Abstract
There are few effective therapies for unresectable or metastatic hepatocellular carcinoma. Recent data have demonstrated efficacy of immune checkpoint blockade in this difficult to treat disease; however, clinical experience is limited. We report a case of hepatocellular carcinoma displaying pseudoprogression followed by a late response with novel magnetic resonance imaging features following treatment with the anti‐programmed cell death protein 1 agent pembrolizumab. (Hepatology Communications 2018;2:148–151)
Abbreviations
- CTLA4
cytotoxic T‐lymphocyte associated protein 4
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- PD‐1
programmed cell death protein 1
Introduction
Immune checkpoint blockade has emerged as a novel powerful class of therapeutic agents for multiple solid tumors, including hepatocellular carcinoma (HCC).1 Response evaluation of immune checkpoint blockade may be complicated by the phenomenon of pseudoprogression. Clinical experience with this class of drugs is still limited in HCC, and serial magnetic resonance changes in HCC following anti‐ programmed cell death protein 1 (PD‐1) therapy have not been reported.
Case Presentation
An 82‐year‐old Caucasian man without chronic liver disease was incidentally found to have a large liver lesion on ultrasonography. Abdominal magnetic resonance imaging (MRI) showed a large 14.8 × 11.5 × 14.9 cm mass with heterogeneous arterial hyperenhancement and washout in the right hemiliver along with enhancing osseous lesions in T10 and L3 vertebrae and a subcarinal mass. Liver function tests and alpha‐fetoprotein were normal, and the viral hepatitis panel was negative. Sampling of the liver lesion revealed well‐differentiated HCC, with metastatic disease confirmed in the subcarinal mass by positive hepatocyte paraffin 1 immunohistochemical staining (Fig. 1A).
Figure 1.
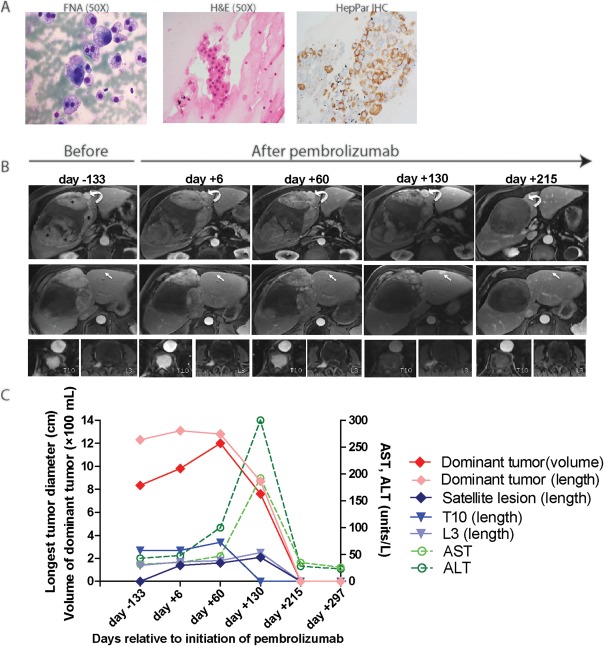
Tumor characteristics.(A) Modified Giemsa (Diff‐Quick) stain of the smear preparation (left) and H&E stain of the cell block (middle) from fine needle aspiration of the subcarinal mass, showing atypical hepatocytes. Immunostain for HepPar‐1 performed on cell block (right) confirms spread from liver primary. (B) MR images from five separate MR examinations performed 133 days prior to initiation of pembrolizumab, at the start of pembrolizumab therapy, 60 days later after 3 cycles, after an additional 3 cycles 130 days later, and after a total of 9 cycles 215 days later. Top row shows large dominant HCC occupying the right hemiliver (asterisks indicate arterially enhancing viable tumor). Curved arrow demonstrates one of many arterially enhancing satellite HCCs. On day +215, no arterially enhancing tumor remains within the dominant HCC (linear areas of hyperintensity were present on the precontrast images, not shown) and the satellite tumor is no longer present. Middle row shows a more inferior portion of the dominant HCC, with arrow depicting one of several arterially enhancing lesions in the left hemiliver, not present prior to therapy, increasing in size during therapy, and completely resolved on day +215. Bottom row shows arterially enhancing osseous lesions in the T10 vertebral body and the right L3 pedicle. Enhancement in T10 resolved and is ill‐defined in L3 on February 3, 2017. Hyperintensity in these osseous lesions on day +215 was present on precontrast images. (C) Serial changes in size (maximal dimension of arterial enhancement) of dominant tumor, a satellite tumor, and subcarinal metastasis as well as liver enzymes over time. Serum AFP remained within normal limits from diagnosis until present. Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FNA, fine need aspiration; H&E, hematoxylin and eosin; MR, magnetic resonance.
