Abstract
Introduction
Regulatory agencies recommend a stepwise approach for demonstrating biosimilarity between a proposed biosimilar and reference biological product emphasizing for functional and structural characterization to trace if there is any difference which may impact safety and efficacy. We studied the comparative structural and biological attributes of recombinant human chorionic gonadotropin (rhCG), SB005, with reference product, Ovidrel® and Ovitrelle®. Recombiant hCG was approved in 2000 by the US Food and Drug Administration for the induction of final follicular maturation, early luteinization in infertile women as part of assisted reproductive technology program. It is also indicated for the induction of ovulation and pregnancy in ovulatory infertile patients whose cause of infertility is not due to ovarian failure.
Materials and methods
Primary structure was studied by intact mass analysis, peptide fingerprinting, peptide mass fingerprinting and sequence coverage analysis. Higher order structure was studied by circular dichroism, ultraviolet-visible spectroscopy, fluorescence spectroscopy, and disulfide bridge analysis. Different isoforms of reference product and SB005 were identified using capillary isoelectric focusing and capillary zone electrophoresis. Glycosylation was studied by N-glycan mapping using LC-ESI-MS, point of glycosylation, released glycan analysis using ultra performance liquid chromatography and sialic acid analysis. Product related impurities such as oligomer content analysis and oxidized impurities were studied using size exclusion chromatography and reverse phase high performance liquid chromatography, respectively. Biological activity in term of potency of reference product and SB005 was studied by in vivo analysis.
Results and Conclusion
In this study we have compared analytical similarity of recombinant rhCG (SB005) produced at Sun Pharmaceuticals with the reference product with respect to its primary, higher order structure, isoforms, charge variants, glycosylation, sialyation pattern, pharmacodynamic and in vivo efficacy. Our studies show that the in house produced rhCG has a high degree of structural and functional similarity with the reference product available in the market.
Keywords: human chorionic gonadotropin, rhCG, biosimilarity, characterization, comparability, Ovidrel®, Ovitrelle®
Introduction
Human chorionic gonadotropin (hCG) is a glycoprotein hormone composed of 237 amino acids with a molecular mass of 25.7 kDa. It is involved in the maintenance of the corpus luteum in early pregnancy. hCG is a heterodimer composed of alpha (hCG alpha) subunit, which is 92 amino acids long, and beta (hCG beta) subunit, which is 145 amino acids long. Its alpha subunit is identical to that of luteinizing hormone, follicle stimulating hormone and thyroid stimulating hormone, and beta subunit is unique to hCG1.
Gonadotropin use when derived from either animal or human tissues has, however, not always been without clinical danger2 (e.g., antibody formation from pregnant mare serum gonadotropin; Creutzfeld–Jacob disease from human pituitary gonadotropin). However with the rise of recombinant DNA technology, it is possible to increase both a protein’s purity and safety. Recombinant DNA technology enables production of uniform and specific product that reduces the dependence on the urine collection and hormone extraction. It would also allow the commercial production to be adjusted according to market requirements.3 Recombinant hCG (rhCG) had been initially manufactured by transfecting non-human cell lines (Chinese hamster ovary cells) with genetic material capable of replicating identical amino acid sequences to the human compound and developed as a pharmaceutical product named Ovidrel® (Merck Serono, Geneva, Switzerland).4 Recombinant hCG has been approved in the year 2000 by the US Food and Drug Administration (US FDA) for the induction of final follicular maturation and early luteinization in infertile women who have undergone pituitary desensitization and who have been appropriately pre-treated with follicle-stimulating hormones as part of an Assisted Reproductive Technology program such as in vitro fertilization and embryo transfer. rhCG is also indicated for the induction of ovulation and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to primary ovarian failure.2 It is marketed in India as Ovitrelle®.
Biosimilars are approved versions of original “innovator” product by government regulators of respective countries like the US FDA, European Medicines Agency, Indian Central Drug Standard Control Organization and can be manufactured when the original product’s patent expires.5 While approving biosimilar, reference to the innovator product is important in terms of analytical similarity between the innovator and biosimilar product. Many a times manufacturing process defines the biological product in terms of complex glycosylation, sialylation, etc. However, biosimilar manufacturers usually do not have access to the innovator molecule’s original cell bank, or the exact fermentation and purification process, nor to the active drug substance, although they do have access to the commercialized final formulated innovator product. So, an important strategy in development of biosimilar product is to follow an extensive state-of-the-art physicochemical, analytical and functional comparison of the biosimilar molecule and the innovator molecule. General requirement under 351(k) application include, among other things, information demonstrating biosimilarity based upon data derived from analytical studies, animal studies and clinical studies.
