Abstract
Epithelial-mesenchymal transition (EMT) allows neoplastic cells to gain the invasive phenotype and become migratory, which is required for cancer progression and metastasis. In the present study, the expression of EMT-associated biomarkers and their association with clinicopathological parameters in laryngeal squamous cell carcinoma (LSCC) was investigated. E-cadherin, N-cadherin, β-catenin and zinc finger E-box binding homeobox 2 (ZEB2) protein expression was evaluated with immunohistochemistry in a cohort of 76 patients with operable LSCC. The association between these transition markers, clinicopathological parameters and their prognostic impact in LSCC was analyzed. Immunohistochemical analysis revealed that EMT-associated proteins were differentially expressed between LSCC and adjacent non-neoplastic laryngeal tissue. Negative E-cadherin expression and positive N-cadherin, β-catenin and ZEB2 expression were associated with a later tumor (T) stage, decreasing tumor differentiation and a reduced overall survival (OS) time (OS: E-cadherin, P=0.016; N-cadherin, P=0.003; β-catenin, P=0.002; ZEB2, P=0.0003). E-cadherin/β-catenin co-expression was significantly associated with the majority of clinicopathological parameters assessed, including lymph node metastases, T stage and tumor cell differentiation (P=0.004, P=0.005, and P<0.001, respectively). Multivariate analysis indicated that T stage and the positive expression of β-catenin and ZEB2 were independent risk factors for OS in LSCC (P=0.014, P=0.025 and P=0.003, respectively). It was concluded that EMT mediates tumor progression, and reduces OS time in patients with LSCC. E-cadherin/β-catenin co-expression may be associated with clinicopathological parameters. T stage, and the positive co-expression of β-catenin and ZEB2 may be independent predictors of prognosis in LSCC.
Keywords: epithelial-mesenchymal transition, laryngeal carcinoma, prognosis
Introduction
Laryngeal squamous cell carcinoma (LSCC) is a common malignancy of the head and neck that is increasing in morbidity and mortality worldwide (1). As biomarkers for various cancers, tumor-node-metastasis stage and grade may be insufficient to indicate a prognosis and treatment for LSCC. The identification of prognostic and predictive biomarkers of LSCC may help clinicians select more appropriate treatment for individual patients. Invasion and metastasis are considered the major clinical challenges in the treatment of cancer; the cellular process of epithelial-mesenchymal transition (EMT), which is characterized by the loss of epithelial markers (including adherens junction proteins E-cadherin, α- and β-catenin) and increased expression of mesenchymal markers (including N-cadherin and vimentin), promotes the aggressive behavior of cancer (2–4).
Although certain molecular markers of the EMT processes have been considered in numerous cancer cell models in vitro (5–7), the association between EMT-associated molecular alterations with LSCC clinicopathological characteristics and prognosis requires further investigation. E-cadherin binding to β-catenin on the cytomembrane represses tumor progression by maintaining cellular adhesion to prevent EMT, cell motility and tumor metastasis (8). Downregulation or loss of E-cadherin and β-catenin from the cytomembrane and nuclear β-catenin expression are frequently observed in multiple cancer types, including head and neck cancer (9–11). EMT has also been demonstrated to be induced by the expression of other EMT-associated proteins, including N-cadherin and zinc finger E-box binding homeobox 2 (ZEB2; also known as SIP1) in various different cancer types, including head and neck squamous cell carcinoma (HNSCC) (12). The decreased expression of membranous E-cadherin accompanied by a simultaneous increase in the expression of N-cadherin (termed the ‘cadherin switch’) has been reported as a phenomenon that is associated with lymph node metastasis in HNSCC, and disease recurrence in LSCC (13–15). Furthermore, ZEB2, a major repressor of E-cadherin, is associated with the initial stage of EMT, and promotes tumor cell migration and invasion (11,12). The overexpression of ZEB2 has been reported in different cancer types and metastatic lymph nodes in HNSCC tissues, and has been suggested as a candidate biomarker for poor prognosis (5,6,16).
Therefore, the primary objective of the present study was to examine the expression patterns of EMT-associated markers (E-cadherin, N-cadherin, β-catenin and ZEB2) in a cohort of patients with LSCC treated with surgery, with and without lymph node metastasis, using immunohistochemical analyses. The results of the present study indicated significant differences in the expression of the four EMT-associated markers between LSCC and the adjacent non-neoplastic laryngeal tissue. The association of these biomarkers with LSCC clinicopathological phenotype and prognosis was analyzed. In particular, the clinicopathological significance of the co-expression of E-cadherin/N-cadherin, E-cadherin/β-catenin, and E-cadherin/ZEB2 in LSCC was assessed.
Materials and methods
Patient cohort
This retrospective study included 76 patients with stage I–IVa LSCC treated from February 2007 to November 2013 at the Ear, Nose, and Throat (ENT) Department of Drum Tower Hospital, Nanjing University (Nanjing, China). All of the patients were male and aged 34–87 years; none of the patients had been previously treated. The data collected, including tumor characteristics and the age at diagnosis, are reported in Table I, using the American Joint Committee on Cancer Staging Manual (2002) (17). All of the patients in the study provided written informed consent; the study protocol was performed in accordance with institutional bioethics guidelines and was approved by the Research and Ethics Committee of Drum Tower Hospital.
