Abstract
Lung is one of the most common sites for bladder cancer to metastasize. Although the involvement of the epithelial-to-mesenchymal transition (EMT) in bladder cancer progression has been established, the mechanism of EMT induction remains unclear. In order to investigate this, T24-parental (P) and T24-lung (L) bladder cancer cells were obtained from primary tumors and lung metastatic sites of an animal model with orthotopic spontaneous metastatic bladder cancer, according to a protocol previously described. Compared with T24-P cells, mesenchymal-like T24-L cells exhibited an increased ability in tumor invasion and metastasis, as well as an increased expression of hypoxia-inducible factor (HIF)-1α, zinc finger E-box-binding homeobox 1 (ZEB1), vimentin and N-cadherin and lower level of cytokeratin 18 were observed. Mechanistically, it was identified that HIF-1α increases ZEB1 expression and subsequently regulates the expression of EMT-related genes in both HIF-1α knocking down by siRNA and gain-in HIF-1α by hypoxia culture cell models. In addition, the expression of HIF-1α and ZEB1 in bladder cancer tissues were increased compared with normal bladder epithelial tissues, as well as significantly increased in the high-grade, invasive and metastatic bladder cancer tissues compared with low-grade, superficial and non-metastatic bladder cancer tissues by using immune-histochemical staining assay. Notably, the protein level of HIF-1α was positively associated with that of ZEB1 in bladder cancer tissues. Results from the present study indicate that HIF-1α promotes ZEB1 expression and EMT in the T24-L human bladder cancer lung metastasis animal model, suggesting that HIF-1α serves an important function in the metastasis of bladder cancer, and HIF-1α and ZEB1 may be potential targets for inhibiting bladder metastasis in the future.
Keywords: bladder cancer, epithelial-to-mesenchymal transition, metastasis
Introduction
Urothelial carcinoma of the bladder (UCB) is the fifth most common malignancy worldwide (1). In total, ~70–80% of patients diagnosed with well-differentiated or moderately-differentiated non-muscle invasive bladder cancer (2). Despite treatment, cancer recurs in 60–70% of patients, of which, 10–30% eventually develop muscle-invasive bladder cancer or metastatic bladder cancer. The lung is one of the most common metastatic sites and is associated with a high frequency of recurrence and mortality, which contributes to a poor prognosis for patients (3,4). Therefore, to develop better therapeutic strategies and decrease the morbidity and mortality associated with bladder cancer, it is imperative to clarify the mechanism of bladder cancer invasion and metastasis.
Clinical investigations have indicated hypoxia to be a common feature of most solid tumors (5), owing to rapid proliferation of cancer cells and/or compression of tumor blood vessels. As a cancer progresses, cancer cells acquire the ability to adapt to hypoxic environments while also resist to apoptosis, increase angiogenesis, enhance the invasive and metastatic potential which makes them more aggressive. A previous study suggests that one of the key factors regulating the response to hypoxia is the heterodimer hypoxia-inducible factor-1 (HIF-1) (6). Under hypoxic conditions, the alpha subunit is not destroyed, and will activate transcription more than 100 gene products that take part in the tumor aggressiveness (6). Its expression is associated to an increased metastatic potential that has been demonstrated in both animal models and human tumors by promotes a perpetual epithelial to mesenchymal transition (EMT) (7).
The transcription factor Zinc-finger E-box-binding homeobox 1 (ZEB1) is a known driver of EMT, and our previous reports (8) confirmed that ZEB1 is an important regulatory factor of bladder cancer invasion and metastasis in vitro and in vivo. Furthermore, ZEB1 high expression is closely associated with markers associated with invasion and metastasis in clinical tumor specimens (9). However, little is known about the association between HIF-1α and ZEB1 protein in bladder cancer, and whether there is an interaction between HIF-1α and ZEB1 in the process of invasion and metastasis of bladder cancer.
To address these issues, an orthotopic animal model was established by injecting human bladder cancer T24-tumorigenic (T24-t) cells into a mouse bladder. Subsequently, the primary tumor and lung metastases were excised and plated on tissue culture dishes with G418 (400 µg/ml) to derive the sublines T24-parental (T24-P) and T24-t-lung (T24-L), which were outlined in our previous study (10,11). In the present study, the previously described sublines T24-P and T24-L, were used to investigate the role of downstream gene regulation of HIF-1α and EMT by mimicking human bladder cancer metastasis (10). In addition, the molecular mechanisms of the lung metastasis of bladder cancer were explored, focusing on the effect of HIF-1α expression changes in bladder cancer cells on ZEB1 expression, and the invasion and metastasis of bladder cancer cells.
Materials and methods
Human tissue specimens
All the paraformaldehyde-fixed and paraffin-embedded primary bladder cancer tissues (n=79) and adjacent histologically normal tissues (n=11) were obtained from the Department of Urology, The First Affiliated Hospital of Xi'an Jiaotong University, (Xi'an, China). All the tissues were either obtained from transurethral resection of bladder tumor (TURBT) or Radical cystectomy between 2,006.1 and 2,011.9. The histopathology of the specimens was examined and classified by pathologists of Medical School, Xi'an Jiaotong University. Sixty-six patients were men and thirteen were women. Mean patient age was 63 years (range, 35–82 years). Bladder carcinomas were staged according to the tumor-node-metastasis system based on the International Union against Cancer (12). Genitourinary pathologists determined tumor stage as: Ta (n=1); T1 (n=41); T2 (n=17); T3 (n=17); T 4 (n=3), and according to World Health Organization (1973) standard for pathological grade (13): grade I (n=26), grade II (n=32), grade III (n=21). This study was approved by the Committee for the Protection of Human Subjects of the First Affiliated Hospital of Xi'an Jiaotong University, and informed consent was obtained from all patients.
