Abstract
Blindness in glaucoma is the result of death of Retinal Ganglion Cells (RGCs) and their axons. RGC death is generally preceded by a stage of reversible dysfunction and structural remodeling. Current treatments aimed at reducing intraocular pressure (IOP) are ineffective or incompletely effective in management of the disease. IOP-independent neuroprotection or neuroprotection as adjuvant to IOP lowering in glaucoma remains a challenge as effective agents without side effects have not been identified yet. We show in DBA/2J mice with spontaneous IOP elevation and glaucoma that the lifespan of functional RGCs can be extended by preconditioning RGCs with retrobulbar lidocaine in one eye at four months of age that temporary blocks RGC axonal transport. The contralateral, PBS-injected eye served as control. Lidocaine-induced impairment of axonal transport to superior colliculi was assessed by intravitreal injection of cholera toxin B. Long-term (nine months) effect of lidocaine were assessed on RGC electrical responsiveness (PERG), IOP, expression of relevant protein (BDNF, TrkB, PSD95, GFAP, Synaptophysin, and GAPDH) and RGC density. While lidocaine treatment did not alter the age-related increase of IOP, TrkB expression was elevated, GFAP expression was decreased, RGC survival was improved by 35%, and PERG function was preserved. Results suggest that the lifespan of functional RGCs in mouse glaucoma can be extended by preconditioning RGCs in early stages of the disease using a minimally invasive treatment with retrobulbar lidocaine, a common ophthalmologic procedure. Lidocaine is inexpensive, safe and is approved by Food and Drug Administration (FDA) to be administered intravenously.
Keywords: glaucoma, mouse, retinal ganglion cells, lidocaine, neuroprotection
1. Introduction
Blindness in glaucoma is the result of death of Retinal Ganglion Cells (RGCs) and their axons [1]. RGC death is generally preceded by a stage of reversible dysfunction and structural remodeling [2]. Current treatments aimed at reducing intraocular pressure (IOP) are ineffective or incompletely effective in management of the disease [3]. IOP-independent neuroprotection or neuroprotection as adjuvant to IOP lowering in glaucoma remains a challenge as effective agents without side effects have not been identified yet [4]. The purpose of this study is to test the hypothesis that the lifespan of functional RGCs in mouse glaucoma can be extended by preconditioning RGCs in early stages of the disease using a minimally invasive treatment with retrobulbar lidocaine, a common ophthalmologic procedure. In vitro and in vivo studies have shown that lidocaine is neuroprotective against hypoxia and ischemia [5]. The primary action of retrobulbar lidocaine on the optic nerve is to inhibit transmembrane ionic fluxes [6] resulting in reduction of afferent electrical activity to thalamic targets of the visual pathway as well as inhibition of bi-directional axonal transport [7,8]. Axon transport plays a critical role in supplying materials for a variety of neuronal functions such as morphogenetic plasticity, synaptic transmission, and cell survival [9]. Previous results of our group in mice showed that retrobulbar lidocaine temporarily reduces RGC electrical responsiveness, which however rapidly recovers baseline values [10]. Chou and Porciatti [10] proposed that lidocaine-induced, temporary reduction of RGC electrical responsiveness is secondary to inhibition of axonal transport of target-derived factors that retrogradely sustain RGC function [11,12]. Here we show that short-term retrobulbar lidocaine administration in early stages of mouse glaucoma has a long-term effect on the expression of relevant retinal proteins and sparing of functional RGCs.
