Abstract
Acute kidney injury (AKI) is associated with subsequent chronic kidney disease (CKD), but the mechanism is unclear. To clarify this, we examined the association of AKI and new-onset or worsening proteinuria during the 12 months following hospitalization in a national retrospective cohort of United States Veterans hospitalized between 2004–2012. Patients with and without AKI were matched using baseline demographics, comorbidities, proteinuria, estimated glomerular filtration rate, blood pressure, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker (ACEI/ARB) use, and inpatient exposures linked to AKI. The distribution of proteinuria over one year post-discharge in the matched cohort was compared using inverse probability sampling weights. Subgroup analyses were based on diabetes, pre-admission ACEI/ARB use, and AKI severity. Among the 90,614 matched AKI and non-AKI pairs, median estimated glomerular filtration rate was 62 mL/min/1.73m2. The prevalence of diabetes and hypertension were 48% and 78%, respectively. The odds of having one plus or greater dipstick proteinuria was significantly higher during each month of follow-up in patients with AKI than in patients without AKI (odds ratio range 1.20–1.39). Odds were higher in patients with Stage II or III AKI (odds ratios 1.32–1.81) than in Stage I AKI (odds ratios 1.18–1.32), using non-AKI as the reference group. Results were consistent regardless of diabetes status or baseline ACEI/ARB use. Thus, AKI is a risk factor for incident or worsening proteinuria, suggesting a possible mechanism linking AKI and future CKD. The type of proteinuria, physiology, and clinical significance warrant further study as a potentially modifiable risk factor in the pathway from AKI to CKD.
Keywords: Acute kidney injury, proteinuria
INTRODUCTION
Acute kidney injury (AKI) is increasingly recognized as a common complication of acute illness and a risk factor for morbidity and mortality.1–10 AKI is associated with the development or progression of chronic kidney disease (CKD), but the mechanisms are not well-understood.11–18 Proteinuria is also a well-established predictor of future loss of kidney function,19–22 and better understanding of the longitudinal association between AKI and proteinuria may help reveal a mechanism linking AKI and progression of CKD.
Preclinical data demonstrate that animals subjected to renal ischemia-reperfusion injury develop proteinuria even after serum creatinine has returned to baseline.23 Prior studies in children demonstrate increased risk of developing proteinuria and CKD after AKI, but these studies are limited by lack of appropriate controls and pre-AKI proteinuria data.24–27 Similarly, a recent study in adults with dialysis-requiring AKI demonstrates a 42% prevalence of albuminuria during the follow-up period, but lacks information on pre-hospitalization proteinuria.28 Since proteinuria is a known risk factor for AKI,29,30 it is unclear whether baseline proteinuria is an unaccounted for confounder predisposing these patients to AKI, or whether AKI itself increases the risk for developing incident or progressive proteinuria.
Because proteinuria is a prognostic indicator and a modifiable risk factor in CKD progression, determining whether AKI is associated with incident or progressive proteinuria is an important step toward understanding the link between AKI and future CKD, and formulating therapeutic strategies for AKI survivors. We hypothesized that AKI is associated with both incident and progressive proteinuria. To test our hypothesis, we conducted this study within a large national, observational, matched cohort of U.S. Veterans hospitalized between 2004 and 2012.
RESULTS
Patient Characteristics
A total of 657,840 patients were eligible for the study, 115,467 of whom experienced AKI. Cohort selection is shown in Figure 1, and cohort characteristics prior to matching are presented in Supplementary Table S1. Optimal-distance-matching resulted in a total of 181,228 patients, with 90,614 experiencing AKI, and 90,614 not experiencing AKI. AKI and non-AKI groups were well matched (Table 1), except for severe sepsis (standardized difference 19.4%). The proportions of patients experiencing Stage I, II, or III AKI were 84% (n=75,862), 10% (n=9,575), and 6% (n=5,177), respectively. Median patient age was 66 (IQR, 59–77) years, 98% were male, 75% Caucasian, 48% had diabetes mellitus, and 78% had hypertension. Median baseline eGFR was 62 (IQR, 48–77) mL/min/1.73m2. Fifty-six percent of the cohort had ACEI/ARB prescriptions within 90 days prior to admission. Pre-admission proteinuria levels in the AKI and non-AKI groups were well-matched, with 61% of patients in each group having no proteinuria at baseline, 17% with trace, 12% with 1+, 7% with 2+, and 3% with 3+ proteinuria. During the 12-month follow-up, 46% of patients had one urine dipstick measurement, 27% had two measurements, and 27% underwent three or more assessments.
Figure 1.