Reagents and antibodies
A HIF-1α siRNA transfection kit was purchased from Shanghai GenePharma Co, Ltd (Shanghai, China). Matrigel was purchased from BD Transduction Laboratories (Franklin Lakes, NJ, USA). Antibodies used for western blot were as follows: mouse monoclonal antibodies to cytokeratin18 (1:1,000; cat. no. 4546; Cell Signaling Technology, Beverly, MA, USA), goat polyclonal antibody to Vimentin (1:500; cat. no. sc-7557; Santa Cruz Biotechnology, Santa Cruz, CA, USA), GAPDH (1:1,2000; cat. no. KC-5G4; Kang Chen Bio-technology, Shanghai, China), rabbit polyclonal antibody to N-cadherin (1:1,000; cat. no. 13116; Cell Signaling Technology), HIF-1α (1:1,000; cat. no. ab113642; Abcam, Cambridge, UK) and rabbit monoclonal antibody to ZEB1 (1:1,000; cat. no. 3396; Cell Signaling Technology). Rabbit polyclonal antibody to HIF-1α for immunohistochemical staining (1:500; cat. no. 04-006; Millipore Corporation, Billerica, MA, USA), rabbit monoclonal antibody to ZEB1 for immunohistochemical staining (1:500; cat. no. A301-922A; Bethyl Laboratories, Montgomery, TX, USA) were used.
Immunohistochemical (IHC) staining
The standard two-step Envision method of IHC staining was used to assess the expression of HIF-1α and ZEB1. Briefly, 5 µm sections were deparaffinized, rehydrated and subjected to antigen retrieval in citrate buffer (10 mM, pH 6.0) for 5 min at high temperature (121°C), and then endogenous peroxidase and alkaline phosphatase activity were and blocked by incubating in 0.3% H2O2 for 30 min. Slides were then incubated overnight at 4°C with IHC-specific HIF-1α and ZEB1 antibodies (dilution, 1:200) in a moist chamber. Following a wash with PBS, the slides were incubated with horseradish peroxidase-labelled anti-rabbit (dilution, 1:100; cat. no. K4002; Dako; Agilent Technologies, Inc.) for 30 min at room temperature. Rinsed with PBS, signals were detected by adding substrate hydrogen peroxide using diaminobenzidine as a chromogen followed by hematoxylin counterstaining, dehydrated, air-dried, and mounted. Negative control slices were prepared by omitting the primary antibody. HIF-1α and ZEB1 expression in human TCC tumors was semiquantitatively evaluated according to the intensity of the staining (0, 1+, 2+ and 3+) and the percentage of positive cells [0 (≤10%), 1 (10–25%), 2 (25–50%), 3 (50–75%) and 4 (≥75%)]. The staining result was considered higher expression when intensity was 2+ or 3+ and the percentage category was 2–4, and lower expression when intensity was 0 or 1+, or if intensity was >1 and the percentage category was 0 or 1. All sections were evaluated blindly by 2 of the authors.
Cell line and cell culture
All components for cell culture were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). T24-parental (P) and T24-lung (L) bladder cancer cells were obtained from primary tumors and lung metastases according to a protocol previously described (10,11). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific Inc.) and 400 mg/l G418 in a humidified incubator with 5% CO2, 95% air at 37°C. While, for mimicking the hypoxia conditions, the cells were cultured in the atmosphere with 1% O2 and 99% N2 in 37°C (10,11).
Small interfering RNA (siRNA) transfection
The sequence of siRNA for HIF-1α was as follows: Sense 5′-CCAGCAGACUCAAAUACAATT-3′, antisense 5′-UUGUAUUUGAGUCUGCUGGTT-3′ (Shanghai GenePharma, Shanghai, China). A total of 5×105 cells were seeded in a 6-well plate and grown to 70–80% confluence prior to transfection. Cells were transfected, according to the manufacturer's protocol, with oligonucleotide duplexes (200 nM) premixed with Oligofectamine (Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM-I (Invitrogen; Thermo Fisher Scientific, Inc.) for 4 h. Cells were treated with oligofectamine and scrambled siRNA served as a negative control (NC-siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense, 5′-ACGUGACACGUUCGGAGAATT-3′; Dharmacon, Lafayette, CO, USA). Transfection studies were performed in duplicates according to the manufacturer's protocol.
RNA extraction and quantitative RT-PCR
Total cellular RNA was extracted using the Highly Pure RNA Isolation kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the protocol provided by the manufacturer, and was quantified by absorbance at 260 nm. Total RNA (2 µg) was reverse transcribed using a Revert Aid™ First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Primers for the amplification of HIF-1α and ZEB1 were constructed with the following sequences: HIF-1α forward, 5′-TCAAAGTCGGACAGC-CTCA-3′, reverse, 5′-CCCTGCAGTAGGTTTCT-GCT-3′, 460 bp product; ZEB1 forward, 5′-TTCAAACCCATAGTGGTTGCT-3′, reverse, 5′-TGGGAGATACCAAACCAACTG-3′, 151 bp product; β-actin forward, 5′-ATCATGTTTGAGACCTTCAACA-3′, reverse, 5′-CATCTCTTGCTCGAAGTCCA-3′, 318 bp product.