The biosimilar approval process requires the quality assessment of the product by a comparability exercise with the innovator product. While showing the comparability between the innovator product and biosimilar product, it does not necessarily mean that the quality attributes of the biosimilar and innovator product are identical but they should be highly similar and that the existing knowledge is sufficiently predictive to ensure that any differences in quality attributes have no adverse impact upon safety and efficacy of the biosimilar product. Here we report the analytical characterization of a proposed biosimilar rhCG and reference product. Primary and higher order structures of proposed biosimilar (SB005) and reference product were analyzed using a variety of methods based on high-performance liquid chromatography (HPLC), liquid chromatography-electron spray ionization-mass spectroscopy (LC-ESI-MS), capillary electrophoresis (CE), circular dichroism (CD) and ultraviolet-visible (UV-Vis) spectrophotometer.
Materials and methods
Materials
Lots of rhCG reference products were sourced from USA and India and stored as per manufacturer’s instructions. The proposed biosimilar product, Sun Choriogonadotropin alpha (rhCG) SB005, was produced in-house at Sun Pharmaceutical Industries Limited, Vadodara, India, and used for all the analyses. All the reagents used for analysis were of analytical grade.
Methods
State-of-the-art approach was used for demonstration of analytical comparability of SB005 with the reference product approved in the USA and India.6,7 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analysis for reference product and SB005 were performed to evaluate identification and purity. Primary structure of reference product and SB005 was studied by intact mass analysis, peptide fingerprinting, peptide mass fingerprinting and sequence coverage analysis. Higher order structure was studied by CD, UV-Vis spectroscopy, fluorescence spectroscopy and disulfide bridge analysis using LC-ESI-MS. Different isoforms of reference product and SB005 were identified using capillary isoelectric focusing and capillary zone electrophoresis (CZE). Glycosylation was studied by N-glycan mapping using LC-ESI-MS, point of glycosylation, released glycan analysis using ultra performance liquid chromatography (UPLC) and sialic acid analysis. Product-related impurities such as oligomer content analysis and oxidized impurities were studied using size exclusion chromatography (SEC) and reverse phase HPLC, respectively. Biological activity in terms of potency of reference product and SB005 was studied by in vivo analysis.
SDS-PAGE and western blot analysis
SB005 and reference product were analyzed by reducing and non-reducing SDS-PAGE. Samples were dialyzed against phosphate buffer (2.5 mM, pH 7.0 containing 0.1 mg/mL methionine) followed by quantification at 277 nm. In total, 3 μg of the samples was loaded per well on 12% denaturing polyacrylamide gels. Gels were run at constant current (15 mA) for ~75 minutes, using Mini-PROTEAN tetra cell system (Bio-Rad, 165-4130) and 1X SDS gel running buffer (25 mM Tris, 192 mM glycine and 0.1% SDS). Gels were stained with Coomassie Brilliant Blue R-250 and documented using G-Box Imaging system (Syngene, Cambridge, UK).
SB005 and reference product were prepared for SDS-PAGE and separated under non-reducing condition followed by blot transfer to an activated PVDF membrane using Mini trans blot cell (Bio-Rad, 170-3930) at 250 mA for 2 hours at 2–8°C. The membrane was blocked with 5% skim milk for 1 hour with gentle shaking at RT (25°C). After blocking and washing, the blot was incubated with 1:500 diluted anti-hCG primary antibody (Medix Biochemica, Espoo, Finland) for 1 hour with gentle shaking at RT (25°C). After washing, the blot was incubated with HRP-conjugated anti-mouse IgG (Sigma-Aldrich Co., St Louis, MO, USA; A4416-1ML, 1:2500) for 1 hour with gentle shaking at RT (25°C). Blot was developed using diaminobenzidine/NiCl2/H2O2 solution and documented using G-Box chemiXX6 imaging system (Syngene).
Intact mass analysis
The intact mass of SB005 and reference product was determined using LC-ESI-MS (AB SCIEX Triple TOF® 5600+) (SCIEX, Framingham, WA, USA). In total, 10 μg of intact protein sample was injected in time of flight-mass spectroscopy (TOF-MS) through Acquity UPLC (Waters®; Waters Corporation, Milford, MA, USA). The acquired protein spectra were deconvoluted using Bayesian tool of Analyst TF 1.6 software (SCIEX).