2. Results
2.1. Retrobulbar Lidocaine Impairs Axon Transport
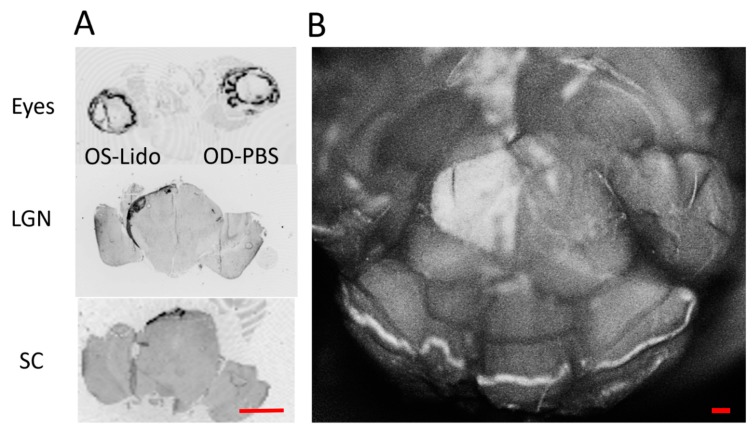
To confirm previous reports [7] that retrobulbar lidocaine impairs axonal transport, we intravitreally injected Cholera toxin b-subunit conjugated to AlexaFluor488 (CTB) in both eyes immediately after lidocaine retrobulbar injection in the left eye and retrobulbar phosphate-buffered saline (PBS) injection in the right eye. The next day, the entire brain was fixed with 4% paraformaldehyde in 0.5 M phosphate buffer pH 7.35 and dissected from the skull to expose the Superior Colliculi (SC). Confocal Scanning Laser images (Spec-CAM-11796-S3610) of the brain surface were taken to assess fluorescence of SC. As shown in Figure 1B, only the left SC (contralateral to the PBS-injected right eye) displayed marked fluorescence, whereas no fluorescence was appreciable in the right SC (contralateral to the lidocaine-injected eye). Then, consecutive tissue sections from the eyes to the brain were imaged with a fluorescent scanner (Typhoon, Amersham). Representative sections displayed in Figure 1A show that while CTB fluorescence was present in both eyes, fluorescence was only present in the outer shell of the lateral geniculate nucleus (LGN) receiving the PBS-injected contralateral axons; the inner core of the LGN receiving the lidocaine-injected ipsilateral axons did not fluoresce. In addition, only the SC contralateral to the PBS-injected eye displayed fluorescence. Altogether, results summarized in Figure 1 demonstrate that retrobulbar lidocaine inhibits axon transport to thalamic and midbrain targets.
Figure 1.
Retrobulbar lidocaine blocks axon transport. (A) Representative consecutive tissue sections from the eyes to the brain one day after retrobulbar lidocaine in the left eye, immediately followed by intravitreal injection of Alexa Fluor 488 Cholera Toxin B in both eyes. Fluorescence is evident in both eyes but it is absent in the lateral geniculate nucleus (LGN) and Superior Colliculi (SC) contralateral to the left eye. Fluorescence is present in the LGN and SC contralateral to the phosphate-buffered saline (PBS)-injected right eye; scale bar, 200 µm. (B) Confocal scanning laser ophthalmoscopy of the midbrain surface. The entire surface of the right SC receiving afferents from the lidocaine- injected right eye does not fluoresce, indicating impairment of axon transport in the lidocaine-treated eye; scale bar, 200 µm.
2.2. Retrobulbar Lidocaine Reversibly Impairs RGC Function
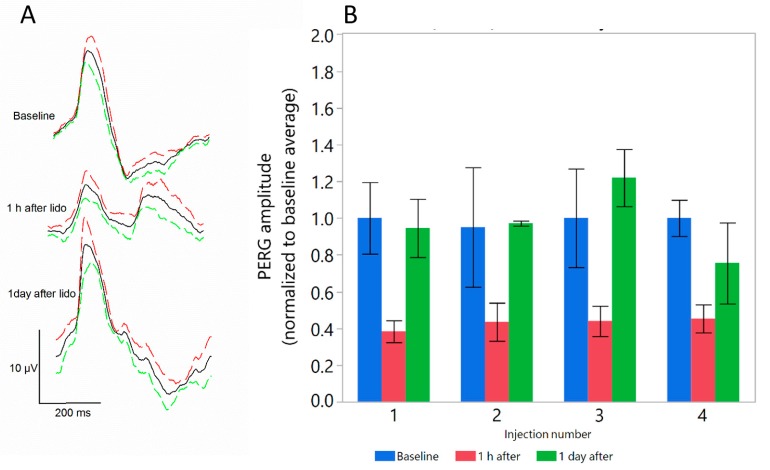
Figure 2 shows that 1 h after retrobulbar lidocaine injection, RGC function measured with the Pattern Electroretinogram (PERG) [13] was reduced by more than 50%, however it fully recovered the next day. After PERG recovery, further lidocaine injections produced similar results. The temporary effect of lidocaine on PERG can be explained assuming impairment of retrograde signaling to the retina of factors that sustains RGC responsiveness [10]. Altogether, results summarized in Figure 2 indicate that the effect of lidocaine on PERG is repeatable, and that multiple lidocaine injections do not appear to be toxic to the retina as shown by unaltered PERG.
Figure 2.
Retrobulbar lidocaine reversibly reduces Pattern Electroretinogram (PERG). (A) PERG waveforms in DBA/2J mice before and at different times after injection. Continuous black lines represent the grand-average waveform of all mice tested, and colored dashed lines represent the superior (red) and inferior (green) 95% confidence interval of the mean; (B) Peak-to-trough PERG amplitude decreases by about 60% one hour after lidocaine but recovers baseline values one day after injection. The effect is repeatable after four injections four days apart. Bars represent the mean ± SEM (N = 5).