Table I.
Patient clinicopathological characteristics.
| Variable | Value |
|---|---|
| Total, n | 76 |
| Age (years) | |
| Range | 34–87 |
| Median | 64 |
| Lymph node metastasis, n (%) | |
| Positive | 38 (50.00) |
| Negative | 38 (50.00) |
| Tumor stage, n (%) | |
| T1–3 | 45 (59.21) |
| T4 | 31 (40.79) |
| Tumor cell differentiation, n (%) | |
| Good | 8 (10.53) |
| Moderate | 52 (68.42) |
| Poor | 16 (21.05) |
| Localization, n (%) | |
| Supraglottic | 14 (18.42) |
| Glottic | 46 (60.53) |
| Subglottic | 7 (9.21) |
| Hypopharynx invaded | 9 (11.84) |
| Surgery type, n (%) | |
| Partial laryngectomy | 3 (3.95) |
| Total laryngectomy | 73 (96.05) |
All of the patients underwent primary partial or total laryngectomy, and unilateral or bilateral cervical lymph node dissection at the ENT Department of Drum Tower Hospital. All of the collected pathology materials were reviewed after excision by the Department of Pathology of Drum Tower Hospital to confirm the diagnosis of LSCC and assess the degree of differentiation. Adjacent non-neoplastic laryngeal tissues were used as controls.
Immunohistochemistry
Representative tissue sections of 2-µm thickness were dewaxed in xylene and rehydrated in graded ethanol. Antigen retrieval was performed by pressure heating the slides in 0.01 M pH 6.0 citrate buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Endogenous peroxidase activity was quenched by treatment with 3% H2O2 for 15 min at room temperature, followed by incubation with non-specific protein blocking solution 1% bovine serum albumin (cat. no., 11021037; Thermo Fisher Scientific, Waltham, USA) in PBS for 45 min at room temperature. Sections were subsequently incubated with primary antibodies against E-cadherin (mouse monoclonal; cat. no., ab1416), N-cadherin (rabbit polyclonal; cat. no., ab18203), β-catenin (rabbit monoclonal; cat. no., ab32572), and ZEB2 (rabbit polyclonal; cat. no., ab138222; all Abcam, Cambridge, UK) overnight at 4°C. The secondary reactions for all antibodies were performed using the Polink-1 HRP DAB Detection System kit (OriGene Technologies, Inc., Beijing, China). The slides were rinsed, counterstained with Harris hematoxylin for 15 sec at room temperature, dehydrated and mounted. For negative controls, blocking solution was added instead of the primary antibody.
Immunohistochemical evaluation
All slides were assessed by the following evaluation method, based on other studies regarding LSCC, HNSCC and oral squamous cell carcinoma (OSCC) (18–20). In the assessment of E-cadherin and β-catenin staining, the focus was on the cell membranes. For E-cadherin, the staining was considered negative, and therefore, ‘low expression’, if <90% of the cells were positive for membranous staining. For β-catenin, the staining of the cell membranes was evaluated as negative, with weak-to-extensive staining in the cytoplasm and nucleus considered positive. When considering N-cadherin, the cells were negative, and therefore exhibited ‘low expression,’ if <20% of the cells were stained. Finally, for ZEB2, the intensity of nuclear staining was evaluated as follows: 0= no staining, 1= weakly positive, 2= moderately positive and 3= strongly positive, and the extent of staining was based on the percentage of positive cells, where 1=1–25%, 2=26–50%, 3=51–75%, and 4=7 6–100%. The total ZEB2 immunoreactivity score was calculated as the product of the scores for the intensity of nuclear staining and the extent of staining. The scores were then divided into negative (<3) and positive (≥3).
Statistical analysis
Statistical analysis was performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). The statistical associations of protein expression levels with clinicopathological parameters were analyzed with Pearson's χ2, or Fisher's exact test for nominal data. Survival probability differences were compared with the log-rank test, and the association of survival rates with the four EMT biomarkers was illustrated using Kaplan-Meier survival curves. A multivariate analysis was performed using the Cox proportional hazards model. P<0.05 was considered to indicate a statistically significant result.
Results
Clinicopathological characteristics and outcomes of patients with LSCC
The main clinicopathological characteristics of the patients with LSCC in the present study are included in Table I. The follow-up survival data were available for 70/76 patients (92.11%), with a median survival time of 38.5 months, and a maximum follow-up period of 98 months.
