Abstract
We report on a novel autoantigen expressed in human macular tissues, identified following an initial Western blot (WB)-based screening of sera from subjects with age-related macular degeneration (AMD) for circulating auto-antibodies (AAbs) recognizing macular antigens. Immunoprecipitation, 2D-gel electrophoresis (2D-GE) and liquid chromatography-tandem mass spectrometry (LC-MS/MS), direct enzyme-linked immunosorbent assays (ELISA), WBs, immunohistochemistry (IHC), human primary and ARPE-19 immortalized cell cultures were used to characterize this novel antigen. An approximately 40-kDa autoantigen in AMD was identified as the scavenger receptor CD5 antigen-like protein (CD5L), also known as apoptosis inhibitor of macrophage (AIM). CD5L/AIM was localized to human RPE by IHC and WB methods and to retinal microglial cells by IHC. ELISAs with recombinant CD5L/AIM on a subset of AMD sera showed a nearly 2-fold higher anti-CD5L/AIM reactivity in AMD vs. control sera (p=0.000007). Reactivity ≥0.4 was associated with 18-fold higher odds of having AMD (χ2=21.42, p=0.00063). Circulating CD5L/AIM levels were also nearly 2-fold higher in AMD sera compared to controls (p=0.0052). The discovery of CD5L/AIM expression in the RPE and in retinal microglial cells adds to the known immunomodulatory roles of these cells in the retina. The discovery of AAbs recognizing CD5L/AIM identifies a possible novel disease biomarker and suggest a potential role for CD5L/AIM in the pathogenesis of AMD in situ. The possible mechanisms via which anti-CD5L/AIM AAbs may contribute to AMD pathogenesis are discussed. In particular, since CD5L is known to stimulate autophagy and to participate in oxidized LDL uptake in macrophages, we propose that anti-CD5L/AIM auto-antibodies may play a role in drusen biogenesis and inflammatory RPE damage in AMD.
Keywords: CD5L, retinal pigment epithelium, microglia, age-related macular degeneration, auto-antibody, scavenger receptor, autoimmunity
Graphical Abstract
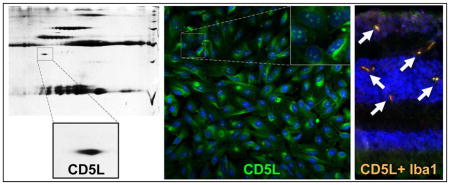
Sera of subjects with age-related macular degeneration exhibit auto-antibodies directed against an antigen that, following 2D gel and mass spectrometry experiments, was discovered to be the CD5-like (CD5L) protein, a secreted scavenger receptor known to be produced by macrophages. Immunohistochemical experiments reveal that this antigen is expressed also throughout the cytoplasm and, more discretely, at the nuclear level (inset) by human retinal pigment epithelium cell lines. Confocal immunohistochemical microscopy experiments show also that CD5L reactivity in human macular neuroretinal tissue section colocalizes with reactivity for Iba1, a known microglial specific marker.
1. Introduction
Age-related macular degeneration (AMD) is a highly prevalent, multifactorial polygenic complex disease. Increased AMD risk is associated with advanced age as well as with modifiable factors such as smoking (Age-Related Eye Disease Study Research Group, 2000). Cholesterol and oxidized lipids are well known key components of drusen (Malek et al., 2003; Curcio et al., 2005a; Curcio et al., 2005b), which represent the clinical hallmark of AMD. Over the past two decades, there has been a significant increase in the understanding of how and why the lipidic component of these deposits may accumulate underneath the retinal pigment epithelium from a histochemical, biochemical, genetic and mechanistic point of view (Curcio et al., 2001; Zareparsi et al., 2004; Li et al., 2005; Curcio et al., 2009; Chen et al., 2010; Curcio et al., 2010; Neale et al., 2010; Wang et al., 2010; Curcio et al., 2011; Yu et al., 2011a; Yu et al., 2011b; Cougnard-Gregoire et al., 2014; Oak, Messinger and Curcio, 2014; Pikuleva and Curcio, 2014).
At the same time, a role for inflammation and the immune system in the pathogenesis of AMD has also become far better appreciated, especially since the seminal studies on drusen composition by Hageman et al. (Hageman et al., 2001) and Crabb et al. (Crabb et al., 2002) paved the way to the identification of complement factor H (CFH) genetic variants associated to greater odds of having AMD (Edwards et al., 2005; Hageman et al., 2005; Haines et al., 2005; Klein et al., 2005). Since then, numerous other loci, many of which are involved with the complement pathways and regulation of inflammation and of the immune system, have been linked to increased (Montezuma, Sobrin and Seddon, 2007; Fritsche et al., 2013) as well as reduced (“protective variants”) odds of having AMD (Spencer et al., 2007a,2007b). Statistical genetic approaches to AMD have consistently confirmed the strong role of genetic factors in AMD (Swaroop et al., 2007; Hageman et al., 2011; Grassmann et al., 2012; Yu et al., 2012), yet these approaches do not achieve full discrimination of AMD vs. control samples even when environmental modifiable factors are included in the models, especially when population samples without clear-cut, preexisting advanced AMD are included in the analyses (Spencer et al., 2011). This is at least in part because there are additional layers of complexity underlying AMD pathogenesis than genetic and environmental/lifestyle factors alone that need to be investigated and taken into account (Newman et al., 2012). Among these, even though AMD certainly cannot be characterized as an autoimmune disease, a potential role for autoantibodies (AAbs) as biomarkers of AMD of possible pathogenetic relevance has also become increasingly appreciated following the first reports thereof in the 1990s (Penfold et al., 1990). For example, autoreactivity against carboxy-ethyl-pyrrole (CEP) and CEP serum levels has been strongly linked to increased odds of exudative AMD (Gu et al., 2003), CEP-modified proteins have been shown to be proangiogenic (Ebrahem et al., 2006), the disease pathogenicity of developing autoreactivity towards CEP adducts has been demonstrated in animal models (Hollyfield et al., 2008; Hollyfield, Perez and Salomon, 2010), and the combined use of genomic and serological biomarkers like CEP and anti-CEP AAbs has been shown to be a better predictor of AMD odds than genomic biomarkers alone (Gu et al., 2009).
We have recently expanded and refined studies of autoimmunity in AMD by screening serum samples from over 350 elderly subjects with and without AMD for AAbs recognizing antigens from human macular tissue lysates (Iannaccone et al., 2012). Comparative analyses by Western blot (WB) criteria identified a number of bands that are significantly more frequent and/or more intensely positive in AMD sera [Lenchik N, et al. Identification of Human Macular Tissue Antigens Recognized by Serum Auto-Antibodies (auto-Abs) in Patients with Age-Related Macular Degeneration (AMD). Invest Ophthalmol Vis Sci 2013; 54(6): E-Abstract 4103] and (Iannaccone et al., 2015). By 2D gel electrophoresis (2D-GE) and liquid chromatography-tandem mass spectrometry (LC-MS/MS), these studies have recently led us to identify conclusively five human macular autoantigens targeted by AAbs in the serum of AMD subjects that, because of their known physiological roles in ocular tissues and their altered expression levels in AMD human tissues, appear to be involved with AMD pathogenesis: two members of the HSP70 family, HSPA8 and HSPA9; HSPB4/CRYAA; Annexin A5; and Protein S100-A9/calgranulin B. By ELISA testing with recombinant proteins, we have confirmed that AMD subjects have AAbs against these specific antigens (Iannaccone et al., 2015). Herein, we present additional and novel evidence that also the secreted scavenger receptor CD5 antigen-like protein/apoptosis inhibitor of macrophage (CD5L/AIM) is physiologically expressed in the retinal pigment epithelium (RPE) and human retinal microglial cells, that CD5L/AIM is another autoantigen involved in autoreactivity in AMD, and that its circulating serum levels are also elevated in AMD. CD5L/AIM is a glycosylated, secreted protein member of the scavenger receptor cysteine-rich (SRCR) class B subfamily of proteins and is a pattern recognition receptor (PRR) known in the literature also as Spα, IgM-associated peptide, and Api6 (Sarrias et al., 2005; Li et al., 2011). Finally, we propose a hypothetical framework via which CD5L/AIM – and AAbs directed against it – may contribute to AMD pathogenesis in ways that could bring together, at least in part, the lipidic, inflammatory and immunological sides of the disease.
2. Methods
Our study was conducted with the approval of the University of Tennessee Health Science Center Institutional Review Board and in compliance with the Declaration of Helsinki. Written informed consent was obtained from each participant before enrollment.