Sequence coverage analysis
Sequence coverage analysis is done for obtaining extensive sequence information over the entire length of a protein in a single series of experiments using high-resolution and high mass accuracy tandem mass spectrometry (MS). SB005 and reference product were denatured using 8 M urea, reduced using 10 mM DTT and alkylated using 40 mM IAM followed by digestion with trypsin and deglycosylation with enzyme PNGase-F in ammonium bicarbonate buffer. The generated peptides were analyzed using LC-ESI-MS. ProteinPilot software was used to get the percentage sequence coverage. In addition, N-terminal peptide sequence along with “b” and “y” ions coverage was studied.
Peptide fingerprinting
The primary structures of SB005 and the reference product were compared using the peptide fingerprinting method. Proteins were digested separately with trypsin and endoproteinase Asp-N (Sigma-Aldrich Co.) for 20 hours, and peptide mixtures were separated on reverse phase liquid chromatography using Acquity UPLC (Waters).
Peptide mass fingerprinting
The orthogonal approach was adapted to compare the primary structure of SB005 and reference product. Protein samples were digested with trypsin and deglycosylated with enzyme PNGase-F (Prozyme, Hayward, CA, USA). Theoretically calculated peptide masses were extracted using XIC manager and compared with those of reference product.
UV/Vis spectroscopy
The conformational integrity of SB005 and reference product was determined using UV/Vis spectroscopy. SB005 and reference product at 0.2 mg/mL concentration of 10 mM phosphate buffer (pH 6.8) were subjected to a spectral scan from 200 to 800 nm, with path length of 10 mm using Spectramax M3 (Molecular Devices LLC, Sunnyvale, CA, USA).
CD spectroscopy
The conformation of the secondary structure of SB005 and reference product was determined using CD spectrophotometer JASCO J-815 spectrometer (Jasco, Easton, MD, USA). CD spectra were recorded at a temperature of 25°C, with a scanning speed of 1 nm/min and band width of 1 mm. Far UV spectra were recorded with for the range of 190–240 nm at a concentration of 0.1 mg/mL in 10 mM phosphate buffer (pH 6.8).
Fluorescence spectroscopy
Fluorescence studies of SB005 and reference product were carried out using Spectramax M3 at 0.1 mg/mL concentration. These samples were subjected to emission spectra scan from 300 to 800 nm, with path length of 10 mm.
Capillary isoelectrofocusing
Isoforms of SB005 and reference product were separated by capillary isoelectrofocusing using CE (PA800 Plus; Beckman Coulter, Brea, CA, USA). SB005 and reference product at 2.0 mg/mL concentration were mixed with ampholytes and stabilizers. The isoforms were focused by ampholytic pH gradient through an applied electric field. Chemical mobilization was applied to mobilize the pH gradient across the detection window, and separated isoforms were detected at 280 nm. The samples were spiked with internal pI markers, and migration time of individual peak was recorded.
Capillary zone electrophoresis
Charge heterogeneity in biomolecule was measured by CZE using CE. SB005 and reference product (2 mg/mL) were separated in 50 μm × 50.0 cm dynamically coated bare fused silica capillary (AB SCIEX, effective length: 40.2 cm) using Tricine as buffer agent, urea as denaturant and putrescine dihydrochloride as wall modifier. The heterogeneity was detected at 214 nm.
N-glycan mapping using LC-ESI-MS
N-glycans were released from SB005 and reference product using PNGase F enzyme (Prozyme; P0705L) at optimum conditions. The sample mixture was treated with cold ethanol to precipitate the protein, and clear supernatant containing the glycan moieties was collected by centrifugation. Extracted glycans were labeled with a fluorescent 2-aminobenzamide (2-AB) dye (Prozyme; GKK-404). The excess amounts of dye were removed from the mixture by using GlycoClean S cartridges (Prozyme; GKI-4726). Purified labeled glycans were analyzed by LC-ESI-MS in negative mode. The N-glycan masses were extracted using XIC manager, and product ion spectrum of individual mass was checked to confirm glycan species. Further, structural confirmation of each N-glycan species was done using SimGlycan® software.
Released N-glycan analysis
Extracted glycans from SB005 and reference product were labeled with a fluorescent 2-AB dye. The excess amounts of dye were removed from the mixture by using GlycoClean S cartridges. Purified glycans were injected and separated using HPLC on TSKgel DEAE-5PW column (TOSOH, Tokyo, Japan). The individual peaks of SB005 and reference product were integrated and compared.