EMT-related markers are differentially expressed between LSCC and non-neoplastic tissues
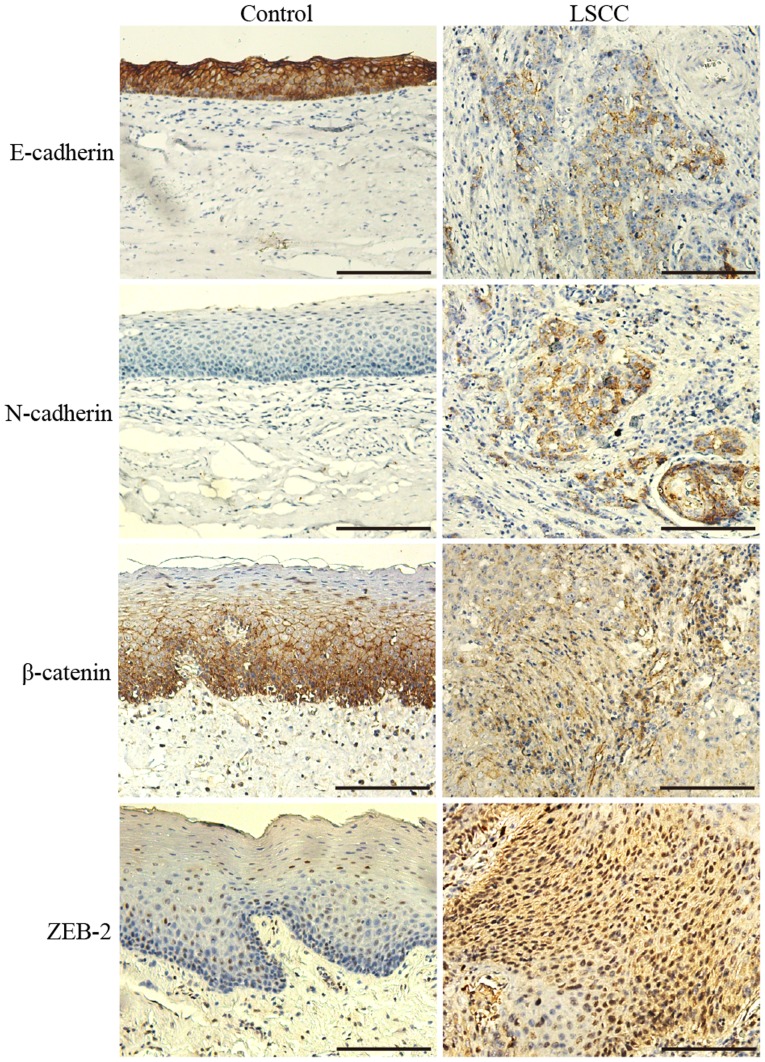
E-cadherin, N-cadherin, β-catenin and ZEB2 were differentially expressed between LSCC and non-neoplastic tissues (Fig. 1). E-cadherin and β-catenin were highly expressed in a membranous pattern in non-neoplastic laryngeal tissues. In the majority of the cells of the tumor tissue samples, their expression on the membrane was predominantly reduced, and cytoplasmic expression patterns were diffuse (Fig. 1).
Figure 1.
Representative images of the immunostaining of E-cadherin, N-cadherin, β-catenin and ZEB2 in non-neoplastic tissues and LSCC. Magnification, ×200. LSCC, laryngeal squamous cell carcinoma; ZEB2, zinc finger E-box binding homeobox 2.
According to the immunohistochemical evaluation, positive E-cadherin expression was observed in significantly fewer LSCC tissue samples (42.11%; 32/76) than non-neoplastic tissue samples (100%; 76/76; P<0.001).
The β-catenin staining pattern was also significantly different between LSCC and non-neoplastic tissues (Fig. 1); 40.79% (31/76) of the LSCC tissues were considered to exhibit positive staining localized in the cytoplasm and nucleus, whereas only 1.32% (1/76) of the non-neoplastic tissues demonstrated similar staining (P<0.001).
N-cadherin expression was observed in the cytoplasm of the cells in a number of LSCC tissue samples (11/76), with a positive rate of 14.47%, whereas its expression was not identified in any of the non-neoplastic tissues (P<0.001).
The rate of the positive expression of ZEB2 in LSCC tissue samples was 48.68% (37/76); there was frequently strong staining in the nucleus and cytoplasm. However, the positive rate in the control group was only 9.21% (7/76), which was significantly different than the rate in the LSCC group (P<0.001); there was weak nuclear staining in a limited number of cells in the non-neoplastic tissues.
Correlation of EMT marker expression with clinicopathological parameters
As included in Table II, E-cadherin and β-catenin expression, a hallmark of EMT, was significantly associated with lymph node metastases, T stage and differentiation status (P=0.020, P=0.002; P=0.004, P=0.003; P=0.028, P<0.001, respectively). It was identified that relatively reduced E-cadherin staining in the membrane and increased β-catenin staining in the cytoplasm and nucleus were observed in the majority of LSCC cases with lymph node metastases, stage T4 or poor differentiation. N-cadherin expression was significantly associated with T stage and differentiation (P=0.003; P=0.010).
Table II.