2.1. AMD participants and human serum samples
A selection of 28 AMD samples was used for the 2D-GE to achieve antigen identification via LC-MS/MS, and 18 of these for the ELISA experiments. Subjects with AMD were 63–91 years old (mean ± SD, 77.3 ± 5.5), 64% female, and all but two participants were White. Controls (n=21 for the 2D-GE and LC-MS/MS experiments, and n=15 for the ELISA experiments) were 69–84 (75.0 ± 4.5) years old, 76% female, and all but one participant were White (please see Supplemental Methods and Supplemental Tables 1 and 2 for further details). Serum samples used in this investigation were selected out of a repository of 131 AMD subjects and 231 controls who participated in the Age-Related Maculopathy Ancillary (ARMA) Study, ancillary to the NIA-sponsored Health ABC Study. The Health ABC Study is a study of health, age, and body composition in highly functional elderly subjects conducted at two sites, Memphis and Pittsburgh. ARMA is an approved ancillary study of a subset of participants from the Memphis site that was designed to improve our understanding of the relationship between inflammation (and, in this case, autoimmunity) and nutritional factors in macular aging and ARM. Details about, and findings from the ARMA Study population have been published previously (Gallaher et al., 2007; Iannaccone et al., 2007; Spencer et al., 2011; Vishwanathan et al., 2014). To minimize confounding, all ARMA participants were chosen to be free of diabetes, rheumatologic or autoimmune diseases, any history of or ongoing inflammatory systemic or ocular disorders, glaucoma, and/or retinal diseases other than AMD. Sera were collected from all participants according to the Health ABC study protocol, as previously reported (Iannaccone et al., 2007). Additional details about patient demographics and serum collection methods are provided under Supplemental Methods and Supplemental Tables 1 and 2. The age and gender of the participants included in this sub-investigation was representative of the average age range of the entire ARMA Study population, and there was no statistically significant difference in these parameters between the 2DGE and ELISA sub-samples or between AMD and control samples (p>0.05 in all cases). All AMD cases were classified according to the 2001 AREDS classification scheme based on the original study protocol and were from AMD patients AREDS grade ≥3 (which encompasses a range of early-to-mid AMD, mainly depending on abundance of drusen) (Age-Related Eye Disease Study Research Group, 2001). All AMD subjects had at least one eye with grade-3 disease, and 16 of them had at least one eye with advanced AMD (grade 4), 10 of whom had bilateral advanced disease. All subjects meeting AREDS grading criteria 0–2 (thus, including subjects with hard drusen, which were always presented in limited fashion, and/or RPE changes but no soft drusen) were grouped in this study as controls (Age-Related Eye Disease Study Research Group, 2001). Please see the Supplemental methods section and Supplemental Tables 1 and 2 for further details on the participant population and the grading of their fundus changes.
2.2. Preparation and processing of human donor eyes, immunoprecipitation (IP), 2D gel electrophoresis (2D-GE) and liquid chromatography tandem mass spectrometry (LC-MS/MS)
Human eyes (n=17) from donors age 62–94 years old were obtained from the Mid-South Eye Bank, (Memphis, TN), the Arkansas Lions Eye Bank (Little Rock, AR), the Eye Bank for Sight Restoration (New York, NY), and the National Disease Research Interchange (NDRI, Philadelphia, PA). All donor eyes used for this investigation were from Caucasian donors, and free of AMD. Death-to-collection time for the donor eyes ranged between 3.7 and 12.0 hrs (mean ± SD: 7.79 ± 3.03 hrs). The procedures we used to prepare human donor macular tissue homogenates have been described previously [(Iannaccone et al., 2012; Iannaccone et al., 2015) and Lenchik N, et al. Identification of Human Macular Tissue Antigens Recognized by Serum Auto-Antibodies (auto-Abs) in Patients with Age-Related Macular Degeneration (AMD). Invest Ophthalmol Vis Sci 2013; 54(6): E-Abstract 4103] and are further detailed in the Supplemental Methods. Macular tissue homogenates harvested from these donor tissues were pooled, and aliquots were immunoprecipitated with AMD and control sera, separated on 2D-GE gels, stained by SYPRO-Ruby Gel Stain (BioRad) and analyzed with Progenesis software (Nonlinear USA). The 2D-GE protein spots of interest were digested with trypsin as described previously (Giorgianni et al., 2004; Pabst et al., 2008; Iannaccone et al., 2015), and the digests were analyzed with LC-MS/MS performed on an ion trap tandem mass spectrometer. Further details are provided in (Iannaccone et al., 2015) and in the Supplemental Methods section.
2.3. Estimation of serum autoreactivity by enzyme-linked immunosorbent assays (ELISA)
To confirm the reactivity of serum AAbs against the identified protein (CD5L/AIM, see results), aliquots of the serum samples used in the 2D-GE and LC-MS/MS experiments were tested at 1:500 dilution in ELISA for reactivity against recombinant CD5L/AIM protein (R&D Systems). Immobilon IV 96-well plates (Millipore) were coated with 5 μg recombinant human CD5L/AIM and probed in duplicate with 0.2 μl serum from either normal or AMD subjects for 2 hrs at room temperature. Plates were then washed and probed with 1:3,000 goat HRP-conjugated anti-human IgG secondary Ab (ThermoFisher Scientific), which does not cross react with human IgM. ELISA reactivities were measured in triplicate on all samples with a microQuant Spectrophotometer (BioTek) and expressed as optical density (OD450) values relative to background values, as previously reported (Iannaccone et al., 2015). Wells coated with purified rabbit IgG (ThermoFisher Scientific) probed as above were used as negative control (see Supplemental Methods section for additional details).
2.4. Tissue expression and localization procedures
From mouse EST and in situ hybridization data, CD5L/AIM had been reported as expressed in the eye, including the retina (Mouse Genomics Institute and NEIBank). However, no information existed on its cellular localization, or on its expression in the human retina or RPE. Thus, a series of experiments were performed using a variety of techniques to specifically confirm the expression of the CD5L/AIM protein in human macular tissues.
Since CD36, another member of the scavenger receptor superfamily and binding partner of CD5L/AIM, is known to be expressed in the RPE (Ryeom, Sparrow and Silverstein, 1996; Kauppinen et al., 2012; Niu, Zhang and McNutt, 2013), is a receptor for CD5L/AIM, and mediates CD5L/AIM endocytosis in macrophages (Kurokawa et al., 2010; Amezaga et al., 2014; Sanjurjo et al., 2015b; Sanjurjo et al., 2015c), we sought to determine whether RPE cells expressed CD5L/AIM. To test this specific hypothesis, two approaches were taken. First, total protein was extracted from two distinct primary human RPE cell lines and from ARPE-19 cells via sonication for WB use. Cells were cultured in serum free medium (SFM) to avoid potential confounding from any serum-derived CD5L/AIM. Fluorescent IHC by confocal microscopy was performed on SFM-cultured ARPE-19 cells to localize the expression of CD5L/AIM at the subcellular level. In brief, cells were probed using polyclonal rabbit anti-CD5L primary Abs raised against a 16 amino acid peptide from near the N-terminal region of human CD5L (ab45408, Abcam). Cells were washed and probed with goat anti-rabbit IgG secondary antibodies conjugated to AlexaFluor488 (LifeTechnologies, green stain). Nuclei were stained using DAPI (blue stain). Further details about the methods used for these cell culture studies are provided in the Supplemental Methods section.
Secondly, we sought to confirm CD5L/AIM expression at the mRNA level via end-point PCR from ARPE-19 cells grown in 10-mm plates until confluency. mRNA was extracted with a Qiagen kit. After purification, the mRNA concentration was measured by the nano-drop method. Before PCR amplification, the mRNA solution was treated with DNAse I (Invitrogen). The PCR amplification reaction (40 cycles) was performed using the following conditions: initial denaturation at 95°C for 5 min, 95°C for 30 s, 65°C for 30 s and 72°C for 1min. The following primer pairs were used: 5′-TGG GAC ATT AAG GAC GTG GC -3′ (forward) and 5′-CGG TCT CTG AAG GAG GGA GA -3′ (reverse) for CD5L/AIM, and 5′-ACC ACA GTC CAT GCC ATC AC -3′ (forward) and 5′-TCC ACC ACC CTG TTG CTG TA -3′ (reverse) for GAPDH. The PCR product was analyzed by agarose gel electrophoresis. After gel electrophoresis, the CD5L/AIM DNA band was cut from the gel and analyzed by DNA sequencing to confirm the expected CD5L/AIM transcript.