Point of glycosylation (glycopeptide analysis)
The post-translational modification site was identified in SB005 and reference product by tryptic-chymotryptic digestion method. The peptides generated were analyzed on LC-ESI-MS. The m/z of extracted ions was recorded and loaded in SimGlycan software to identify the point of glycosylation.
Oxidized impurity profiling using reverse phase chromatography
Oxidized impurities present in SB005 and reference product were analyzed on HPLC using C4 column (Waters). Samples (5 μg) were injected, and UV detection was carried out at 220 nm. The chromatograms were integrated, and oxidized impurities were determined.
Oligomer analysis using SEC
Oligomer content of SB005 and reference product was determined using SEC. The analysis was performed using BioSep-SEC-S2000 (Phenomenex, Torrance, CA, USA). The chromatograms were integrated and oligomer content was determined.
In vivo assay
In vivo bioassay of SB005 and reference product was performed as per British Pharmacopoeia, 2012. Male Sprague-Dawley rats (aged 19–21 days at the time of dosing) were used in study. Animals were weighed and divided into groups with 7 animals in each group. SB005 and reference product were administered subcutaneously (3, 9 and 27 IU/kg body weight) to experimental animals (Sprague-Dawley rats, male) for 4 days. On the fifth day, seminal vesicles were removed and weighed. The result of assay was calculated by considering the weight of the seminal vesicles as the response. Potency was calculated based on response.
Animal experiments were conducted according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment, Forests and Climate Change, Government of India and approved by the Institutional Animal Ethics Committee of Sun Pharmaceutical Advanced Research Center, Vadodara, India. All applicable guidelines for the care and use of animals were followed for in vivo bioassay experiments.
Results
SDS-PAGE and western blot analysis
SB005 and reference product were analyzed by reducing and non-reducing SDS-PAGE to evaluate fragmentation, aggregation and purity. No fragmentation or aggregation was observed in the gel after staining with Coomassie Brilliant Blue R-250. Electrophoretic mobility band pattern and molecular weight were found similar in SB005 and reference product in reducing and non-reducing gel (Figure 1A). Similar band pattern and immunoreactivity towards antibody specific to rhCG were observed in western blot experiments (Figure 1B).
Figure 1.

(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of SB005 and reference products in reducing and non-reducing conditions. Lane 1: Molecular weight marker (315-10 kDa). Lane 2: Reference product-1; Lane 3: Reference product-2; Lane 4: SB005. (B) Western blot analysis of SB005 and reference products. Lane 1: Molecular weight marker (315-10 kDa); Lane 2: Reference product-1; Lane 3: Reference product-2; Lane 4: SB005.
Intact mass analysis
Intact mass analysis of SB005 and reference product revealed molecular mass of rhCG. The deconvoluted spectra of SB005 and reference product were tiled horizontally in Analyst software to compare the mass of individual isoform of alpha and beta subunits. The mass profile of SB005 and reference product was found to be similar (Tables 1 and 2).
Table 1.
Intact mass of the isoforms observed in alpha subunits of SB005 and reference products
| Sample name | Alpha subunit
|
|||||
|---|---|---|---|---|---|---|
| Isoforms (Da)
| ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Reference product-1 | 13773.3 | 14024.8 | 14316.3 | 14607.5 | 14681.1 | 14972.4 |
| Reference product-2 | 13733.4 | 14024.7 | 14316.4 | 14607.5 | 14681.2 | 14972.2 |
| SB005 | 13733.5 | 14025.1 | 14316.3 | 14606.9 | 14681.7 | 14972.2 |
Table 2.
Intact mass of the isoforms observed in beta subunits of SB005 and reference products
| Sample name | Beta subunit
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoforms (Da)
| |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Reference product-1 | 21154.1 | 21444.5 | 21736.3 | 22027.6 | 22394.3 | 22684.5 | 23068.6 | 23360.6 | 23651.9 | 23942.7 | 24307.9 | 24454.7 | 24599.7 |
| Reference product-2 | 21154.1 | 21445.8 | 21736.7 | 22027.4 | 22393.5 | 22684.6 | 23069.1 | 23359.9 | 23651.8 | 23942.1 | 24307.5 | 24454.1 | 24600.5 |
| SB005 | 21154 | 21445.9 | 21736.8 | 22028.4 | 22393.5 | 22683.6 | 23069.6 | 23360.7 | 23651.8 | 23943 | 24309.5 | 24454.6 | 24600.4 |
Peptide fingerprinting
Peptide fingerprinting is one of the techniques to characterize a biomolecule in context of its sequence similarity and primary structure. Protein digestion by trypsin and endoproteinase Asp-N gives specific peptide fragments digested from defined site. Any modification in amino acid will change the peptide hydrophobicity and differences will be captured by peptide profile generated on reverse phase chromatography. The chromatogram of SB005 and reference product was integrated, and fingerprint profile of SB005 and reference product was found to be similar (Figures 2 and 3).