Differential expression of epithelial-mesenchymal transition-associated biomarkers in laryngeal squamous cell carcinoma and non-neoplastic tissues.
| A, Association with E-cadherin | ||||
|---|---|---|---|---|
| Clinicopathological parameter | Positive, n | Negative, n | χ2 | P-value |
| Lymph node metastasis | 5.398 | 0.020 | ||
| Positive | 11 | 27 | ||
| Negative | 21 | 17 | ||
| T stage | 8.188 | 0.004 | ||
| T1–3 | 25 | 20 | ||
| T4 | 7 | 24 | ||
| Tumor differentiation | 7.164 | 0.028 | ||
| Good | 2 | 14 | ||
| Moderate | 26 | 26 | ||
| Poor | 3 | 5 | ||
| Localization | 0.758 | 0.860 | ||
| Supraglottic | 5 | 9 | ||
| Glottic | 21 | 25 | ||
| Subglottic | 3 | 4 | ||
| Hypopharynx invasion | 3 | 6 | ||
| B, Association with N-cadherin | ||||
| Clinicopathological parameter | Positive, n | Negative, n | χ2 | P-value |
| Lymph node metastasis | 0.516a | |||
| Positive | 7 | 31 | ||
| Negative | 4 | 34 | ||
| T stage | 0.006a | |||
| T1–3 | 2 | 43 | ||
| T4 | 9 | 22 | ||
| Tumor differentiation | 9.199 | 0.010 | ||
| Good | 6 | 10 | ||
| Moderate | 5 | 47 | ||
| Poor | 0 | 8 | ||
| Localization | 3.069 | 0.381 | ||
| Supraglottic | 2 | 12 | ||
| Glottic | 5 | 41 | ||
| Subglottic | 1 | 6 | ||
| Hypopharynx invasion | 3 | 6 | ||
| C, Association with β-catenin | ||||
| Clinicopathological parameter | Positive, n | Negative, n | χ2 | P-value |
| Lymph node metastasis | 9.207 | 0.002 | ||
| Positive | 22 | 16 | ||
| Negative | 9 | 29 | ||
| T stage | 9.111 | 0.003 | ||
| T1–3 | 12 | 33 | ||
| T4 | 19 | 12 | ||
| Tumor differentiation | 23.985 | <0.001 | ||
| Good | 15 | 1 | ||
| Moderate | 13 | 39 | ||
| Poor | 3 | 5 | ||
| Localization | 4.678 | 0.197 | ||
| Supraglottic | 8 | 6 | ||
| Glottic | 17 | 29 | ||
| Subglottic | 1 | 6 | ||
| Hypopharynx invasion | 5 | 4 | ||
| D, Association with zinc finger E-box binding homeobox 2 | ||||
| Clinicopathological parameter | Positive, n | Negative, n | χ2 | P-value |
| Lymph node metastasis | 23.227 | <0.001 | ||
| Positive | 29 | 9 | ||
| Negative | 8 | 30 | ||
| T stage | 13.637 | <0.001 | ||
| T1–3 | 14 | 31 | ||
| T4 | 23 | 8 | ||
| Tumor differentiation | 8.626 | 0.013 | ||
| Good | 13 | 3 | ||
| Moderate | 21 | 31 | ||
| Poor | 3 | 5 | ||
| Localization | 2.128 | 0.546 | ||
| Supraglottic | 9 | 5 | ||
| Glottic | 20 | 26 | ||
| Subglottic | 3 | 4 | ||
| Hypopharynx invasion | 5 | 4 | ||
Fisher's exact test was performed instead of Pearson's χ2 test. T, tumor.
As ZEB2 may repress E-cadherin expression (5,16), it is reasonable to expect its increased expression to be associated with the tumor characteristics associated with E-cadherin loss. Indeed, the expression of ZEB2 was associated with the lymph node metastasis status, T stage and the differentiation status of tumor cells in the present study (P<0.001; P<0.001; P=0.013).
In addition, the association of the co-expression of E-cadherin and the other three EMT-associated biomarkers with clinicopathological parameters was assessed in the present study, as included in Table III. E-cadherin/β-catenin co-expression was significantly associated with the lymph node metastasis status (P=0.004), T stage (P=0.005) and differentiation (P<0.001), whereas E-cadherin/N-cadherin or E-cadherin/ZEB2 co-expression was significantly associated with only two of the clinicopathological parameters (T stage, P=0.001; differentiation, P=0.012). However, the co-expression of E-cadherin with the other three EMT-associated biomarkers individually was not associated with the localization of LSCC. Additionally, there was no significant association between the individual expression of the four EMT-associated biomarkers and tumor localization.
Table III.
Co-expression of E-cadherin and the other three epithelial-mesenchymal transition-associated biomarkers in laryngeal squamous cell carcinoma.