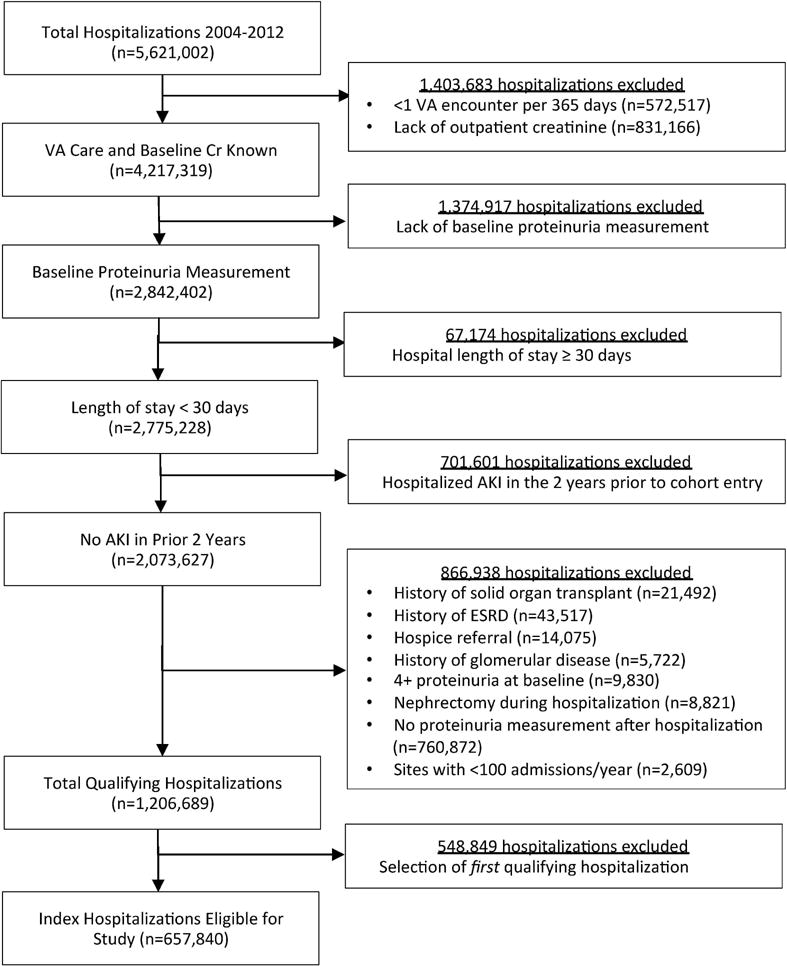
Flow diagram depicting selection of eligible hospitalizations for the study cohort.
Table 1.
Baseline and inpatient characteristics of matched cohort
| Characteristic | Total (n=181,228) |
No AKI (n=90,614) |
AKI (n=90,614) |
Standardized Difference |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Age | 66 (59–77) | 66 (59–77) | 67 (59–77) | <0.01 |
| Gender (% male) | 177,266 (98%) | 88,688 (98%) | 88,578 (98%) | <0.01 |
| Race (% white) | 136,239 (75%) | 68,556 (76%) | 67,683 (75%) | 0.02 |
| Baseline characteristics | ||||
| Preadmission low dipstick proteinuria (Negative) | 122,700 (68%) | 61,130 (67%) | 61,570 (68%) | <0.01 |
| Preadmission high dipstick proteinuria (3+) | 9,750 (5%) | 4,672 (5%) | 5,078 (6%) | 0.04 |
| Median (baseline) pre-admission dipstick proteinuria (Negative) | 110,460 (61%) | 55,230 (61%) | 55,230 (61%) | <0.01 |
| Baseline eGFR mL/min/1.73m2 | 62 (48–77) | 62 (48–77) | 62 (47–77) | 0.01 |
| Mean preadmission systolic blood pressure | 134 (125–144) | 134 (125–143) | 135 (125–144) | 0.05 |
| ACEI/ARB use within 90 days | 101,322 (56%) | 49,416 (55%) | 51,906 (57%) | 0.06 |
| Prescription NSAID use within 90 days | 36,787 (20%) | 17,098 (19%) | 19,689 (22%) | 0.07 |
| Diuretic use within 90 days | 78,215 (43%) | 36,800 (41%) | 41,415 (46%) | 0.10 |
| Number of creatinine measurements in year prior to admission | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) | 0.08 |
| Number of urine dipstick proteinuria measurements in year prior to admission | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 0.08 |
| Comorbidities | ||||
| Diabetes mellitus | 87,429 (48%) | 42,200 (47%) | 45,229 (50%) | 0.07 |
| Hypertension | 141,162 (78%) | 70,555 (78%) | 70,607 (78%) | <0.01 |
| Congestive heart failure | 19,103 (11%) | 8,999 (10%) | 10,104 (11%) | 0.04 |
| Coronary artery disease | 51,765 (29%) | 25,465 (28%) | 26,300 (29%) | 0.02 |
| Liver disease / cirrhosis | 3,765 (2%) | 1,835 (2%) | 1,930 (2%) | <0.01 |
| Peripheral vascular disease | 7,244 (4%) | 3,487 (4%) | 3,757 (4%) | 0.02 |
| Dementia | 5,971 (3%) | 2,859 (3%) | 3,112 (3%) | 0.02 |
| Cerebrovascular disease | 23,844 (13%) | 11,090 (12%) | 12,754 (14%) | 0.05 |
| Chronic obstructive pulmonary disease | 11,857 (7%) | 5,411 (6%) | 6,446 (7%) | 0.05 |
| Peptic ulcer disease | 4,016 (2%) | 1,991 (2%) | 2,025 (2%) | <0.01 |
| Cancer (except non-melanoma skin cancer) | 32,960 (18%) | 15,394 (17%) | 17,566 (19%) | 0.06 |
| Acuity | ||||
| ICU hospitalization | 22,582 (12%) | 9,730 (11%) | 12,852 (14%) | 0.10 |
| AKI characteristics | ||||
| Stage 1 | N/A | N/A | 75,862 (84%) | N/A |