Figure 2.
Peptide fingerprinting of SB005 and reference products using trypsin enzyme.
Figure 3.
Peptide fingerprinting of SB005 and reference products using endoproteinase Asp-N enzyme.
Peptide mapping
Peptide mapping is an identified methodological tactic for confirmation of the primary structure of protein. The method is designed to identify any modification or alternation in the primary structure. Generated peptides after digestion were analyzed on UPLC system that was connected to Triple TOF-MS 5600 system to generate the total ion chromatogram (TIC) of peptide mixture. The list of theoretical masses was created in XIC manager and all desired ions were extracted from TIC (Figure 4). The presence of “b” and “y” ions was confirmed in MS-MS spectra. The peptide mass of SB005 was found similar to that of reference product.
Figure 4.
Comparative total ion chromatogram (TIC) profile of SB005 and reference products.
Sequence coverage analysis
Sequence coverage analysis was used for obtaining extensive sequence information over the entire length of a protein in a single series of experiments using high-resolution and high mass accuracy tandem MS. The data acquired from MS and MS/MS were loaded in ProteinPilot software to get the percentage sequence coverage. The overall combined results provided 100% coverage for alpha and beta subunits of SB005 and reference product. In addition, ProteinPilot software facilitated the N-terminal peptide sequence along with “b” and “y” ion coverage.
UV/CD/fluorescence spectroscopy
For UV spectroscopy, the protein concentration was adjusted to 0.5 mg/mL and scanned in UV/Vis range of 200–800 nm. UV/Vis analysis of reference product and SB005 showed similar spectroscopic profile (Figures S1 and S2).
CD is a spectrophotometric tool for determination of the secondary structure and folding properties of proteins. Secondary structure of protein is sensitive to its environment, temperature or pH. CD spectroscopy can be used to study the effect of environmental conditions and interaction of protein with other molecules on secondary structure of protein. CD can be also used to study protein interactions. Far UV-CD spectroscopy analysis of reference product and SB005 showed a similar spectroscopic profile, indicating consistency in the protein with respect to the conformation attributes of the protein (Figure 5).
Figure 5.
Comparative far ultraviolet circular dichroism spectra of SB005 and reference products.
Fluorescence spectroscopy is used to measure intrinsic fluorescence of the molecule, which states about the conformational state of a protein. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of tryptophan residues, with some emissions due to tyrosine and phenylalanine. Tryptophan typically has a wavelength of maximum absorption of 280 nm and an emission peak ranging from 300 to 350 nm depending on the polarity of the local environment. Disulfide bonds also have some absorption in this wavelength range. Tryptophan fluorescence is strongly influenced by the proximity of other amino acid residues therefore it is a sensitive measurement of the conformational state of a protein. SB005 and reference product showed similar fluorescence spectroscopic profile.
Released N-glycan analysis
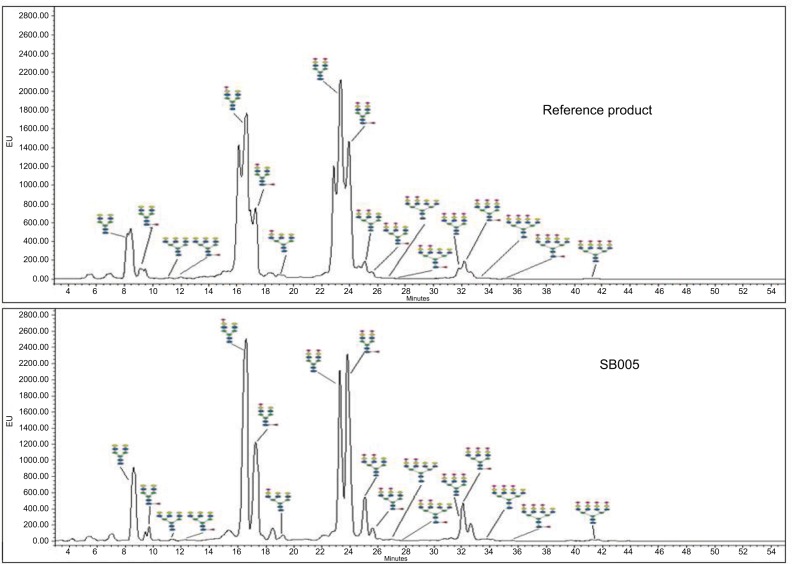
Role of glycans are well understood in protein folding, secretion and plasma half-life of the glycoprotein hormones. In vivo biological activity is dependent on the presence of glycan species. Complete understanding of content and type of glycan is crucial in glycoprotein characterization. The individual peaks of chromatogram of glycans of SB005 and reference product were integrated. The percentage area of neutral, monosialylated, disialylated, trisialylated and tetrasialylated species were determined, and Z-number was calculated. Z-number obtained from SB005 and reference product was found to be similar (Table 3).