2.3. Lidocaine Treatment Does not Induce Long-Term Changes of IOP and RGC Function
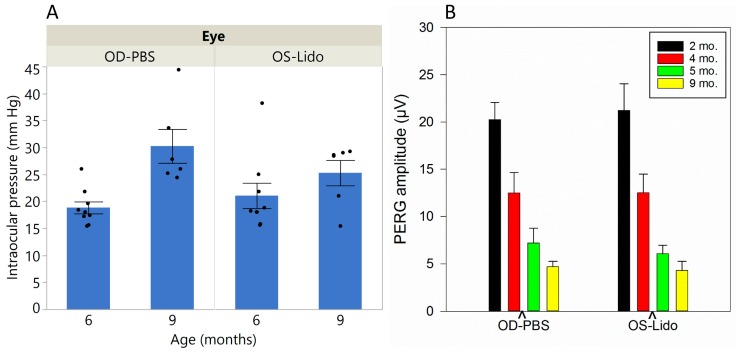
In DBA/2J mice, IOP is known to progressively increase with age while PERG amplitude declines [14,15]. Results shown in Figure 3 confirm that this was also the case in our sample, with IOP increasing from about 20 mm Hg at six months of age to about 25 mm Hg at nine months of age (Figure 3A). However, repeated retrobulbar lidocaine injections in the left eye at four months of age did not cause significant IOP changes in the treated eye compared to the control eye (two-way ANOVA: effect of Age, p < 0.0018; interaction between age and treatment, p = 0.12). Figure 3B shows that PERG amplitude declined with increasing age. However, lidocaine treatment at four months of age did not induce further RGC dysfunction at five and nine months of age (2-way ANOVA: effect of age, p < 0.001, interaction between age and treatment, p = 0.6) Results suggests that lidocaine treatment at a young age does not have long-term toxic effects on RGC function as shown by a sensitive measure such as the PERG.
Figure 3.
Intraocular pressure (IOP) and PERG amplitude as function of age in Lidocaine-treated and PBS-treated D2 mice. (A) IOP increases with age with no apparent differences between Lidocaine-treated and PBS-treated D2 mice. Bars represent the mean ± SEM (6 mo., N = 9; 9 mo., N = 6); black dots represent measurements in individual mice; (B) PERG amplitude declines with increasing age, with no apparent differences between Lidocaine-treated and PBS-treated D2 mice. Bars represent the mean ± SEM (2 mo., N = 7; 4 mo., N = 20; 5 mo., N = 9; 9 mo., N = 17).
2.4. Short-Term Lidocaine Treatment Induces Long-Term Changes of Protein Expression in DBA/2J Glaucoma
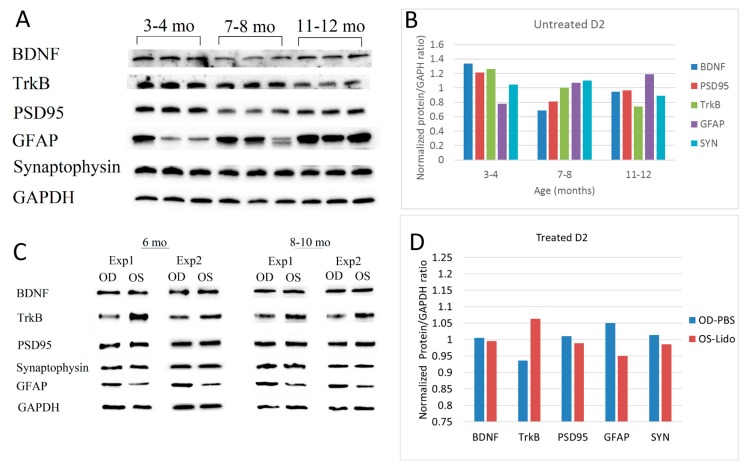
Previous analyses using microarrays, RT-PCR, and RNAseq, have shown marked changes of gene expression with increasing age in DBA/2J mice [15,16]. Western Blot analysis displayed in Figure 4A,B shows that in untreated DBA/2J mice, protein expression changed substantially with age. In particular, expression of TrkB progressively decreased with increasing age whereas expression of GFAP increased and expression of Synaptophysin was virtually invariant. Expression of BDNF and PSD95 tended to decrease in a nonlinear manner. However, when DBA/2J four month old received repeated retrobulbar Lidocaine injections, protein expression substantially was altered in the treated eye compared to the control eye 2–6 months later. Figure 4C,D shows that in the lidocaine treated eye, compared to the PBS control, the expression of TrkB was relatively increased while that of GFAP was relatively reduced, in countertendency with the natural history of age-related changes shown in Figure 4B. The expression of other proteins appeared unaltered.
Figure 4.
