Abstract
The term “liver tissue engineering” summarizes one of the ultimate goals of modern biotechnology: the possibility of reproducing in total or in part the functions of the liver in order to treat acute or chronic liver disorders and, ultimately, create a fully functional organ to be transplanted or used as an extracorporeal device. All the technical approaches in the area of liver tissue engineering are based on allocating adult hepatocytes or stem cell‐derived hepatocyte‐like cells within a three‐dimensional structure able to ensure their survival and to maintain their functional phenotype. The hosting structure can be a construct in which hepatocytes are embedded in alginate and/or gelatin or are seeded in a pre‐arranged scaffold made with different types of biomaterials. According to a more advanced methodology termed three‐dimensional bioprinting, hepatocytes are mixed with a bio‐ink and the mixture is printed in different forms, such as tissue‐like layers or spheroids. In the last decade, efforts to engineer a cell microenvironment recapitulating the dynamic native extracellular matrix have become increasingly successful, leading to the hope of satisfying the clinical demand for tissue (or organ) repair and replacement within a reasonable timeframe. Indeed, the preclinical work performed in recent years has shown promising results, and the advancement in the biotechnology of bioreactors, ex vivo perfusion machines, and cell expansion systems associated with a better understanding of liver development and the extracellular matrix microenvironment will facilitate and expedite the translation to technical applications. (Hepatology Communications 2018;2:131–141)
Abbreviations
- 3D
three‐dimensional
- ECM
extracellular matrix
- iPSC
inducible pluripotent stem cell
Introduction
Chronic liver diseases affect more than 500 million people worldwide and cause 2% of all deaths.1, 2 In addition, liver‐related deaths are progressively increasing, with cirrhosis anticipated to be the twelfth leading cause of death in 2020.3 Liver transplant is the only definitive cure, but there is a huge discrepancy between the need for transplantation and the availability of donor organs. As a result, a substantial number of patients die while on the waiting list.4 Among various approaches proposed to increase the number of available grafts is the use of marginal donors, i.e., cases in which the cadaver graft has been adversely affected by factors such as pressure requirement, hypernatremia, and hepatic steatosis. The use of marginal donor organs is associated with a higher incidence of primary nonfunction and early graft impairment as well as a poorer long‐term outcome.5 Therefore, novel alternative strategies to overcome these limitations are urgently needed.
Hepatocyte transplantation was first introduced to replace a lacking essential enzymatic activity in patients with a hepatic inborn error of metabolism or to improve liver function in patients with liver failure. However, this approach has failed to show long‐term clinical benefits due to poor cell engraftment and time‐limited survival of the transplanted hepatocytes.6
Over the last 20‐30 years, a variety of methods have been developed to improve or replace, at least temporarily, essential hepatic metabolic functions.7 These have included extracorporeal bioartificial liver devices8, 9, 10 and cell therapy.11, 12, 13 Along these lines, major progress has been made with the development of bioengineering models combining primary or stem cell‐derived cells by using three‐dimensional (3D) scaffolds attempting to reproduce the complexity of tissue architecture.14 More recently, efforts to engineer a cell microenvironment recapitulating the dynamic native extracellular matrix (ECM) have become increasingly successful, leading to the hope of satisfying the clinical demand for tissue (or organ) repair and replacement within a reasonable timeframe. Several pioneering studies performed employing a variety of native tissue and cell‐culture techniques have highlighted the need for precise information on 3D architectural/biomechanic features, ECM biochemical composition, and the potential array of signals derived from the cell–biomaterial interactions.
The current aim is to technically achieve more effective and permanent interventions, such as implantable liver constructs and whole‐organ engineering, for total or partial organ function replacement (Fig. 1). This review article summarizes the recent history and the current achievements in the field of liver bioengineering, recapitulating the cultural and technical basis for a hopefully rapid utilization in clinical practice.
Figure 1.
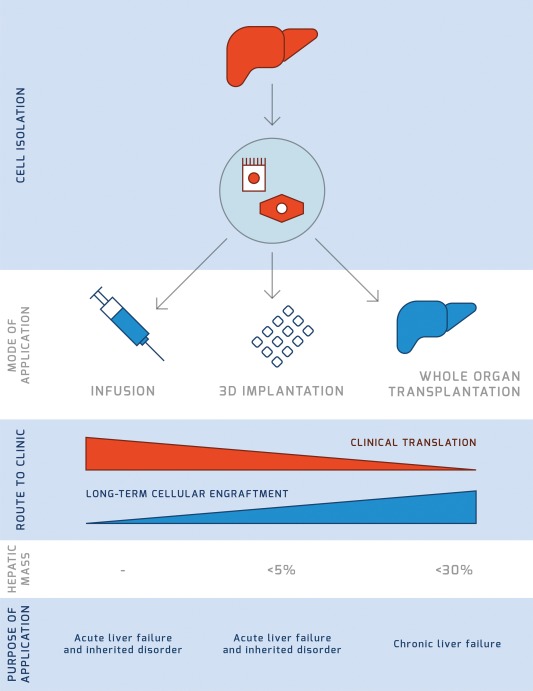
Applications of liver tissue engineering. The direct infusion of hepatocytes in humans is an established methodology proposed to treat inborn errors of metabolism but is characterized by short‐term clinical benefits. Alternative strategies have been developed, including implantation of 3D constructs and tissue/whole‐organ engineering. At present, the clinical applicability of these strategies is inversely proportional to the long‐term cellular engraftment, which is indeed the ultimate goal. In addition, implantation of 3D constructs of liver cells can achieve a replacement of the hepatic mass below 5% and is therefore indicated only for inborn errors of metabolism and to a much lesser extent for acute liver failure. Based on current technological development, engineering of large portions of liver tissue (e.g., the left liver lobe) or even of the whole organ is able to provide less than 30% of the liver mass and could be used to treat acute and even chronic liver failure as an extracorporeal device.
