by a pairwise comparison analysis (32). DDR1 was overexpressed in ductal carcinomas, as confirmed by immunohistochemistry, in which DDR1 was positive in 96.2% of ductal carcinomas compared with only 13.8% of lobular carcinomas (33). Considering this, DDR1 may represent a novel tissue marker in the differentiation of ductal and lobular breast carcinoma as an addition to the well-established marker E-cadherin (32–34). In triple-negative breast carcinoma, a DDR1low/DDR2high subtype has been identified that may be more invasive and associated with a worse prognosis (13). In human breast cancer stem cells with the CD44highCD24low phenotype, DDR1 expression was reduced (35–37). In other histological types of breast carcinoma, the expression level of DDR1 is lower in the more mesenchymal and invasive Basal B type cell lines, a subtype with enhanced invasive properties (38). Overall, the DDR1 expression profiles of different histological types of breast carcinoma may vary, as summarized in Table I.
Table I.
Expression levels of DDR1 in different types of breast carcinoma.
| DDR1 expression | ||
|---|---|---|
| Breast cancer type | High | Low |
| Metastasis-containing lymph nodes | (83) | |
| Ductal carcinoma in situ | (13,84) | |
| Invasive ductal carcinoma | (32,48,85) | |
| Invasive lobular carcinoma | (32,84,85) | |
| Middle to high-grade carcinoma | (54) | |
| Triple-negative breast carcinoma | (13,84) | |
DDR1, discoidin domain receptor 1.
4. DDR1 association with EMT in breast carcinoma
The epithelial-to-mesenchymal transition (EMT) program promotes cell motility, invasion and metastasis (39–41). EMT is characterized by an increase in cell motility, invasiveness and stem cell-like properties. Tumor cells that undergo EMT express fewer epithelial markers, including E-cadherin and cytokeratins, but express more mesenchymal markers, including vimentin and N-cadherin, with a possible switch in DDR expression from DDR1 (epithelial) to DDR2 (mesenchymal) (42,43). DDR1, as opposed to DDR2, is downregulated to induce the expression of the EMT transcription factors Twist and Snail in breast epithelial cells, suggesting a differential regulation of DDRs during the development of EMT (2,44,45). In breast carcinoma cells, DDR1 negatively regulates EMT. DDR1 is expressed predominantly in regions of cell-cell contact, where it interacts with and stabilizes E-cadherin in normal epithelial cells (46–49). Studies on tissue samples of patients with breast cancer also demonstrate a negative correlation between DDR1 and zinc finger E-box-binding homeobox 1 (ZEB1) expression. ZEB1 is a key regulator of the EMT program in human breast cancer cells and can directly suppress the transcription of E-cadherin to promote EMT (33). Therefore, when the expression level of DDR1 is high, it may inhibit ZEB1 and the EMT program. Furthermore, the overexpression of DDR1a or DDR1b reduces the invasive phenotype and regulates the F-actin cytoskeletal organization of breast cancer cells (46). Therefore, in breast cancer cells, DDR1 serves a negative function in the EMT program.