In addition, since CD5L/AIM is also known to be expressed by macrophages, we sought to determine if the resident immunocompetent cells of the retina, the microglia, expressed CD5L/AIM as well. To test this specific hypothesis, formalin-fixed human macular 8-μm thick cryosections collected 5 hrs. post-mortem by the National Disease Research Interchange (NDRI, Philadelphia, PA) from a 74-year old White female donor on charged slides were obtained. The donor died from cancer-related complications, and had no significant ocular history other than cataract surgery. Sections were probed with a goat polyclonal anti-CD5L/AIM antibody (Santa Cruz Biotech) and a microglial-specific marker, a rabbit polyclonal anti-Iba-1 (Wako Chemicals), followed by applying Alexa-fluor conjugated secondary antibodies, an anti-goat IgG conjugated to Alexa-Fluor 488 (green CD5L/AIM stain) and an anti-rabbit IgG conjugated to Alexa-Fluor 555 (red stain for Iba-1). Nuclei were stained with TOPRO3 (Life Technologies, blue stain). See Supplemental Methods section for further details about the methods used.
2.5. Estimation of serum CD5L levels by Western blot
Lastly, since CD5L/AIM is a secreted protein, and since elevated circulating levels of another important autoimmune biomarker of AMD, CEP, have also been reported in conjunction with elevated anti-CEP AAb titers (Gu et al., 2003; Gu et al., 2009, 2010), we performed fluorescent WBs on the sera of our participants who underwent ELISA testing for anti-CD5L AAbs to measure also the circulating levels of CD5L/AIM and test the hypothesis that the latter could be elevated in AMD sera as well. Briefly, 1.5 μL of serum was run per lane on a 15% SDS-PAGE. Electrophoretically separated proteins were then transferred to nitrocellulose. Following blocking in 3% bovine serum albumin, blots were incubated in a 1:500 of rabbit anti-CD5L/AIM (Abcam-ab45408) and goat anti-transferrin (Abcam-ab19177) to serve as a loading control followed by incubation in 1:10,000 of secondary Abs. Additional details about the methods used are provided in the Supplemental Methods section. Blots were then imaged on Odyssey Imaging System (Licor) and bands densitometrically quantitated using ImageJ software (Rasband WS, ImageJ, U. S. National Institutes of Health, Bethesda, MD, http://imagej.nih.gov/ij/, 1997–2014).
2.6. Statistical Analyses
Quantitative data are presented as mean ± 1 standard deviation (SD) of the OD450 values (ELISA for anti-CD5L AAbs) or the intensity of the WB bands (serum CD5L) quantified as above and expressed in arbitrary units (A.U.). Statistical comparisons of these reactivities were conducted by means of two-tailed, unpaired student’s t-test for equal or unequal variances, as applicable. The spot intensity on 2D-GE was evaluated automatically by the Progenesis protein quantification software by one-way ANOVA. The 2x2 contingency table analysis for ELISA reactivity yielding the χ2 value, the odds ratio (OR), and the 95% CI, sensitivity, specificity, Positive Predictive Value (PPV), estimating the probability of a true positive test result (i.e., for ELISA reactivities ≥0.4, i.e., 2SD above control reactivity) and Negative Predictive Value (NPP) estimating the probability of a true negative test result (i.e., for ELISA reactivity <0.4) were conducted with SAS statistical software (Cary, NC). The p-value for the 2x2 contingency table was estimated by Fisher exact test. An alpha level of 0.05 was used for all analyses.
3. Results
3.1. Identification of CD5L/AIM as a target of serum antibodies
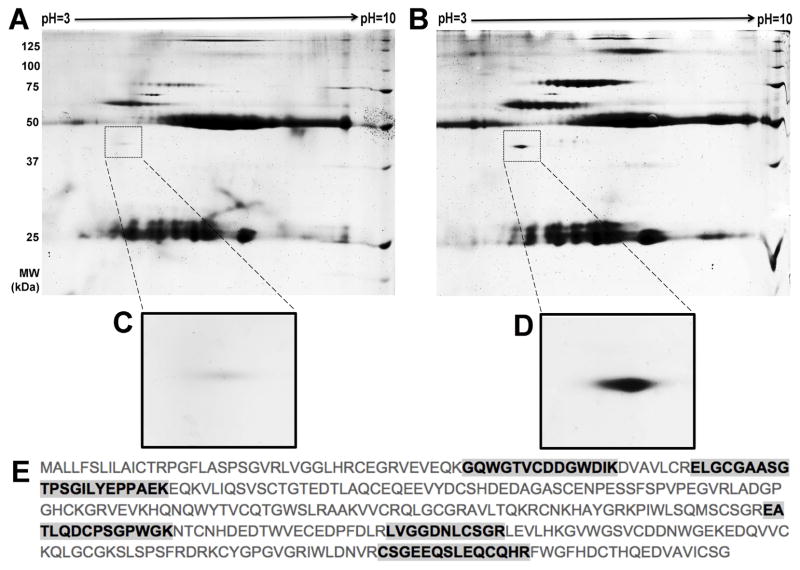
2D-GE revealed various spots from AMD sera that were more intense than those from unaffected subjects and/or uniquely present in the former (Fig. 1A, B). This is consistent with our recent observation that other AAbs exist in AMD sera (Iannaccone et al., 2015). From this analysis, we identified in multiple serum samples a specific spot that migrated at approximately 40 kDa and with an isoelectric point of approximately 5.2, which stained more intensely (by one-way ANOVA performed with Progenesis protein quantification software, p=0.041) in lysates immunoprecipitated with AMD sera. This finding is in line with our previously reported frequent and intense autoreactivity at or around 38–40kDa by WB criteria as well (Iannaccone et al., 2015). The protein corresponding to the spot seen by 2D-GE, highlighted in Fig. 1C–D, was identified as the 38kDa CD5L/AIM protein. A total of five unique peptides were mapped by LC-MS/MS, with 23% protein sequence coverage (Fig. 1E) and an overall Sequest HT protein identification score of 32.89. Additional details about the outcome of the LC-MS/MS analyses are presented in Supplemental Table 2 in the Supplemental Results section. Identification of CD5L/AIM by LC-MS/MS was confirmed from several distinct 2D-GE gels.
Figure 1. Example of 2D gel electrophoresis (2D-GE) findings and mass spectrometry (LC-MS/MS) results.
Human macular lysates from donor eyes were immunoprecipitated with whole serum from age-matched participants that were either affected or unaffected with age-related macular degeneration (AMD). These precipitates were run on 2D gels and spots unique to the AMD sera gels were identified by mass spectrometry. Panel A shows a representative unaffected participant serum gel and Panel B shows a representative affected (AMD) serum gel. The insets in Panels C and D show the area of the gel in which the spot later identified as CD5L/AIM migrated. Panel E displays the protein sequence of human CD5L/AIM (UniProt accession number O43866). The peptides identified by LC-MS/MS are highlighted in bold font. Note that other spots appear differentially present on the AMD 2D-GE shown in B compared to the control one in A. This is consistent with our finding that additional autoreactivities exist in AMD sera, and that different AMD sera will exhibit qualitatively and quantitatively distinct (i.e., different in identity, relative intensity, or both) autoreactivity profiles.
3.2. Confirmation of serum IgG binding with ELISA and Estimation of circulating CD5L/AIM levels
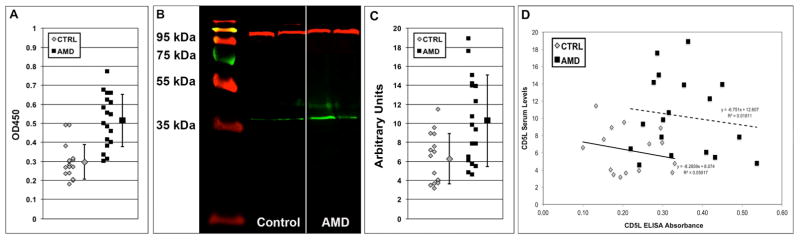
ELISA using recombinant human CD5L/AIM protein was used to confirm that the antigen identified by LC-MS/MS was truly CD5L/AIM. In this assay (Fig. 2A), AAbs from AMD patient sera (n=18) bound to CD5L/AIM protein at a ratio 1.73-fold higher than sera from unaffected subjects (n=15). This difference was highly significant (p=0.000007). Reactivity in negative control experiments was always negligible. An ELISA reactivity ≥0.4 against CD5L/AIM was associated with 18-fold higher likelihood of being from an AMD sample [χ2=21.42; p=0.00063; odds ratio (OR)=18.2, 95% confidence interval (CI)=2.99, 110.69], and had good sensitivity (point estimate=0.72, 95% CI=0.46, 0.89) and high specificity (point estimate=0.88, 95% CI=0.60, 0.98) in discriminating between AMD and control samples. The Positive Predictive Value (PPV), estimating the probability of a true positive test result (i.e., for ELISA reactivities ≥0.4) was 0.87 (95% CI=0.58, 0.98) and the Negative Predictive Value (NPV) estimating the probability of a true negative test result (i.e., for ELISA reactivities <0.4) was 0.74 (95% CI=0.49, 0.90).
