Abstract
In this study, we tested the effect of the stilbene resveratrol on life span, body composition, locomotor activity, stress response, and the expression of genes encoding proteins centrally involved in ageing pathways in the model organism Drosophila melanogaster. Male and female w1118 D. melanogaster were fed diets based on sucrose, corn meal, and yeast. Flies either received a control diet or a diet supplemented with 500 µmol/L resveratrol. Dietary resveratrol did not affect mean, median, and maximal life span of male and female flies. Furthermore, body composition remained largely unchanged following the resveratrol supplementation. Locomotor activity, as determined by the climbing index, was not significantly different between control and resveratrol-supplemented flies. Resveratrol-fed flies did not exhibit an improved stress response towards hydrogen peroxide as compared to controls. Resveratrol did not change mRNA steady levels of antioxidant (catalase, glutathione-S-transferase, NADH dehydrogenase, glutathione peroxidase, superoxide dismutase 2) and longevity-related genes, including sirtuin 2, spargel, and I’m Not Dead Yet. Collectively, present data suggest that resveratrol does not affect life span, body composition, locomotor activity, stress response, and longevity-associated gene expression in w1118 D. melanogaster.
Keywords: resveratrol, Drosophila, healthy ageing, life span, longevity
1. Introduction
Diet is an important determinant of health and disease prevention. Epidemiological data on the consumption of foods rich in fruits and vegetables suggests that secondary plant metabolites may favour health and successful ageing [1].
The traditional Asian and the Mediterranean diets are rich in fruits and vegetables [2]. There are specific plant bioactives, which predominantly occur in the Mediterranean (e.g., resveratrol from red wine, hydroxytyrosol from olives) and in the Asian diets (e.g., isoflavones from soybean and epigallocatechin gallate from green tea). In this context, we have recently introduced the concept of the so-called “MediterrAsian” diet combining foods of the traditional Asian as well as Mediterranean diet as a promising dietary strategy in chronic disease prevention [2].
Drosophila melanogaster is widely used as a model organism in ageing studies. Drosophila exhibits a relatively short life span of 60 to 90 days, which makes it particularly attractive for life span studies [3,4]. Furthermore, in recent years, the fruit fly has also been increasingly recognised as a model organism in nutrition research. Feed intake, body composition, locomotor activity, gut function, composition of the microbiota, ageing, as well as life span can be systematically determined in Drosophila in response to dietary factors [5,6,7,8,9,10,11]. Moreover, diet-induced pathophysiological mechanisms including both intestinal and systemic inflammatory processes [12,13,14,15,16,17], and stress response against various triggers like reactive oxygen species, alcohol, acids, or heat [6,12,18,19] may be evaluated in the fruit fly under defined experimental conditions. We have recently shown that secondary plant metabolites including isoflavones [5], green tea catechins [10], and isothiocyanates [20] are capable of improving health status and survival in male D. melanogaster.
The stilbene trans-resveratrol (3,4′,5-trihydroxystilbene) has been widely suggested as a putative “anti-ageing” molecule, e.g., in Saccharomyces cerevisiae [21,22,23], Caenorhabditis elegans [24,25,26], and killifish [27,28,29]. However, literature is contradictory regarding the life span modulating properties of resveratrol in D. melanogaster [24,30,31,32]. Furthermore, resveratrol mostly failed to improve life span in studies conducted in mice [33]. Several mechanisms, including induction of autophagy and sirtuins [34,35,36,37,38,39], modulation of IGF signalling [26,40,41], improvement of stress response [42,43,44,45,46], endogenous antioxidant defence [43,47,48], mitochondrial function [41,49,50,51], as well as anti-inflammatory properties [52,53,54,55,56,57,58,59], have been suggested by which resveratrol may counteract the ageing process. Moreover, there is literature data indicating that resveratrol may affect body weight [60,61,62,63,64,65], body composition [62,64,66], and metabolism [65,66,67,68] in different species—however, data are partly contradicting.
Although resveratrol has been shown to increase the life span in short-lived species like worms (C. elegans) [24,25] and killifish [27,28], the role of resveratrol in the fruit fly is less clear. Therefore, the aim of the present study was to systematically investigate the effect of dietary resveratrol on life span, body composition, stress response, and longevity-associated gene expression in D. melanogaster.
2. Results
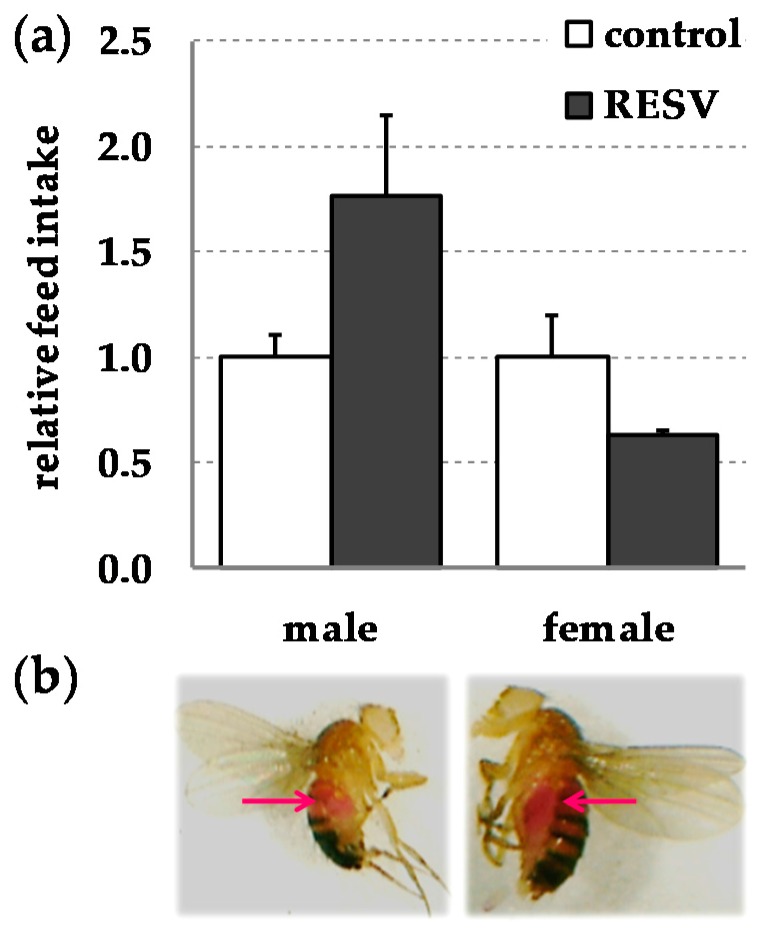
Since feed intake may affect body composition, metabolism, locomotor activity, and life span, we monitored the feed intake of D. melanogaster in the presence and absence of resveratrol by the food-dye-based “sulforhodamine B gustatory assay” [5,69]. Under the conditions investigated there were no significant differences in feed intake between resveratrol-supplemented flies and controls both in males (p = 0.162) and females (p = 0.126) (Figure 1).
Figure 1.
Dietary resveratrol (RESV; 500 µmol/L) does not affect feed intake in w1118 D. melanogaster. (a) Relative feed intake in male and female flies following a five-day feeding period with a RESV-supplemented or a control diet. Flies were administered the experimental diets for five days prior to administration of a sulforhodamine B (0.2% w/v)-supplemented medium for 8 h. Feed intake was quantified via fluorometric measurements. Feed intake of RESV-treated males and females was normalised to the feed intake of their control fed counterparts, respectively. Bars show means ± SEM comprising 60–100 flies/group. Statistics: 2-sided Student’s t-test and Mann–Whitney U.; (b) Bright-field pictures of male and female w1118 flies administered with sulforhodamine B for 8 h. Arrows point to pink-coloured body parts due to the sulforhodamine B ingestion.