Implantable Technologies for Liver Therapies
Clinical trials on hepatocyte transplantation have recently demonstrated long‐term safety, but donor hepatocyte engraftment and restoration of failing host livers have not been adequate to reduce the need for organ transplantation.6 The development of implantable engineered hepatic tissue is a promising strategy for the treatment of liver disease due to the possibility of overcoming the limitations of the current cell‐therapy strategies, including lack of engraftment, poor long‐term cell survival, and the inherent lag phase before a clinical benefit is achieved.15 Implantable engineered hepatic tissues are typically developed by immobilizing or encapsulating hepatic cells in scaffolds made of different biomaterials in conjunction with strategies to optimize hepatocyte survival and function, thus leading to the in vitro generation of liver‐like tissue prior to implantation. The key step to develop a therapeutic product for the treatment of liver failure requires the presence of functional hepatocytes with efficient transport of nutrients and secretion of key hepatic factors, i.e., albumin and coagulation factors, within the engineered hepatic tissue. In addition, the long‐term survival of the implanted engineered tissue within the host after transplantation is needed. A variety of biomaterials have been recently developed with potentially adequate physiochemical, biomechanical, and 3D properties.16, 17 Furthermore, relevant environmental factors, like cell–cell interactions in coculture systems, cell–matrix interactions, and paracrine factors, can be incorporated in the structure of implantable tissues. Different methodologies can be employed for the production of implantable hepatic tissues, such as cell encapsulation, 3D printing, and decellularization–recellularization technologies.
CELL ENCAPSULATION
The key feature of the microencapsulation technique is that cells are embedded in a semipermeable polymerized structure with the aim of protecting them from a host immune attack while allowing the diffusion of nutrients, oxygen, and metabolic products that ensure cell function and survival.18, 19 Primary human hepatocytes cultured on alginate microbeads in vitro for 3 days showed albumin and urea production. In addition, the intraperitoneal transplantation of hepatocyte microbeads improved liver function up to 7 days in an animal model of acute liver failure.20 Despite these encouraging results, it is necessary to achieve more scientific insights and technical validation of both long‐term in vitro culture and in vivo implantation before this technology can be proposed for clinical applications. In addition, key challenges have been highlighted and include the risk of an inflammatory reaction against the biomaterial and a significant reduction in cell viability caused by the use of crosslinking agents used for the preparation of the hepatocyte microbeads. Recently, human hepatocyte‐like cells derived from inducible pluripotent stem cells (iPSCs) were encapsulated in alginate beads together with human hepatic stellate cells. This promoted an evident hepatic differentiation of iPSCs when compared to single‐cell culture conditions. In addition, human cocultured encapsulated cells were transplanted in immunocompetent mice without causing immune rejection for at least 24 days.21 However, the actual applicability of the encapsulated coculture system approach needs to be further explored in specific disease models where additional parallel strategies aimed at reducing potential foreign body fibrotic reactions22, 23 and improving neovascularization24, 25 should be considered.
3D PRINTING
The assembly of 3D structures by employing 3D bioprinting relies on printing programs that allow the precise positioning of living cells within a 3D structure of biocompatible material, i.e., the “ink,” able to support cell differentiation and function.26 Different manufacturing techniques have been used to 3D print hepatic‐like structures, including biomimicry (i.e., identical reproduction of the cellular and extracellular components of a tissue or organ) and minitissue building blocks (i.e., cell sphere assembled in a more complex 3D structure).27 Cells of the hepatic cell line Hepg2 were printed with alginate as the crosslinking agent, but cell viability was reduced when a high‐extrusion pressure from the printing device was applied.28 Because alginate is characteristically bioinert to mammalian cells and therefore not supportive of cell differentiation and survival, other biomaterials have been explored.29 For example, the use of gelatin as a base material ensures the control of ink thickness and higher printability.27 Indeed, addition of gelatin to alginate allowed a primary hepatocyte‐laden ink to be extruded at low temperature and subsequently stabilized with calcium chloride.30 Pure hepatocyte‐gelatin solutions have been printed into large (>2 mm in height) structures, but this process required postprinting stabilization with a harsh glutaraldehyde wash, which is known to be cytotoxic.31 Recently, 3D‐printed tissues were fabricated using mouse iPSC‐derived hepatocytes mixed with alginate hydrogels. These constructs gradually increased the level of metabolic function during 28 days of in vitro culture and maintained metabolic activity upon transplantation in animal models with liver damage.32 However, the central challenge is still the need to reproduce the complex microarchitecture and biochemistry of the many ECM components and multiple cell types in sufficient resolution to recapitulate the integrated biological functions typical of a certain tissue.