Figure 2. Serum from subjects with AMD reacts specifically with CD5L/AIM and exhibits also elevated circulating CD5L/AIM levels.
Circulating AAbs from a selection of AMD-affected study participants (n=18) specifically reacted with recombinant CD5L/AIM protein in a direct enzyme-linked immunosorbence assay (ELISA) over unaffected subjects (CTRL, n=15). The individual reactivities are shown as a scatterplot in Panel A. The average reactivity ± 1SD is also shown next to the scatterplots. The difference in anti-CD5L/AIM AAbs was highly significant (p=0.000007). An ELISA autoreactivity ≥0.4 against CD5L/AIM was associated with 18-fold higher likelihood of being from an AMD sample. The serum levels of free circulating CD5L/AIM measured by Western blots (WB) were also elevated in the serum of these AMD participants compared to the unaffected controls. Representative WB examples are illustrated in Panel B [red bands: transferrin (control); green bands: CD5L/AIM] and the individual reactivities are shown as a scatterplot in Panel C. The average reactivity ± 1SD is also shown next to the scatterplots. The difference was significant (p=0.0052). A linear correlation analysis between the AAb levels as detected by ELISA and the serum CD5L/AIM levels as measured by WB is plotted in Panel D, showing a weak trend towards an inverse correlation for both data subsets (AMD and control sera).
Furthermore, WBs on the same serum samples (Fig. 2B) showed that AMD sera exhibited 1.64-fold higher circulating levels of secreted CD5L/AIM (10.26 ± 4.82, mean ± SD A.U.) compared to control subjects (6.28 ± 2.65 A.U.). Although significant overlap between AMD and control sera was seen (Fig. 2C), also this difference was statistically significant (p=0.0052).
Lastly, in an attempt to glean insight into the mechanisms leading up to production of AAb against CD5L, we explored whether elevated CD5L serum levels could be correlated either directly or inversely with anti-CD5L levels. Plotting one vs. the other in this limited discovery dataset among either the AMD or the control subjects, we observed only a weak trend towards lower serum CD5L levels in subjects with higher anti-CD5L reactivity in both subgroups (Fig. 2D).
3.3. CD5L/AIM expression in the human RPE and retina
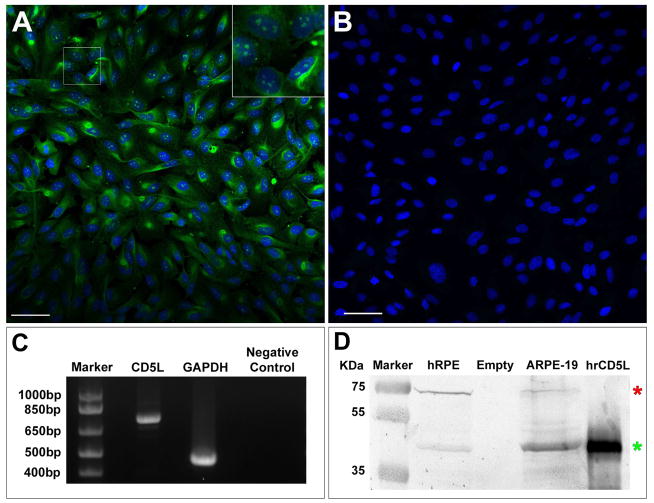
Cellular localization experiments on cultured immortalized ARPE-19 cells by IHC confocal microscopy showed (Fig. 3A) that, in SFM culture conditions as described above, CD5L/AIM is discretely expressed at both the nuclear level and, in a more diffuse and granular pattern, throughout the cytoplasm of the cell (green stain – see also inset in Fig. 3A). No expression was seen in negative control conditions (Fig. 3B). We confirmed further these findings by performing end-point PCR, demonstrating expression of the expected CD5L/AIM transcript in ARPE-19 cells (Fig. 3C) and by performing WBs on human RPE-specific protein lysate from human primary RPE cell lines and immortalized ARPE-19 identically cultured in SFM, which showed CD5L/AIM expression in both (Fig. 3D, green asterisk, CD5L/AIM band; red asterisk, RPE65 control band). Sequencing of the band seen by end-point PCR shown in Fig. 3C confirmed the expected CD5L/AIM mRNA sequence.
Figure 3. Expression of CD5L/AIM in the retinal pigment epithelium.
Panel A, confocal microscopy on immortalized human ARPE-19 cells cultured in serum-free medium (SFM). CD5L/AIM immunofluorescence is shown in green (blue, DAPI nuclear stain). Note the diffuse granular cytoplasmic reactivity seen in the ARPE-19 cells and the more discrete, clump-like nuclear localization of the reactivity (see also magnified view in the inset in the top right hand corner, corresponding to the cropped area in Panel A). Panel B, negative control for ARPE-19 cells cultured in SFM labeled only with secondary antibody and DAPI for blue nuclear stain. Bar in Panels A and B = 50μm. Panel C, results of end-point PCR demonstrate expression of the expected CD5L/AIM transcript in ARPE-19 cells, which was further confirmed by sequencing of the band (GAPDH, positive control; empty lane, negative control). Panel D, results of Western blots performed on cell culture lysates of a primary human RPE cell line (hRPE) and an immortalized ARPE-19 cell line (green asterisk: CD5L/AIM band), confirming expression of CD5L/AIM also in both cell lines (red asterisk: RPE65 control band; last lane: human recombinant CD5L/AIM, positive control).
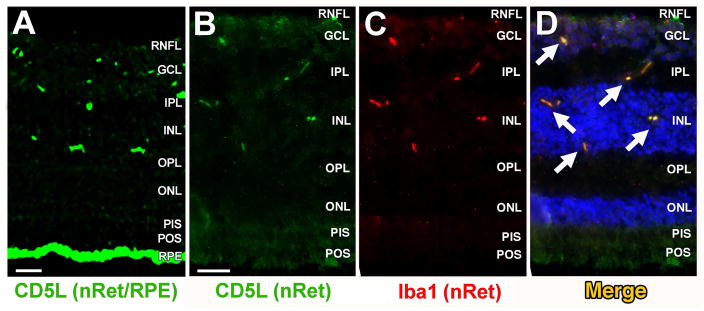
Retinal CD5L/AIM localization experiments were first performed on human macular retinal tissue sections at relatively low magnification to generally confirm expression. In these experiments, CD5L/AIM positive staining was seen both in a discrete pattern in the inner half of the neuroretina and, very prominently, at the RPE level (Fig. 4A). Confocal microscopy IHC experiments via co-staining for CD5L/AIM and the microglial cell marker, Iba1, were repeated on neuroretina-only tissue sections at higher magnification, and showed a CD5L/AIM staining pattern that was consistent with what seen in the initial experiments (Fig. 4B) and that coincided with that for Iba-1 (Fig. 4C–D). This finding suggests that CD5L/AIM is physiologically expressed by resident retinal microglial cells.
Figure 4. Expression of CD5L/AIM in retinal microglial cells.
Immunostaining of a healthy human donor macular section with CD5L/AIM-specific Ab (green, Panel A) shows discrete staining in the inner half of the retina and very strong RPE staining, confirming the findings from Fig. 3. Note that a portion of the RPE signal was due to age-related autofluorescence and seen (in lesser amount) also in control experiments (not shown). The experiments summarized in Fig. 3 were conducted in part to overcome this issue. Subsequent confocal, higher magnification microscopy experiments were conducted on macular neuroretinal sections only (no RPE) also for this reason. Co-staining of a healthy human donor macular neuroretinal section with the same CD5L/AIM-specific Ab (green, Panel B) or the microglial specific marker Iba1 (red, Panel C) and a fluorescence-conjugated secondary Ab shows that CD5L/AIM colocalizes with Iba1 (merge, Panel D) in the same discrete fashion at the GCL, IPL and ONL level as seen in Panel A. DAPI (blue) was used as a nuclear stain. Abbreviations, nRet: neuroretina; PIS/POS: photoreceptor inner segment/outer segment; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer. Bar in Panels A and B = 20μm.