Accordingly, resveratrol-supplemented and control-fed flies exhibited similar fat, protein, and glucose contents (Table 1), whereby flies showed a rather heterogeneous response to the dietary resveratrol treatment as revealed by higher standard errors. Solely the protein content was slightly increased in resveratrol-fed males compared to controls. Thus, overall body composition of D. melanogaster remained largely unchanged in response to dietary resveratrol supplementation.
Table 1.
Changes in body weight and body composition of male and female w1118 D. melanogaster in dependence of dietary resveratrol (RESV; 500 µmol/L) administration for ten days compared to controls.
| Parameter | Male | Female | ||||
|---|---|---|---|---|---|---|
| Control | RESV 1 | p-Value | Control | RESV 1 | p-Value | |
| Body weight (µg/fly) | 778 ± 46 | 774 ± 42 | 0.953 | 1222 ± 43 | 1223 ± 77 | 0.989 |
| Triglycerides (% control) | 100 ± 9.0 | 165 ± 31 | 0.083 | 100 ± 5.1 | 95.5 ± 13 | 0.750 |
| Protein (% control) | 100 ± 1.8 | 121 ± 7.5 | 0.035 | 100 ± 2.0 | 103 ± 5.3 | 0.679 |
| Glucose (% control) | 100 ± 12 | 115 ± 21 | 0.500 | 100 ± 14 | 156 ± 34 | 0.100 |
All data are shown as means ± SEM from three independent experiments comprising 178–224 flies/group (body weight) and 20–35 flies/group (body composition). Outliers were removed. Statistics: 2-sided Student’s t-test and Mann–Whitney U. 1 RESV: resveratrol (500 µmol/L).
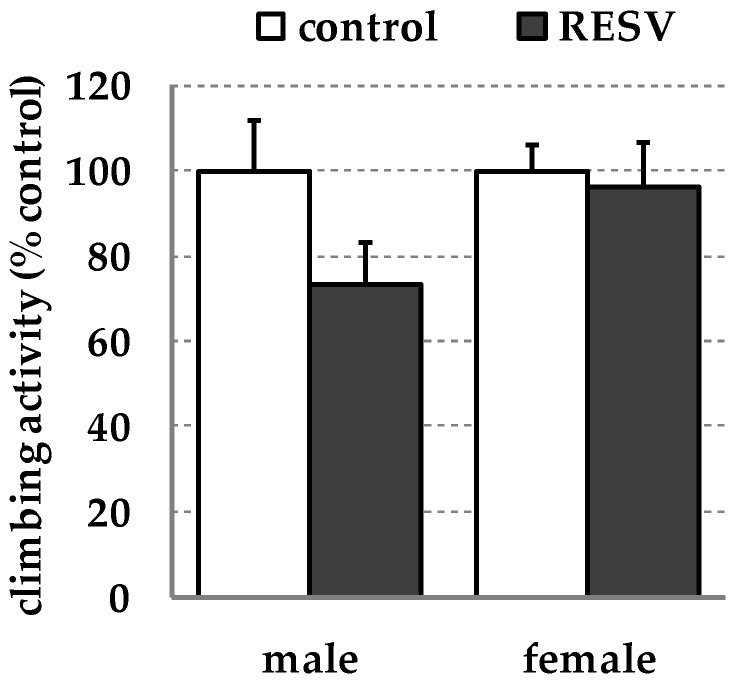
Locomotor activity of D. melanogaster was determined by calculating the climbing score applying the so-called RING assay [70,71]. Under the conditions investigated, locomotor activity was similar between control and resveratrol fed flies both in males (p = 0.092) and females (p = 0.743) as shown in Figure 2.
Figure 2.
Dietary resveratrol (RESV; 500 µmol/L) does not affect locomotor activity in w1118 D. melanogaster. Relative climbing activity of male and female flies following a thirty-day feeding period in the presence or absence of resveratrol. Locomotor activity was quantified via the rapid iterative negative geotaxis (RING) assay. Bars show means ± SEM from 20 measurements/group derived from two independent experiments. Statistics: Mann–Whitney U. RESV: resveratrol (500 µmol/L).
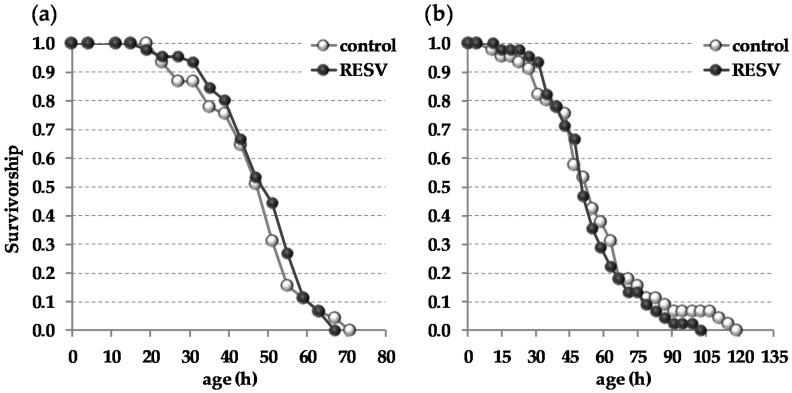
The hydrogen peroxide-based stress resistance assay is well established and suitable to examine both direct and indirect antioxidant effects of secondary plant metabolites in fruit flies [6,72,73,74,75]. In order to test flies for stress resistance against reactive oxygen species, male and female w1118 were challenged with hydrogen peroxide (10% w/v diluted in a 5% w/v sucrose solution) following a ten-day feeding period with a resveratrol-supplemented or a control diet. The hydrogen peroxide administration dramatically increased mortality of both male and female D. melanogaster as reported in the literature. However, there was no significant advantage for survival when flies received dietary resveratrol prior to hydrogen peroxide challenge as compared to controls (Figure 3). Both male and female flies did not benefit from dietary resveratrol supplementation or even displayed slightly reduced mean and median survival rates compared to their control-fed counterparts.
Figure 3.
Dietary resveratrol (RESV; 500 µmol/L) does not improve stress resistance of w1118 D. melanogaster against reactive oxygen species. Flies received a resveratrol-supplemented or a control diet for ten days prior to the exposure to hydrogen peroxide (10% w/v diluted in a 5% w/v sucrose solution). Dead flies were steadily counted every four hours. (a) Survival curve of male and (b) female flies. The stress resistance experiment was independently performed three times with 45 flies/group each revealing similar results. Statistics: Log-Rank.
Accordingly, mRNA expression levels of genes encoding antioxidant enzymes were not significantly modulated by resveratrol ingestion (Table 2).
Table 2.