5. Complex role of DDR1 in migration
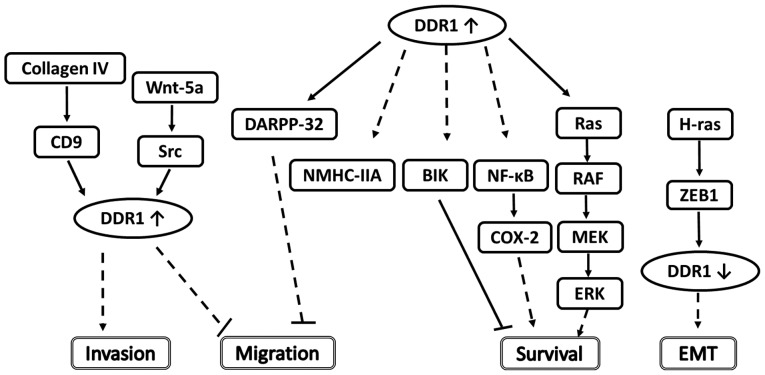
Studies have demonstrated that DDR1 functions in the regulation of cell adhesion and migration in tumors (50–53). Neuhaus et al (54) used chemokine-driven transwell migration assays to assess the migration of small interfering RNA (siRNA)-transfected cells and detected a marked reduction of cell migration following the knockdown of DDR1 in T47D and MDA-MB-468 breast cancer cell lines; T47D cell migration was reduced by 23% and MDA-MB-468 migration by 57%. It was concluded that when DDR1 is downregulated, the migration ability is also decreased. A study has demonstrated that DDR1 can mediate cell migration by means of regulating the migration suppressor Syk kinase (55), providing further evidence for a pro-migratory role of DDR1. Castro-Sanchez et al (56) demonstrated that in MDA-MB-231 breast cancer cells, DDR1 mediates matrix metalloproteinase (MMP)-2 and-9 secretion and invasion induced by native type IV collagen. In NIH3T3 fibroblasts and MCF7 breast cancer cells, DDR1 was demonstrated to inhibit cell spreading, but to promote migration, via interaction with non-muscle myosin heavy chain-IIA, a contractile protein associated with cell spreading (57). Furthermore, native type IV collagen induces a transient increase of CD9-cell surface levels and cell migration through a DDR1 and CD9-dependent pathway in MDA-MB-231 breast cancer cells (58). Therefore, numerous studies demonstrate that DDR1 performs a pro-migratory function (Fig. 1).
Figure 1.
Reported DDR1-associated signaling pathways in breast cancer cells. The mechanisms for the effect of ZEB1, COX-2, DARPP-32 and Wnt-5a on the migration, survival, EMT and invasion regulatory networks are illustrated. Solid lines indicate direct interactions or effects, whereas dashed lines indicate indirect interactions or effects through one or more intermediate steps. Pointed and flat arrows indicate activating and inhibiting effects, respectively. DDR1, discoidin domain receptor 1; ZEB1, zinc finger E-box-binding homeobox 1; COX-2, cyclooxygenase-2; DARPP-32, dopamine- and cAMP-regulated neuronal phosphoprotein; EMT, epithelial-to-mesenchymal transition; CD9, cluster of differentiation 9; NMHC-IIA, non-muscle myosin heavy chain-IIA; BIK, Bcl-interacting killer; NF-κB, nuclear factor-κB; MEK, ERK activator kinase; ERK, extracellular signal-regulated kinase.
However, Hansen et al (59) reported an opposite effect of DDR1; they identified that in the MCF-7 and MDA-MB-231 breast cancer cell lines, DDR1 suppressed migration when co-expressed with its interacting partner, dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32). In the presence of DDR1, DARPP-32 was confined to a restricted subcellular location at the plasma membrane, which induced an anti-migratory function in various breast cancer cell lines (59). However, this anti-migration function did not require DDR1 activation, in contrast with the study by Neuhaus et al (54). Furthermore, Jonsson et al (52) reported that a reduced extent of DDR1 tyrosine phosphorylation in Wnt-5a antisense cells promoted cell scattering, enhanced cell motility and impaired cell-collagen interaction. Wnt-5a may activate DDR1 to inhibit migration in non-malignant human mammary epithelial cells (52). Wnt gene families encode signaling glycoproteins that are associated with embryogenesis and the regulation of normal and pathological cell processes (60–62). Wnts are implicated in tumor formation; the endogenous expression of Wnt-5a is sufficient to enable the collagen-induced phosphorylation of DDR1 receptors in MCF-7 (63–65). Roarty and Serra (66) demonstrated that the negative interference of transforming growth factor-β signaling not only affected the expression of Wnt-5a, but also the phosphorylation of DDR1, a downstream target of Wnt-5a associated with cell migration. Overall, the effect of DDR1 on migration may depend on interacting factors.