| A, Co-expression of E-cadherin and N-cadherin | ||||||
|---|---|---|---|---|---|---|
| E-cadherin/N-cadherin status, n | ||||||
| Clinicopathological parameter | +/− | +/+ | −/− | −/+ | χ2 | P-value |
| Lymph node metastasis | 5.733 | 0.125 | ||||
| Positive | 10 | 1 | 21 | 6 | ||
| Negative | 20 | 1 | 14 | 3 | ||
| T stage | 16.101 | 0.001 | ||||
| T1–3 | 25 | 0 | 18 | 2 | ||
| T4 | 5 | 2 | 17 | 7 | ||
| Tumor differentiation | 16.265 | 0.012 | ||||
| Good | 2 | 0 | 8 | 6 | ||
| Moderate | 24 | 2 | 23 | 3 | ||
| Poor | 4 | 0 | 4 | 0 | ||
| Localization | 5.363 | 0.802 | ||||
| Supraglottic | 5 | 0 | 7 | 2 | ||
| Glottic | 20 | 1 | 21 | 4 | ||
| Subglottic | 3 | 0 | 3 | 1 | ||
| Hypopharynx invasion | 2 | 1 | 4 | 2 | ||
| B, Co-expression of E-cadherin and β-catenin | ||||||
| E-cadherin/β-catenin status, n | ||||||
| Clinicopathological parameter | +/− | +/+ | −/− | −/+ | χ2 | P-value |
| Lymph node metastasis | 13.444 | 0.004 | ||||
| Positive | 9 | 2 | 7 | 20 | ||
| Negative | 17 | 4 | 12 | 5 | ||
| T stage | 12.749 | 0.005 | ||||
| T1–3 | 22 | 3 | 11 | 9 | ||
| T4 | 4 | 3 | 8 | 16 | ||
| Tumor differentiation | 25.386 | <0.001 | ||||
| Good | 0 | 2 | 1 | 13 | ||
| Moderate | 23 | 3 | 16 | 10 | ||
| Poor | 3 | 1 | 2 | 2 | ||
| Localization | 7.495 | 0.586 | ||||
| Supraglottic | 3 | 2 | 3 | 6 | ||
| Glottic | 17 | 4 | 12 | 13 | ||
| Subglottic | 3 | 0 | 3 | 1 | ||
| Hypopharynx invasion | 3 | 0 | 1 | 5 | ||
| C, Co-expression of E-cadherin and ZEB2 | ||||||
| E-cadherin/ZEB2 status, n | ||||||
| Clinicopathological parameter | +/− | +/+ | −/− | −/+ | χ2 | P-value |
| Lymph node metastasis | 27.44 | <0.001 | ||||
| Positive | 7 | 4 | 2 | 25 | ||
| Negative | 17 | 4 | 13 | 4 | ||
| T stage | 16.590 | <0.001 | ||||
| T1–3 | 20 | 5 | 11 | 9 | ||
| T4 | 4 | 3 | 4 | 20 | ||
| Tumor differentiation | 12.303 | 0.056 | ||||
| Good | 1 | 1 | 2 | 12 | ||
| Moderate | 20 | 6 | 11 | 15 | ||
| Poor | 3 | 1 | 2 | 2 | ||
| Localization | 4.558 | 0.871 | ||||
| Supraglottic | 3 | 2 | 2 | 7 | ||
| Glottic | 16 | 5 | 10 | 15 | ||
| Subglottic | 2 | 1 | 2 | 2 | ||
| Hypopharynx invasion | 3 | 0 | 1 | 5 | ||
T, tumor; ZEB2, zinc finger E-box binding homeobox 2.
Association of EMT-associated marker expression and clinicopathological parameters with clinical outcome
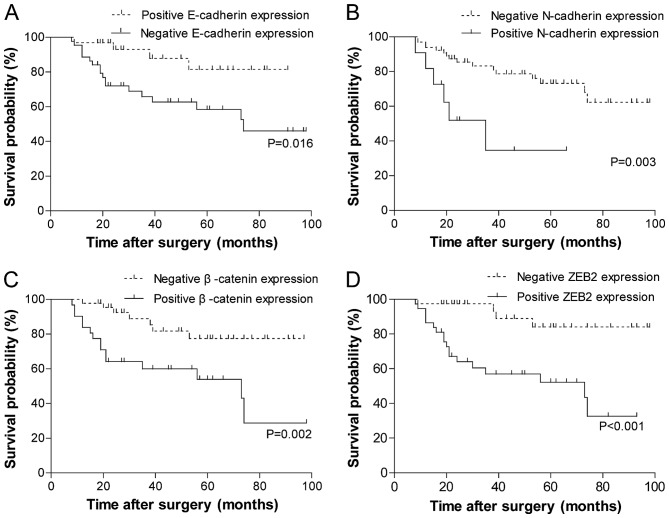
The results of the univariate analysis of the biomarkers for overall survival (OS) are summarized in Fig. 2. Patients whose tumors exhibited the negative membrane expression of E-cadherin (Fig. 2A; P=0.016) or positive expression of the other three biomarkers experienced significantly reduced OS time (Fig. 2B-D; N-cadherin, P=0.003; β-catenin, P=0.002; ZEB2, P<0.001). The association between the clinicopathological parameters and the clinical outcome was also examined by log-rank analysis (data not shown). OS time was significantly improved among patients with no lymph node metastases, an early T stage (T1-3) and strong differentiation of tumor cells (χ2=4.873, P=0.027; χ2=9.567, P=0.023; χ2=6.126, P=0.047, respectively). Tumor localization was not significantly associated with OS (χ2=1.420, P=0.701).
Figure 2.
Association of four epithelial-mesenchymal transition-associated markers with overall survival. Kaplan-Meier overall survival curves stratified by the expression of (A) E-cadherin, (B) N-cadherin, (C) β-catenin and (D) ZEB2. P-values were calculated using the log-rank test. ZEB2, zinc finger E-box binding homeobox 2.
Multivariate analysis
In the multivariate analysis, all of the analyzed factors were those identified as significant in the univariate analyses, with the exception of tumor localization, using the Cox proportional hazards model (data not shown). The result revealed that T stage and the positive expression of β-catenin or ZEB2 were independent risk factors for OS in LCSS (HR, 3.004; 95% CI, 1.24–7.25; P=0.014; HR, 2.877; 95% CI, 1.15–7.23; P=0.025; HR, 5.278; 95% CI, 1.77–15.70; P=0.003; respectively).