Relative mRNA expression of antioxidant enzymes in female w1118 D. melanogaster in dependence of resveratrol (RESV; 500 µmol/L) administration for ten days compared to controls.
| Target | mRNA Expression Level vs. Control |
p-Value 6 |
|---|---|---|
| Cat 1 | 0.87 ± 0.15 | 0.700 |
| GstD2 2 | 1.02 ± 0.22 | 0.948 |
| ND-75 3 | 0.99 ± 0.22 | 0.963 |
| PHGPx 4 | 0.89 ± 0.17 | 0.741 |
| Sod2 5 | 1.03 ± 0.18 | 0.930 |
Data are shown as means ± SEM comprising 40 flies/group from two independent experiments; α-Tubulin at 84B (αTub84B) and Ribosomal protein L32 (RpL32) served as the housekeeping genes. Statistics: 2-sided Student’s t-test and Mann–Whitney U. 1 Cat: Catalase; 2 GstD2: Glutathione S transferase D2; 3 ND-75: NADH dehydrogenase (ubiquinone) 75 kDa subunit; 4 PHGPx: PHGPx with glutathione peroxidase activity; 5 Sod2: Superoxide dismutase 2 (Mn). 6 p-value indicates differences in relative mRNA expression in whole body RNA extracts of resveratrol treated flies compared to controls.
Several studies suggest that resveratrol may affect the life span of model organisms. Therefore, we determined mean, median, and maximum life span of flies in response to the resveratrol treatment. However, dietary resveratrol did not change mean, median, and maximum life span of male and female w1118 D. melanogaster in general (Table 3). Resveratrol ingestion rather decreased than increased mean, medium, and maximum life span in both males and females compared to the respective control groups in the majority of the assays performed within this study (Table 3), although resveratrol-dependent differences in life span remained mostly non-significant.
Table 3.
Differences in mean, median, and maximum survival rates (%) of male and female w1118 D. melanogaster in dependence of life-long dietary resveratrol (RESV; 500 µmol/L) administration compared to controls.
| Trial | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Max. 1 | p-Value 2 | Mean | Median | Max. 1 | p-Value 2 | |
| No. 1 | +15.2 | +2.50 | +1.03 | 0.307 | −8.20 | −7.50 | −14.1 | 0.066 |
| No. 2 | −11.8 | −19.6 | +3.04 | 0.345 | −14.7 | −46.2 | −1.04 | 0.099 |
| No. 3 | −33.1 | −56.3 | −9.35 | <0.001 | +15.9 | ±0.00 | +1.06 | 0.341 |
The life span experiment was independently performed three times (Trial No. 1–3) comprising 150 flies/group in each experiment. Changes in mean, medium, and maximum life span (% control) of RESV treated males and females in comparison to the respective control group are shown for each single experiment. RESV did not improve the life span of male and female w1118 D. melanogaster. Statistics: Log-Rank. RESV: resveratrol (500 µmol/L); data are shown as means. 1 max.: maximum lifespan defined as the mean survival of the longest-lived 10%; 2 p-value indicates differences in overall survival benefit of resveratrol treated flies compared to controls.
Furthermore, we monitored mRNA steady levels of various genes encoding proteins that have been reported to be related to ageing and longevity in the fruit fly. Thus, mRNA levels of the health and life span associated genes I’m Not Dead Yet (INDY) [76], sirtuin (Sir2) [77], and spargel (srl) [78] were determined by quantitative real time PCR in whole body homogenates. Similar to our life span data, none of these genes were significantly regulated on the transcript level following dietary resveratrol supplementation. Relative transcript levels are summarized in Table 4.
Table 4.
Relative mRNA expression of longevity-associated genes in male and female w1118 D. melanogaster in dependence of resveratrol (RESV; 500 µmol/L) administration for ten days compared to controls.
| Sex | Transcript | |||||
|---|---|---|---|---|---|---|
| Sir2 1 | p-Value 4 | INDY 2 | p-Value 4 | Srl 3 | p-Value 4 | |
| Male | 0.90 ± 0.19 | 0.700 | 1.13 ± 0.15 | 0.437 | 1.77 ± 0.74 | 0.375 |
| Female | 0.83 ± 0.12 | 0.405 | 1.02 ± 0.14 | 0.913 | 1.12 ± 0.19 | 0.593 |
Data are shown as means ± SEM comprising 40 flies/group in total from two independent experiments; α-Tubulin at 84B (αTub84B) and Ribosomal protein L32 (RpL32) served as the housekeeping genes. Statistics: 2-sided Student’s t-test and Mann–Whitney U. 1 Sir2: Sirtuin 2; 2 INDY: I’m Not Dead Yet; 3 srl: spargel; 4 p-value indicates differences in relative mRNA expression in whole body RNA extracts of resveratrol-treated flies compared to controls.
Resveratrol displays a rather low bioavailability; hence tissue distribution may affect its efficacy to modulate metabolism and life span [79]. Therefore, we determined resveratrol concentrations in the whole body homogenates of male and female flies. We recovered substantial quantities of resveratrol in the whole body homogenates of resveratrol-treated flies, whereas no resveratrol was detected in homogenates of controls (limit of detection 0.49 nmol/g flies). These data suggest that resveratrol was readily taken up from the diet. Interestingly, female flies fed the resveratrol-supplemented diet exhibited a 2.2-fold higher resveratrol body concentration as compared to male flies (20.98 ± 0.44 vs. 9.60 ± 2.80 nmol/g fly; means ± SD; N = 54 flies/group following a ten-day feeding period).
3. Discussion
Several studies in lemurs, mice, honey bees, and humans suggest that resveratrol may affect food intake due to its bitter taste [65,80,81,82,83], which may in turn affect stress response and metabolism. However, in the current fly study, we did not observe significant differences in feed intake between groups (Figure 1). Although literature data suggest that resveratrol may affect body weight and body composition [60,61,62,63,64,65,66,84], we did not find any significant changes in these parameters, except for the slight increase in protein content in males, following dietary resveratrol supplementation (Table 1).
Furthermore, we did not observe changes in both locomotor activity and life span in resveratrol-fed flies as compared to controls (Figure 2; Table 3). One limitation of our present study is that only one dietary resveratrol concentration has been investigated and no dose-response analysis has been performed. Thus, we cannot fully exclude the possibility that higher or lower dietary resveratrol concentrations may modulate overall health status and life span in D. melanogaster as indicated by other but rather contradictory studies [24,30,31,85,86]. Therefore, further studies may include additional resveratrol concentrations and treatment periods for body weight and composition measurements, transcript analyses, and oxidative stress resistance evaluation. Due to comparable high variances in the treatment groups, the inclusion of further fly strains should be also considered.
We have recently conducted a systematic literature review concerning the effect of resveratrol on life span in various model organisms. Interestingly, resveratrol supplementation has been reported to increase life span in approximately 60% of the studies conducted in model organisms [33]. However, literature data is rather inconsistent, suggesting that the life span effects of resveratrol vary in relation to the model organism. Furthermore, it should be considered that other factors such as dose, gender, genetic background, and diet composition [35,87] may contribute to the high variance in the life span prolonging ability of resveratrol in the fruit fly.
Several plant bioactives, including lutein, epicatechin, epigallocatechin gallate, and apple polyphenols, have been reported to counteract reactive oxygen species induced mortality in D. melanogaster [6,88,89]. It is suggested that the protective action of these plant bioactives may be partly mediated via the induction of the transcription factor cnc, which represents an ortholog of mammalian Nrf2 in the fruit fly [90], and its target genes exhibiting antioxidant activity [89,91,92]. However, under the conditions investigated resveratrol did not improve the survival of flies treated with hydrogen peroxide as compared to controls (Figure 3). Accordingly, the relative transcript levels of cnc target genes and further antioxidant enzymes such as glutathione-S-transferase, glutathione peroxidase, NADH dehydrogenase, superoxide dismutase, and catalase remained largely unchanged in response to the resveratrol treatment (Table 2).