A logical approach to identify the ideal composition of the bio‐ink is to analyze the composition and distribution of ECM proteins in decellularized tissue scaffolds.33, 34, 35 The ability to image, map, and reproduce complex 3D structures composed of biologically relevant ECM proteins would represent a major technical advancement. In this direction, ECM derived from decellularized tissues could be a useful biomaterial for bioprinting applications. Along these lines, liver ECM derived from decellularized tissue has been employed as bio‐ink for 3D cell printing, improving the differentiation of bone marrow‐derived stem cells into hepatocyte‐like cells as well as enhancing HepG2 cell metabolic function when compared to cells cultured in monolayers of collagen type I.36
ARTIFICIAL AND NATURAL SCAFFOLDS
One of the most exploited systems for the development of 3D platforms for in vitro culture consists of seeding cells into 3D scaffolds. These scaffolds can be derived from both synthetic and biological sources. Synthetic scaffolds can be easily manufactured but lack some key features, such as the physiological bioactivity and the biomechanics of the natural ECM. The most common artificial matrices used for engineering biological tissues are synthetic polymers (e.g., polylactide‐co‐glycolide, polyethylene glycol, and polycaprolactone)37, 38 and natural‐derived hydrogels (e.g., alginates, celluloses, polyethylene).39 In addition, 3D scaffolds can be developed by using biological ECM‐derived materials. For instance, several substrates have been developed using basement membrane gels or type I collagen gels. However, the use of one or more ECM components does not recapitulate the biochemical and architectural complexity of a fully assembled natural ECM microenvironment and is in general characterized by limited hepatocyte viability and function.40 Therefore, functional substrates and scaffolds capable of providing a more appropriate microenvironment should be developed for the use of hepatocytes in liver tissue engineering, cell therapy, and transplantation.
In order to resolve these issues, attention has been directed at the development of biomaterials for functional tissue engineering by employing acellular tissues derived from the decellularization of tissues and organs. This process involves the complete removal of cellular material from the tissue while maintaining ECM protein composition, topography, and mechanical properties of the native tissue.41 Alternatively or in addition, the use of hydrogels reproducing the biochemistry of tissue‐specific ECM proteins has been proposed. ECM hydrogels were derived from decellularized rat livers and employed for both 2D‐plate coating and in vivo hepatocyte transplantation. Primary rat hepatocytes cultured on a liver ECM hydrogel‐coated substrate exhibited higher viability and improved hepatic functions compared to cells cultured on a noncoated or collagen type I‐coated substrate. In addition, liver ECM hydrogels engineered with rat hepatocytes maintained the hepatic phenotype and functions after in vivo transplantation.41 Decellularized tissues have also been used as a carrier for hepatocyte transplantation. This approach resulted in longer hepatocyte survival and higher metabolic activity compared to the infusion of unsupported hepatocyte suspensions.42
An implantable engineered tissue represents a novel approach to overcome limitations of cell therapy and to provide small hepatic mass (<5%) to improve metabolic function. However, in order to replace the vital functions of a human liver and allow patient survival, a much larger mass (>25%) is needed.43 This ambitious goal is the core aim of the whole organ decellularization–recellularization technology that is covered in the next section.
Decellularized 3D ECM Scaffolds for Tissue/Whole Organ Engineering
Decellularization of tissues and even whole organs represents a novel approach for the development of perfusable ECM‐derived scaffolds with preserved vascular integrity. Over the past decade, several studies have demonstrated the appropriateness of using naturally occurring ECM scaffolds derived by decellularized human or animal tissues for tissue engineering. In this context, liver bioengineering could be used for transplantation44 and for drug toxicity testing in 3D in vitro cultures.45 There is convincing experimental evidence that decellularization–recellularization technologies provide a valuable platform for liver bioengineering through the repopulation of liver ECM scaffolds with parenchymal and nonparenchymal liver cells, thus recapitulating, at least in part, natural tissue complexity.
The decellularization of whole organs was first introduced by Ott et al.33 in 2008 with the aim to develop an acellular heart from mice. This pioneering work entailed the removal of cellular material while preserving the vascular network, ECM composition, and 3D architecture of native tissue. The preservation of those physiologic features allowed the functional engraftment of cardiomyocytes with restoration of contractility. The perfusion protocol employed by Ott and colleagues was characterized by retrograde coronary perfusion at constant pressure. Afterward, Uygun et al.46 adapted this protocol to develop the first whole‐organ rodent liver scaffold. In this case, investigators used an antegrade perfusion through the portal vein at a constant flow rate and were able to obtain a translucent acellular tissue within several days. Subsequently, several protocols have been developed to obtain nonhuman liver scaffolds.47, 48, 49, 50 The resulting 3D ECM scaffolds have been shown to provide an excellent environment for the in vitro growth of multiple liver cell types retaining excellent functionality.51, 52 Notably, in 2010, the repopulation of an acellular rat liver scaffold with 50 million mature rat hepatocytes was achieved by cell perfusion through the portal vein. Importantly, hepatocytes migrated beyond the matrix barrier to reach the decellularized sinusoidal spaces.46 In 2012, a further step onward was made with the repopulation of a pig liver scaffold with human fetal hepatocytes and stem cells.53 Shortly after, larger size livers were decellularized, including ferret in 201154 and porcine in 2012.53 Although the use of xenogeneic livers is widely discussed and proposed as a base for applications ranging from transplantation to tissue engineering, there is concern about the relevant differences in the 3D architecture when compared to human liver, in addition to biocompatibility and immunogenicity issues. In particular, the differences in the vascular structure between human liver and liver obtained from other species may lead to hemodynamic consequences incompatible with the preservation of the transplanted engineered liver tissue. Indeed, the ideal biomaterials for liver tissue engineering should be derived from human liver. The first successful decellularization of a human liver (left lobe and whole organ) was achieved by our research group in 201534 by using a novel retrograde, two‐step, perfusion flow‐rate methodology able to preserve the fine 3D hepatic architecture and the liver ECM biochemical composition as confirmed by scanning electron microscopy and proteomic analysis, respectively.