6. DDR1 in breast carcinoma invasion
Regarding the role of DDR1 in invasion, accumulating evidence produced with matrigel invasion assays indicates that DDR1 can promote invasion in a number of types of human cancer cell line, including breast (56), lung (8), prostate (9), pituitary adenoma (67), hepatocellular carcinoma (14) and glioma (68,69). The pro-invasive function of DDR1 may be mediated by the upregulation of the expression of MMPs, particularly MMP-2 and MMP-9. The elevated expression of MMPs contributes to the degradation of extracellular matrix components, facilitating cancer cell invasion (56). Products that can suppress the pro-invasion function of DDR1 have been developed; Santa Cruz Biotechnology have developed two novel antibodies that can inhibit DDR1a-mediated Matrigel invasion (68) and DDR1 activation in human MDA-MB-231 cells (58). Therefore, previous studies predominantly support that DDR1 promotes invasion in breast cancer.
7. Effects of DDR1 on the apoptosis or survival of breast cancer cells
Varying DDR1 expression levels have been identified in a variety of types of human cancer. Assent et al (70) identified that in breast cancer, DDR1 performed a role in monitoring the cellular microenvironment and triggering apoptosis via the induction of Bcl-2-interacting killer. To confirm that apoptosis could be induced by DDR1, they used siRNA to reduce the DDR1 expression level in MCF-7 cells and incubated them with collagen gels. They determined that the downregulation of DDR1 inhibited apoptosis by ~60% in breast cancer cells. A further study identified that the catalytic activity of membrane type-1-MMP (MT1-MMP) impaired this DDR1-initiated apoptotic program, although the mechanisms by which MT1-MMP may interfere with DDR1-initiated signaling remain unclear (70).
DDR1 can induce pro- (5,44,69,71,72) and anti- (73–75) proliferative effects depending on the cell type. It was previously demonstrated that in KRAS-driven lung adenocarcinoma, DDR1 and Notch co-inhibition suppressed the activation of critical tumor survival-promoting signaling pathways (76). High DDR1 expression may also exhibit a positive effect in the proliferation and/or survival of breast cancer cells (46,77). Ongusaha et al (78) demonstrated that the DDR1 receptor could function as a survival effector in wild-type p53-containing breast cancer cells exposed to genotoxic drugs. Furthermore, Fanale et al (79) reported that the DDR1 pathway is likely to be an alternative to the established pro-growth and survival signaling pathways in tumor cells, as activated DDR1 significantly increased tumor cell survival in vitro. Therefore, we hypothesize that in breast carcinoma, the DDR1 pathway may be pro-apoptotic or pro-survival, depending on the microenvironment.
8. DDR1 enhances the chemoresistance to genotoxic drugs
Previous studies have demonstrated that DDR1 is a direct transcriptional target of p53. In wild-type p53-containing cells exposed to genotoxic drugs, DDR1 can function as a survival effector (78). During genotoxic stress, the inhibition of DDR1 function led to the markedly increased apoptosis of wild-type p53-containing cells via a caspase-dependent pathway (78). Das (80) reported that DDR1 induced cyclooxygenase-2 (COX-2) expression, resulting in enhanced chemoresistance in MDA-MB-435 and T47D breast cancer cells. Subsequent to using short hairpin RNA against DDR1 to eliminate DDR1-mediated COX-2 induction, they identified that the chemosensitivity of the breast cancer cells was increased. They also demonstrated that DDR1 activated the nuclear factor-κB (NF-κB) pathway under genotoxic stress. When they inhibited the activation of the NF-κB pathway, the level of DDR1-induced COX-2 was reduced, leading to enhanced breast cancer cell chemosensitivity. Therefore, DDR1-mediated COX-2 induction was NF-κB-dependent (80). However, the effect of DDR1 on genotoxic drug resistance requires further study.