Age-related changes of relevant protein expression in untreated (A,B) and Lidocaine-treated (C,D) DBA/2J mice. (A) Western Blot images of Brain Derived Neurotrophic Factor (BDNF) (28 kDa), Tyrosine Receptor Kinase B (TrkB) (140 kDa), PSD95 (95 kDa), Synaptophysin (SYN) (38 kDa), Glial Fibrillary Acidic Protein (GFAP) (50 kDa) and Glyceraldehyde 3-phosphate Dehydrogenase GAPDH (37 kDa) proteins assessed in untreated mice of different ages (3 mice for age group, pooled retinas of both eyes); (B) Average protein expression (normalized protein/GAPDH ratio) as as function of age in untreated mice. Bars represent the average of 6 retinas for age group; (C) Representative Western Blot images of relevant retinal proteins in mice 6 and 8–10 old that received 4 retrobulbar injections of Lidocaine in the left eye and PBS in the right eye at 4 months of age; (D) Average protein expression in Lidocaine-treated (OS) and PBS-treated (OD) eyes. Bars represent the average ratios of 9 D2 mice of 6–10 months of age.
2.5. Short-Term Lidocaine Treatment Results in Improved Long-Term Survival of Functional RGCs
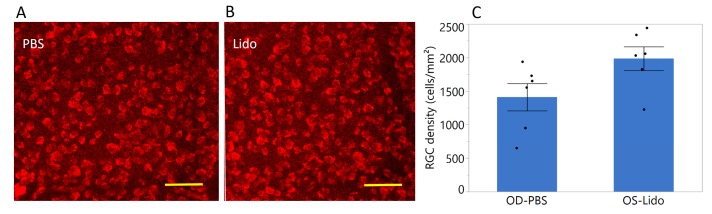
At 9 months of age, early manifest RGC death is normally present in D2 mice [17]. Figure 5 shows that RGC survival in nine-month-old D2 mice was significantly improved after short-term lidocaine treatment performed at four months of age. In Lidocaine-treated eyes, RGC density was higher by 35% compared to the PBS-treated eyes (p = 0.04, N = 6).
Figure 5.
Retrobulbar lidocaine is neuroprotective in early DBA/2J glaucoma. (A,B) examples of confocal images of RBPMS-positive RGCs in whole-mounted retinas of one nine-month-old D2 that received four retrobulbar injections of lidocaine at four months of age in one eye (B) and an equal volume of PBS in the other eye (A), scale bar, 50 µm; (C) Mean RGC density in D2 mice nine mo. old treated with retrobulbar lidocaine in one eye and PBS in the contralateral eye at four months of age. RGC density is higher in lidocaine-treated eyes by 35% (p = 0.04). Error bars represent the SEM, N = 6. Black dots represent measurements in individual mice.
3. Discussion
Previous studies have shown that local anesthetic lidocaine is neuroprotective in in vitro and in vivo models of hypoxia and ischemia, as well as after spinal cord injury [5]. It has been suggested that reduction of neuroinflammation and inhibition of apoptosis contribute to neuroprotection. The present study investigated the potential role of lidocaine in the well-established DBA/2J mouse model of glaucoma, which has the hallmarks of human glaucoma including elevated IOP and chronic/progressive degeneration of retinal ganglion cells. Ischemia and neuroinflammation also may contribute to the disease [18,19,20].
The main result of the present study is that repeated retrobulbar lidocaine performed at four months of age—before the onset of IOP elevation and RGC dysfunction and death in DBA/2J glaucoma—resulted in long-term neuroprotection of functional RGCs. At nine months of age, when RGC death is normally present in about 30% of DBA/2J mice [17], lidocaine-treated eyes had RGC density significantly higher by ~35% on average compared to control, PBS-treated eye. The amplitude of the PERG, a sensitive measure of RGC function, was similar in the two eyes. This indicates that repeated lidocaine treatment at pre-glaucomatous ages was not toxic to RGCs, and that surviving RGCs were still functional. Intraocular lidocaine has been previously shown not to alter the ERG of rabbits [21].
One contributing factor to the neuroprotective effect of lidocaine might have been the upregulation of TrkB, which promotes cell survival [22,23] and is altered in experimental glaucoma [24]. This occurred in countertendency to progressive decline of TrkB expression with increasing age in untreated DBA/2J mice. Another contributing factor might have been the downregulation of GFAP, which indicates reduced astrocytic activation and inflammation [19,25,26], whereas in untreated DBA/2J mice there was a progressive increase of GFAP expression with increasing age. A third contributing factor might have been the marginally lower IOP in the lidocaine-treated eyes compared to the PBS-control eyes at nine months of age. Even though the IOP difference between the two eyes was not significant, a small difference at a high baseline level may have played a role in reducing the likelihood of cell death [27,28]. Intravenous lidocaine has been shown to attenuate the increase in intracranial pressure and IOP of patients receiving succinylcholine prior to intubation [29] and lower IOP in acute primary angle closure glaucoma [30]. Altogether, TrkB upregulation, GFAP downregulation, and IOP lowering may have played a synergistic role in the neuroprotective effect of lidocaine in DBA/2J glaucoma. Further investigation in a larger sample of mice including more advanced stages of the disease is needed to fully establish the neuroprotective effect of lidocaine and contributing factors.