For qPCR, the SYBR Premix Ex Taq™ II system (Takara Biotechnology Co., Ltd., Dalian, China) was used with the CFX96TM Real-time system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction mix tubes contained SYBR Premix Ex Taq II (12.5 µl), 1 µl primer (10 µM,), 200 ng cDNA and 9.5 µl ddH2O. There was one cycle of pre-degeneration at 95°C for 30 sec, then 35 repeats of 95°C for 5 sec followed by 60°C for 30 sec, then a final stage of 95°C for 15 sec followed by 60°C for 30 sec, and 15 sec at 95°C. β-actin was used as an internal control. All experiments were repeated at least twice in duplicate.
Invasion and migration assays
The invasion and migration capability of cells in vitro was determined using a Boyden chamber assay. For the invasion assay, 50 µl of Matrigel was applied to 8 µm pore polycarbonate membrane filters (BD Biosciences, San Jose, CA, USA) in a 24-well plate, and allowed to solidify overnight. Then, 5×104 cells, detached using trypsin-EDTA and resuspended in 200 µl FBS-free DMEM were added to the upper chamber, and 800 µl FBS-free DMEM was added to the lower chamber. Subsequent to incubating the plates at 37°C in 5% CO2 for 24 h, cells on top of the membrane were scraped away using a cotton swab. Cells at the bottom surface of the membrane were fixed with 4% paraformaldehyde for 10 min, stained with crystal violet solution (0.01% in the ethanol) for 15 min at room temperature, then washed three times with PBS (pH 7.4). The cells were counted using an inverted microscope; 5 fields of view were randomly selected at ×100 magnification, and the mean number of cells was determined. For determining cell migration, the Boyden chamber assay was performed as described above, without Matrigel. Presented data are representative of three individual wells.
Wound healing assay
Cells were cultured to a monolayer of 100% confluence in a 6-well plate and washed three times with PBS (pH 7.4) to remove residual FBS. Subsequent to incubating the cells at 37°C in 5% CO2 for 12 h with FBS-free DMEM, the plate was scratched to remove a 400–450 µm strip of cells across the well with a standard 200 µl pipette tip. Wounded monolayers were washed twice with PBS to remove non-adherent cells, and the cells were incubated at 37°C in 5% CO2. The width of the scratches was photographed and measured at 0, 6 and 12 h after scratching. Presented data are representative of three individual wells.
Protein extraction and western blot analysis
Cells were harvested at 70–80% confluence and washed with 4°C PBS three times. Total cellular protein lysates were prepared with radio immunoprecipitation assay buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% NP40 and 0.5% sodium deoxycholate] containing proteinase inhibitors 1% cocktail and 1 mM PMSF, (Sigma Aldrich; Merck KGaA, Darmstadt, Germany). A total of 20–40 µg of protein (Pierce bicinchoninic acid assay protein assay kit; cat. no. 23225; Thermo Fisher Scientific, Inc.) was separated by SDS-PAGE (10% gel) and transferred to nitrocellulose membranes. Following blocking at room temperature for 1 h with 5% skimmed milk in TBS (pH 7.6), the membranes were incubated with primary antibodies (HIF-1α, ZEB1 and N-Cadherin, 1:1,000; CK18, Vimentin, 1:300; GAPDH, 1:15,000) at 4°C overnight, then washed with TBST with Tween-20 (pH 7.6). Membranes were incubated with goat anti-rabbit (1:5,000; cat. no. A0545; Sigma Aldrich; Merck KGaA) or anti-mouse secondary antibody (1:30,000; cat. no. A5278; Sigma Aldrich; Merck KGaA) coupled to the first antibody at room temperature in the dark for 1 h, followed by washing as above in the dark, drying with neutral absorbent paper and scanning by Odyssey detection system (LI-COR Biosciences, Lincoln, NE, USA). MG-132 (Sigma Aldrich, Merck KGaA) was used to inhibit proteasome-dependent degradation if necessary (10 µM, 4 h prior to protein harvesting). Loading differences were normalized using a monoclonal GAPDH antibody.