4. Discussion
CD5L/AIM was initially reported to be expressed exclusively in macrophages (Haruta et al., 2001), but is has been since localized also to the alveolar lung epithelium (Lian et al., 2005; Li et al., 2011). CD5L/AIM has also been detected in human and mouse serum (Gangadharan et al., 2007; Gray et al., 2009), and elevated CD5L/AIM serum levels have been reported in a number of inflammatory diseases (Sanjurjo et al., 2015c). Our study provides evidence that CD5L/AIM is physiologically expressed in the RPE and in resident microglial cells, thus adding another type of epithelial cell and immunocompetent cell to the shortlist of cells expressing CD5L/AIM in the human body. In addition, our data shows that, in AMD, circulating AAbs recognizing CD5L/AIM are present at significantly higher levels compared to control samples, and that also serum levels of secreted CD5L/AIM are elevated in AMD.
Binding of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which are modified (e.g., oxidized) self-antigens (Kauppinen et al., 2012; Niu et al., 2013), is a primary function of the innate immune system to discriminate between self and non-self through PRRs. To date, five major PRR families have been associated with the innate immune system: a) complement-associated receptors; b) toll-like receptors (TLRs); c) advanced glycation end-products (RAGE) receptors; d) NOD-like receptors (NLRs); and e) the scavenger receptor (SR) family to which CD5L/AIM belongs (Martinez et al., 2011).
The role of autoimmunity and inflammation in neurodegenerative diseases has been subject of intense investigation during the past two decades, and especially in more recent years. Although AMD is not considered a classic inflammatory disease, and should not be considered a primary autoimmune one either, it has now been conclusively shown that inflammation and the immune system play an integral role in disease pathogenesis (Hageman et al., 2001; Anderson et al., 2002), that immunocompetent cells such as macrophages, microglia and giant cells localize near drusen at the Bruch’s membrane level and in choroidal neovascular membranes (Hageman et al., 2001; Chen, Forrester and Xu, 2011), and that drusen, an integral biomarker for AMD, represent not just inert waste deposits and debris that cannot be eliminated, but in fact lipid-laden formations that are the likely byproduct of local active inflammatory processes, possibly contributed to by the lipid accumulation itself (Hageman et al., 2001; Anderson et al., 2002; Malek et al., 2003; Rudolf et al., 2008). The discovery of CD5L/AIM physiological expression by the RPE and retinal microglia and of AAbs recognizing this secreted member of the SR protein family expressed by the RPE and retinal microglial cells in the serum of AMD subjects, together with the finding that serum levels of this protein are also elevated, is intriguing because it dovetails nicely with the emerging inflammatory and immune-mediated profile of AMD, and because it offers a plausible link also with the role of lipids in drusen (see below). The reactivity levels in AMD and the capacity of anti-CD5L/AIM autoreactivity to discriminate between AMD and control samples are closely comparable to those exhibited by anti-CEP AAbs, a biomarker of established importance for AMD with highly probable pathogenetic implications that also provides a link between autoimmunity towards a lipid-linked ocular antigen and oxidative damage (Gu et al., 2003; Hollyfield et al., 2008; Gu et al., 2009; Hollyfield et al., 2010; Doyle et al., 2012; Cruz-Guilloty et al., 2013; Renganathan et al., 2013; Wang et al., 2014; Wang et al., 2015). Thus, we propose that anti-CD5L/AIM autoreactivity may represent a novel biomarker of AMD that could also have pathogenic relevance. Interestingly, anti-CD5L/AIM AAbs – and elevated circulating CD5L/AIM levels – were found also in subjects with early to mid-stage AMD. Thus, we conclude that these CD5L/AIM-related serological changes do not reflect an expression of late-stage disease or after-the-fact biomarkers but that, in fact, they could be a biomarker of AMD also in its earlier stages.
How could CD5L/AIM and AAbs directed against this protein play a role in AMD? The RPE plays an essential role in the survival of retinal photoreceptors and in retinal defense mechanisms at the outer blood retinal barrier interface. This interface and the cellular phenotypes expressed by the RPE in AMD have been recently object of elegant combined high resolution ex vivo histological and immunohistochemical and in vivo imaging studies (Pang et al., 2015; Zanzottera et al., 2015a; Zanzottera et al., 2015b). In addition to its role in the regeneration of visual chromophores in the visual cycle, and its photoreceptor nutritional and homeostatic role (Simo et al., 2010; Saari, 2012), the RPE is also responsible for: a) secretion of molecules critical to photoreceptor survival, such as pigment epithelium-derived factor, a potent neutrophin for photoreceptor outer segments and a very important anti-angiogenic factor (Jablonski et al., 2000, 2001; Barnstable and Tombran-Tink, 2004; Becerra, 2006); b) the production of vascular endothelial growth factor, a key molecule in the neovascularization processes that characterize exudative AMD (Miller et al., 2013); c) the release of, and response to various inflammatory and immune-related molecules and stimuli (Elner et al., 1991; Elner et al., 1996; Crane et al., 2000; Bian et al., 2001; Bian et al., 2011; Yang et al., 2011); d) PAMP and DAMP binding (Kauppinen et al., 2012; Niu et al., 2013); and e) phagocytosis of photoreceptor outer segment tips, mediated at least in part via CD36, another member of the scavenger receptor superfamily expressed by the RPE (Ryeom et al., 1996; Kauppinen et al., 2012; Niu et al., 2013). CD36 is also a receptor for CD5L/AIM and mediates CD5L/AIM endocytosis in macrophages (Kurokawa et al., 2010; Amezaga et al., 2014; Sanjurjo et al., 2015b; Sanjurjo et al., 2015c). Hence, it is possible that CD36 may be a receptor for CD5L/AIM also in the RPE. Furthermore, it has been recently demonstrated that CD5L/AIM promotes the clearance of oxidized low-density lipoprotein (oxLDL) (Amezaga et al., 2014). Since oxLDL is known to be a component of drusen (Amaral and Rodriguez, 2011), we propose that CD5L/AIM may physiologically play a role, possibly together with CD36, in the clearance of oxLDL from the sub-RPE environment and, thus, be a potentially important role player in limiting the formation of drusen with age. We further propose that AAbs directed against CD5L/AIM, as the ones we discovered in our investigation, may compromise CD5L/AIM functions in the RPE and/or impede its binding to oxLDL, CD36, or both, and potentially facilitate drusen biogenesis via impaired oxLDL clearance. We have recently obtained in vitro evidence that this mechanism could be indeed at play [Koirala et al. The CD5-like (CD5L) protein is expressed by retinal pigment epithelium (RPE) cells and is a key role player in the uptake of oxidized low-density lipoproteins (oxLDL), Invest. Ophthalmol. Vis. Sci. 2016; 57(5): E-Abstract 5014]. Further studies on this particular aspect are in progress.
In addition, it has been recently shown that CD5L/AIM activates autophagy through CD36 in macrophages (Sanjurjo et al., 2015b). The potential importance of impaired autophagy in AMD and its role in causing phenotypes with multiple features of human AMD in rats and mice with genetically compromised autophagy pathways has been recently revealed (Zigler et al., 2011; Kaarniranta et al., 2013; Celkova, Doyle and Campbell, 2015; Yao et al., 2015). When autophagy is compromised, an activation of the NLRP3 inflammasome, another member of the DAMP/PAMP-recognizing PPR family, occurs. The importance of NLRP3 inflammasome activation in AMD has recently emerged (Celkova et al., 2015). Thus, it is plausible that AAbs directed against CD5L/AIM could impair CD5L/AIM-mediated autophagy activation, promote NLRP3 inflammasome activation, and exacerbate AMD at least in part also via a direct autophagy-mediated mechanism. This additional potential pathogenetic mechanism is not mutually exclusive with the drusen biogenesis one and is particularly appealing, also in light of our recent discovery of other macular autoantigens in AMD, all of which share potential implications with the regulation of autophagy (Iannaccone et al., 2015). Additional studies will be necessary to test this hypothesis further.