Ischemic preconditioning is known to induce retinal tolerance to ischemia and optic nerve injury [31,32,33]. This study shows that the lifespan of functional RGCs in mouse glaucoma can be extended by preconditioning RGCs in early stages of the disease using a minimally invasive treatment with retrobulbar lidocaine, a common ophthalmologic procedure. Lidocaine is inexpensive, safe and is approved by FDA to be administered intravenously [5]. This study also provides further proof of principle that retinal ganglion cells have an intrinsic capacity to react to stress by changing expression of key proteins that sustain their survival [34,35,36]. Boosting endogenous neuroprotection with lidocaine may represent a novel therapeutic avenue in glaucoma.
4. Materials and Methods
4.1. Animals and Husbandry
All procedures were performed in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for use of animals in ophthalmic and vision research. The experimental protocol was approved by the Animal Care and Use Committee of the University of Miami (IACUC Prot. 16-247, 6 July 2017). A total of 89 DBA/2J (D2), mice were used. The D2 mouse is a well-established genetic model of spontaneous glaucoma that has similarities with human pigmentary glaucoma [17,37]. By 5–6 months of age, D2 mice develop pigment dispersion in the iris that impairs aqueous humor outflow, resulting in moderate IOP elevation. By 5–6 months of age RGC function starts decreasing, and by 9–10 months the PERG amplitude is at noise level [14,15]. By 12 months of age D2 mice develop moderate or severe loss of RGC density [14,15,38]. All mice were maintained in a cyclic light environment (12 h light: 50 lux–12 h: dark) and fed with Grain Based Diet (Lab Diet: 500, Opti-diet, PMI Nutrition International, Inc., Brentwood, MO, USA). All procedures and testing were performed under anesthesia by means of intraperitoneal injections (0.5–0.7 mL/kg) of a mixture of ketamine (42.8 mg/mL) and xylazine (8.6 mg/mL). Retrobulbar injections of lidocaine (2 µL, 40 µg/µL) were performed in the left eye with a 23-gauge needle using a supraorbital approach. An equal volume of PBS was injected retrobulbar to the right eye as control. For experiments of long-term effects of lidocaine, repeated injections (n = 4 every 4 days) were done in mice four months old. Protein expression, RGC function and RGC density were quantified in the age range 6–10 months.
4.2. Pattern Electroretinogram (PERG) Recording
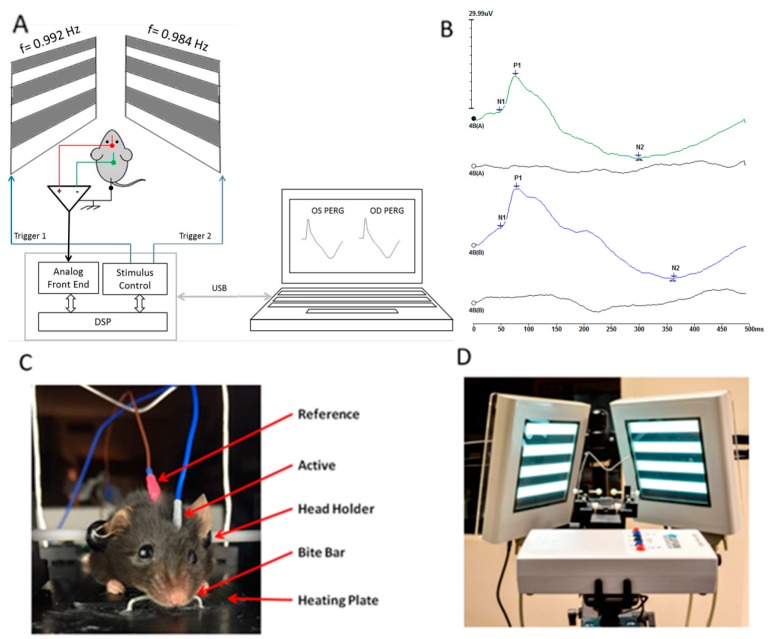
As a sensitive measure of RGC function we used the Pattern Electroretinogram (PERG) [13,39], Figure 6. As previously described [39], anesthetized mice were gently restrained in a custom-made holder that allowed unobstructed vision. The body of the animal was kept at a constant temperature of 37.0 °C using a feedback-controlled heating pad (TCAT-2LV, Physitemp Instruments, Inc., Clifton, NJ, USA). The eyes of anesthetized mice were typically wide open and in a stable position, with optical axes pointing laterally and upwardly [25,26]. Pupils were natural and had a diameter smaller than 1 mm [40]; eyes were not refracted for the viewing distance since the mouse eye with natural pupil has a large depth of focus [41,42]. A small drop of balanced saline was topically applied as necessary to prevent corneal dryness. The PERG was recorded simultaneously from each eye using a common subcutaneous needle in the snout with a commercially available instrument (Jorvec Corp., Miami, FL, USA). Visual stimuli consisted of black-white horizontal bars generated on LED tablets and presented independently to each eye at 10 cm distance (56° vertical × 63° horizontal field; spatial frequency, 0.05 cycles/deg; 98% contrast; 800 cd/sqm mean luminance; right-eye reversal, 0.992 Hz; left-eye reversal, 0.984 Hz). Electrical signals recorded from the common snout electrode were averaged (>1110 epochs), and PERG responses from each eye isolated by averaging at stimulus-specific synchrony [39]. Examples of normal PERG waveforms are shown in Figure 1. PERG amplitude was defined as amplitude difference from the major positive peak (P1) to the major negative trough (N2). PERG latency was defined as the time lag from zero (onset of reversal) to the peak of the P1 wave.