Due to metastatic disease, sorafenib 400 mg twice daily was started. Unfortunately, an MRI 2 months later showed an increase in size of the liver and subcarinal tumors. Sorafenib was discontinued, and the patient underwent palliative transarterial chemoembolization. Over the next 11 months, serial scans revealed a minimal decrease in arterial enhancement within the hepatic mass and increased size of osseous metastatic disease. After informed consent, we began compassionate use of pembrolizumab 2 mg/kg every 3 weeks. After cycle 3 (day +60), MRI showed stable liver lesion length but an increase in arterially enhancing tumor volume (980 mL to 1,200 mL) and an increase in size of arterially enhancing satellite tumors and the T10 lesion (Fig. 1B). The patient reported improved energy and appetite and so treatment was continued. After cycle 6 (day +130), MRI showed a decrease in length of arterial enhancement within the dominant HCC, an increase in length of satellite tumors, and a decrease in total enhancing tumor volume (760 mL). Arterial enhancement in T10 had resolved completely. Liver function tests showed elevated alkaline phosphatase 268, aspartate aminotransferase 215, alanine aminotransferase 298, and total bilirubin 0.4, which could suggest possible liver injury from tumor progression or autoimmune hepatitis. Corticosteroids were not administered for transaminase elevation, and treatment was continued based on clinical judgment. After cycle 12 (day +215), restaging MRIs showed no residual arterial enhancement in any hepatic or osseous lesions and progressive decrease in size of the subcarinal mass; liver function tests had normalized. Serum alpha‐fetoprotein has remained within normal range since diagnosis. The patient currently remains on treatment.
Discussion
Pseudoprogression is a new challenge for oncologists administering immune‐based therapies.2 This case has allowed us to make new important observations. First, our patient developed radiographic patterns of disease progression through 6 cycles (4.5 months) of pembrolizumab and did not have disease control until 9 cycles (6.7 months), which is delayed compared to another report of HCC showing disease progression until 4 months3 and reports of melanoma showing most occurrences of pseudoprogression prior to 3 months of treatment.4 Second, our patient developed a mixed response of individual hepatic lesions. In this regard, arterially enhancing tumor volume consolidates tumor burden as a single value and could potentially be helpful in determining treatment response. Third, our report is the first to show MRI features during pseudoprogression, noting that there is measurable increase in viable tumor during treatment, as evidenced by a) enlarging HCC lesions that demonstrate arterial enhancement and washout and b) new arterially enhancing lesions in the left hemiliver, consistent with new tumor according to the Liver Imaging Reporting and Data System v2017 criteria.5 Pseudoprogression in other solid tumors has implicated immune cell infiltration and necrosis as the etiology of increased lesion size.2 The possibility of tissue necrosis contributing to increased tumor size has been ruled out with MRI, but the contribution of immune infiltration to arterial enhancement is unclear. The Liver Imaging Reporting and Data System imaging criteria may require further examination and validation in response assessment for systemic therapies in HCC, particularly for novel immunotherapy agents that may recruit immune cells to tumors and could alter tumor enhancement patterns independent of progression. Fourth, we show that patients can develop transaminitis during pseudoprogression.
It is noteworthy that our patient developed HCC without underlying hepatitis or cirrhosis, as the vast majority of patients with HCC have underlying liver disease. The presence and nature of underlying liver disease could influence the response to immunotherapy; however, in the largest phase I/II study of checkpoint inhibition in HCC to date, responses to anti‐PD1 therapy were observed independent of underlying viral hepatitis.1
The literature contains few reports of checkpoint blockade immunotherapy for the treatment of HCC. The anti‐PD‐1 checkpoint inhibitor nivolumab was recently evaluated in the phase I/II CheckMate‐040 study treating patients with or without prior sorafenib treatment but with HCC due to underlying hepatitis B, hepatitis C, or without viral hepatitis. At the dose‐expansion phase of nivolumab 3 mg/kg every 2 weeks, an objective response rate of 20% was observed, independent of viral hepatitis or prior exposure to or response to sorafenib. Similar to other tumor types, 69% of responses occurred prior to 3 months of therapy. Toxicity was also not significantly different between groups, with 15%‐20% of patients experiencing transaminase elevation. Unfortunately, toxicity or efficacy data related to the presence or absence of underlying cirrhosis are not available.1
The anti‐cytotoxic T‐lymphocyte associated protein 4 (anti‐CTLA4) agent tremelimumab was evaluated as monotherapy in a phase II study of HCC in the setting of hepatitis C virus‐induced cirrhosis. In a cohort of 21 patients, a disease control rate of 76% was observed. Notably, 45% and 25% of patients experienced >grade 3 elevation in aspartate aminotransferase and alanine aminotransferase, respectively, peaking on cycle 1 between days 15‐30 and remaining elevated for approximately 45 days, the resolution of which occurred without corticosteroids.6 This time course stands in contrast to our patient, wherein maximal transaminase elevation occurred approximately 130 days after the initiation of pembrolizumab and remained elevated for a duration of approximately 75 days. The mechanism of transaminase elevation secondary to anti‐CTLA4 and anti‐PD‐L1/PD‐1 therapy may be distinct, which will require further study. Notably in both scenarios, transaminase elevation resolved without administration of corticosteroids.
In summary, our case and additional reports in the literature highlight the variable kinetics of patient response to checkpoint blockade immunotherapy and suggest the need for additional biomarkers of response or progression to prevent premature and inappropriate therapy discontinuation. We expect this information to benefit other oncologists using immunotherapy in HCC.
Authors names in bold designate shared co‐first authorship.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. El‐Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015;33:3541‐3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mamdani H, Wu H, O'Neil BH, Sehdev A. Excellent response to anti‐PD‐1 therapy in a patient with hepatocellular carcinoma: case report and review of literature. Discov Med 2017;23:331‐336. [PubMed] [Google Scholar]
- 4. Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American College of Radiology . CT/MRI LI‐RADS® September, 2017. https://www.acr.org/Quality‐Safety/Resources/LIRADS/LIRADS‐v2017.
- 6. Sangro B, Gomez‐Martin C, de la Mata M, Inarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA‐4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81‐88. [DOI] [PubMed] [Google Scholar]