9. Conclusions
The present review described the complex functions of DDR1 in regulating EMT, migration, invasion, apoptosis, survival and chemoresistance to genotoxic drugs in breast carcinoma, as well as illustrating the identified up/downstream signaling molecules that mediate these effects (Table II, Fig. 1). The effects of DDR1 expression in breast carcinoma may depend on the histological type, grade and hormone receptor status of the tumor (Table I). Considering the critical role of collagen-induced DDR1 in the migration, invasion, apoptosis and chemoresistance of breast carcinoma cells, the associated molecular mechanisms require further investigation.
Table II.
In vitro functions of discoidin domain receptor 1 in breast carcinoma progression.
| Process | Positive regulator | Negative regulator |
|---|---|---|
| Proliferation/survival | MCF-7 (78) | MCF-7 and ZR-75-1 (70,86) |
| MDA-MB-435 and T47D (80) | ||
| Migration | MCF-7 (57) | MCF-7 (59) |
| MDA-MB-231 (58) | MDA-MB-231 (46,59) | |
| MDA-MB-468 and T47D (54) | Hs578T (46) | |
| Invasion | MDA-MB-231 (58,87) | Not reported |
| Epithelial-to-mesenchymal transition | Not reported | Hs578T, MCF-7 and MDA-MB-231 (46) |
In summary, regulation via DDR1 may be critical for breast tumor suppression or promotion and therefore, the development of small-molecule drugs targeting DDR1 may be a novel strategy for anticancer therapy according to the histological type, grade and hormone receptor status of the breast tumor. To the present day, studies have identified imatinib, nilotinib and dasatinib as DDR1 inhibitors (81,82).
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81301806), Jiangsu Planned Projects for Postdoctoral Research Funds (grant no. 1501060A), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents and the Jiangsu Province Graduate Innovation Project (grant no. SJLX15_0727).
References
- 1.Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: An update on discoidin domain receptor function. Cell Signal. 2006;18:1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: New players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/S1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 4.Playford MP, Butler RJ, Wang XC, Katso RM, Cooke IE, Ganesan TS. The genomic structure of discoidin receptor tyrosine kinase. Genome Res. 1996;6:620–627. doi: 10.1101/gr.6.7.620. [DOI] [PubMed] [Google Scholar]
- 5.Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan J, Yahata T, Adachi S, Yoshihara K, Tanaka K. Identification of receptor tyrosine kinase, discoidin domain receptor 1 (DDR1), as a potential biomarker for serous ovarian cancer. Int J Mol Sci. 2011;12:971–982. doi: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakada M, Kita D, Teng L, Pyko IV, Watanabe T, Hayashi Y, Hamada J. Receptor tyrosine kinases: Principles and functions in glioma invasion. Adv Exp Med Biol. 2013;986:143–170. doi: 10.1007/978-94-007-4719-7_8. [DOI] [PubMed] [Google Scholar]
- 8.Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS, Chung MJ. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24:311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- 9.Shimada K, Nakamura M, Ishida E, Higuchi T, Yamamoto H, Tsujikawa K, Konishi N. Prostate cancer antigen-1 contributes to cell survival and invasion though discoidin receptor 1 in human prostate cancer. Cancer Sci. 2008;99:39–45. doi: 10.1111/j.1349-7006.2007.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65:195–203. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- 11.Willenbrock K, Kuppers R, Renne C, Brune V, Eckerle S, Weidmann E, Bräuninger A, Hansmann ML. Common features and differences in the transcriptome of large cell anaplastic lymphoma and classical Hodgkin's lymphoma. Haematologica. 2006;91:596–604. [PubMed] [Google Scholar]
- 12.Song J, Chen X, Bai J, Liu Q, Li H, Xie J, Jing H, Zheng J. Discoidin domain receptor 1 (DDR1), a promising biomarker, induces epithelial to mesenchymal transition in renal cancer cells. Tumour Biol. 2016;37:11509–11521. doi: 10.1007/s13277-016-5021-2. [DOI] [PubMed] [Google Scholar]