To date, the only published work on whole liver engineering has been based on perfusion decellularization–recellularization strategies, with no recorded work on whole liver reconstruction with synthetic or biological polymers. There are several advantages in using decellularized organs as a platform for whole liver engineering; the use of the decellularized liver bioscaffold provides not only a 3D‐vascularized scaffold for nutrient delivery but also retains the environmental cues necessary for progenitor hepatic and endothelial cells to grow, differentiate, and maintain functionality.55, 56, 57
The three major obstacles to be addressed to produce large‐volume bioengineered tissues and organs are (i) the selection of appropriate cell types, (ii) the route of cell administration, and (iii) the cell‐seeding protocol. Uygun et al.46 achieved for the first time the recellularization of a whole rat liver scaffold by perfusing rat hepatocytes through the portal vein. This work highlighted key limitations, such as (i) a slow flow rate is unable to spread the hepatocytes deep into the liver lobes and a fast flow rate would cause the hepatocytes to aggregate, thereby obstructing the vessels; (ii) once transplanted in the experimental animal, the bioengineered liver was rejected as a result of extensive liver intravascular thrombosis.
To further investigate the efficiency of cell seeding into the liver scaffolds, Soto‐Gutierrez et al.52 evaluated three different methods to reintroduce adult mouse hepatocytes into a decellularized rat liver: (i) direct parenchymal injection, (ii) continuous perfusion, and (iii) multistep infusion. All three methods used a total of 10 million to 50 million cells and a slow perfusion rate of 2 mL/minute. After extensive evaluation of the integrity, attachment, function, and distribution of engrafted cells, it was found that the multistep infusion technique presented the most suitable results. However, these studies highlighted the fundamental need of providing an adequate re‐endothelization before reseeding the scaffolds with hepatocytes. Indeed, when exposed to the systemic circulation, repopulated scaffolds missing an appropriate endothelial lining are prone to thrombosis induced by platelet activation due to exposure to the basement membrane.
In an attempt to provide an answer to the key questions raised by previous studies and to better understand the role of re‐endothelization on decellularized liver scaffolds, Baptista et al.54 reported the engraftment of fetal liver cells cocultured with human umbilical cord endothelial cells in decellularized ferret liver scaffolds and the key importance of the direction of perfusion flow in the localization of endothelial cells within the liver. Cells seeded through the portal vein (i.e., by antegrade perfusion) were distributed throughout the liver microcirculation while cells seeded through the vena cava (i.e., retrograde perfusion) were found to be localized predominantly in large‐ and medium‐caliber vessels throughout the liver.
Strategies involving heparinized scaffolds have also been tested to reduce posttransplantation thrombosis. Bao et al.58 treated decellularized rat livers with heparin using a layer‐by‐layer self‐assembly technique prior to hepatocyte seeding. Ex vivo perfusion with whole blood showed reduced platelet activation and adhesion in heparin‐treated bioengineered livers, with a consequent reduction of thrombotic events.
In addition to the need for an endothelial lining, increasing evidence suggests that repopulation also with other nonparenchymal liver cells could improve the functionality of the bioengineered ECM scaffold. In this direction, Barakat et al.53 successfully improved the engraftment of hepatocytes by coculturing them with hepatic stellate cells in porcine livers. In this study, human fetal stellate cells seeded 1‐2 days prior to human fetal hepatocytes actively produced fibronectin, which assisted hepatocyte engraftment within the liver parenchyma.
Another critical component of native livers is the biliary tree. It is estimated that a healthy human liver produces 750 mL of bile daily, the majority of which is secreted by hepatocytes.59 Efforts to address this aspect were first addressed by Baptista et al.54 who showed the ability of decellularized livers to support the differentiation of fetal hepatoblasts into biliary and hepatocytic lineages. The fetal liver cells were seeded through the portal vein and vena cava but showed no accurate distribution of the various differentiated cells to the correct location within the liver lobules. Moreover, Ogiso et al.60 demonstrated the existence of organ‐specific cell–ECM communication, which promotes the maturation of engrafted fetal hepatocytes into both hepatocyte and cholangiocyte lineages, without the addition of any prodifferentiation signals. In addition, it was found that using the biliary tree to seed the fetal hepatocytes resulted in a more accurate distribution of differentiated cells as well as an enhanced distribution of hepatocytes into the parenchyma compared to seeding through the vena cava.
Overall, the work so far performed has increased our awareness on the challenges we are facing to translate a truly functional bioengineered liver into clinic. In addition to these challenges, one key aspect that still needs to be answered is the enormous number of cells needed. A hepatic function below 30% of normal is hardly compatible with life. Accordingly, an average human of 70 kg would need approximately 84 billion hepatocytes to achieve at least 30% liver function.61 Although many groups have attempted to overcome this problem by using fetal liver cells or stem cells (Table 1), the production of such enormous numbers of hepatocytes is still far from our technical capability.
Table 1.