Cell culture as well as in vivo studies suggest that resveratrol may modulate the expression of genes encoding proteins that are centrally involved in ageing related pathways and suppresses DNA damage, thereby affecting the ageing process [93,94,95,96]. Among others, sirtuins seem to be important molecular targets of resveratrol [49,97,98]. Sir2 is an important modulator of the ageing process and locomotor activity in the fruit fly [77,86,99]. Moderate induction of Sir2 expression is associated with life span elongation in D. melanogaster in response to both nutrient composition as well as dietary plant bioactives [5,77,99,100,101,102]. In contrast, mutant fly strains with diminished or absent Sir2 expression display a markedly shortened life span and lack responsiveness to life span extending dietary interventions [31,99,101]. However, in the present study, Sir2 mRNA levels were not affected in whole body homogenates of D. melanogaster. The same holds true for other genes previously reported to affect ageing and life span in the fruit fly, including INDY and srl (Table 4), being in accordance with the ineffectiveness of resveratrol to prolong life span in w1118 males and females (Table 3). It should be considered that INDY expression is not inevitably associated with longevity in the w1118 D. melanogaster strain [103]. Moreover, it needs to be taken into account that, in the present study, gene expression was determined in whole body homogenates and not in specific tissues and organs of D. melanogaster. Thus, it could be possible that resveratrol may have affected gene expression in distinct tissues of the fly, which was however beyond the scope of the current study.
Unlike in the present study in w1118 D. melanogaster, resveratrol was shown to increase life span in the short-lived killifish [27,28] and in laboratory mice in dependence of their genetic background [41,104]. Besides differences in the dietary resveratrol concentrations and durations of the experimental trials, there may be also species-specific differences in resveratrol metabolism [33].
Importantly, resveratrol whole body concentrations in both male and female flies were elevated in response to dietary resveratrol supplementation. Interestingly, female flies exhibited a whole body resveratrol concentration more than twice as high as male flies, which warrants further investigations into the underlying mechanisms. Studies by Wang et al. and Zou et al. [32,87] suggested that female but not male D. melanogaster may benefit from dietary resveratrol supplementation in terms of life span prolongation in dependence of the macronutrient composition of the diet. This may be partly related to a higher resveratrol body concentration in female vs. male flies as observed in the present study. The storage of fat diverges in male and female fruit flies starting in the early adulthood while the extent of the sex-specific deviation depends on the fly strain [105,106]. Thus, difference in whole body resveratrol content between male and female flies may also partly depend on the higher fat content in w1118 females compared to males (triglyceride level in ten-day-old males: 13.2 ± 0.7 ng/µg fly; females: 32.7 ± 2.8 ng/µg fly; p < 0.001) resulting in an increased accumulation of the primarily fat-soluble resveratrol.
In conclusion, present data suggest that dietary resveratrol, although sufficiently absorbed, does not affect life span, body composition, stress response, and longevity-related gene expression in male and female w1118 D. melanogaster.
4. Materials and Methods
4.1. Fly Strain and Husbandry
The Drosophila melanogaster strain w1118 (Bloomington Drosophila Stock Center #5905, Indiana University, Bloomington, IN, USA) was used for all experiments. w1118 flies were maintained on a standard diet at 25 °C and 60% humidity with a 12/12 h light–dark cycle. Standard medium was prepared as described previously [5]. The experimental diet consisted of sucrose (5% w/v; Carl Roth, Karlsruhe, Germany), Agar Type II (0.5% w/v; Dutscher Scientific, Grays, UK), corn meal (8.6% w/v; Dutscher Scientific), and inactive dry yeast (5% w/v; Dutscher Scientific), while Tegosept (0.3% w/v; Dutscher Scientific) and propionic acid (0.3% v/v; Carl Roth) served as preservatives. Resveratrol was purchased from Carl Roth and was dissolved in dimethyl sulfoxide (DMSO; Carl Roth) at a concentration of 100 mmol/L. This resveratrol stock solution was stored at −80 °C until supplementation of the experimental diet at a final concentration of 500 µmol/L. This concentration was chosen based on contradictory literature data reporting both beneficial [30,35,85] and detrimental [24,32] effects of oral resveratrol administration on the fruit fly that were observed in a concentration range between 100 and 1000 µmol/L. To ensure sufficient supply and absorption of resveratrol, 500 µmol/L were orally applied in this study. DMSO (0.5% v/v) served as the vehicle control. For all experiments, flies received either a resveratrol-supplemented (500 µmol/L) or a control diet and were reared on the experimental diets at a population density of 25 flies/vial.
4.2. Life Span Analyses
Newly enclosed synchronized flies were permitted to mate for two days as described previously [5]. Sexual activity may act as a confounding variable in life span experiments [107,108] as frequent mating shortens life expectancy in D. melanogaster [107,109]. Therefore, flies were separated according to sex two days past enclosure and were housed in single-sex cohorts. The flies were fed the resveratrol-supplemented or the control diet life-long while transferred to fresh medium three times a week. The number of dead flies was recorded at each transfer until all flies were dead. The experiment was independently performed three times with 150 flies/group, respectively.
4.3. Gustatory Assay
Sulforhodamine B (0.2% w/v; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was used to determine food intake in both male and female flies fed a resveratrol-supplemented or a control diet. Flies were reared on the experimental diet for five days prior performing the food-dye-based gustatory assay according to the protocol described elsewhere [5,69]. This feeding period was chosen as food consumption in older flies remarkably declines in comparison to early-life feeding levels [110]. The gustatory assay was independently performed two times.
4.4. Body Weight and Body Composition
Flies received a resveratrol-supplemented or a control diet for ten days. Body weight was estimated as described in [5], the same applies to the quantification of whole body triglyceride, protein and glucose levels. Average body weight of male and female w1118 was calculated from two independent experiments with 51–137 flies/group, respectively (178–224 flies/group in total). Body composition data were collected from three independent experiments comprising 5–15 flies/group, respectively (20–35 flies/group in total).
4.5. Locomotor Activity
Locomotor activity can be easily evaluated in D. melanogaster by applying the rapid iterative negative geotaxis (RING) assay [70,71] indicating the health status of fruit flies. Climbing speed was determined in both male and female flies by performing the RING assay as previously described in detail in [5] following oral administration of a resveratrol-supplemented or a control diet for thirty days. As locomotor activity declines with age [11] and senescence is functionally associated with the flies’ climbing ability reflecting the functional status of muscle and locomotor function [111], flies were pre-treated for thirty days to investigate a putative anti-ageing effect of resveratrol in D. melanogaster. The experiment was performed two times comprising ten repetitive measurements each (N = 100).
4.6. Oxidative Stress Resistance
Resistance against reactive oxygen species was investigated by applying the hydrogen peroxide stress test as described previously [12]. As H2O2 generates hydroxyl radicals in the presence of metal ions [6], it was used to assess the resistance of male and female w1118 flies that were pre-fed with a resveratrol (500 µmol/L)-supplemented or a control diet for ten days. This pre-feeding period was chosen as flies exhibited adequate whole body resveratrol concentrations that allowed the investigation of putative resveratrol-dependent antioxidant effects in vivo. The experiment was independently performed three times including 45 flies/group, respectively.