Table 3.
Comparative Z-number of SB005 and reference products
| Sample name | Z number |
|---|---|
| Reference product-1 | 141.40 |
| Reference product-2 | 143.87 |
| SB005 | 145.88 |
Labeled and purified glycans were also separated and analyzed using LC-ESI-MS. The N-glycan masses were extracted using peak view software. The product ion spectrum of individual mass was checked for desired product ion to confirm the presence of expected glycan species. Further, structural confirmation of each N-glycan species was done by SimGlycan software. Using MS, 22 species were identified in SB005 and reference product (Figure 6).
Figure 6.
Comparative N-glycan spectra of SB005 and reference product analyzed using ultra performance liquid chromatography (UPLC).
Site of glycosylation by LC-MS
Eight glycosylation sites were identified in SB005 and reference product. Peptide mapping confirmed two glycosylation sites at Asn_52 and Asn_78 in alpha subunit and six glycosylation sites at Asn_105, Asn_122, Ser_213, Ser_219, Ser_224 and Ser_230 in beta subunit of SB005 and reference product.
Product-related impurities
Product-related impurities in term of oligomer content analysis and oxidized impurities in the sample of SB005 and reference product were studied using HPLC. Both alpha and beta subunit sequences of rhCG have amino acids like methionine and asparagine, which are prone to oxidation and deamidation, respectively. These modifications may alter the product efficacy. Hence, close monitoring is required to have control on product quality. It is well known that the oxidative change decreases hydrophobicity of protein molecule and gets eluted earlier than the unchanged molecule. In contrast to oxidation, deamidation causes an increase in hydrophobicity of protein molecule and gets eluted later than the unchanged species. The total percentage of oxidized impurity was found to be less than 1% for SB005 and reference product (Table 4).
Table 4.
Oxidized impurities present in SB005 and reference products
| Sample name | Area of oxidized impurities (%) |
|---|---|
| Reference product-1 | 0.28 |
| Reference product-2 | 0.22 |
| SB005 | 0.94 |
The molecule may undergo unexpected modifications like oxidation and deamidation during production process. These changes may impact critically on aggregation of a biomolecule. SEC separates molecule on the basis of its hydrodynamic volume; higher molecular weight molecules are eluted earlier than the molecules having comparatively lower molecular weight. Thus, SEC may be an accurate method for analysis of protein aggregation (oligomer), which indicates purity of protein. The oligomer content in the SB005 and reference product was found to be less than 1% (Table 5).
Table 5.
Oligomer content present in SB005 and reference products
| Sample name | % Area of oligomer |
|---|---|
| Reference product-1 | 0.36 |
| Reference product-2 | 0.26 |
| SB005 | 0.92 |
Capillary isoelectrofocusing
Capillary isoelectrofocusing analysis is a highly resolving technique to separate the proteins primarily on the basis of difference in their isoelectric points (pI). The isoforms of proteins were focused by ampholytic pH gradient through an applied electric field. The obtained profile was integrated and seven isoforms were observed. The migration time of individual isoform and spiked pI marker was recorded. A graph of migration time was plotted against isoelectric point of spiked pI marker. From the equation derived from the graph, the pI of individual isoform observed in SB005 and reference product was calculated. The isoelectric point of individual isoform observed in SB005 was compared to reference product and found to be similar (Table 6).
Table 6.