Statistical analysis
All statistical analyses were performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Quantitative data are presented as the mean ± standard deviation from ≥3 independent experiments. The statistical significance of differences among multiple groups was one-way analysis of variance followed by Bonferroni's multiple comparisons test. When the comparison involved only 2 groups, a 2-sided Student's t-test was used. IHC statistical analysis was performed with the χ2 test. The analysis of HIF-1α and ZEB1 association was performed using Spearman's correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of HIF-1α and ZEB1 in human bladder cancer tissues and normal bladder tissues
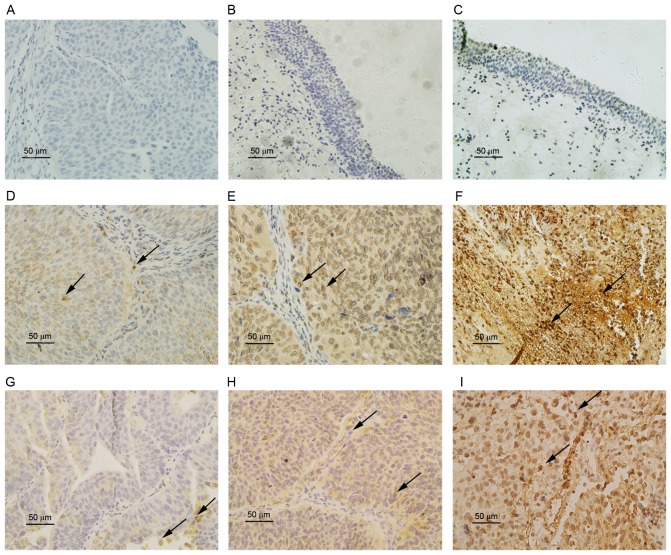
To investigate the association between the expression of HIF-1α and ZEB1 in bladder carcinoma, IHC staining for HIF-1α and ZEB1 was performed on 79 UCB tissues and 11 normal bladder epithelial control tissues. The staining results (Fig. 1) indicated that HIF-1α and ZEB1 expression occurred in the cytoplasm and nucleolus in bladder cancer tissues. The expression of HIF-1α (P=0.005) and ZEB1 (P=0.007) in UCB tissues was significantly higher than in normal bladder epithelium (Table I). The expression score and rate of HIF-1α and ZEB1 were also significantly higher in the high-grade, invasive and metastatic UCB than in low-grade, superficial and non-metastatic UCB (P<0.05). However, a Mann-Whitney U test identified no significant differences (P>0.05) in HIF-1α and ZEB1 expression levels according to age (<60 and ≥60 years) and sex (Table II).
Figure 1.
Representative immunohistochemical staining images of (A) bladder cancer negative staining control; (B) normal epithelium stained for HIF-1α; (C) normal epithelium stained for ZEB1; grade (D) G1, (E) G2 and (F) G3 tumor tissue stained for HIF-1α; grade (G) G1, (H) G2 and (I) G3 tumor tissue stained for ZEB1. Certain positively stained cells are indicated by black arrows. Scale bar, 50 µm. HIF-1α, hypoxia inducible factor-1α; ZEB1, zinc-finger E-box binding homeobox 1.
Table I.
HIF-1α and ZEB1 expression in bladder tumor and normal tissues as determined with immunohistochemistry analysis.
| HIF-1α expression score | ZEB1 expression score | |||||
|---|---|---|---|---|---|---|
| Tissue type | Mean | SD | P-value | Mean | SD | P-value |
| Tumor | 3.75 | 0.44 | 0.005 | 2.81 | 0.39 | 0.007 |
| Normal | 2.02 | 0.81 | 2.01 | 0.79 | ||
HIF-1α, hypoxia inducible factor-1α; ZEB1, zinc-finger E-box-binding homeobox 1; SD, standard deviation.
Table II.
Association between the IHC expression of HIF-1α and ZEB1 with clinical characteristics.
| HIF-1α expression | ZEB1 expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | n | (−), n | (+), n | R value | P-value | (−), n | (+), n | R value | P-value |
| Age | −0.143 | 0.825 | −0.117 | 0.753 | |||||
| ≤60 | 31 | 14 | 17 | 16 | 15 | ||||
| >60 | 48 | 18 | 30 | 24 | 24 | ||||
| Sex | −0.132 | 0.439 | 0.115 | 0.557 | |||||
| Male | 66 | 26 | 40 | 36 | 30 | ||||
| Female | 13 | 6 | 7 | 4 | 9 | ||||
| Grade | 0.462 | 0.003 | 0.381 | 0.004 | |||||
| G1 | 26 | 21 | 5 | 22 | 4 | ||||
| G2-3 | 53 | 11 | 42 | 18 | 35 | ||||
| Stage | 0.387 | 0.002 | 0.452 | <0.001 | |||||
| Ta-1 | 42 | 30 | 12 | 32 | 10 | ||||
| T2-4 | 37 | 2 | 35 | 8 | 29 | ||||
| Lymphatic metastasis | 0.213 | <0.001 | 0.314 | 0.003 | |||||
| Yes | 10 | 0 | 10 | 1 | 9 | ||||
| No | 69 | 32 | 37 | 39 | 30 | ||||
HIF-1α, hypoxia inducible factor-1α; ZEB1, zinc-finger E-box-binding homeobox 1.
In the 79 UCB tissues, the positive expression rate of HIF-1α was 59.49% (47/79), and that of ZEB1 was 49.37% (36/79). The positive expression rate of ZEB1 was 76.6% (36/47) in HIF-1α positive expression group. In the HIF-1α-negative group, the ZEB1 positive expression rate was 9.38% (3/32). Spearman's rank correlation analysis indicated a significant positive association between the expression of HIF-1α and ZEB1 (r=0.337, P<0.05, Table II).
HIF-1α promotes bladder cancer cell migration and invasion in vitro
It was reported in our previous study that the T24-P and T24-L sublines had a similar growth rate; however, the growth rate of orthotopic and metastatic xenograft bladder tumors of T24-L cells was slightly higher than that of T24-P cells. In addition, the incidences of distant metastasis observed in the T24-P and T24-L group were 9 and 36%, respectively.