4.7. RNA Isolation and qRT-PCR
Flies were orally administered a resveratrol (500 µmol/L)-supplemented or a control diet for ten days. Whole flies (ten per sample) were homogenised in a TissueLyser II (Qiagen, Hilden, Germany) prior to total RNA isolation with the help of TriFast reagent (peqlab, Erlangen, Germany) according to the manufacturer’s protocol. RNA concentration was determined via NanoDrop measurements (NanoDrop2000c; ThermoScientific, Waltham, MA, USA). mRNA expression was quantified by qRT-PCR measurements using the SensiFast SYBR No-ROX One-Step Kit (Bioline, London, UK) and a Rotor-Gene 6000 real-time PCR cycler (Corbett/Qiagen). Relative mRNA concentrations were calculated using a standard curve. The expression of the longevity associated genes (INDY, F: GATTGGTTGTGTTCCTGGTG, R: CGTCACATAGAGAGGCAAGG; Sir2, F: CCGTTACTGAGGAGGAGCTG, R: GTAGATCGCACACGTCCTTG; srl, F: CTCTTGGAGTCCGAGATCCGCAA, R: GGGACCGCGAGCTGATGGTT [78]), and of the antioxidant enzymes (Cat, F: CCTCTGATTCCTGTGGGCAA, R: GACGACCATGCAGCATCTTG; GstD2, F: GTCTACTTCGCAGGCATCAC, R: CTTCTCGATCCAGGCCTTGA; ND-75, F: CGGACATTAACTACACGGGC, R: CAATCTCGGAGGCGAAACG; PHGPx, F: CCTCAACTTCCCGTGCAATC, R: CCATTCACATCGACCTTGGC; Sod2, F: AACGCAGATATGTTCGTGGC, R: GGTGATGCAGCTCCATGATC) was normalized to the expression of the housekeeping genes α-Tubulin at 84B (αTub84B) and Ribosomal protein L32 (RpL32), respectively. Experiment was independently performed two times with 20 flies/group, respectively.
4.8. Whole Body Resveratrol Concentration
Newly hatched two-day-old flies were orally administered a resveratrol (500 µmol/L)-supplemented or a control diet for ten days. Whole flies were stored at −80 °C until analysis. For analysis, whole flies (27 flies/sample) were mixed with 200 µL ice-cold aqueous triethylammonium acetate solution (1 mol/L, pH = 7.0) followed by adding 1 mL of an ice-cold acetonitril–methanol mixture (1:1, v/v). Afterwards, 1-mm silica spheres were added and flies were treated with a FastPrep homogenizer (3 × 15 s, 4 m/s) with intermediate cooling periods on ice (FastPrep-24, MP Biomedicals, Solon, OH, USA). Homogenates were transferred to a new sample tube. The residual silica spheres were washed with 100 µL of an ice-cold acetonitrile–methanol mixture (1:1, v/v) and this washing fraction was combined with the transferred homogenates. Next, homogenates were first stirred on a vortex mixer for 10 s and then treated in an ultrasonic bath for 10 min. Once again, homogenates were stirred on a vortex mixer for 10 s followed by a centrifugation of the samples (23,100× g; 10 min; 0 °C). Supernatants were transferred to new sample tubes und evaporated to dryness using a SpeedVac (SPD131; Thermo Electron LED GmbH, Langenselbold, Germany). Residues were first dissolved in 70 µL of 30% (v/v) methanol in water and then centrifuged at 23,100× g for 10 min. Supernatants were transferred to a HPLC vial and analysed by LC-MS. A TripleTOF 5600 mass spectrometer (AB Sciex, Darmstadt, Germany) equipped with a 1290 Infinity LC system (Agilent, Waldbronn, Germany) was used. The LC-MS system was controlled by the software Analyst TF 1.6.0. LC separation was carried out on an Agilent Eclipse Plus C18 column (150 mm × 3.0 mm internal diameter, 3.5 µm; Agilent). Eluent A was an aqueous ammonium formiate buffer (25 mmol/L, pH = 3.0) and eluent B was an acetonitrile–methanol mixture (1:1, v/v). A linear gradient was used with a flow rate of 0.6 mL/min and the following elution profile: 0–1 min, isocratic with 10% B; 1–19 min, from 10 to 28% B; 19–33 min from 28 to 70% B; 33–34 min, from 70 to 95% B; 34–39 min, isocratic with 95% B; 39–40 min, from 95 to 10% B; and 40–45 min, isocratic with initial conditions. The column oven was set to 40 °C and the injection volume was 20 µL. The DuoSpray source was operated in negative ESI mode using the following source parameters: curtain gas, 35 psi; ion spray voltage, −4500 V; ion source gas 1, 60 psi; ion source gas 2, 70 psi; ion source gas 2 temperature, 650 °C. The MS full scans were recorded from m/z 100 to 800 with an accumulation time of 200 ms, a declustering potential of −110 V and a collision energy voltage of −10 V. The MS/MS spectra (product ion) were recorded from m/z 50 to 600 in the high-sensitivity mode with an accumulation time of 80 ms, a collision energy voltage of −45 V, and a collision energy spread of 25 V. Nitrogen was used as collision gas. Analysis of data was performed with the MultiQuant 2.1.1 and PeakView 1.2.0.3 software (AB Sciex, Darmstadt, Germany). trans-Resveratrol was identified by retention time and MS/MS spectrum. Accurate XICs (10 mDa extraction width) were used to monitor and quantify the analytes. In detail, trans-resveratrol was quantified by an external calibration using commercial reference compound (Sigma-Aldrich, Deideshofen, Germany). Therefore, control flies were spiked with 5 µL of the specific standard solution (concentration range between 0.8 and 100 µmol/L trans-resveratrol in DMSO) and these spiked samples were worked up as described above. A best fit line was obtained by linear regression using a weighting of 1/x. Whole body resveratrol measurements comprised 54 flies/group, respectively.
4.9. Statistics
Life span and survival rates following H2O2 treatment were analysed using the DLife software (Winchecker version 3.0 [112]). Values are given as means and were statistically compared via a Log-Rank Test based on R (i386 version 3.1.0). All other data are given as means ± SEM, except otherwise mentioned, and were statistically analysed by applying SPSS (version 24; SPSS Inc., Munich, Germany). Data were tested for normality of distribution (Kolmogorov–Smirnov and Shapiro–Wilk) and mean comparisons were carried out using a 2-sided Student’s t-test. If the assumption of a normal distribution was violated, the non-parametric Mann–Whitney U. test was used. Significance was accepted at p-values < 0.05.
Acknowledgments
Sources of funding for research work: University of Kiel (federate state Schleswig-Holstein, Germany). For covering the costs to publish in open access: We acknowledge financial support by Land Schleswig-Holstein within the funding programme “Open Access Publikationsfonds”.