Discussion
EMT, a cellular program in which epithelial cells develop the motile and invasive properties typical of mesenchymal cells, is an important process in the progression, invasion and metastasis of cancer (1–3). At the molecular level, EMT, which is indicated by changes in the expression of specific proteins, involves the downregulation of epithelial-type markers, including adherens junction proteins, and the expression of mesenchymal proteins, including EMT-associated transcription factors (21–23). EMT was previously reported to be associated with aggressive behavior and a poor prognosis for several types of tumor, including HNSCC, and other studies have investigated the involvement of EMT specifically in LSCC (3,8,24,25). The present study was conducted to provide preliminary clinicopathological data on this topic. At present, the occurrence of EMT has been investigated in LSCC in studies that largely focused on the loss of membranous E-cadherin and the overexpression of cytoplasmic β-catenin (7,15,19,26,27). In the present study, the expression of an extended panel of EMT-associated markers, including E-cadherin, N-cadherin, β-catenin and ZEB2, was examined in a larger cohort of patients with LSCC. Additionally, analysis of the association between the expression of these markers and clinicopathological and follow-up data was performed to determine important prognostic information. The findings of the present study indicated that four of these EMT-associated proteins were differentially expressed between LSCC and non-neoplastic mucosal epithelium. E-cadherin expression was significantly reduced in the membrane, and there was a diffuse cytoplasmic staining pattern in tumor tissue. Previous studies reported a decrease in expression of the epithelial marker E-cadherin in LSCC cell lines and resected samples, and some provided evidence of cytoplasmic E-cadherin expression only in LSCC (26,28–30). The results of the present study indicated that the E-cadherin expression pattern was altered in LSCC, and are therefore, was in accord with the majority of previous studies.
E-cadherin is a cell surface glycoprotein which mediates intercellular adhesion through the interactions of its extracellular and cytoplasmic domains with β-catenin (31). The destabilization of cadherin/catenin complex formation that results from the downregulation or loss of E-cadherin expression may serve a role in tumor invasion and metastasis (32,33). A previous study identified the loss of membranous E-cadherin and β-catenin expression, in addition to increases in cytoplasmic expression, irrespective of the lymph node or distant metastasis status, in HNSCC (34). β-catenin expression was reported in the membrane and cytoplasm in LSCC cells by Goulioumis et al and Galera-Ruiz et al (14,33), who observed a significant association between β-catenin expression and localization (glottis and supraglottis LSCC). β-catenin exhibited significantly different expression between LSCC and non-carcinoma tissue in the present study, with positive staining identified in the cytoplasm and nucleus of tumor tissue. Significant associations were identified between β-catenin expression and lymph node metastases, T stage and tumor cell differentiation, but not with tumor localization.
It has been reported that cadherin switching (a decrease in E-cadherin with an increase in N-cadherin) is a feature of EMT in numerous types of malignant tumor and that an association exists between cadherin switching and lymph node metastasis in a number of tumor types, including HNSCC (4,11,13,14). In the present study, it was only partially expressed in LSCC samples, while N-cadherin expression was negative in the control group. Although the N-cadherin positive rate of 14.47% detected in LSCC tissue was low, the result of statistical analysis revealed that there was a significant difference in expression between the LSCC and non-neoplastic tissues, and N-cadherin expression was significantly associated with T stage and differentiation. Furthermore, there was no significant association between N-cadherin expression and lymph node metastasis, whereas N-cadherin expression was associated with T stage, tumor differentiation and poor OS. Greco et al (27) previously reported that N-cadherin expression was associated with the tumor histological grade, but not OS. Taken together, these results indicate that the cadherin switch between E-cadherin and N-cadherin may be a classical phenomenon in tumor-associated EMT rather than an individual criterion in LSCC, perhaps due to the low rate of positive N-cadherin expression in this study.
ZEB2 is associated with EMT, and is therefore proposed to be involved in this key step of the progression of different types of tumor; as a repressor of E-cadherin, the expression of ZEB2 is inversely associated with it (5,35). Furthermore, it has been reported that the co-expression of ZEB2 and other EMT-related protein markers is associated with poor prognosis in HNSCC and OSCC (6,20); however, to the best of our knowledge, no clinicopathological research has been conducted on the importance of ZEB2 in LSCC. The present study confirmed that ZEB2 expression was significantly increased in tumor tissue compared with non-carcinoma tissue, and was directly associated with the status of lymph node metastases, T stage and tumor cell differentiation in LSCC. It was also observed that positive ZEB2 expression was associated with a poor prognosis in patients with LSCC; therefore, it is reasonable to consider ZEB2 as an EMT biomarker in LSCC oncogenesis, development and metastasis based on the conclusions of the present study.
EMT is a complex process that often involves several types of EMT-associated proteins during malignant tumor progression and metastasis in patients (22,36). The four EMT biomarkers in the present study exhibited significantly different expression between the LSCC and control tissues. Considering E-cadherin to be a hallmark of EMT progression, the co-expression of E-cadherin and the other three EMT-related biomarkers was also taken into account. Among the three types of co-expression, E-cadherin/β-catenin had the most significant association with the clinicopathological characteristics of lymph node metastases, T stage and tumor cell differentiation. To the best of our knowledge, this is the first study to investigate EMT in LSCC by assessing the co-expression of two biomarkers.