REPRESENTIVE WHOLE LIVER RECELLULARIZATION TECHNIQUES
| Authors | Year | Species | Cell Source(s) | Recellularization Techniques | In Vitro Culture | In Vivo Transplantation |
|---|---|---|---|---|---|---|
| Uygun et al.46 | 2010 | Rat | 2 × 107 adult rat hepatocytes | 4‐step infusion through the PV | 7 days | 8 hours |
| Bao et al.58 | 2011 | Rat | 1 × 108 adult rat hepatocyte spheroids | 1‐step infusion through the PV | 0.25 days | 72 hours |
| Baptista et al.54 | 2011 | Ferret | 7 × 107 human fetal liver cells + 3 × 107 human umbilical vein endothelial cells | Co‐infusion through the PV over a period of 16 hours | 7 days | ‐ |
| Gutierrez et al.52 | 2011 | Mouse | 10‐50 × 106 mouse hepatocytes | Direct PIs vs. continuous perfusion vs. multistep perfusion through the PV | 7 days | ‐ |
| Barakat et al.53 | 2012 | Pig | 3.5 × 108 human fetal stellate cells + 1 × 109 human fetal hepatocytes | 1‐step infusion through the PV | 13 days | ‐ |
| Yagi et al.70 | 2013 | Pig | 1 × 109 porcine hepatocytes | 3‐step infusion through the PV | 7 days | ‐ |
| Kadota et al.71 | 2014 | Rat | 5 × 107 rat hepatocytes + 1 × 107 bone marrow‐derived rat MSCs | 3‐step co‐infusion through the PV | 6 days | 1 hour |
| Jiang et al.72 | 2014 | Mouse | 5 × 107 bone marrow‐derived mouse MSCs | 5‐step infusion through the PV | 28 days | ‐ |
| Navarro‐Tableros et al.73 | 2015 | Mouse | 0.8‐1 × 108 adult human liver stem‐like cells | 4‐step infusion through the PV, IVC, SVC, CD | 21 days | ‐ |
| Ko et al.74 | 2015 | Pig | 5 × 107 mouse vascular endothelial cells expressing GFP protein (MS1) | 1‐step infusion through the PV | 3 days | 24 hours |
| Bruinsma et al.75 | 2015 | Rat | 8 × 107 adult rat hepatocytes | 4‐step direct PIs | 5 days | 24 hours |
| Zhou at al.76 | 2016 | Rat | 2 ×107 rat normal liver cell line (BRL) + 5 × 106 endothelial progenitor cells | 10‐step direct PIs + 1‐step PV perfusion | 7 days | ‐ |
| Park et al.77 | 2016 | Mouse | 2 × 107 porcine iPSC‐Heps | 4‐step infusion through the PV | 5 days | 1‐8 hours |
| Hussein et al.78 | 2016 | Pig | 4.5 × 108 human liver hepatoblastoma (HepG2) + 3.5 × 108 and 1.5 × 108 human endothelial cell line | 3‐step infusion through the PV + 1‐step PV and HA perfusion | 10 days | 1 hour |
| Ogiso et al.60 | 2016 | Rat | 6 × 106 mouse fetal hepatocytes | 1‐step infusion through the BD | 7 days | ‐ |
| Wen et al.79 | 2016 | Mouse | 2 × 106 mouse hepatocytes | 4‐step infusion through the PV | 7 days | ‐ |
Abbreviations: BD, bile duct; BRL, CD, cystic duct; GFP, green fluorescent protein; IVC, inferior vena cava; MSC, murine stem cell; PI, parenchymal injections; PV, portal vein; SVC, superior vena cava.
Conclusions
Liver tissue engineering is a fast growing field with the ambitious goal of shaping the field of hepatology and liver transplant. Several technical standards to achieve have been identified and are summarized in Fig. 2. The preclinical work performed in recent years is showing promising results, and the advancement in the biotechnology of bioreactors, ex vivo perfusion machines, and cell expansion systems associated with a better understanding of liver development and the ECM environment will facilitate and expedite the move of tissue engineering technologies in clinic.
Figure 2.
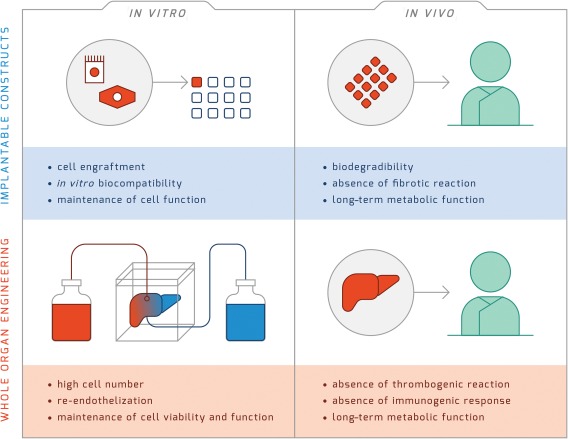
Technical standards for liver tissue engineering. In vitro cell engraftment, biocompatibility, and maintenance of cell function are the key requisites for the clinical use of implantable liver constructs. After in vivo implantation, engineered constructs need long‐term maintenance of their metabolic function associated with biodegradability and absence of fibrotic reaction. The whole‐organ engineering approach presents more challenges compared to implantable constructs. Indeed, this approach requires a high cell number for recellularization, extensive or complete re‐endothelization, and maintenance of cell viability and function. In addition, before the engineered tissue can be proposed for clinical use, preclinical studies need to demonstrate the absence of thrombogenic reaction and the absence of an immunogenic response in addition to the long‐term maintenance of metabolic function.