Figure 6.
(A) Mouse PERG recording layout; (B) PERG waveforms simultaneously recorded from each eye using one common electrode in the snout; (C) Mouse with non-corneal subcutaneous needle electrodes resting on a feedback-controlled thermostatic plate; (D) Patterned visual stimuli generated on high luminance (800 cd/sqm) LED displays and presented at each eye independently with slightly different reversal frequency (right eye: 0.984 Hz; left eye: 0.992 Hz). Asynchronous averaging allows isolation of monocular PERGs.
4.3. Western Blots
Expression of relevant retinal neurotrophic expression (BDNF, TrkB), synaptic markers (PSD95, synaptophysin) and glial markers (GFAP) was performed with Western Blot. Eyes were harvested, and retinae dissected from the pigment epithelium. Retinae from each eye were pooled separately and homogenized in RIPA buffer containing 1XHALT protease inhibitor cocktail (Pierce, Rockford, IL, USA). The protein concentrations of retinal homogenates were estimated using DC protein assay (Bio-Rad, Hercules, CA, USA). Equal concentration of the total protein samples (40 µg) were added to the Laemmli buffer, boiled at 95 °C for 5 min ad further electrophoresed on 4–20% Mini-Protean TGX precast gels (Bio-Rad). The separated proteins were transferred onto a 0.2 μm polyvinylidene difluoride membranes (PVDF, Bio-Rad). Membranes were blocked in 5% milk in 1× Tris-buffered saline + Tween (TBST) for 1 h at room temperature. The blocked membranes were incubated with primary antibody for overnight at 4 °C. Membranes were washed three times for 15 min, and then incubated with secondary antibody for 60 min at room temperature. For each sample, three gel runs were performed. To normalize for protein loading, membranes were stripped and then reprobed with constitutively expressing housekeeping gene (GAPDH). Primary were: BDNF (Novus, Littleton, CO, USA) NBP2-36705, 2 µg/mL; TrkB (Cell Signaling Technology, Danvers, MA, USA) #4603, 1:1000; PSD95 (Abcam, Cambridge, MA, USA) ab76115, 1:1000; GFAP (Cell Signaling Technology #12389, 1:1000), Synaptophysin (Cell Signaling Technology, #5461, 1:1000) and GAPDH (Cell Signaling Technology, #8884, 1:1000). Membranes were washed three times for 15 min and developed with enhanced chemiluminescence (ECL; Bio-Rad) solution. The developed bands were quantified using ImageJ software for gel densitometry (provided in the public domain by the National Institutes of Health, available online: http://rsbweb.nih.gov/ij/download.html). The relative density of protein bands was normalized to the levels of GAPDH (protein/GAPDH) within each sample by dividing the percent value for each sample by the percent value for the GAPDH standard.
4.4. RGC Density
RGC density was assessed in whole-mounted retinas immunostained with RBPMS primary antibody (PhosphoSolutions, Aurora, CO, USA) 1832-RBPMS, 1:500 plus Alexa 594 secondary antibody (Abcam, ab150188, 1:500) that stains all RGCs [43], and manually quantified with confocal microscopy images.
Acknowledgments
NIH R01EY019077 (VP), NIH P30EY014801 (VP), unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness, Inc., Cure Glaucoma Foundation (VP).