Abbreviations
| Cat | Catalase |
| GstD2 | Glutathione S transferase D2 |
| INDY | I’m Not Dead Yet |
| Max. | maximum |
| ND-75 | NADH dehydrogenase (ubiquinone) 75 kDa subunit |
| PHGPx | PHGPx with glutathione peroxidase activity |
| RESV | resveratrol |
| Sir2 | Sirtuin 2 |
| Sod2 | Superoxide dismutase 2 (Mn) |
| srl | spargel |
Author Contributions
Anika E. Wagner and Stefanie Staats conceived and designed the experiments; Bianca Kowalewski performed the experiments; Bianca Kowalewski, Stefanie Staats, Florian T. Rieck, and Sebastian T. Soukup analysed the data; Gerald Rimbach and Sabine E. Kulling contributed reagents, materials, and analysis tools; Gerald Rimbach, Stefanie Staats, Sebastian T. Soukup, and Sabine E. Kulling wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
References
- 1.Pallauf K., Duckstein N., Rimbach G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2016;76:145–162. doi: 10.1017/S0029665116000720. [DOI] [PubMed] [Google Scholar]
- 2.Pallauf K., Giller K., Huebbe P., Rimbach G. Nutrition and healthy ageing: Calorie restriction or polyphenol-rich “MediterrAsian” diet? Oxid. Med. Cell. Longev. 2013;2013:707421. doi: 10.1155/2013/707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandison R.C., Wong R., Bass T.M., Partridge L., Piper M.D.W. Effect of a Standardised Dietary Restriction Protocol on Multiple Laboratory Strains of Drosophila melanogaster. PLoS ONE. 2009;4:e4067. doi: 10.1371/journal.pone.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang H.-L., Benzer S., Min K.-T. Life extension in Drosophila by feeding a drug. Proc. Natl. Acad. Sci. USA. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piegholdt S., Rimbach G., Wagner A.E. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016;30:948–958. doi: 10.1096/fj.15-282061. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Han S., Wang H., Wang T. Lutein extends the lifespan of Drosophila melanogaster. Arch. Gerontol. Geriatr. 2014;58:153–159. doi: 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ja W.W., Carvalho G.B., Zid B.M., Mak E.M., Brummel T., Benzer S. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc. Natl. Acad. Sci. USA. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Li Y.M., Lei L., Liu Y., Wang X., Ma K.Y., Chen Z.Y. Cranberry anthocyanin extract prolongs lifespan of fruit flies. Exp. Gerontol. 2015;69:189–195. doi: 10.1016/j.exger.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A.E., Piegholdt S., Rabe D., Baenas N., Schloesser A., Eggersdorfer M., Stocker A., Rimbach G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget. 2015;6:30568–30578. doi: 10.18632/oncotarget.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon A.F., Liang D.T., Krantz D.E. Differential decline in behavioral performance of Drosophila melanogaster with age. Mech. Ageing Dev. 2006;127:647–651. doi: 10.1016/j.mad.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Piegholdt S., Rimbach G., Wagner A.E. Effects of the isoflavone prunetin on gut health and stress response in male Drosophila melanogaster. Redox Biol. 2016;8:119–126. doi: 10.1016/j.redox.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stramer B., Wood W. Inflammation and wound healing in Drosophila. Methods Mol. Biol. 2009;571:137–149. doi: 10.1007/978-1-60761-198-1_9. [DOI] [PubMed] [Google Scholar]
- 14.Vlisidou I., Wood W. Drosophila blood cells and their role in immune responses. FEBS J. 2015;282:1368–1382. doi: 10.1111/febs.13235. [DOI] [PubMed] [Google Scholar]
- 15.Panayidou S., Apidianakis Y. Regenerative inflammation: Lessons from Drosophila intestinal epithelium in health and disease. Pathogens. 2013;2:209–231. doi: 10.3390/pathogens2020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apidianakis Y., Rahme L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman N., Maniatis T. NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 18.Clark A.G., Fucito C.D. Stress tolerance and metabolic response to stress in Drosophila melanogaster. Heredity. 1998;81:514–527. doi: 10.1046/j.1365-2540.1998.00414.x. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen J.G., Nielsen M.M., Kruhoffer M., Justesen J., Loeschcke V. Full genome gene expression analysis of the heat stress response in Drosophila melanogaster. Cell Stress Chaperones. 2005;10:312–328. doi: 10.1379/CSC-128R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baenas N., Piegholdt S., Schloesser A., Moreno D.A., García-Viguera C., Rimbach G., Wagner A.E. Metabolic Activity of Radish Sprouts Derived Isothiocyanates in Drosophila melanogaster. Int. J. Mol. Sci. 2016;17:251. doi: 10.3390/ijms17020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 22.Yang H., Baur J.A., Chen A., Miller C., Sinclair D.A. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6:35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarolim S., Millen J., Heeren G., Laun P., Goldfarb D.S., Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5:169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Bass T.M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Lee J., Kwon G., Park J., Kim J.-K., Lim Y.-H. Brief Communication: SIR-2.1-dependent lifespan extension of Caenorhabditis elegans by oxyresveratrol and resveratrol. Exp. Biol. Med. 2016;241:1757–1763. doi: 10.1177/1535370216650054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viswanathan M., Kim S.K., Berdichevsky A., Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Genade T., Lang D.M. Resveratrol extends lifespan and preserves glia but not neurons of the Nothobranchius guentheri optic tectum. Exp. Gerontol. 2013;48:202–212. doi: 10.1016/j.exger.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Valenzano D.R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. Resveratrol Prolongs Lifespan and Retards the Onset of Age-Related Markers in a Short-Lived Vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 29.Valenzano D.R., Cellerino A. Resveratrol and the Pharmacology of Aging: A New Vertebrate Model to Validate an Old Molecule. Cell Cycle. 2006;5:1027–1032. doi: 10.4161/cc.5.10.2739. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramani S.P., Mohan J., Chatterjee A., Patnaik E., Kukkupuni S.K., Nongthomba U., Venkatasubramanian P. Pomegranate Juice Enhances Healthy Lifespan in Drosophila melanogaster: An Exploratory Study. Front. Public Health. 2014;2:245. doi: 10.3389/fpubh.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayashima Y., Katayanagi Y., Tanaka K., Fukutomi R., Hiramoto S., Imai S. Alkylresorcinols activate SIRT1 and delay ageing in Drosophila melanogaster. Sci. Rep. 2017;7:43679. doi: 10.1038/srep43679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C., Wheeler C.T., Alberico T., Sun X., Seeberger J., Laslo M., Spangler E., Kern B., de Cabo R., Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age. 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallauf K., Rimbach G., Rupp P.M., Chin D., Wolf I.M. Resveratrol and Lifespan in Model Organisms. Curr. Med. Chem. 2016;23:4639–4680. doi: 10.2174/0929867323666161024151233. [DOI] [PubMed] [Google Scholar]
- 34.Pallauf K., Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013;12:237–252. doi: 10.1016/j.arr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Wood J.G., Rogina B., Lavu S., Howitz K., Helfand S.L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 36.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. 2010;6:186–188. doi: 10.4161/auto.6.1.10817. [DOI] [PubMed] [Google Scholar]