Each of the four EMT markers examined in the present study had been previously demonstrated to have clinical implications in other types of tumor (4–13), and they were all further demonstrated to have prognostic implications for OS by univariate analysis in LSCC in the present study. In addition, patients with the loss of E-cadherin, expression of N-cadherin and overexpression of β-catenin experienced a significantly reduced OS time, in accord with previous results derived from other tumors and LSCC (25,27,32). Furthermore, the effect of ZEB2 on LSCC prognosis was elucidated for the first time. By employing a Cox proportional hazards model, it was identified that T-stage and positive β-catenin and ZEB2 expression were independent risk factors for adverse OS in the multivariate analysis. Lopez-Gonzalez et al (36) reported that the overexpression of cytoplasmic β-catenin was associated with poor tumor differentiation. Greco et al (27) revealed that the reduced expression of cytoplasmic β-catenin was associated with high histological grade; however, they identified that cytoplasmic β-catenin overexpression corresponded to significantly improved disease-specific survival in certain patients with LSCC. Increasing tumor histological grade should generally correspond to reduced survival time, which is in accord with the results of the present study, which identified that patients with the overexpression of β-catenin experienced worse OS. Furthermore, in the study by Greco et al T stage was also an independent prognostic predictor in the multivariate analysis, which is consistent with the present study's results.
The present study, with a cohort of 76 patients, was, to the best of our knowledge, the first systematic investigation of LSCC that utilized immunohistochemistry analysis to identify that positive ZEB2 expression is also an independent risk factor for LSCC prognosis. ZEB2, a transcriptional repressor, induces EMT by suppressing the expression of E-cadherin and contributes to the invasiveness of malignant tumors; therefore, it has been considered as a predictor of prognosis in numerous types of cancer, including head and neck cancer; the high expression of ZEB2 predicted a poor prognosis (6,37–39). The results of the present study also indicated that ZEB2 expression could also be a critical factor in predicting the prognosis of LSCC. Further junctional proteins were identified as potential ZEB2 targets, and targeted treatment should be investigated in a clinical setting.
In conclusion, EMT, which is mediated by several biomarkers, serves a role in the prognosis of LSCC by increasing the risk of tumor metastasis, therefore reducing OS time. The reduction in membranous E-cadherin expression, and the increase in cytoplasmic β-catenin expression, may be hallmarks of the EMT process in LSCC. ZEB2 expression, as an independent prognostic predictor, combined with its association with clinicopathological parameters and OS, should be considered as an EMT biomarker in LSCC based on the results of the present study. N-cadherin was also indicated as an EMT biomarker on account of its association with oncogenesis, development and metastasis in LSCC; however, there is still controversy in the literature regarding how these biomarkers affect survival. Therefore, more research is required to elucidate the association between molecular biomarkers and the clinicopathological characteristics of patients with LSCC.
Acknowledgements
The study was supported by the Nanjing Medical Science Technology Development Programme (grant nos. YKK14062 and QRX17012), the Project of Invigorating Health Care through Science, Technology and Education (grant no. ZDXKB2016015). Additionally, the authors would like to thank the Core Medical Laboratory of Drum Tower Hospital, Nanjing University Medical School.
Glossary
Abbreviations
- LSCC
laryngeal squamous cell carcinoma
- EMT
epithelial-mesenchymal transition
- ZEB2
zinc finger E-box binding homeobox 2
- ENT
ear, nose, and throat
- OS
overall survival
- HNSCC
head and neck squamous cell carcinoma
- T
tumor
- OSCC
oral squamous cell carcinoma
References
- 1.de Vincentiis M, De Virgilio A, Bussu F, Gallus R, Gallo A, Bastanza G, Parrilla C, Greco A, Galli J, Turchetta R, et al. Oncologic results of the surgical salvage of recurrent laryngeal squamous cell carcinoma in a multicentric retrospective series: Emerging role of supracricoid partial laryngectomy. Head Neck. 2015;37:84–91. doi: 10.1002/hed.23563. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Kang Y, Massague J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, Wu BC, Chen YW, Huang PI, Lo WL. Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poor prognosis and maintains tumor-initiating properties in head and neck cancer. Oral Oncol. 2013;49:34–41. doi: 10.1016/j.oraloncology.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Zhang GH, Yang XM, Li SS, Liu X, Yang QT, Li Y, Ye J. Reduced E-cadherin expression is associated with lymph node metastases in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2012;39:186–192. doi: 10.1016/j.anl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial-mesenchymal transition programming in invasion and metastasis: A clinical perspective. Cancer Manag Res. 2013;5:187–195. doi: 10.2147/CMAR.S35171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiery JP, Chua K, Sim WJ, Huang R. Epithelial mesenchymal transition during development in fibrosis and in the progression of carcinoma. Bull Cancer. 2010;97:1285–1295. doi: 10.1684/bdc.2010.1206. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PT, Kudo Y, Yoshida M, Kamata N, Ogawa I, Takata T. N-cadherin expression is involved in malignant behavior of head and neck cancer in relation to epithelial-mesenchymal transition. Histol Histopathol. 2011;26:147–156. doi: 10.14670/HH-26.147. [DOI] [PubMed] [Google Scholar]