Author Contributions
Tsung-Han Chou and Vittorio Porciatti conceived and designed the experiments; Tsung-Han Chou and Giovanni Luca Romano performed the PERG and IOP experiments and harvested the tissue; Ganeswara Rao Musada performed the Western Blots, Elizabeth Bolton performed RGC counts, Vittorio Porciatti and Tsung-Han Chou analyzed the data; Vittorio Porciatti wrote the paper, and all co-authors contributed to review it.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012;31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Porciatti V., Ventura L.M. Retinal ganglion cell functional plasticity and optic neuropathy: A comprehensive model. J. Neuroophthalmol. 2012;32:354–358. doi: 10.1097/WNO.0b013e3182745600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jampel H.D., Chon B.H., Stamper R., Packer M., Han Y., Nguyen Q.H., Ianchulev T. Effectiveness of intraocular pressure-lowering medication determined by washout. JAMA Ophthalmol. 2014;132:390–395. doi: 10.1001/jamaophthalmol.2013.7677. [DOI] [PubMed] [Google Scholar]
- 4.Levin L.A., Crowe M.E., Quigley H.A. Neuroprotection for glaucoma: Requirements for clinical translation. Exp. Eye Res. 2017;157:34–37. doi: 10.1016/j.exer.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leng T., Gao X., Dilger J.P., Lin J. Neuroprotective effect of lidocaine: Is there clinical potential? Int. J. Physiol. Pathophysiol. Pharmacol. 2016;8:9–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Cummins T.R. Setting up for the block: The mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. J. Physiol. 2007;582:11. doi: 10.1113/jphysiol.2007.136671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagiolini M., Caleo M., Strettoi E., Maffei L. Axonal transport blockade in the neonatal rat optic nerve induces limited retinal ganglion cell death. J. Neurosci. 1997;17:7045–7052. doi: 10.1523/JNEUROSCI.17-18-07045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanai A., Hiruma H., Katakura T., Sase S., Kawakami T., Hoka S. Low-concentration lidocaine rapidly inhibits axonal transport in cultured mouse dorsal root ganglion neurons. Anesthesiology. 2001;95:675–680. doi: 10.1097/00000542-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Inman D.M., Harun-Or-Rashid M. Metabolic vulnerability in the neurodegenerative disease glaucoma. Front. Neurosci. 2017;11:146. doi: 10.3389/fnins.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou T.H., Park K.K., Luo X., Porciatti V. Retrograde signaling in the optic nerve is necessary for electrical responsiveness of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2013;54:1236–1243. doi: 10.1167/iovs.12-11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kafitz K.W., Rose C.R., Konnerth A. Neurotrophin-evoked rapid excitation of central neurons. Prog. Brain Res. 2000;128:243–249. doi: 10.1016/S0079-6123(00)28021-7. [DOI] [PubMed] [Google Scholar]
- 12.Blum R., Kafitz K.W., Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel NaV1.9. Nature. 2002;419:687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- 13.Porciatti V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015;141:164–170. doi: 10.1016/j.exer.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleh M., Nagaraju M., Porciatti V. Longitudinal evaluation of retinal ganglion cell function and iop in the DBA/2J mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2007;48:4564–4572. doi: 10.1167/iovs.07-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams P.A., Harder J.M., Foxworth N.E., Cochran K.E., Philip V.M., Porciatti V., Smithies O., John S.W. Vitamin b3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science. 2017;355:756–760. doi: 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele M.R., Inman D.M., Calkins D.J., Horner P.J., Vetter M.L. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 17.Libby R.T., Anderson M.G., Pang I.H., Robinson Z.H., Savinova O.V., Cosma I.M., Snow A., Wilson L.A., Smith R.S., Clark A.F., et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- 18.Schuettauf F., Rejdak R., Walski M., Frontczak-Baniewicz M., Voelker M., Blatsios G., Shinoda K., Zagorski Z., Zrenner E., Grieb P. Retinal neurodegeneration in the dba/2j mouse-a model for ocular hypertension. Acta Neuropathol. 2004;107:352–358. doi: 10.1007/s00401-003-0816-9. [DOI] [PubMed] [Google Scholar]
- 19.Wilson G.N., Inman D.M., Dengler Crish C.M., Smith M.A., Crish S.D. Early pro-inflammatory cytokine elevations in the dba/2j mouse model of glaucoma. J. Neuroinflamm. 2015;12:176. doi: 10.1186/s12974-015-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder J.M., Braine C.E., Williams P.A., Zhu X., MacNicoll K.H., Sousa G.L., Buchanan R.A., Smith R.S., Libby R.T., Howell G.R., et al. Early immune responses are independent of rgc dysfunction in glaucoma with complement component c3 being protective. Proc. Natl. Acad. Sci. USA. 2017;114:e3839–e3848. doi: 10.1073/pnas.1608769114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang C., Peyman G.A., Sun G. Toxicity of intraocular lidocaine and bupivacaine. Am. J. Ophthalmol. 1998;125:191–196. doi: 10.1016/S0002-9394(99)80091-9. [DOI] [PubMed] [Google Scholar]