- 13.Toy KA, Valiathan RR, Núñez F, Kidwell KM, Gonzalez ME, Fridman R, Kleer CG. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150:9–18. doi: 10.1007/s10549-015-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, Beckebaum S. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 16.Maskarinec G, Pagano IS, Little MA, Conroy SM, Park SY, Kolonel LN. Mammographic density as a predictor of breast cancer survival: The Multiethnic Cohort. Breast Cancer Res. 2013;15:R7. doi: 10.1186/bcr3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tice JA, O'Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density and the risk of breast cancer. J Natl Cancer Inst. 2013;105:1043–1049. doi: 10.1093/jnci/djt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ameli F, Rose IM, Masir N. Expression of DDR1 and DVL1 in invasive ductal and lobular breast carcinoma does not correlate with histological type, grade and hormone receptor status. Asian Pac J Cancer Prev. 2015;16:2385–2390. doi: 10.7314/APJCP.2015.16.6.2385. [DOI] [PubMed] [Google Scholar]
- 19.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Current Opin Cell Biol. 2010;22:697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/S1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 21.Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- 22.Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- 23.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283:6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: Binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- 26.L'Hote CG, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: Effect of adhesion on DDR1 phosphorylation. FASEB J. 2002;16:234–236. doi: 10.1096/fj.01-0414fje. [DOI] [PubMed] [Google Scholar]
- 27.Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580:15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol Biol Cell. 2006;17:2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Müller K, Kramer K, Kuster B. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics. 2012;75:3465–3477. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Nascimento AF, Raut CP, Fletcher CD. Primary angiosarcoma of the breast: Clinicopathologic analysis of 49 cases, suggesting that grade is not prognostic. Am J Surg Pathol. 2008;32:1896–1904. doi: 10.1097/PAS.0b013e318176dbc7. [DOI] [PubMed] [Google Scholar]
- 31.Petridis C, Brook MN, Shah V, Kohut K, Gorman P, Caneppele M, Levi D, Papouli E, Orr N, Cox A, et al. Genetic predisposition to ductal carcinoma in situ of the breast. Breast Cancer Res. 2016;18:22. doi: 10.1186/s13058-016-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turashvili G, Bouchal J, Baumforth K, Wei W, Dziechciarkova M, Ehrmann J, Klein J, Fridman E, Skarda J, Srovnal J, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. doi: 10.1186/1471-2407-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res. 2008;68:537–544. doi: 10.1158/0008-5472.CAN-07-5682. [DOI] [PubMed] [Google Scholar]
- 34.Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103:344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- 35.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam N, Kwon SC, Senie R, Kathuria N. Breast and cervical cancer screening among South Asian women in New York city. J Immigr Minor Health. 2006;8:211–221. doi: 10.1007/s10903-006-9325-y. [DOI] [PubMed] [Google Scholar]
- 37.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44 (hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 39.Sheen YY, Kim MJ, Park SA, Park SY, Nam JS. Targeting the transforming growth factor-β signaling in cancer therapy. Biomol Ther (Seoul) 2013;21:323–331. doi: 10.4062/biomolther.2013.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 41.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 42.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 43.Savagner P, Boyer B, Valles AM, Jouanneau J, Thiery JP. Modulations of the epithelial phenotype during embryogenesis and cancer progression. Cancer Treat Res. 1994;71:229–249. doi: 10.1007/978-1-4615-2592-9_12. [DOI] [PubMed] [Google Scholar]