More favorable funding routes should be implemented at the academic level for researchers working in the field of regenerative medicine. Tissue engineering research and regenerative medicine research is currently underfunded, receiving less than $500 million annually in the United States compared to $5 billion for cancer and $2.8 billion for human immunodeficiency virus/acquired immune deficiency syndrome.62 Similarly, biotechnology companies active in the field of tissue engineering are facing several difficulties in bringing forward tissue‐engineered products toward market authorization because of the large costs for production and challenges to scaling up the production.63, 64, 65
The regulatory framework of tissue engineering and regenerative medicine products is continuously moving toward a more favorable environment, allowing the rapid commercialization of innovative medical products and for improved access for patients in need. Expedited‐approval pathways and programs, priority review, or programs alternative to the standard review processes for medical products have been developed, and legislation has been enacted by the U.S. Food and Drug Administration and the European Medicines Agency.66, 67 Recently, the Japanese government reformed its pharmaceutical affairs legislation and created a new regulation called the Pharmaceuticals, Medical Devices, and Other Therapeutic Products Act in November 2014.68 The new Act introduces conditional and time‐limited approval for regenerative medicine products, which are still in early phase of clinical trial (i.e., safety data confirmed). Along this line, one product has already been granted conditional and time‐limited authorization based on the probable benefit that was demonstrated by pilot clinical trial data.69
Potential conflict of interest: Dr. Mazza consults and owns stock in Engitix and consults for Promethera; he owns stock in 3P‐Sense. Dr. Pinzani consults and owns stock in Engitix and consults for NeuroVive; he owns stock in 3P‐Sense. Dr. Al‐Akkad consults and owns stock in Engitix. Dr Rombouts consults and owns stocks in Engitix Ltd.
Supported by the National Institute for Health Research, University College London Biomedical Research Centre (to G.M.) and a Ph.D. studentship from The Royal Free Charity and The Fiorina Foundation (to W.A.A.). Funding was also provided by Innovate UK (to K.R.).
REFERENCES
- 1. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197‐2223. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448‐457. [DOI] [PubMed] [Google Scholar]
- 3. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: Global Burden of Disease Study. Lancet 1997;349:1498‐1504. [DOI] [PubMed] [Google Scholar]
- 4. Dutkowski P, Oberkofler CE, Bechir M, Mullhaupt B, Geier A, Raptis DA, et al. The model for end‐stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl 2011;17:674‐684. [DOI] [PubMed] [Google Scholar]
- 5. Attia M, Silva MA, Mirza DF. The marginal liver donor‐‐an update. Transpl Int 2008;21:713‐724. [DOI] [PubMed] [Google Scholar]
- 6. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol 2010;7:288‐298. [DOI] [PubMed] [Google Scholar]
- 7. Mazza G, De Coppi P, Gissen P, Pinzani M. Hepatic regenerative medicine. J Hepatol 2015;63:523‐524. [DOI] [PubMed] [Google Scholar]
- 8. Selden C, Spearman CW, Kahn D, Miller M, Figaji A, Erro E, et al. Evaluation of encapsulated liver cell spheroids in a fluidised‐bed bioartificial liver for treatment of ischaemic acute liver failure in pigs in a translational setting. PLoS One 2013;8:e82312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van de Kerkhove MP, Hoekstra R, Chamuleau RA, van Gulik TM. Clinical application of bioartificial liver support systems. Ann Surg 2004;240:216‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology 2001;34:447‐455. [DOI] [PubMed] [Google Scholar]
- 11. Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. Cell therapies for liver diseases. Liver Transpl 2012;18:9‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler‐Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 1998;338:1422‐1426. [DOI] [PubMed] [Google Scholar]
- 13. Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, et al. Hepatocyte transplantation in a 4‐year‐old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow‐up. Transplantation 2003;76:735‐738. [DOI] [PubMed] [Google Scholar]
- 14. Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med 2012;4:160rv12. [DOI] [PubMed] [Google Scholar]
- 15. Lee SW, Wang X, Chowdhury NR, Roy‐Chowdhury J. Hepatocyte transplantation: state of the art and strategies for overcoming existing hurdles. Ann Hepatol 2004;3:48‐53. [PubMed] [Google Scholar]
- 16. Jain E, Damania A, Kumar A. Biomaterials for liver tissue engineering. Hepatol Int 2014;8:185‐197. [DOI] [PubMed] [Google Scholar]
- 17. O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today 2011;14:88‐95. [Google Scholar]
- 18. Orive G, Santos E, Poncelet D, Hernández RM, Pedraz JL, Wahlberg LU, et al. Cell encapsulation: technical and clinical advances. Trends Pharmacol Sci;36:537‐546. [DOI] [PubMed] [Google Scholar]
- 19. Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TM, De Vos P, et al. Cell encapsulation: promise and progress. Nat Med 2003;9:104‐107. [DOI] [PubMed] [Google Scholar]