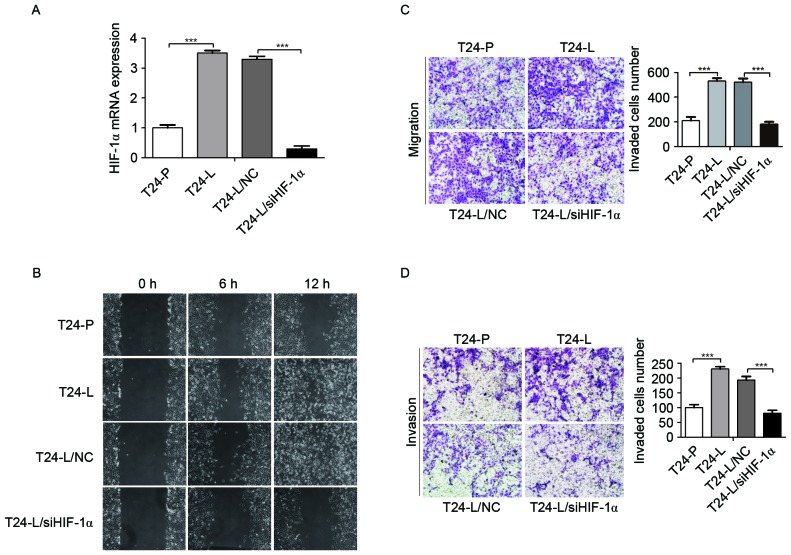
HIF-1α is known to serve an important role in tumor metastasis. To identify the roles of HIF-1α in bladder cancer invasion and metastasis, the expression of HIF-1α in T24-P and T24-L cells was examined; it was identified that HIF-1α mRNA level was higher in T24-L cells than that in T24-P cells using an RT-qPCR assay (Fig. 2A). In addition, to further identify the function of HIF-1α in T24-L cells, HIF-1α expression was knocked down by transfecting HIF-1α and scrambled siRNA into T24-L cells. As demonstrated in Fig. 2A, the HIF-1α expression level was significantly reduced by transfection with siRNA against HIF-1α compared with the negative control group in T24-L cells, as determined by RT-qPCR analysis (P<0.001).
Figure 2.
Knockdown of HIF-1α by siRNA reduces the malignancy of T24-L cells. (A) Reverse transcriptase-quantitative polymerase chain reaction was used to demonstrate the expression of HIF-1α in T24-L and T24-P, and the efficiency of siRNA against HIF-1α in T24-L. The expression of HIF-1α is significantly increased in T24-L. ***P<0.001 si HIF-1α vs. NC. (B) Wound-healing assay to demonstrate that T24-L cells exhibited higher migration activity than T24-P cells, and T24-L control cells exhibited higher migration activity compared with HIF-1α knockdown T24-L cells. Boyden chamber assays to demonstrate that (C) the migration and (D) the migration and invasion ability of T24-L cells was greater compared with T24-P cells, and that HIF-1α knockdown impaired the migration and invasion capacity of T24-L cells. Magnification, ×100. ***P<0.001. HIF-1α, hypoxia inducible factor-1α; siRNA, small interfering RNA; T24-L, T24 lung metastasis cells; T24-P, T24 parental cells; NC, negative control.
To determine whether HIF-1α knockdown affects the migration and/or invasion of bladder cancer cell lines, wound-healing and transwell assays were performed. It was identified that the migration and invasion ability of T24-L cells were greater than T24-P cells (Fig. 2B, C and D; P<0.001); in addition, HIF-1α knockdown by siRNA significantly reduced the cell motility and invasion of T24-L cells (P<0.001). These results indicated that HIF-1α promoted the migration and invasion of bladder cancer cells.
HIF-1α increases ZEB1 expression and promotes EMT in bladder cancer cells
EMT may be a crucial step in the initiation of the metastatic spread of tumor cells into distal organs (7); ZEB1 may be a key driver of bladder cancer invasion and metastasis (14). Therefore, it was examined whether the expression of HIF-1α modulates ZEB1 expression or the process of EMT in bladder cancer cells. As demonstrated in Fig. 3A, ZEB1 mRNA expression in T24-L cells was significantly higher than that in T24-P cells, as detected by RT-qPCR, and significantly reduced in T24-L cells transfected with HIF-1α siRNA. Furthermore, as determined by western blot analysis, HIF-1 α, ZEB1, N-cadherin and vimentin protein expression was higher in T24-L cells than in T24-P cells, whereas cytokeratin-18 protein level was lower in T24-L cells. In T24-L cells transfected with HIF-1α siRNA, HIF-1α, ZEB1, N-cadherin and vimentin protein was downregulated whereas the expression of cytokeratin-18 was upregulated (Fig. 3B). These results indicate that HIF-1α expression increased the expression of ZEB1 and promoted the process of EMT in bladder cancer cells.
Figure 3.