- 22.Kimura A., Namekata K., Guo X., Harada C., Harada T. Neuroprotection, growth factors and bdnf-trkb signalling in retinal degeneration. Int. J. Mol. Sci. 2016;17:e1584. doi: 10.3390/ijms17091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta V.K., You Y., Gupta V.B., Klistorner A., Graham S.L. Trkb receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013;14:10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pease M.E., McKinnon S.J., Quigley H.A., Kerrigan-Baumrind L.A., Zack D.J. Obstructed axonal transport of bdnf and its receptor trkb in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2000;41:764–774. [PubMed] [Google Scholar]
- 25.Chong R.S., Martin K.R. Glial cell interactions and glaucoma. Curr. Opin. Ophthalmol. 2015;26:73–77. doi: 10.1097/ICU.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tezel G., Yang X., Luo C., Cai J., Powell D.W. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2012;53:4220–4233. doi: 10.1167/iovs.11-9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuettauf F., Quinto K., Naskar R., Zurakowski D. Effects of anti-glaucoma medications on ganglion cell survival: The dba/2j mouse model. Vis. Res. 2002;42:2333–2337. doi: 10.1016/S0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- 28.Sawada K., Hiraoka M., Ohguro H. Effect of antiglaucoma medicine on intraocular pressure in dba/2j mice. Ophthalmic Res. 2016;55:205–211. doi: 10.1159/000444057. [DOI] [PubMed] [Google Scholar]
- 29.Lev R., Rosen P. Prophylactic lidocaine use preintubation: A review. J. Emerg. Med. 1994;12:499–506. doi: 10.1016/0736-4679(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 30.Jin X., Xue A., Zhao Y., Qin Q., Dong X.D., Qu J. Efficacy and safety of intravenous injection of lidocaine in the treatment of acute primary angle-closure glaucoma: A pilot study. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:1611–1616. doi: 10.1007/s00417-007-0572-y. [DOI] [PubMed] [Google Scholar]
- 31.Roth S., Li B., Rosenbaum P.S., Gupta H., Goldstein I.M., Maxwell K.M., Gidday J.M. Preconditioning provides complete protection against retinal ischemic injury in rats. Investig. Ophthalmol. Vis. Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- 32.Liu X., Liang J.P., Sha O., Wang S.J., Li H.G., Cho E.Y.P. Protection of retinal ganglion cells against optic nerve injury by induction of ischemic preconditioning. Int. J. Ophthalmol. 2017;10:854–861. doi: 10.18240/ijo.2017.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Roth S., Laser M., Ma J.X., Crosson C.E. Retinal preconditioning and the induction of heat-shock protein 27. Investig. Ophthalmol. Vis. Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- 34.Yang X., Chou T.H., Ruggeri M., Porciatti V. A new mouse model of inducible, chronic retinal ganglion cell dysfunction not associated with cell death. Investig. Ophthalmol. Vis. Sci. 2013;54:1898–1904. doi: 10.1167/iovs.12-11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson E.C., Jia L., Cepurna W.O., Doser T.A., Morrison J.C. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison J.C., Cepurna W.O., Tehrani S., Choe T.E., Jayaram H., Lozano D.C., Fortune B., Johnson E.C. A period of controlled elevation of iop (CEI) produces the specific gene expression responses and focal injury pattern of experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2016;57:6700–6711. doi: 10.1167/iovs.16-20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John S.W., Smith R.S., Savinova O.V., Hawes N.L., Chang B., Turnbull D., Davisson M., Roderick T.H., Heckenlively J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 38.Howell G.R., Libby R.T., Jakobs T.C., Smith R.S., Phalan F.C., Barter J.W., Barbay J.M., Marchant J.K., Mahesh N., Porciatti V., et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou T.H., Bohorquez J., Toft-Nielsen J., Ozdamar O., Porciatti V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Investig. Ophthalmol. Vis. Sci. 2014;55:2469–2475. doi: 10.1167/iovs.14-13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaraju M., Saleh M., Porciatti V. Iop-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 2007;48:4573–4579. doi: 10.1167/iovs.07-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remtulla S., Hallett P.E. A schematic eye for the mouse, and comparisons with the rat. Vis. Res. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 42.Schmucker C., Schaeffel F. A paraxial schematic eye model for the growing c57bl/6 mouse. Vis. Res. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez A.R., de Sevilla Muller L.P., Brecha N.C. The rna binding protein rbpms is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014;522:1411–1443. doi: 10.1002/cne.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]