- 37.Park D., Jeong H., Lee M.N., Koh A., Kwon O., Yang Y.R., Noh J., Suh P.-G., Park H., Ryu S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016;6:21772. doi: 10.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding S., Jiang J., Zhang G., Bu Y., Zhang G., Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE. 2017;12:e0183541. doi: 10.1371/journal.pone.0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saini A., Al-Shanti N., Sharples A.P., Stewart C.E. Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Exp. Physiol. 2012;97:400–418. doi: 10.1113/expphysiol.2011.061028. [DOI] [PubMed] [Google Scholar]
- 41.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosutti A., Degens H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015;5:8093. doi: 10.1038/srep08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghanim H., Sia C.L., Korzeniewski K., Lohano T., Abuaysheh S., Marumganti A., Chaudhuri A., Dandona P. A Resveratrol and Polyphenol Preparation Suppresses Oxidative and Inflammatory Stress Response to a High-Fat, High-Carbohydrate Meal. J. Clin. Endocrinol. Metab. 2011;96:1409–1414. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristl J., Teskač K., Caddeo C., Abramović Z., Šentjurc M. Improvements of cellular stress response on resveratrol in liposomes. Eur. J. Pharm. Biopharm. 2009;73:253–259. doi: 10.1016/j.ejpb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Xiang L., Nakamura Y., Lim Y.M., Yamasaki Y., Kurokawa-Nose Y., Maruyama W., Osawa T., Matsuura A., Motoyama N., Tsuda L. Tetrahydrocurcumin extends life span and inhibits the oxidative stress response by regulating the FOXO forkhead transcription factor. Aging. 2011;3:1098–1109. doi: 10.18632/aging.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danilov A., Shaposhnikov M., Shevchenko O., Zemskaya N., Zhavoronkov A., Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6:19428–19444. doi: 10.18632/oncotarget.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee L.-C., Weng Y.-T., Wu Y.-R., Soong B.-W., Tseng Y.-C., Chen C.-M., Lee-Chen G.-J. Downregulation of proteins involved in the endoplasmic reticulum stress response and Nrf2-ARE signaling in lymphoblastoid cells of spinocerebellar ataxia type 17. J. Neural Transm. 2014;121:601–610. doi: 10.1007/s00702-013-1157-z. [DOI] [PubMed] [Google Scholar]
- 48.Robb E.L., Winkelmolen L., Visanji N., Brotchie J., Stuart J.A. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem. Biophys. Res. Commun. 2008;372:254–259. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Hui Y., Lu M., Han Y., Zhou H., Liu W., Li L., Jin R. Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem. 2017;119:392–399. doi: 10.1016/j.acthis.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Zhang B., Xu L., Zhuo N., Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem. Biophys. Res. Commun. 2017;493:373–381. doi: 10.1016/j.bbrc.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Karuppagounder V., Arumugam S., Thandavarayan R.A., Pitchaimani V., Sreedhar R., Afrin R., Harima M., Suzuki H., Nomoto M., Miyashita S., et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014;23:617–623. doi: 10.1016/j.intimp.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Kjær T.N., Thorsen K., Jessen N., Stenderup K., Pedersen S.B. Resveratrol Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. PLoS ONE. 2015;10:e0126599. doi: 10.1371/journal.pone.0126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bereswill S., Muñoz M., Fischer A., Plickert R., Haag L.-M., Otto B., Kühl A.A., Loddenkemper C., Göbel U.B., Heimesaat M.M. Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation. PLoS ONE. 2010;5:e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larrosa M., Yañéz-Gascón M.J., Selma M.V., González-Sarrías A., Toti S., Cerón J.J., Tomás-Barberán F., Dolara P., Espín J.C. Effect of a Low Dose of Dietary Resveratrol on Colon Microbiota, Inflammation and Tissue Damage in a DSS-Induced Colitis Rat Model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 56.Jeong S.I., Shin J.A., Cho S., Kim H.W., Lee J.Y., Kang J.L., Park E.-M. Resveratrol attenuates peripheral and brain inflammation and reduces ischemic brain injury in aged female mice. Neurobiol. Aging. 2016;44:74–84. doi: 10.1016/j.neurobiolaging.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Tran H.T., Liong S., Lim R., Barker G., Lappas M. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS ONE. 2017;12:e0173373. doi: 10.1371/journal.pone.0173373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao W., Li A., Feng X., Hou T., Liu K., Liu B., Zhang N. Metformin and resveratrol ameliorate muscle insulin resistance through preventing lipolysis and inflammation in hypoxic adipose tissue. Cell. Signal. 2016;28:1401–1411. doi: 10.1016/j.cellsig.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Bariani M.V., Correa F., Leishman E., Domínguez Rubio A.P., Arias A., Stern A., Bradshaw H.B., Franchi A.M. Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth. MHR Basic Sci. Reprod. Med. 2017;23:571–581. doi: 10.1093/molehr/gax036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S., Jin Y., Choi Y., Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Chang C.C., Lin K.Y., Peng K.Y., Day Y.J., Hung L.M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2016;63:169–178. doi: 10.1507/endocrj.EJ15-0545. [DOI] [PubMed] [Google Scholar]
- 62.Mendez-del Villar M., Gonzalez-Ortiz M., Martinez-Abundis E., Perez-Rubio K.G., Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014;12:497–501. doi: 10.1089/met.2014.0082. [DOI] [PubMed] [Google Scholar]
- 63.Jeon S.-M., Lee S.-A., Choi M.-S. Antiobesity and Vasoprotective Effects of Resveratrol in ApoE-Deficient Mice. J. Med. Food. 2014;17:310–316. doi: 10.1089/jmf.2013.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 65.Dal-Pan A., Blanc S., Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010;10:11. doi: 10.1186/1472-6793-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Um J.-H., Park S.-J., Kang H., Yang S., Foretz M., McBurney M.W., Kim M.K., Viollet B., Chung J.H. AMP-Activated Protein Kinase–Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ran G., Ying L., Li L., Yan Q., Yi W., Ying C., Wu H., Ye X. Resveratrol ameliorates diet-induced dysregulation of lipid metabolism in zebrafish (Danio rerio) PLoS ONE. 2017;12:e0180865. doi: 10.1371/journal.pone.0180865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimenez-Gomez Y., Mattison J.A., Pearson K.J., Martin-Montalvo A., Palacios H.H., Sossong A.M., Ward T.M., Younts C.M., Lewis K., Allard J.S., et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on a high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahadorani S., Bahadorani P., Phillips J.P., Hilliker A.J. The effects of vitamin supplementation on Drosophila life span under normoxia and under oxidative stress. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:35–42. doi: 10.1093/gerona/63.1.35. [DOI] [PubMed] [Google Scholar]
- 70.Bazzell B., Ginzberg S., Healy L., Wessells R.J. Dietary composition regulates Drosophila mobility and cardiac physiology. J. Exp. Biol. 2013;216:859–868. doi: 10.1242/jeb.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gargano J.W., Martin I., Bhandari P., Grotewiel M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Zou Y., Liu Y., Ruan M., Feng X., Wang J., Chu Z., Zhang Z. Cordyceps sinensis oral liquid prolongs the lifespan of the fruit fly, Drosophila melanogaster, by inhibiting oxidative stress. Int. J. Mol. Med. 2015;36:939–946. doi: 10.3892/ijmm.2015.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schriner S.E., Katoozi N.S., Pham K.Q., Gazarian M., Zarban A., Jafari M. Extension of Drosophila lifespan by Rosa damascena associated with an increased sensitivity to heat. Biogerontology. 2012;13:105–117. doi: 10.1007/s10522-011-9357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minois N., Carmona-Gutierrez D., Bauer M.A., Rockenfeller P., Eisenberg T., Brandhorst S., Sigrist S.J., Kroemer G., Madeo F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and -independent pathways. Cell Death Dis. 2012;3:e401. doi: 10.1038/cddis.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou Y.X., Ruan M.H., Luan J., Feng X., Chen S., Chu Z.Y. Anti-Aging Effect of Riboflavin via Endogenous Antioxidant in Fruit fly Drosophila Melanogaster. J. Nutr. Health Aging. 2017;21:314–319. doi: 10.1007/s12603-016-0752-8. [DOI] [PubMed] [Google Scholar]