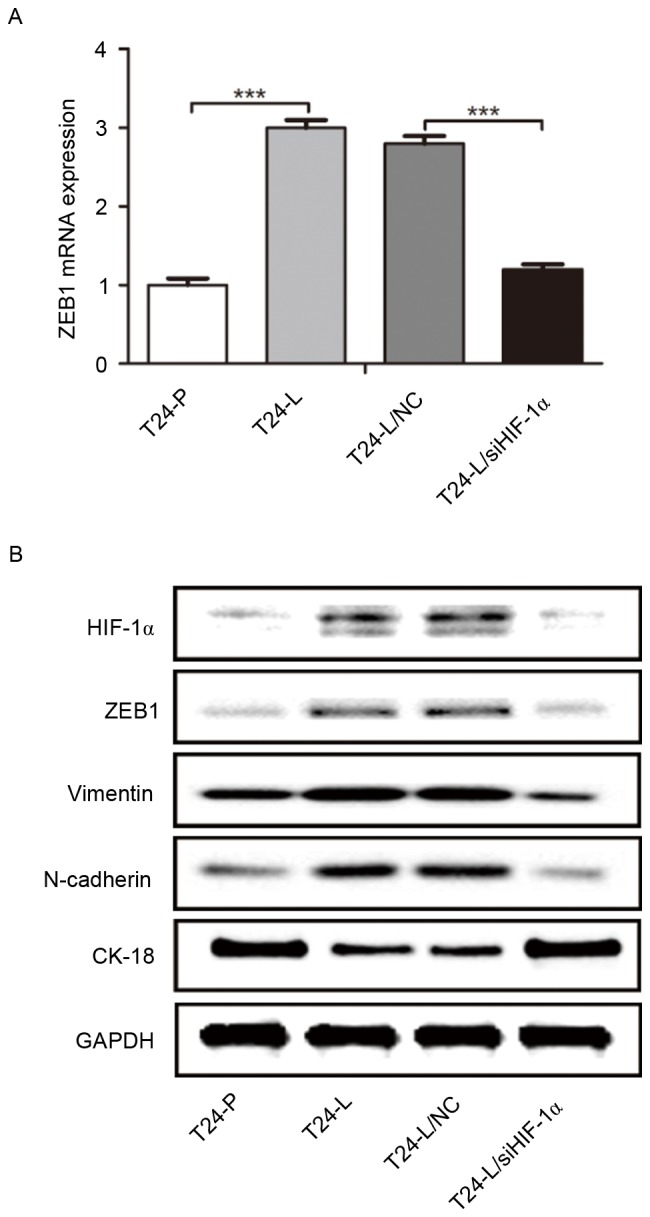
ZEB1 expression is regulated by HIF-1α, and promotes epithelial-mesenchymal transition. (A) Reverse transcription-quantitative polymerase chain reaction was used to determine the level of ZEB1 mRNA expression in T24-P/T24-L/T24-NC/T24-L/siHIF-1α. ZEB1 was significantly elevated in T24-L, and transfection with siHIF-1α downregulated ZEB1 expression in T24-L cells, compared with the control. (B) Western blot analysis indicated that the protein level of HIF-1α, ZEB1, vimentin and N-cadherin was higher in T24-L cells than T24-P cells, whereas CK-18 expression was lower in T24-L cells. siHIF-1α transfection in T24-L led to the downregulation of ZEB1, Vimentin and N-Cadherin, but the upregulation of CK-18. ***P<0.001. ZEB1, zinc-finger E-box-binding homeobox 1; HIF-1α, hypoxia inducible factor-1α; T24-L, T24 lung metastasis cells; siHIF-1α, small interfering RNA against HIF-1α; T24-P, T24 parental cells; CK-18, cytokeratin-18; NC, negative control.
It was then determined whether the induction of HIF-1α expression by hypoxia also regulated ZEB1 expression. As displayed in Fig. 4, hypoxia significantly increased the mRNA level of HIF-1α and ZEB1 in T24-P cells, as detected by RT-qPCR. Furthermore, hypoxia increased the protein expression of HIF-1α, ZEB1, N-cadherin and vimentin in T24-P cells, whereas cytokeratin18 protein expression was reduced, as identified by western blot analysis. These results confirm that HIF-1α can increase the expression of ZEB1 and suggest that hypoxia promotes cell migration and invasion through HIF-1α and ZEB1 expression in bladder cancer.
Figure 4.
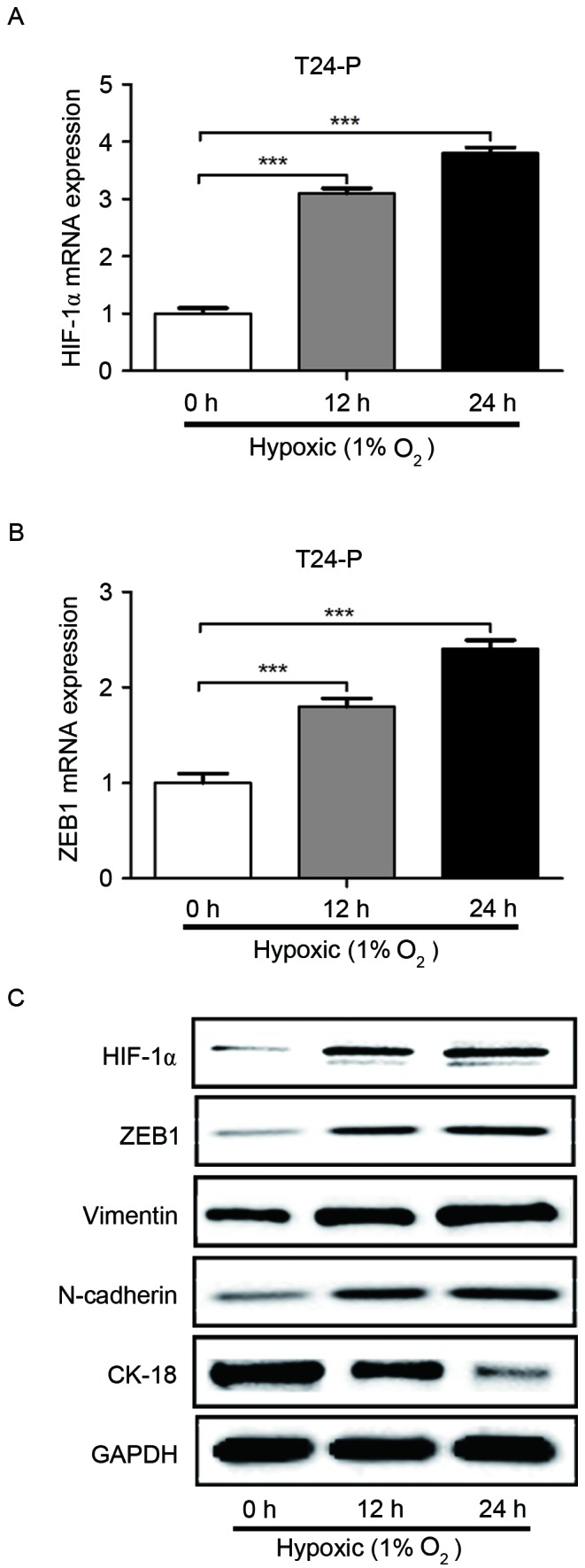
Hypoxia increases the expression of HIF-1α and ZEB1 in T24-P cells, and promotes the process of epithelial-mesenchymal transition. Induced hypoxia increased the expression of (A) HIF-1α and (B) ZEB1 in T24-P cells. (C) Western blotting revealed that HIF-1α, ZEB1, N-cadherin and vimentin were upregulated when T24-P cells were grown in hypoxic conditions, but that CK-18 was downregulated. ***P<0.001. HIF-1α, hypoxia inducible factor-1α; ZEB1, zinc-finger E-box-binding homeobox 1; T24-P, T24 parental cells; CK-18, cytokeratin-18.