The biological significance of the finding of elevated CD5L/AIM circulating levels in AMD sera is uncertain. It is not presently known whether the observed increase would precede the formation of the anti-CD5L/AIM AAbs. It is also unknown whether it may reflect the generalized inflammatory state underlying AMD as seen in other inflammatory diseases (Sanjurjo et al., 2015c), and thus may be a trigger for AAb formation, or whether CD5L/AIM-secreting cells may increase CD5L/AIM release as a compensatory phenomenon following the development of the AAbs. There is precedent for an autoimmune response against a ubiquitous self-antigen to result in tissue-specific manifestations. For example, in systemic lupus erythematosus, anti-DNA AAbs arise and lead to immune deposits in the kidneys, which, over time, impairs the function of the basement membrane and results in glomerulonephritis (Bonanni et al., 2015). Thus, anti-CD5L/AIM AAbs could contribute to the gradual damage of macular tissues in ways other than a direct effect on CD5L/AIM hypothesized functions within the RPE and retinal microglial cells. This hypothesis will require further testing at the clinical, preclinical and in vitro level.
Microglial cells are normally quiescent resident retinal immunocompetent cells involved in both innate and adaptive immunity (Langmann, 2007; Karlstetter, Ebert and Langmann, 2010; Karlstetter and Langmann, 2012, 2014). Aging microglial cells aggregate in the outer retina, accumulate large amounts of by-products of lipid oxidation, and develop significantly reduced mobility (Ma et al., 2013). Retinal microglial cells can also act as antigen presenting cells (Gregerson, Sam and McPherson, 2004) and, in autoimmune eye disease, microglia both initiate disease and limit subsequent inflammation (Chen, Yang and Kijlstra, 2002). Microglial cells activated by retinal injury transform into microglial foam cells, and migrate to the injured outer retina (Combadiere et al., 2007), where they participate in the phagocytosis of debris (Egensperger et al., 1996). Activated microglial cells, however, can also produce proinflammatory cytokines and chemokines that create a neurotoxic milieu that can contribute to disease progression (Langmann, 2007). There is robust evidence indicating that retinal microglial cells may play a role in the pathogenesis of both retinal dystrophies and AMD (Gupta, Brown and Milam, 2003; Langmann, 2007; Karlstetter et al., 2010; Adamus et al., 2012; Karlstetter et al., 2014; Indaram et al., 2015; Lad et al., 2015). Recently, much attention has been given to the discovery that modulation of microglial cell activation in retinal degenerative diseases may have immediate therapeutic implications (Zhao et al., 2015). To our knowledge, the role of CD5L/AIM in retinal microglial cells has not yet been studied, but its role in inflammatory cells elsewhere in the body, and especially in circulating macrophages, has been well characterized (Arai et al., 2005; Chawla, Nguyen and Goh, 2011). Early studies have demonstrated the importance of CD5L/AIM for macrophage survival when challenged by either a microbial load (Joseph et al., 2004) or oxidized products of cellular metabolism (Arai et al., 2005; Hamada et al., 2014). More recent work has provided new evidence for a role of CD5L/AIM in clearance of oxLDL (Amezaga et al., 2014) and autophagy in macrophages (Sanjurjo et al., 2015a). Thus, AAbs directed against CD5L/AIM could interfere with the local immunoregulatory role of microglial cells in the human retina and contribute at least in part to AMD pathogenesis via this route. How exactly this may be possible, though, remains to be elucidated and warrants further investigations.
Lastly, ARPE-19 cells showed a dispersed, granular immunofluorescence pattern throughout the cytoplasm and prominent clumpy nuclear staining (Fig. 3A). Since CD5L/AIM is a secreted protein, there are at least two possible mechanisms for how CD5L/AIM could accumulate in the nucleus of RPE cells. One possible route could use alternatively spliced isoforms of CD5L/AIM, provided at least one lacks the secretion leader peptide and is therefore translated into a cytoplasmic protein. If a mechanism for nuclear entry exists, CD5L/AIM could accumulate at certain sites in the nucleus. Alternatively, CD5L/AIM may be taken up again by the cells after having been secreted. The engulfed protein may utilize an incompletely characterized mechanism to escape the endocytic vesicle and migrate from there to the cytoplasm and then to the nucleus. Support for the second potential mechanism comes from studies by Kurokawa et al. (Kurokawa et al., 2010). In their study, these authors used cd5l/aim−/− mice as recipients for intravenously injected recombinant CD5L/AIM. The recombinant protein was found to enter monocytic cells in these animals and, by confocal microscopy, it was shown that CD5L/AIM emerged from endosomes prior to their fusion with lysosomes. Some of the nuclei in these cells also exhibited anti-CD5L/AIM staining. Now that the intranuclear localization of CD5L/AIM is confirmed, attention should focus also on the potential nuclear functions of this protein.
5. Conclusions
In summary, our discovery of the in situ expression of CD5L/AIM by human RPE and retinal microglia and the finding of anti-CD5L/AIM AAbs (alongside elevated circulating CD5L/AIM protein levels) in AMD suggest a plausible role for CD5L/AIM in human eye physiology and disease, identify a potential novel serum biomarker for AMD, and provide a possible link between AMD and immune-mediated processes, oxLDL metabolism, and autophagy that may be of mechanistic and pathogenetic relevance for drusen biogenesis and AMD development.
Supplementary Material
Highlights.
Human retinal pigment epithelium and retinal microglia express the CD5 antigen-like protein (CD5L), also known as apoptosis inhibitor of macrophages (AIM)
AMD patients exhibit autoantibodies against CD5L/AIM
Anti-CD5L/AIM ELISA reactivity ≥0.4 is associated with 18-fold higher odds of AMD
Anti-CD5L/AIM autoreactivity could be a potential novel AMD biomarker with possible pathogenic potential
Acknowledgments
We gratefully acknowledge the use of the Licor Odyssey instrumentation to quantitate Western blot reactivities in the laboratory of Dr. Yates, UTHSC Dept. of Pharmaceutical Sciences. The primary human RPE cell lines used in the study were a generous gift by Dr. Ed Chaum, UTHSC Ophthalmology/Hamilton Eye Institute. Preliminary findings from this investigation have been presented in poster format at the 2014 ARVO meeting, Orlando, FL, May 4–8, 2014, and the 2014 American Academy of Ophthalmology meeting, Chicago, IL, October 19–22, 2014.