- 14.Zidar N, Boštjančič E, Gale N, Kojc N, Poljak M, Glavač D, Cardesa A. Down-regulation of microRNAs of the miR-200 family and miR-205, and an altered expression of classic and desmosomal cadherins in spindle cell carcinoma of the head and neck-hallmark of epithelial-mesenchymal transition. Hum Pathol. 2011;42:482–488. doi: 10.1016/j.humpath.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Cappellesso R, Marioni G, Crescenzi M, Giacomelli L, Guzzardo V, Mussato A, Staffieri A, Martini A, Blandamura S, Fassina A. The prognostic role of the epithelial-mesenchymal transition markers E-cadherin and Slug in laryngeal squamous cell carcinoma. Histopathology. 2015;67:491–500. doi: 10.1111/his.12668. [DOI] [PubMed] [Google Scholar]
- 16.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th. Springer; New York, NY: 2002. pp. 47–57. Chapter 2. [Google Scholar]
- 18.Goulioumis AK, Varakis J, Goumas P, Papadaki H. Differential beta-catenin expression between glottic and supraglottic laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1573–1578. doi: 10.1007/s00405-010-1249-4. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed RA, Shawky Ael-A, Hamed RH. Prognostic significance of cyclin D1 and E-cadherin expression in laryngeal squamous cell carcinoma. Pathol Oncol Res. 2014;20:625–633. doi: 10.1007/s12253-014-9741-6. [DOI] [PubMed] [Google Scholar]
- 20.Kong YH, Syed Zanaruddin SN, Lau SH, Ramanathan A, Kallarakkal TG, Vincent-Chong VK, Wan Mustafa WM, Abraham MT, Abdul Rahman ZA, Zain RB, Cheong SC. Co-Expression of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated with poor survival. PLoS One. 2015;10:e0134045. doi: 10.1371/journal.pone.0134045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376. doi: 10.1146/annurev-cellbio-092910-154036. [DOI] [PubMed] [Google Scholar]
- 22.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 24.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: Mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz KA, Hoffman HT, Zimmerman MB, Robinson RA. Decreased E-cadherin but not beta-catenin expression is associated with vascular invasion and decreased survival in head and neck squamous carcinomas. Otolaryngol Head Neck Surg. 2006;134:142–146. doi: 10.1016/j.otohns.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Psyrri A, Kotoula V, Fountzilas E, Alexopoulou Z, Bobos M, Televantou D, Karayannopoulou G, Krikelis D, Markou K, Karasmanis I, et al. Prognostic significance of the Wnt pathway in squamous cell laryngeal cancer. Oral Oncol. 2014;50:298–305. doi: 10.1016/j.oraloncology.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Greco A, De Virgilio A, Rizzo MI, Pandolfi F, Rosati D, de Vincentiis M. The prognostic role of E-cadherin and β-catenin overexpression in laryngeal squamous cell carcinoma. Laryngoscope. 2016;126:E148–E155. doi: 10.1002/lary.25736. [DOI] [PubMed] [Google Scholar]
- 28.Goulioumis AK, Fuxe J, Varakis J, Repanti M, Goumas P, Papadaki H. Estrogen receptor-beta expression in human laryngeal carcinoma: Correlation with the expression of epithelial-mesenchymal transition specific biomarkers. Oncol Rep. 2009;22:1063–1068. doi: 10.3892/or_00000537. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Li HZ, Lu SM, Tian JJ, Ma JK, Wang HB, Xu W. Down-regulation of TWIST decreases migration and invasion of laryngeal carcinoma Hep-2 cells by regulating the E-cadherin, N-cadherin expression. J Cancer Res Clin Oncol. 2011;137:1487–1493. doi: 10.1007/s00432-011-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/β-catenin pathway in laryngeal cancer Hep-2 cells. Tumour Biol. 2014;35:7965–7973. doi: 10.1007/s13277-014-2045-3. [DOI] [PubMed] [Google Scholar]
- 31.Stappert J, Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun. 1994;2:319–327. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- 32.Joo YE, Rew JS, Choi SK, Bom HS, Park CS, Kim SJ. Expression of e-cadherin and catenins in early gastric cancer. J Clin Gastroenterol. 2002;35:35–42. doi: 10.1097/00004836-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Kallakury BV, Sheehan CE, Winn-Deen E, Oliver J, Fisher HA, Kaufman RP, Jr, Ross JS. Decreased expression of catenins (alpha and beta), p120 CTN, and E-cadherin cell adhesion proteins and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer. 2001;92:2786–2795. doi: 10.1002/1097-0142(20011201)92:11<2786::AID-CNCR10128>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Andrews NA, Jones AS, Helliwell TR, Kinsella AR. Expression of the E-cadherin-catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer. 1997;75:1474–1480. doi: 10.1038/bjc.1997.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 36.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding W, You H, Dang H, LeBlanc F, Galicia V, Lu SC, Stiles B, Rountree CB. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology. 2010;52:945–953. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]