Discussion
Owing to incomplete blood vessel networks and the imbalance between proliferation and angiogenesis, hypoxia is a common feature of the microenvironment in various solid tumors, including bladder cancer (15,16). Hypoxia serves a critical role in various cellular and physiologic events, including cell proliferation, survival, angiogenesis (17), immune-surveillance, metabolism, and tumor invasion and metastasis, and it is often associated with a poor prognosis (18). Investigating the biology of tumor cells in hypoxic conditions may be important for improving therapeutic efficacy and for eradication of cancer. Hypoxia activates relevant gene expression through HIFs, transcription factors from a family that includes HIF-1, HIF-2 and HIF-3. HIF is a heterodimer composed of an alpha and a beta subunit, in which the HIF-1α protein is a master regulator of the hypoxic response in bladder urothelial carcinomas (6). HIF-1α may be associated with clinicopathological parameters with prognostic value, including tumor stage, grade, proliferation index and microvessel density (19). Our previous study provided novel insights into the mechanism of HIF-1α/matrix metalloproteinase-1 in the process of distant metastasis of bladder cancer, offering a potential therapeutic target for metastatic bladder cancer therapy (10). In the present study, it was identified that HIF-1α promotes cell migration and cell invasion, and that hypoxia may induce EMT through HIF-1α in bladder cancer, suggesting that HIF-1α serves an important role in bladder cancer metastasis.
As a potent suppressor of epithelial marker, transcription factor ZEB1 is one of the key inducers of EMT; its expression promotes the tumorigenesis and metastasis of various types of carcinoma (20). Our previous study (21) revealed a novel mechanism facilitating metastatic bladder cancer cell re-colonization into bone, in which phosphoinositide 3-kinase/Akt targeted glycogen synthase kinase 3β/β-catenin and regulated the expression of ZEB1. Furthermore, the results provided a molecular and clinicopathological basis for the role of ZEB1 in bladder cancer invasion and metastasis. Therefore, ZEB1 could be a potential prognostic marker and a drug target for muscle-invasive or metastatic bladder cancer (21).
It was previously reported that dysregulated HIF-1 activity resulting from VHL loss of function in RCC4 cells induces the expression of multiple known repressors of CDH1 (ZEB1) gene transcription directly or indirectly, so as to promote tumor progression, invasion and metastasis (17). However, there is limited data regarding the association between HIF-1α and ZEB1 protein in bladder cancer tissue, and whether there is interaction between HIF-1α and ZEB1 in the process of invasion and metastasis of bladder cancer. In the present study, the expression of HIF-1α and ZEB1 in bladder transitional cell carcinoma tissues were significantly increased compared with normal bladder epithelium tissues. Furthermore, the expression score and rate of both HIF-1α and ZEB1 were significantly higher in the high-grade, invasive and metastatic bladder cancer compared with low-grade, superficial and non-metastatic bladder cancer (P<0.05) and expression of both proteins were positively associated with each other in bladder carcinoma tissues. To our knowledge, this is the first study involved in the association between HIF-1α and ZEB1 protein expression in bladder cancer tissue.
To further investigate the interaction between HIF-1 α and ZEB1 in the process of invasion and metastasis of bladder cancer, a novel model of TCC metastasis was adopted, consisting of two isogenetic T24-t sublines, T24-P and T24-L we adopt a novel model of bladder cancer metastasis consisting of two isogenetic T24-t sublines, designated T24-P and T24-L, generated through successive in vivo passaging and in vitro subculture (11).
The sublines were previously demonstrated to exhibit a similar in vitro growth rate, and share several of the same numerical and structural abnormalities of karyotypes. Cytogenetic evaluation of T24-P cells (having the same molecular features of bladder cancer in situ) and T24-L cells (having the same molecular features with cells of bladder cancer lung metastasis) revealed that these cell lines are indeed associated; however, exhibit specific cytogenetic abnormalities (10,11). For example, T24-L cells exhibited more invasive and metastatic abilities than orthotopic T24-P cells in vitro; the T24-L subline acquired more mesenchymal-like characteristics and the T24-P subline was more epithelial-like.