Funding
This investigation was supported by NEI/NIH grants 1R21EY018416 (AI) and 1R01EY022706 (AI/FG) and by Research to Prevent Blindness, Inc. New York, NY (Physician Scientist Award to AI and unrestricted grant to UTHSC Ophthalmology). The LC-MS/MS portion of this project was funded in part also by a UTHSC College of Pharmacy Seed Grant (FG). Funds for the LTQ mass spectrometer were provided by NIH grant S10RR16679, and by the UTHSC College of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamus G, Wang S, Kyger M, Worley A, Lu B, Burrows GG. Systemic immunotherapy delays photoreceptor cell loss and prevents vascular pathology in Royal College of Surgeons rats. Mol Vis. 2012;18:2323–37. [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–32. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- Amaral J, Rodriguez IR. 7-ketocholesterol Induced Ocular Angiogenesis: A Potential Age-related Risk Factor in the Pathogenesis of Age-related Macular Degeneration (AMD) Investigative Ophthalmology & Visual Science. 2011;52:2303–03. [Google Scholar]
- Amezaga N, Sanjurjo L, Julve J, Aran G, Perez-Cabezas B, Bastos-Amador P, Armengol C, Vilella R, Escola-Gil JC, Blanco-Vaca F, Borras FE, Valledor AF, Sarrias MR. Human scavenger protein AIM increases foam cell formation and CD36-mediated oxLDL uptake. J Leukoc Biol. 2014;95:509–20. doi: 10.1189/jlb.1212660. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004;23:561–77. doi: 10.1016/j.preteyeres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Becerra SP. Focus on Molecules: Pigment epithelium-derived factor (PEDF) Exp Eye Res. 2006;82:739–40. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Khanna H, Murga-Zamalloa CA, Patil S, Elner VM. Expression and functional roles of caspase-5 in inflammatory responses of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:8646–56. doi: 10.1167/iovs.11-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1beta and TNF-alpha-induced chemokine expression in human retinal pigment epithelial cells. Exp Eye Res. 2001;73:111–21. doi: 10.1006/exer.2001.1019. [DOI] [PubMed] [Google Scholar]
- Bonanni A, Vaglio A, Bruschi M, Sinico RA, Cavagna L, Moroni G, Franceschini F, Allegri L, Pratesi F, Migliorini P, Candiano G, Pesce G, Ravelli A, Puppo F, Martini A, Tincani A, Ghiggeri GM. Multi-antibody composition in lupus nephritis: isotype and antigen specificity make the difference. Autoimmun Rev. 2015;14:692–702. doi: 10.1016/j.autrev.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Celkova L, Doyle SL, Campbell M. NLRP3 Inflammasome and Pathobiology in AMD. J Clin Med. 2015;4:172–92. doi: 10.3390/jcm4010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YS. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology. 2011;11:738–49. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang P, Kijlstra A. Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm. 2002;10:27–39. doi: 10.1076/ocii.10.1.27.10328. [DOI] [PubMed] [Google Scholar]
- Chen M, Forrester JV, Xu H. Dysregulation in Retinal Para-Inflammation and Age-Related Retinal Degeneration in CCL2 or CCR2 Deficient Mice. PLoS ONE. 2011;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–6. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougnard-Gregoire A, Delyfer MN, Korobelnik JF, Rougier MB, Le Goff M, Dartigues JF, Barberger-Gateau P, Delcourt C. Elevated high-density lipoprotein cholesterol and age-related macular degeneration: the Alienor study. PLoS One. 2014;9:e90973. doi: 10.1371/journal.pone.0090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane IJ, Wallace CA, McKillop-Smith S, Forrester JV. Control of chemokine production at the blood-retina barrier. Immunology. 2000;101:426–33. doi: 10.1046/j.1365-2567.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, et al. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflam. 2013;2013:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009;28:393–422. doi: 10.1016/j.preteyeres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res. 2010;51:451–67. doi: 10.1194/jlr.R002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–45. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:265–74. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005a;81:731–41. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005b;80:761–75. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O’Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18:791–8. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, Salomon RG, Crabb JW, Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:13480–4. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Egensperger R, Maslim J, Bisti S, Hollander H, Stone J. Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Muller cells) Brain Res Dev Brain Res. 1996;97:1–8. doi: 10.1016/s0165-3806(96)00119-8. [DOI] [PubMed] [Google Scholar]
- Elner SG, Strieter RM, Elner VM, Rollins BJ, Del Monte MA, Kunkel SL. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab Invest. 1991;64:819–25. [PubMed] [Google Scholar]
- Elner VM, Elner SG, Standiford TJ, Lukacs NW, Strieter RM, Kunkel SL. Interleukin-7 (IL-7) induces retinal pigment epithelial cell MCP-1 and IL-8. Exp Eye Res. 1996;63:297–303. doi: 10.1006/exer.1996.0118. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, et al. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433–9. 39e1–2. doi: 10.1038/ng.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher K, Mura M, Todd WA, Harris TL, Kenyon E, Harris T, Satterfield S, Johnson KC, Kritchevsky SB, Iannaccone A. Estimation of Macular Pigment Optical Density in the Elderly. Test-Retest Variability and Effect of Optical Blur in Pseudophakic Subjects. Vision Res. 2007;47:1253–59. doi: 10.1016/j.visres.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan B, Antrobus R, Dwek RA, Zitzmann N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem. 2007;53:1792–9. doi: 10.1373/clinchem.2007.089144. [DOI] [PubMed] [Google Scholar]
- Giorgianni F, Cappiello A, Beranova-Giorgianni S, Palma P, Trufelli H, Desiderio DM. LC-MS/MS analysis of peptides with methanol as organic modifier: improved limits of detection. Anal Chem. 2004;76:7028–38. doi: 10.1021/ac0493368. [DOI] [PubMed] [Google Scholar]
- Grassmann F, Fritsche LG, Keilhauer CN, Heid IM, Weber BH. Modelling the genetic risk in age-related macular degeneration. PLoS One. 2012;7:e37979. doi: 10.1371/journal.pone.0037979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Chattopadhyay D, Beale GS, Patman GL, Miele L, King BP, Stewart S, Hudson M, Day CP, Manas DM, Reeves HL. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer. 2009;9:271. doi: 10.1186/1471-2407-9-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson DS, Sam TN, McPherson SW. The antigen-presenting activity of fresh, adult parenchymal microglia and perivascular cells from retina. J Immunol. 2004;172:6587–97. doi: 10.4049/jimmunol.172.11.6587. [DOI] [PubMed] [Google Scholar]
- Gu J, Pauer GJ, Yue X, Narendra U, Sturgill GM, Bena J, Gu X, Peachey NS, Salomon RG, Hagstrom SA, Crabb JW. Assessing susceptibility to age-related macular degeneration with proteomic and genomic biomarkers. Mol Cell Proteomics. 2009;8:1338–49. doi: 10.1074/mcp.M800453-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Pauer GJ, Yue X, Narendra U, Sturgill GM, Bena J, Gu X, Peachey NS, Salomon RG, Hagstrom SA, Crabb JW. Proteomic and Genomic Biomarkers for Age-Related Macular Degeneration. Adv Exp Med Biol. 2010;664:411–17. doi: 10.1007/978-1-4419-1399-9_47. [DOI] [PubMed] [Google Scholar]
- Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, Salomon RG. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–71. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Gehrs K, Lejnine S, Bansal AT, Deangelis MM, Guymer RH, Baird PN, Allikmets R, Deciu C, Oeth P, Perlee LT. Clinical validation of a genetic model to estimate the risk of developing choroidal neovascular age-related macular degeneration. Hum Genomics. 2011;5:420–40. doi: 10.1186/1479-7364-5-5-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hamada M, Nakamura M, Tran MT, Moriguchi T, Hong C, Ohsumi T, et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat Commun. 2014;5:3147. doi: 10.1038/ncomms4147. [DOI] [PubMed] [Google Scholar]
- Haruta I, Kato Y, Hashimoto E, Minjares C, Kennedy S, Uto H, Yamauchi K, Kobayashi M, Yusa S, Muller U, Hayashi N, Miyazaki T. Association of AIM, a novel apoptosis inhibitory factor, with hepatitis via supporting macrophage survival and enhancing phagocytotic function of macrophages. J Biol Chem. 2001;276:22910–4. doi: 10.1074/jbc.M100324200. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–8. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone A, Giorgianni F, New DD, Hollingsworth TJ, Umfress AC, Alhatem AH, et al. Circulating Autoantibodies in Age-Related Macular Degeneration Recognize Human Macular Tissue Antigens Implicated in Autophagy, Immunomodulation, and Protection from Oxidative Stress and Apoptosis. PLoS ONE. 2015;10:e0145323. doi: 10.1371/journal.pone.0145323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone A, Mura M, Gallaher K, Todd WA, Kenyon E, Harris TL, Harris T, Satterfield S, Johnson KC, Kritchevsky SB. Macular Pigment Optical Density in the Elderly. Findings in a Large Biracial Mid-South Sample. Invest Ophthalmol Vis Sci. 2007;48:1458–65. doi: 10.1167/iovs.06-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone A, Neeli I, Krishnamurthy P, Lenchik NI, Wan H, Gerling IC, Desiderio DM, Radic MZ. Autoimmunity in Age-Related Macular Degeneration: A Possible Role Player in Disease Development and Progression. Adv Exp Med Biol. 2012;723:11–16. doi: 10.1007/978-1-4614-0631-0_2. [DOI] [PubMed] [Google Scholar]