- 44.Maeyama M, Koga H, Selvendiran K, Yanagimoto C, Hanada S, Taniguchi E, Kawaguchi T, Harada M, Ueno T, Sata M. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. 2008;113:2823–2831. doi: 10.1002/cncr.23900. [DOI] [PubMed] [Google Scholar]
- 45.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh M, Woo Y, Valiathan RR, Jung HY, Park SY, Kim YN, Kim HR, Fridman R, Moon A. Discoidin domain receptor 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-Ras-induced epithelial to mesenchymal transition. Int J Cancer. 2015;136:E508–E520. doi: 10.1002/ijc.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh YC, Wu CC, Wang YK, Tang MJ. DDR1 triggers epithelial cell differentiation by promoting cell adhesion through stabilization of E-cadherin. Mol Biol Cell. 2011;22:940–953. doi: 10.1091/mbc.E10-08-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eswaramoorthy R, Wang CK, Chen WC, Tang MJ, Ho ML, Hwang CC, Wang HM, Wang CZ. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. J Cell Physiol. 2010;224:387–397. doi: 10.1002/jcp.22134. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 2005;65:10289–10297. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. FASEB J. 2001;15:2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- 52.Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Sci. 2001;114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- 53.Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90:1147–1149. doi: 10.1161/01.RES.0000022166.74073.F8. [DOI] [PubMed] [Google Scholar]
- 54.Neuhaus B, Bühren S, Böck B, Alves F, Vogel WF, Kiefer F. Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell Mol Life Sci. 2011;68:3757–3770. doi: 10.1007/s00018-011-0676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dejmek J, Leandersson K, Manjer J, Bjartell A, Emdin SO, Vogel WF, Landberg G, Andersson T. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11:520–528. [PubMed] [Google Scholar]
- 56.Castro-Sanchez L, Soto-Guzman A, Guaderrama-Diaz M, Cortes-Reynosa P, Salazar EP. Role of DDR1 in the gelatinases secretion induced by native type IV collagen in MDA-MB-231 breast cancer cells. Clin Exp Metastasis. 2011;28:463–477. doi: 10.1007/s10585-011-9385-9. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y, Arora P, McCulloch CA, Vogel WF. The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. J Cell Sci. 2009;122:1637–1646. doi: 10.1242/jcs.046219. [DOI] [PubMed] [Google Scholar]
- 58.Castro-Sanchez L, Soto-Guzman A, Navarro-Tito N, Martinez-Orozco R, Salazar EP. Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells. Eur J Cell Biol. 2010;89:843–852. doi: 10.1016/j.ejcb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Hansen C, Greengard P, Nairn AC, Andersson T, Vogel WF. Phosphorylation of DARPP-32 regulates breast cancer cell migration downstream of the receptor tyrosine kinase DDR1. Exp Cell Res. 2006;312:4011–4018. doi: 10.1016/j.yexcr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Dierick H, Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol. 1999;43:153–190. doi: 10.1016/S0070-2153(08)60381-6. [DOI] [PubMed] [Google Scholar]
- 61.Pruitt MM, Letcher EJ, Chou HC, Bastin BR, Schneider SQ. Expression of the wnt gene complement in a spiral-cleaving embryo and trochophore larva. Int J Dev Biol. 2014;58:563–573. doi: 10.1387/ijdb.140084ss. [DOI] [PubMed] [Google Scholar]
- 62.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 64.Katoh M. WNT/PCP signaling pathway and human cancer (Review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 65.Yang Y. Wnt signaling in development and disease. Cell Biosci. 2012;2:14. doi: 10.1186/2045-3701-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida D, Teramoto A. Enhancement of pituitary adenoma cell invasion and adhesion is mediated by discoidin domain receptor-1. J Neurooncol. 2007;82:29–40. doi: 10.1007/s11060-006-9246-6. [DOI] [PubMed] [Google Scholar]