Isoelectric point (pI) values of individual isoforms of SB005 and reference products
| Sample name | pI values of individual isoforms
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Reference product-1 | 5.4 | 4.9 | 4.8 | 4.4 | 4.0 | 3.8 | 3.2 |
| Reference product-2 | 5.5 | 5.0 | 4.7 | 4.5 | 4.1 | 4.0 | 3.4 |
| SB005 | 5.5 | 5.0 | 4.7 | 4.5 | 4.2 | 3.8 | 3.4 |
Capillary zone electrophoresis
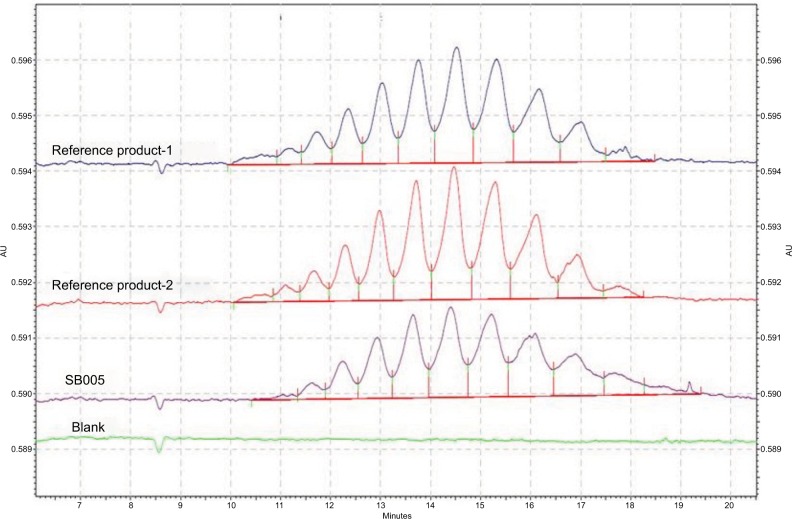
CZE is one of the characterization tools used to observe the charge heterogeneity in glycosylated biomolecule. rhCG is a member of glycoprotein hormone family. These carbohydrate modifications account for 30–35% of the mass of the hormone and result in several different glycoforms. CZE was used to compare the charge distribution and microheterogeneity between SB005 and reference product. The profile and number of isoforms of SB005 and reference product were compared and found to be similar (Figure 7).
Figure 7.
Comparative capillary zone electrophoresis (CZE) profile of SB005 and reference products.
Potency
Potency determination is very important for biopharmaceutical drugs. In vivo bioassay determination is an essential tool for biological characterization of biopharmaceuticals. Bioassay provides biological activity and potency of the drug in the living organism. The result of assay was calculated by considering the weight of the seminal vesicles as the response in Sprague-Dawley rats. The in vivo potency of SB005 was compared to reference product (Table 7) and was found to be similar (p>0.5).
Table 7.
In vivo potency of SB005 and reference products
| Batch name | In vivo potency (IU/mg) |
|---|---|
| Reference product-1 | 29901.18 |
| Reference product-2 | 24528.80 |
| SB005 | 27037.40 |
Discussion
A biosimilar is a biological product that is similar to an existing approved biological drug already approved by regulatory agencies. It should demonstrate similarity to the innovator product with regards to biological characteristics, activity, safety and efficacy. It can enter the market as and when the original product goes off patent.8 Biologicals being expensive, it is anticipated that biosimilar will deliver price reductions of 15–30%.9 Manufacturing process during development of biosimilar can lead to small changes in the structure of biologics, which can affect their function. These might be due to the environment in which the cells grow and post-translational modifications (like glycosylation, phosphorylation, acetylation, etc.). The glycosylation profile of biologics is very important and should be well characterized due to their potential impact on clinical outcome.10 Thus, a complete analytical characterization of the biosimilar being developed needs to be performed in comparison to the reference product, which is already approved by regulatory agencies.11 Physiochemical and biological assays are performed for biosimilar product characterization and they provide information about the structure, function, biological efficacy and safety of the product, helping in biosimilar advancement.12
Advances in analytical methods allow for the complete and accurate characterization of biosimilars. LC, MS, CD, UV spectroscopy and CZE are some of the techniques used for verifying the presence of sequence variants, post-translational modifications, (such as glycosylation profile), product isoforms and higher order structures. In vivo assays provide valuable information about the potency, biological efficacy and safety of the drug product. Regulatory requirements exist to compare the similarity between biosimilar with existing innovator drugs.13,14 Biosimilar product should not have clinically meaningful differences in terms of safety and effectiveness from the reference product. Only minor differences in clinically inactive components are allowable in biosimilar products. Establishing a high level of structural and functional similarity with reference product at the biosimilar development process may be relevant to clinical outcomes.
Regulatory agencies recommend a stepwise approach for demonstrating biosimilarity between a proposed biosimilar product and reference biological product emphasizing for functional and structural characterization of the proposed biosimilar product to trace if there is any difference that may impact safety and efficacy. The analytical comparability studies are followed by clinical PK/PD and immunogenicity studies. The regulatory agencies expect to conduct clinical comparative efficacy studies if any ambiguity is found in analytical, PK/PD and immunogenicity. Hence, the analytical comparability in functional and structural characterization plays a big role in establishing biosimilarity of the product. Analytical comparability of SB005 and reference product with respect to its primary and higher order structure, isoforms and charge variants, glycosylation and in vivo efficacy was evaluated and was found to be similar.