- Indaram M, Ma W, Zhao L, Fariss RN, Rodriguez IR, Wong WT. 7-Ketocholesterol increases retinal microglial migration, activation, and angiogenicity: a potential pathogenic mechanism underlying age-related macular degeneration. Sci Rep. 2015;5:9144. doi: 10.1038/srep09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–57. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal Muller cell development and glutamine synthetase expression after removal of the retinal pigment epithelium. Glia. 2001;35:14–25. doi: 10.1002/glia.1066. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–84. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Ebert S, Langmann T. Microglia in the healthy and degenerating retina: insights from novel mouse models. Immunobiology. 2010;215:685–91. doi: 10.1016/j.imbio.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Langmann T. Immune mechanisms in retinal degeneration. Klin Monbl Augenheilkd. 2012;229:221–6. doi: 10.1055/s-0031-1282050. [DOI] [PubMed] [Google Scholar]
- Karlstetter M, Langmann T. Microglia in the aging retina. Adv Exp Med Biol. 2014;801:207–12. doi: 10.1007/978-1-4614-3209-8_27. [DOI] [PubMed] [Google Scholar]
- Kauppinen A, Niskanen H, Suuronen T, Kinnunen K, Salminen A, Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—implications for age-related macular degeneration (AMD) Immunology letters. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, Ose R, Mori M, Kubota N, Kadowaki T, Oike Y, Koga H, Febbraio M, Iwanaga T, Miyazaki T. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11:479–92. doi: 10.1016/j.cmet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Lad EM, Cousins SW, Van Arnam JS, Proia AD. Abundance of infiltrating CD163+ cells in the retina of postmortem eyes with dry and neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2015;253:1941–5. doi: 10.1007/s00417-015-3094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–51. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch’s membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005;46:2576–86. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Li Y, Qu P, Wu L, Li B, Du H, Yan C. Api6/AIM/Spalpha/CD5L overexpression in alveolar type II epithelial cells induces spontaneous lung adenocarcinoma. Cancer Res. 2011;71:5488–99. doi: 10.1158/0008-5472.CAN-10-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X, Yan C, Qin Y, Knox L, Li T, Du H. Neutral lipids and peroxisome proliferator-activated receptor-{gamma} control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am J Pathol. 2005;167:813–21. doi: 10.1016/s0002-9440(10)62053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Coon S, Zhao L, Fariss RN, Wong WT. A2E accumulation influences retinal microglial activation and complement regulation. Neurobiol Aging. 2013;34:943–60. doi: 10.1016/j.neurobiolaging.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–25. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011;63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–14. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age related macular degeneration. Semin Ophthalmol. 2007;22:229–40. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci U S A. 2010;107:7395–400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Gallo NB, Hancox LS, Miller NJ, Radeke CM, Maloney MA, Cooper JB, Hageman GS, Anderson DH, Johnson LV, Radeke MJ. Systems-level analysis of age-related macular degeneration reveals global biomarkers and phenotype-specific functional networks. Genome Med. 2012;4:16. doi: 10.1186/gm315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N, Zhang J, McNutt MA. Endogenous IgG Affects the Cell Biology of RPE Cells and Involves the TLR4 Pathway. Investigative ophthalmology & visual science. 2013;54:7045–52. doi: 10.1167/iovs.13-12531. [DOI] [PubMed] [Google Scholar]
- Oak AS, Messinger JD, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014;34:825–6. doi: 10.1097/IAE.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Pabst MJ, Pabst KM, Handsman DB, Beranova-Giorgianni S, Giorgianni F. Proteome of monocyte priming by lipopolysaccharide, including changes in interleukin-1beta and leukocyte elastase inhibitor. Proteome Sci. 2008;6:13. doi: 10.1186/1477-5956-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CE, Messinger JD, Zanzottera EC, Freund KB, Curcio CA. The Onion Sign in Neovascular Age-Related Macular Degeneration Represents Cholesterol Crystals. Ophthalmology. 2015;122:2316–26. doi: 10.1016/j.ophtha.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1990;228:270–4. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- Pikuleva IA, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res. 2014;41:64–89. doi: 10.1016/j.preteyeres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan K, Gu J, Rayborn ME, Crabb JS, Salomon RG, Collier RJ, Kapin MA, Romano C, Hollyfield JG, Crabb JW. CEP biomarkers as potential tools for monitoring therapeutics. PLoS One. 2013;8:e76325. doi: 10.1371/journal.pone.0076325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49:1200–9. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109(Pt 2):387–95. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr. 2012;32:125–45. doi: 10.1146/annurev-nutr-071811-150748. [DOI] [PubMed] [Google Scholar]
- Sanjurjo L, Amézaga N, Aran G, Naranjo-Gómez M, Arias L, Armengol C, Borràs FE, Sarrias MR. The human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy. 2015a;11:487–502. doi: 10.1080/15548627.2015.1017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjurjo L, Amezaga N, Aran G, Naranjo-Gomez M, Arias L, Armengol C, Borras FE, Sarrias MR. The human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages that modulates inflammatory responses. Autophagy. 2015b;11:487–502. doi: 10.1080/15548627.2015.1017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjurjo L, Aran G, Roher N, Valledor AF, Sarrias MR. AIM/CD5L: a key protein in the control of immune homeostasis and inflammatory disease. J Leukoc Biol. 2015c;98:173–84. doi: 10.1189/jlb.3RU0215-074R. [DOI] [PubMed] [Google Scholar]
- Sarrias MR, Rosello S, Sanchez-Barbero F, Sierra JM, Vila J, Yelamos J, Vives J, Casals C, Lozano F. A role for human Sp alpha as a pattern recognition receptor. J Biol Chem. 2005;280:35391–8. doi: 10.1074/jbc.M505042200. [DOI] [PubMed] [Google Scholar]
- Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Deletion of CFHR3 and CFHR1 Genes in Age-Related Macular Degeneration. Hum Mol Genet. 2007a;17:971–77. doi: 10.1093/hmg/ddm369. [DOI] [PubMed] [Google Scholar]
- Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, Gallins P, Agarwal A, Postel EA, Pericak-Vance MA, Haines JL. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum Mol Genet. 2007b;16:1986–92. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- Spencer KL, Olson LM, Schnetz-Boutaud N, Scott WK, Gallins P, Agarwal A, Pericak-Vance MA, Iannaccone A, Kritchevsky SB, Garcia M, Kenyon E, Nalls M, Newman AB, Haines JL. Using Genetic Variation and Environmental Risk Factor Data to Identify Individuals at High Risk for Age-Related Macular Degeneration. PLoS ONE. 2011;6:e17784. doi: 10.1371/journal.pone.0017784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16(Spec No. 2):R174–82. doi: 10.1093/hmg/ddm212. [DOI] [PubMed] [Google Scholar]
- Vishwanathan R, Iannaccone A, Scott TM, Kritchevsky SB, Jennings BJ, Carboni G, Forma G, Satterfield S, Harris T, Johnson KC, Schalch W, Renzi LM, Rosano C, Johnson EJ. Macular pigment optical density is related to cognitive function in older people. Age Ageing. 2014;43:271–5. doi: 10.1093/ageing/aft210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo J, West XZ, Bid HK, Lu L, Hong L, Jang GF, Zhang L, Crabb JW, Clinical G, Proteomic AMDSG, Linetsky M, Salomon RG. Detection and biological activities of carboxyethylpyrrole ethanolamine phospholipids (CEP-EPs) Chem Res Toxicol. 2014;27:2015–22. doi: 10.1021/tx500216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Linetsky M, Guo J, Choi J, Hong L, Chamberlain AS, Howell SJ, Howes AM, Salomon RG. 4-Hydroxy-7-oxo-5-heptenoic Acid (HOHA) Lactone is a Biologically Active Precursor for the Generation of 2-(omega-Carboxyethyl)pyrrole (CEP) Derivatives of Proteins and Ethanolamine Phospholipids. Chem Res Toxicol. 2015;28:967–77. doi: 10.1021/acs.chemrestox.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA. Abundant lipid and protein components of drusen. PLoS One. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Elner SG, Chen X, Field MG, Petty HR, Elner VM. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:6026–34. doi: 10.1167/iovs.10-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Jia L, Khan N, Lin C, Mitter SK, Boulton ME, Dunaief JL, Klionsky DJ, Guan JL, Thompson DA, Zacks DN. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11:939–53. doi: 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Bhangale TR, Fagerness J, Ripke S, Thorleifsson G, Tan PL, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet. 2011a;20:3699–709. doi: 10.1093/hmg/ddr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Reynolds R, Fagerness J, Rosner B, Daly MJ, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011b;52:4663–70. doi: 10.1167/iovs.10-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Reynolds R, Rosner B, Daly MJ, Seddon JM. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest Ophthalmol Vis Sci. 2012;53:1548–56. doi: 10.1167/iovs.11-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanzottera EC, Messinger JD, Ach T, Smith RT, Curcio CA. Subducted and melanotic cells in advanced age-related macular degeneration are derived from retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2015a;56:3269–78. doi: 10.1167/iovs.15-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanzottera EC, Messinger JD, Ach T, Smith RT, Freund KB, Curcio CA. The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2015b;56:3253–68. doi: 10.1167/iovs.15-16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareparsi S, Reddick AC, Branham KE, Moore KB, Jessup L, Thoms S, Smith-Wheelock M, Yashar BM, Swaroop A. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci. 2004;45:1306–10. doi: 10.1167/iovs.03-1253. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zabel MK, Wang X, Ma W, Shah P, Fariss RN, Qian H, Parkhurst CN, Gan WB, Wong WT. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol Med. 2015 doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, McLeod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the betaA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–31. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.