- 20. Jitraruch S, Dhawan A, Hughes RD, Filippi C, Soong D, Philippeos C, et al. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One 2014;9:e113609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song W, Lu Y‐C, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell‐derived hepatocytes in immunocompetent mice via 3D co‐aggregation and encapsulation. Sci Rep 2015;5:16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thevenot PT, Baker DW, Weng H, Sun M‐W, Tang L. The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials. Biomaterials 2011;32:8394‐8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, et al. Size‐ and shape‐dependent foreign body immune response to materials implanted in rodents and non‐human primates. Nat Mater 2015;14:643‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci U S A 2012;109:5022‐5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler HP, et al. Fibroblast growth factor‐1 (FGF‐1) loaded microbeads enhance local capillary neovascularization. J Surg Res 2010;160:208‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech 2014;32:773‐785. [DOI] [PubMed] [Google Scholar]
- 27. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion‐based bioprinting. Biomaterials 2016;76:321‐343. [DOI] [PubMed] [Google Scholar]
- 28. Jeon H, Kang K, Park SA, Kim WD, Paik SS, Lee S‐H, et al. Generation of multilayered 3D structures of HepG2 cells using a bio‐printing technique. Gut Liver 2017;11:121‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci 2012;37:106‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan Y, Wang X, Xiong Z, Liu H, Liu F, Liu F, et al. Direct construction of a three‐dimensional structure with cells and hydrogel. J Bioact Compat Polym 2005;20:259‐269. [Google Scholar]
- 31. Wang X, Yan Y, Pan Y, Xiong Z, Liu H, Cheng J, et al. Generation of three‐dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng 2006;12:83‐90. [DOI] [PubMed] [Google Scholar]
- 32. Kang K, Kim Y, Lee SB, Kim JS, Park S, Kim WD, et al. Three‐dimensional bio‐printing of hepatic structures with direct‐converted hepatocyte‐like cells. Tissue Eng Part A 2017; doi: 10.1089/ten.TEA.2017.0161. [DOI] [PubMed] [Google Scholar]
- 33. Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion‐decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008;14:213‐221. [DOI] [PubMed] [Google Scholar]
- 34. Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al‐Akkad W, et al. Decellularized human liver as a natural 3D‐scaffold for liver bioengineering and transplantation. Sci Rep 2015;5:13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazza G, Al‐Akkad W, Telese A, Longato L, Urbani L, Robinson B, et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation‐decellularization. Sci Rep 2017;7:5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee H, Han W, Kim H, Ha D‐H, Jang J, Kim BS, et al. Development of liver decellularized extracellular matrix bioink for three‐dimensional cell printing‐based liver tissue engineering. Biomacromolecules 2017;18:1229‐1237. [DOI] [PubMed] [Google Scholar]
- 37. Liu Tsang V, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J 2007;21:790‐801. [DOI] [PubMed] [Google Scholar]
- 38. Rimann M, Graf‐Hausner U. Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol 2012;23:803‐809. [DOI] [PubMed] [Google Scholar]
- 39. Miranda JP, Rodrigues A, Tostoes RM, Leite S, Zimmerman H, Carrondo MJ, et al. Extending hepatocyte functionality for drug‐testing applications using high‐viscosity alginate‐encapsulated three‐dimensional cultures in bioreactors. Tissue Eng Part C Methods 2010;16:1223‐1232. [DOI] [PubMed] [Google Scholar]
- 40. Sharma NS, Nagrath D, Yarmush ML. Adipocyte‐derived basement membrane extract with biological activity: applications in hepatocyte functional augmentation in vitro. FASEB J 2010;24:2364‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee JS, Shin J, Park H‐M, Kim Y‐G, Kim B‐G, Oh J‐W, et al. Liver extracellular matrix providing dual functions of two‐dimensional substrate coating and three‐dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 2014;15:206‐218. [DOI] [PubMed] [Google Scholar]
- 42. Zhou P, Lessa N, Estrada DC, Severson EB, Lingala S, Zern MA, et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transpl 2011;17:418‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg 2012;29:6‐17. [DOI] [PubMed] [Google Scholar]
- 44. Uygun BE, Yarmush ML, Uygun K. Application of whole‐organ tissue engineering in hepatology. Nat Rev Gastroenterol Hepatol 2012;9:738‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mazza G, Al‐Akkad W, Rombouts K. Engineering in vitro models of hepatofibrogenesis. Adv Drug Deliv Rev 2017; 121:147‐157. [DOI] [PubMed] [Google Scholar]
- 46. Uygun BE, Soto‐Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010;16:814‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren H, Shi X, Tao L, Xiao J, Han B, Zhang Y, et al. Evaluation of two decellularization methods in the development of a whole‐organ decellularized rat liver scaffold. Liver Int 2013;33:448‐458. [DOI] [PubMed] [Google Scholar]
- 48. Pan MX, Hu PY, Cheng Y, Cai LQ, Rao XH, Wang Y, et al. An efficient method for decellularization of the rat liver. J Formos Med Assoc 2014;113:680‐687. [DOI] [PubMed] [Google Scholar]
- 49. Nari GA, Cid M, Comin R, Reyna L, Juri G, Taborda R, et al. Preparation of a three‐dimensional extracellular matrix by decellularization of rabbit livers. Rev Esp Enferm Dig 2013;105:138‐143. [DOI] [PubMed] [Google Scholar]
- 50. Kajbafzadeh AM, Javan‐Farazmand N, Monajemzadeh M, Baghayee A. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human‐sized liver tissue. Tissue Eng Part C Methods 2013;19:642‐651. [DOI] [PubMed] [Google Scholar]
- 51. Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, et al. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue‐specific biomatrix scaffolds. Hepatology 2011;53:293‐305. [DOI] [PubMed] [Google Scholar]