Conclusion
In this study, we have compared analytical similarity of recombinant rhCG (SB005) produced at Sun Pharmaceuticals with the reference product with respect to its primary, higher order structure, isoforms, charge variants, glycosylation, sialylation pattern, pharmacodynamic and in vivo efficacy.
A detailed physicochemical and biological comparability of the biosimilar being developed with the reference product provides a foundation for the subsequent demonstration of biosimilarity in pre-clinical and clinical trials. A high degree of structural and functional similarity ensures that pre-clinical and clinical studies will also reveal a comparable safety and efficacy profile for the biosimilar.15 Our studies show that the in-house produced rhCG has a high degree of structural and functional similarity with the reference product available in the market.
Supplementary Materials
UV-visible spectroscopy profile of reference product and SB005.
Abbreviations: OD, optical density; UV, ultra-violet.
Fluorescence spectroscopic profile of reference products and SB005.
Footnotes
Disclosure
The authors of this article are employed at either Sun Pharmaceutical Industries Limited or Sun Pharmaceutical Advanced Research Center Vadodara, India. The authors report no other conflicts of interest in this work.
References
- 1.Fournier T, Guibourdenche J, Evain-Brion D. Placenta. 2015;36(Suppl 1):S60–S65. doi: 10.1016/j.placenta.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Ezcurra D, Humaidan P. A review of luteinising hormone and human chorionic gonadotropin when used in assisted reproductive technology. Reprod Biol Endocrinol. 2014;12:95. doi: 10.1186/1477-7827-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allahbadia G. Recombinant or urinary human chorionic gonadotropin in ovulation induction? J Obstet Gynaecol India. 2011;61(6):621–623. doi: 10.1007/s13224-011-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonjallaz P, Loumaye E. Recombinant hCG (OVIDREL) and recombinant interferon-(beta)1a (REBIF). (No. 13 in a series of articles to promote a better understanding of the use of genetic engineering) J Biotechnol. 2001;87(3):279–281. doi: 10.1016/s0168-1656(01)00238-3. [DOI] [PubMed] [Google Scholar]
- 5.Eleryan MG, Akhiyat S, Rengifo-Pardo M, Ehrlich A. Biosimilars: potential implications for clinicians. Clin Cosmet Investig Dermatol. 2016;9:135–142. doi: 10.2147/CCID.S91691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91(3):405–417. doi: 10.1038/clpt.2011.343. [DOI] [PubMed] [Google Scholar]
- 7.Berkowitz SA, Engen JR, Mazzeo JR, Jones GB. Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov. 2012;11(7):527–540. doi: 10.1038/nrd3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadam V, Bagde S, Karpe M, Kadam V. A comprehensive overview on biosimilars. Curr Protein Pept Sci. 2016;17(8):756–761. doi: 10.2174/1389203717666160226144618. [DOI] [PubMed] [Google Scholar]
- 9.Simoens S. Biosimilar medicines and cost-effectiveness. Clinicoecon Outcomes Res. 2011;3:29–36. doi: 10.2147/CEOR.S12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29(4):310–312. doi: 10.1038/nbt.1839. [DOI] [PubMed] [Google Scholar]
- 11.ICH . Comparability of Biotechnological/Biological Product Subject to Changes in their Manufacturing Process Q5E. 2005. ICH Harmonised Tripartite Guideline. [Google Scholar]
- 12.Tsuruta LR, Lopes dos Santos M, Moro AM. Biosimilars advancements: moving on to the future. Biotechnol Prog. 2015;31(5):1139–1149. doi: 10.1002/btpr.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ICH . Pharmaceutical Development Q8 (R2) 2009. ICH Harmonised Tripartite Guideline. [Google Scholar]
- 14.ICH . Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities) Q11. 2012. ICH Harmonised Tripartite Guideline. [Google Scholar]
- 15.Visser J, Feuerstein I, Stangler T, Schmiederer T, Fritsch C, Schiestl M. Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab. BioDrugs. 2013;27(5):495–507. doi: 10.1007/s40259-013-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UV-visible spectroscopy profile of reference product and SB005.
Abbreviations: OD, optical density; UV, ultra-violet.
Fluorescence spectroscopic profile of reference products and SB005.