Consistently, in the present study, HIF-1α, ZEB1, vimentin, and N-cadherin expression were higher, but cytokeratin-18 expression was lower in T24-L cells compared with T24-P cells. Furthermore, knockdown endogenous HIF-1α expression significantly downregulated the expression of ZEB1, accompanied with a decrease in invasive and metastatic ability in vitro and EMT-related protein changes in T24-L cells. When T24-P cells were cultured under hypoxic conditions, HIF-1α expression was induced responsively; meanwhile, simultaneously, the expression level of ZEB1, N-cadherin, and vimentin increased, and the expression level of cytokeratin 18 decreased. The data from the present study suggests that the promotion of EMT by HIF-1α-mediated induction of ZEB1 may serve a crucial role in the process of lung metastasis.
In the present study, it was identified that the expression of HIF-1α and ZEB1 were associated with each other in bladder cancer tissue. Furthermore, HIF-1α expression increased the expression of ZEB1 and promoted EMT, cell migration and invasion in a bladder cancer cell spontaneous lung metastases model. These results indicate that HIF-1α serves an important role in the metastasis of bladder cancer, and that HIF-1α and ZEB1 may be potential targets for inhibiting bladder metastasis in the future.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81572520), the Key Science and Technology Program of Shaanxi Province, China (grant no. 2015SF176), and the Long-term Scheduled Medical Student Research Fund (grant no. 15YB05; The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. N Engl J Med. 1990;322:1129–1138. doi: 10.1056/NEJM199004193221607. [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–825. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kurian A, Lee J, Born A. Urothelial bladder cancer with cavitary lung metastases. Can Respir J. 2011;18:e46–e47. doi: 10.1155/2011/273241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–111. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Difazio LT, Rolandelli RH, Luján JJ, Haskó G, Csóka B, Selmeczy Z, Németh ZH. HIF-1: A key mediator in hypoxia. Acta Physiol Hung. 2009;96:19–28. doi: 10.1556/APhysiol.96.2009.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, Zhang L, Chen Y, Gao Y, Wang B, et al. Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial-mesenchymal transition and stemness. Cell Signal. 2013;25:2625–2633. doi: 10.1016/j.cellsig.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Kenney PA, Wszolek MF, Rieger-Christ KM, Neto BS, Gould JJ, Harty NJ, Mosquera JM, Zeheb R, Loda M, Darling DS, et al. Novel ZEB1 expression in bladder tumorigenesis. BJU Int. 2011;107:656–663. doi: 10.1111/j.1464-410X.2010.09489.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Fan J, Wu K, Zeng J, Sun K, Guan Z, Wang X, Hiesh JT, He D. Roles of HIF-1α in a novel optical orthotopic spontaneous metastatic bladder cancer animal model. Urol Oncol. 2012;30:928–935. doi: 10.1016/j.urolonc.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Karam JA, Huang S, Fan J, Stanfield J, Schultz RA, Pong RC, Sun X, Mason RP, Xie XJ, Niu G, et al. Upregulation of TRAG3 gene in urothelial carcinoma of the bladder. Int J Cancer. 2011;128:2823–2832. doi: 10.1002/ijc.25631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A, European Association of Urology EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Schned AR, Andrew AS, Marsit CJ, Kelsey KT, Zens MS, Karagas MR. Histological classification and stage of newly diagnosed bladder cancer in a population-based study from the Northeastern United States. Scand J Urol Nephrol. 2008;42:237–242. doi: 10.1080/00365590801948166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai T, Yamada S, Fuchs BC, Fujii T, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK, Kodera Y. Epithelial-to-mesenchymal transition predicts prognosis in clinical gastric cancer. J Surg Oncol. 2014;109:684–689. doi: 10.1002/jso.23564. [DOI] [PubMed] [Google Scholar]
- 15.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 16.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- 18.Rohwer N, Lobitz S, Daskalow K, Jöns T, Vieth M, Schlag PM, Kemmner W, Wiedenmann B, Cramer T, Höcker M. HIF-1alpha determines the metastatic potential of gastric cancer cells. Br J Cancer. 2009;100:772–781. doi: 10.1038/sj.bjc.6604919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deniz H, Karakök M, Yagci F, Güldür ME. Evaluation of relationship between HIF-1alpha immunoreactivity and stage, grade, angiogenic profile and proliferative index in bladder urothelial carcinomas. Int Urol Nephrol. 2010;42:103–107. doi: 10.1007/s11255-009-9590-5. [DOI] [PubMed] [Google Scholar]
- 20.Meng X, Kong DH, Li N, Zong ZH, Liu BQ, Du ZX, Guan Y, Cao L, Wang HQ. Knockdown of BAG3 induces epithelial-mesenchymal transition in thyroid cancer cells through ZEB1 activation. Cell Death Dis. 2014;5:e1092. doi: 10.1038/cddis.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K, Fan J, Zhang L, Ning Z, Zeng J, Zhou J, Li L, Chen Y, Zhang T, Wang X, et al. PI3K/Akt to GSK3β/β-catenin signaling cascade coordinates cell colonization for bladder cancer bone metastasis through regulating ZEB1 transcription. Cell Signal. 2012;24:2273–2282. doi: 10.1016/j.cellsig.2012.08.004. [DOI] [PubMed] [Google Scholar]