Dear Sir
Greenhawt et al. performed a secondary analysis of the Learning Early About Peanut Allergy (LEAP) dataset and questioned the recent ‘Addendum Guidelines for the Prevention of Peanut Allergy in the United States’ (1–3). The Addendum recommends early introduction of peanut-containing foods, starting as early as 4–6 months for infants with severe eczema, egg allergy, or both. Their analysis, which we believe to be flawed, concludes that in the LEAP study, a successful oral food challenge (i.e., peanut allergy prevention) was significantly more likely with peanut introduction between 6 and 11 months as compared to 4–6 months of age.
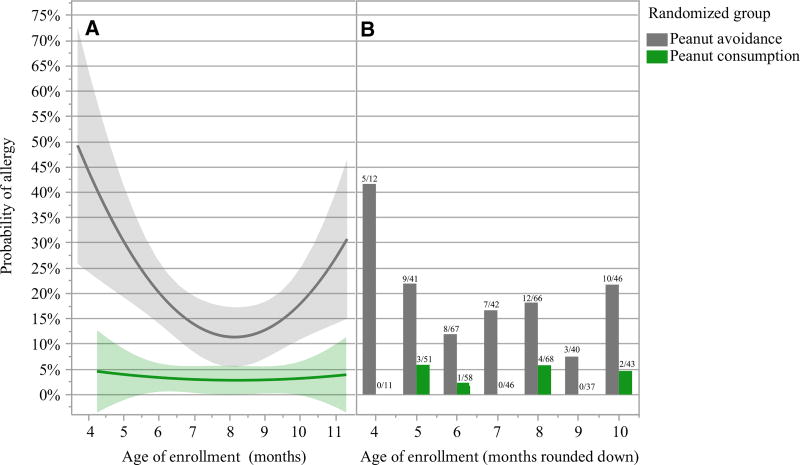
Greenhawt et al. analyzed LEAP data, aggregating both study groups, and found a quadratic relationship between age of enrollment and peanut allergy at 60 months of age. This serves as the basis for their conclusion that peanut allergy prevention was more likely with introduction of peanut beginning at 6 months. However, when similarly analyzed, but separated by study group (Fig. 1, panel A), we see no evidence for an age effect in the peanut consumption group or a lessening of the treatment effect with younger age. Furthermore, the quadratic relationship seen in the avoidance group appears to be driven primarily by a high proportion of subjects who became allergic among the small number of subjects enrolled at 4 months (Fig. 1, panel B). Greenhawt et al. do not consider an alternative interpretation that subjects 4–6 months at enrollment had characteristics putting them at greater risk for allergy, not that efficacy of dietary peanut was less.
Figure 1.
A logistic regression model using a quadratic effect for age is shown in panel (A) for each randomized group (top line, gray for peanut avoidance, bottom line, green for peanut consumption). The actual data are provided as bar charts in panel (B), and the fraction of allergic to total participants is annotated above each bar (left bars, gray for peanut avoidance, right bars, green for peanut consumption). The y-axis displays predicted probabilities for the regression model and the proportion allergic for the bar charts.
The raw data are at odds with the Greenhawt et al. conclusion that introducing peanut prior to 6 months is less efficacious. In infants enrolled before 6 months, 26% of the avoidance group developed peanut allergy vs 5% of the consumption group—an 82% relative reduction in the rate of peanut allergy (OR = 7.06). Considering infants 6 months or older at enrollment, 15% of those in the avoidance group developed peanut allergy vs 3% of those in the consumption group, also an 82% relative reduction (OR = 6.33).
The LEAP study did not enroll a 4-month-old cohort and randomize to begin peanut consumption immediately vs after a delay in treatment, as would be necessary to draw definitive conclusions on the optimal time to introduce peanut. Hence, the best available approach is to determine whether a treatment-by-age interaction (not presented by Greenhawt et al.) is detected within the LEAP dataset.
Accordingly, we fit an additional model: OFC outcome = Treatment + Age + Treatment*Age. The interaction term has a P-value = 0.8 indicating no evidence that the treatment effect differs by age, so that the conclusion of Greenhawt et al. is unsubstantiated. Apart from the lack of an interaction test, we have concerns about the statistical models presented in this publication. The standard for a logistic regression model requires there be ≥10 cases for each covariate. As there were 64 cases of peanut allergy in LEAP, the model of Greenhawt et al., which includes 11 variables, risks overfitting. Considering that several of these variables are correlated, this model is also subject to an elevated risk of false-positive results. Additionally, the authors did not transform the egg- and peanut-specific IgE (sIgE) values as would be appropriate. We ran the model with log-transformed sIgE values and found this substantially changes the results presented in Greenhawt’s table 2a: Race, SCORAD, Egg sIgE (log10), Age, and Age*Age are no longer significant; Peanut sIgE (log10) is now significant (OR = 0.5, P = 0.001); and treatment and peanut wheal size remain significant.
Apart from these statistical concerns, the authors did not discuss the significance of progression of SPT sensitization with age. The LEAP screening data (4) (and unpublished analyses) show a positive correlation between age of enrollment and SPT diameter, arguing that beyond a certain age the therapeutic window of opportunity may have already closed for some children. A further rationale for peanut introduction at 4–6 months for high-risk infants arises from the fact that well-child care visits occur in this interval, but less frequently later in infancy (5). Peanut introduction at this age proved feasible in LEAP and did not affect the duration of breast-feeding nor impact negatively on growth or nutrition (6).
In summary, the LEAP study did not find that peanut introduction between 6 and 11 months of age, as compared to 4–6 months, is more efficacious in preventing peanut allergy.
Footnotes
Conflicts of interest
The authors have no conflict of interest to disclose.
References
- 1.Greenhawt M, Fleischer D, Chan ES, Venter C, Stukus D, Gupta R, et al. LEAPing through the looking glass: secondary analysis of the effect of skin test size and age of introduction on peanut tolerance after early peanut introduction. Allergy. 2016 doi: 10.1111/all.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR, Jr, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131:135–143. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 5.https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html
- 6.Feeney M, Du Toit G, Roberts G, Sayre PH, Lawson K, Bahnson HT, et al. Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition. J Allergy Clin Immunol. 2016;138:1108–1118. doi: 10.1016/j.jaci.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]