- 52. Soto‐Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, et al. A whole‐organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 2011;17:677‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barakat O, Abbasi S, Rodriguez G, Rios J, Wood RP, Ozaki C, et al. Use of decellularized porcine liver for engineering humanized liver organ. J Surg Res 2012;173:e11‐e25. [DOI] [PubMed] [Google Scholar]
- 54. Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011;53:604‐617. [DOI] [PubMed] [Google Scholar]
- 55. Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development 2003;130:2513‐2524. [DOI] [PubMed] [Google Scholar]
- 56. McClelland R, Wauthier E, Uronis J, Reid L. Gradients in the liver's extracellular matrix chemistry from periportal to pericentral zones: influence on human hepatic progenitors. Tissue Eng Part A 2008;14:59‐70. [DOI] [PubMed] [Google Scholar]
- 57. Brown SE, Guzelian CP, Schuetz E, Quattrochi LC, Kleinman HK, Guzelian PS. Critical role of extracellular matrix on induction by phenobarbital of cytochrome P450 2B1/2 in primary cultures of adult rat hepatocytes. Lab Invest 1995;73:818‐827. [PubMed] [Google Scholar]
- 58. Bao J, Shi Y, Sun H, Yin X, Yang R, Li L, et al. Construction of a portal implantable functional tissue‐engineered liver using perfusion‐decellularized matrix and hepatocytes in rats. Cell Transplant 2011;20:753‐766. [DOI] [PubMed] [Google Scholar]
- 59. Boyer JL, Bloomer JR. Canalicular bile secretion in man. Studies utilizing the biliary clearance of (14C)mannitol. J Clin Invest 1974;54:773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ogiso S, Yasuchika K, Fukumitsu K, Ishii T, Kojima H, Miyauchi Y, et al. Efficient recellularisation of decellularized whole‐liver grafts using biliary tree and foetal hepatocytes. Sci Rep 2016;6:35887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sussman NL, Kelly JH. Artificial liver: a forthcoming attraction. Hepatology 1993;17:1163‐1164. [DOI] [PubMed] [Google Scholar]
- 62. Moses H 3rd, Matheson DH, Cairns‐Smith S, George BP, Palisch C, Dorsey ER. The anatomy of medical research: US and international comparisons. JAMA 2015;313:174‐189. [DOI] [PubMed] [Google Scholar]
- 63. Hunsberger J, Harrysson O, Shirwaiker R, Starly B, Wysk R, Cohen P, et al. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl Med 2015;4:130‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Williams DJ, Sebastine IM. Tissue engineering and regenerative medicine: manufacturing challenges. IEE Proc Nanobiotechnol 2005;152:207‐210. [DOI] [PubMed] [Google Scholar]
- 65. Bertram TA, Tentoff E, Johnson PC, Tawil B, Van Dyke M, Hellman KB. Hurdles in tissue engineering/regenerative medicine product commercialization: a pilot survey of governmental funding agencies and the financial industry. Tissue Eng Part A 2012;18:2187‐2194. [DOI] [PubMed] [Google Scholar]
- 66. Jokura Y, Yano K, Yamato M. Comparison of the new Japanese legislation for expedited approval of regenerative medicine products with the existing systems in the USA and European Union. J Tissue Eng Regen Med 2017; doi: 10.1002/term.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bubela T, McCabe C, Archibald P, Atkins H, Bradshaw SE, Kefalas P, et al. Bringing regenerative medicines to the clinic: the future for regulation and reimbursement. Regen Med 2015;10:897‐911. [DOI] [PubMed] [Google Scholar]
- 68. Hara A, Sato D, Sahara Y. New Governmental Regulatory System for Stem Cell–Based Therapies in Japan. Ther Innov Regul Sci 2014;48:681‐688. [DOI] [PubMed] [Google Scholar]
- 69. Barzel A, Paulk NK, Shi Y, Huang Y, Chu K, Zhang F, et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 2015;517:360‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H, et al. Human‐scale whole‐organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 2013;22:231‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kadota Y, Yagi H, Inomata K, Matsubara K, Hibi T, Abe Y, et al. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014;10:268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiang W‐C, Cheng Y‐H, Yen M‐H, Chang Y, Yang VW, Lee OK. Cryo‐chemical decellularization of the whole liver for mesenchymal stem cells‐based functional hepatic tissue engineering. Biomaterials 2014;35:3607‐3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Navarro‐Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A 2015;21:1929‐1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ko IK, Peng L, Peloso A, Smith CJ, Dhal A, Deegan DB, et al. Bioengineered transplantable porcine livers with re‐endothelialized vasculature. Biomaterials 2015;40:72‐79. [DOI] [PubMed] [Google Scholar]
- 75. Bruinsma BG, Kim Y, Berendsen TA, Ozer S, Yarmush ML, Uygun BE. Layer‐by‐layer heparinization of decellularized liver matrices to reduce thrombogenicity of tissue engineered grafts. J Clin Transl Res 2015;1.pii:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou P, Huang Y, Guo Y, Wang L, Ling C, Guo Q, et al. Decellularization and recellularization of rat livers with hepatocytes and endothelial progenitor cells. Artif Organs 2016;40:E25‐E38. [DOI] [PubMed] [Google Scholar]
- 77. Park KM, Hussein KH, Hong SH, Ahn C, Yang SR, Park SM, et al. Decellularized liver extracellular matrix as promising tools for transplantable bioengineered liver promotes hepatic lineage commitments of induced pluripotent stem cells. Tissue Eng Part A 2016;22:449‐460. [DOI] [PubMed] [Google Scholar]
- 78. Hussein KH, Park KM, Kang KS, Woo HM. Heparin‐gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater 2016;38:82‐93. [DOI] [PubMed] [Google Scholar]
- 79. Wen X, Huan H, Wang X, Chen X, Wu L, Zhang Y, et al. Sympathetic neurotransmitters promote the process of recellularization in decellularized liver matrix via activating the IL‐6/Stat3 pathway. Biomed Mater 2016;11:065007. [DOI] [PubMed] [Google Scholar]