- 68.Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76:239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- 69.Yamanaka R, Arao T, Yajima N, Tsuchiya N, Homma J, Tanaka R, Sano M, Oide A, Sekijima M, Nishio K. Identification of expressed genes characterizing long-term survival in malignant glioma patients. Oncogene. 2006;25:5994–6002. doi: 10.1038/sj.onc.1209585. [DOI] [PubMed] [Google Scholar]
- 70.Assent D, Bourgot I, Hennuy B, Geurts P, Noël A, Foidart JM, Maquoi E. A Membrane-Type-1 Matrix Metalloproteinase (MT1-MMP)-discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. PLoS One. 2015;10:e0116006. doi: 10.1371/journal.pone.0116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberts ME, Magowan L, Hall IP, Johnson SR. Discoidin domain receptor 1 regulates bronchial epithelial repair and matrix metalloproteinase production. Eur Respir J. 2011;37:1482–1493. doi: 10.1183/09031936.00039710. [DOI] [PubMed] [Google Scholar]
- 72.Dang N, Hu J, Liu X, Li X, Ji S, Zhang W, Su J, Lu F, Yang A, Han H, et al. CD167 acts as a novel costimulatory receptor in T-cell activation. J Immunother. 2009;32:773–784. doi: 10.1097/CJI.0b013e3181acea46. [DOI] [PubMed] [Google Scholar]
- 73.Vogel WF, Aszódi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Curat CA, Vogel WF. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J Am Soc Nephrol. 2002;13:2648–2656. doi: 10.1097/01.ASN.0000032419.13208.0C. [DOI] [PubMed] [Google Scholar]
- 75.Franco C, Ahmad PJ, Hou G, Wong E, Bendeck MP. Increased cell and matrix accumulation during atherogenesis in mice with vessel wall-specific deletion of discoidin domain receptor 1. Circ Res. 2010;106:1775–1783. doi: 10.1161/CIRCRESAHA.109.213637. [DOI] [PubMed] [Google Scholar]
- 76.Ambrogio C, Gomez-Lopez G, Falcone M, Vidal A, Nadal E, Crosetto N, Blasco RB, Fernández-Marcos PJ, Sánchez-Céspedes M, Ren X, et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22:270–277. doi: 10.1038/nm.4041. [DOI] [PubMed] [Google Scholar]
- 77.Duncan JS, Whittle MC, Nakamura K, Abell AN, Midland AA, Zawistowski JS, Johnson NL, Granger DA, Jordan NV, Darr DB, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149:307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, Lee SW. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22:1289–1301. doi: 10.1093/emboj/cdg129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fanale M. Activated DDR1 increases RS cell survival. Blood. 2013;122:4152–4154. doi: 10.1182/blood-2013-11-534123. [DOI] [PubMed] [Google Scholar]
- 80.Das S. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-B pathway activation. Cancer Res. 2006;66:8123–8130. doi: 10.1158/0008-5472.CAN-06-1215. [DOI] [PubMed] [Google Scholar]
- 81.Rix U, Hantschel O, Duernberger G, Rix Remsing LL, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P, Colinge J, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib, reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 82.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- 83.Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, Dale TC, Gusterson BA, Crompton MR. Expression patterns of the novel receptor-like tyrosine kinase, Ddr, in human breast-tumors. Oncogene. 1995;10:569–575. [PubMed] [Google Scholar]
- 84.Cancer Genome Atlas Network: Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turashvilia G, Bouchala J, Ehrmanna J, Kolara Z, Fridmanb E, Skardaa J. Novel immunohistochemical markers for the differentiation of lobular and ductal invasive breast carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151:59–64. doi: 10.5507/bp.2007.010. [DOI] [PubMed] [Google Scholar]
- 86.Maquoi E, Assent D, Detilleux J, Pequeux C, Foidart JM, Noël A. MT1-MMP protects breast carcinoma cells against type I collagen-induced apoptosis. Oncogene. 2012;31:480–493. doi: 10.1038/onc.2011.249. [DOI] [PubMed] [Google Scholar]
- 87.Juin A, Di Martino J, Leitinger B, Henriet E, Gary AS, Paysan L, Bomo J, Baffet G, Gauthier-Rouvière C, Rosenbaum J, et al. Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol. 2014;207:517–533. doi: 10.1083/jcb.201404079. [DOI] [PMC free article] [PubMed] [Google Scholar]