- 76.Rogina B., Reenan R.A., Nilsen S.P., Helfand S.L. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science. 2000;290:2137–2140. doi: 10.1126/science.290.5499.2137. [DOI] [PubMed] [Google Scholar]
- 77.Whitaker R., Faulkner S., Miyokawa R., Burhenn L., Henriksen M., Wood J.G., Helfand S.L. Increased expression of Drosophila Sir2 extends life span in a dose-dependent manner. Aging. 2013;5:682–691. doi: 10.18632/aging.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tinkerhess M.J., Healy L., Morgan M., Sujkowski A., Matthys E., Zheng L., Wessells R.J. The Drosophila PGC-1α Homolog spargel Modulates the Physiological Effects of Endurance Exercise. PLoS ONE. 2012;7:e31633. doi: 10.1371/journal.pone.0031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim S.J., Lee Y.H., Han M.D., Mar W., Kim W.K., Nam K.W. Resveratrol, purified from the stem of Vitis coignetiae Pulliat, inhibits food intake in C57BL/6J Mice. Arch. Pharm. Res. 2010;33:775–780. doi: 10.1007/s12272-010-0518-5. [DOI] [PubMed] [Google Scholar]
- 81.Rascon B., Hubbard B.P., Sinclair D.A., Amdam G.V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koga C.C., Becraft A.R., Lee Y., Lee S.-Y. Taste Detection Thresholds of Resveratrol. J. Food Sci. 2015;80:S2064–S2070. doi: 10.1111/1750-3841.12976. [DOI] [PubMed] [Google Scholar]
- 83.Gaudette N.J., Pickering G.J. Sensory and chemical characteristics of trans-resveratrol-fortified wine. Aust. J. Grape Wine Res. 2011;17:249–257. doi: 10.1111/j.1755-0238.2011.00144.x. [DOI] [Google Scholar]
- 84.Chandrashekara K.T., Shakarad M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:965–971. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- 85.Bauer J.H., Goupil S., Garber G.B., Helfand S.L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parashar V., Rogina B. dSir2 mediates the increased spontaneous physical activity in flies on calorie restriction. Aging. 2009;1:529–541. doi: 10.18632/aging.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou S., Carey J.R., Liedo P., Ingram D.K., Müller H.-G., Wang J.-L., Yao F., Yu B., Zhou A. The Prolongevity Effect of Resveratrol Depends on Dietary Composition and Calorie Intake in a Tephritid Fruit Fly. Exp. Gerontol. 2009;44:472–476. doi: 10.1016/j.exger.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jimenez-Del-Rio M., Guzman-Martinez C., Velez-Pardo C. The Effects of Polyphenols on Survival and Locomotor Activity in Drosophila melanogaster Exposed to Iron and Paraquat. Neurochem. Res. 2010;35:227–238. doi: 10.1007/s11064-009-0046-1. [DOI] [PubMed] [Google Scholar]
- 89.Peng C., Chan H.Y.E., Huang Y., Yu H., Chen Z.-Y. Apple Polyphenols Extend the Mean Lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- 90.Fuse Y., Kobayashi M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules. 2017;22:436. doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abharzanjani F., Afshar M., Hemmati M., Moossavi M. Short-term High Dose of Quercetin and Resveratrol Alters Aging Markers in Human Kidney Cells. Int. J. Prev. Med. 2017;8:64. doi: 10.4103/ijpvm.IJPVM_139_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu S.-C., Huang S.-M., Chen A., Sun C.-Y., Lin S.-H., Chen J.-S., Liu S.-T., Hsu Y.-J. Resveratrol increases anti-aging Klotho gene expression via the activating transcription factor 3/c-Jun complex-mediated signaling pathway. Int. J. Biochem. Cell Biol. 2014;53:361–371. doi: 10.1016/j.biocel.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 95.Halicka H.D., Zhao H., Li J., Lee Y.S., Hsieh T.C., Wu J.M., Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging. 2012;4:952–965. doi: 10.18632/aging.100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chung H.J., Lee H.K., Kim H.J., Baek S.H., Hong S.T. Gene expression profiles and physiological data from mice fed resveratrol-enriched rice DJ526. Sci. Data. 2016;3:160114. doi: 10.1038/sdata.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulkarni S.S., Cantó C. The molecular targets of resveratrol. Biochim. Biophys. Acta. 2015;1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Bagul P.K., Dinda A.K., Banerjee S.K. Effect of resveratrol on sirtuins expression and cardiac complications in diabetes. Biochem. Biophys. Res. Commun. 2015;468:221–227. doi: 10.1016/j.bbrc.2015.10.126. [DOI] [PubMed] [Google Scholar]
- 99.Rogina B., Helfand S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoffmann J., Romey R., Fink C., Yong L., Roeder T. Overexpression of Sir2 in the adult fat body is sufficient to extend lifespan of male and female Drosophila. Aging. 2013;5:315–327. doi: 10.18632/aging.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banerjee K.K., Ayyub C., Ali S.Z., Mandot V., Prasad N.G., Kolthur-Seetharam U. dSir2 in the Adult Fat Body, but Not in Muscles, Regulates Life Span in a Diet-Dependent Manner. Cell Rep. 2012;2:1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 102.Bauer J.H., Morris S.N., Chang C., Flatt T., Wood J.G., Helfand S.L. dSir2 and Dmp53 interact to mediate aspects of CR-dependent lifespan extension in D. melanogaster. Aging. 2009;1:38–48. doi: 10.18632/aging.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toivonen J.M., Walker G.A., Martinez-Diaz P., Bjedov I., Driege Y., Jacobs H.T., Gems D., Partridge L. No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet. 2007;3:e95. doi: 10.1371/journal.pgen.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gerhardt E., Graber S., Szego E.M., Moisoi N., Martins L.M., Outeiro T.F., Kermer P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS ONE. 2011;6:e28855. doi: 10.1371/journal.pone.0028855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fairbanks L.D., Burch G.E. Rate of water loss and water and fat content of adult Drosophila melanogaster of different ages. J. Insect Physiol. 1970;16:1429–1436. doi: 10.1016/0022-1910(70)90141-1. [DOI] [PubMed] [Google Scholar]
- 106.Ye J., Cui X., Loraine A., Bynum K., Kim N.C., White G., De Luca M., Garfinkel M.D., Lu X., Ruden D.M. Methods for Nutrigenomics and Longevity Studies in Drosophila. In: Tollefsbol T.O., editor. Biological Aging: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2007. pp. 111–141. [DOI] [PubMed] [Google Scholar]
- 107.Partridge L., Farquhar M. Sexual activity reduces lifespan of male fruitflies. Nature. 1981;294:580–582. doi: 10.1038/294580a0. [DOI] [Google Scholar]
- 108.Piper M.D.W., Partridge L. Dietary Restriction in Drosophila: Delayed Aging or Experimental Artefact? PLOS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Partridge L., Green A., Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect Physiol. 1987;33:745–749. doi: 10.1016/0022-1910(87)90060-6. [DOI] [Google Scholar]
- 110.Wong R., Piper M.D.W., Wertheim B., Partridge L. Quantification of Food Intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iliadi K.G., Knight D., Boulianne G.L. Healthy aging—Insights from Drosophila. Front. Physiol. 2012;3:106. doi: 10.3389/fphys.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Linford N.J., Bilgir C., Ro J., Pletcher S.D. Measurement of Lifespan in Drosophila melanogaster. J. Vis. Exp. 2013;71:50068. doi: 10.3791/50068. [DOI] [PMC free article] [PubMed] [Google Scholar]