| Stage 2 | N/A | N/A | 9,575 (10%) | N/A |
| Stage 3 | N/A | N/A | 5,177 (6%) | N/A |
| Inpatient exposures | ||||
| Contrast dye exposure | 12,357 (7%) | 5,778 (6%) | 6,579 (7%) | 0.04 |
| NSAID use | 13,397 (7%) | 6,238 (7%) | 7,159 (8%) | 0.04 |
| Inpatient Conditions | ||||
| Severe Sepsis | 11,377 (6%) | 3,571 (4%) | 7,806 (9%) | 0.19 |
| Congestive heart failure | 23,998 (13%) | 10,699 (12%) | 13,299 (15%) | 0.08 |
| Acute coronary syndrome | 8,204 (5%) | 3,918 (4%) | 4,286 (5%) | 0.02 |
| Major Surgery | ||||
| Abdominal | 4,411 (2%) | 2,197 (2%) | 2,214 (2%) | <0.01 |
| Vascular | 5,302 (3%) | 2,587 (3%) | 2,715 (3%) | <0.01 |
Abbreviations: ACEI/ARB, angiotensin converting enzyme inhibitor or angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; NSAID, non-steroidal anti-inflammatory drugs. Variables used in matching with prevalence <2% not shown in the table include: Connective tissue disease, acute decompensated liver disease, prior stem cell or bone marrow transplant, HIV, mechanical ventilation, aminoglycoside exposure, inpatient gastrointestinal bleed, cardiac surgery during hospitalization, and inpatient chemotherapy. Veterans Integrated Service Network (VISN) location and year of admission were used in matching but omitted from the table due to number of categories.
Categorical variables are presented as absolute numbers and percentages. Continuous variables are reported as median and interquartile range. Standardized difference less than 10% (0.10) represents no significant difference between groups after matching.
Primary Analysis
In each of the 12 follow-up months, the proportion of patients in the AKI group with ≥1+ dipstick proteinuria (median per follow-up month) was significantly higher than at baseline (Figure 2). Using inverse probability sampling weights (IPSW) logistic regression, patients experiencing AKI had higher odds of having ≥1+ proteinuria during each of the 12 months of follow-up than matched non-AKI counterparts (Table 2), with OR’s ranging 1.20–1.39 (P<0.001 for all). This association was strongest at 2 months following hospital discharge, with OR 1.39 (95% CI, 1.33–1.46), but persisted throughout follow-up (full details in Supplementary Table S2).
Figure 2.
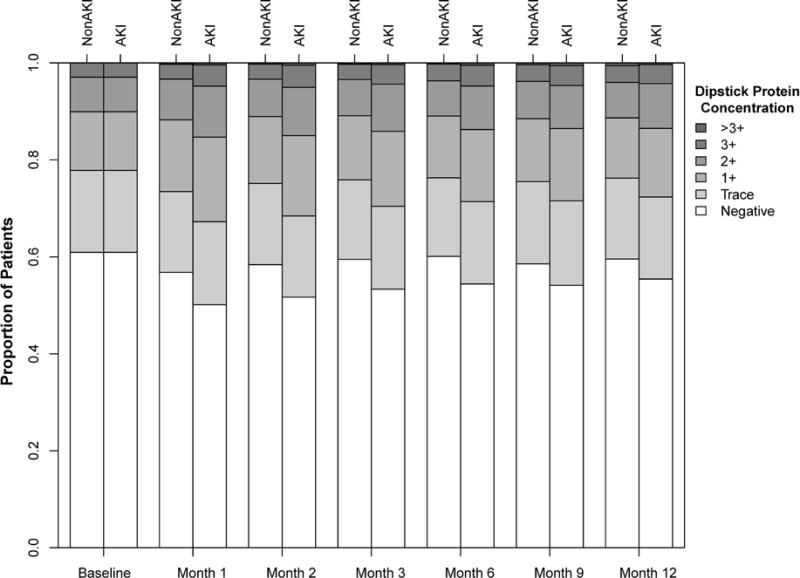
Bar plot depicting degree of proteinuria in patients experiencing versus those not experiencing AKI.
Table 2.
Inverse probability sampling weighted logistic regression for dipstick proteinuria during 12-month follow-up after hospital discharge.
| Month | Odds Ratio (95% CI) | P-value |
|---|---|---|
|
| ||
| 1 | 1.35 (1.29–1.41) | <0.001 |
| 2 | 1.39 (1.33–1.46) | <0.001 |
| 3 | 1.32 (1.26–1.39) | <0.001 |
| 4 | 1.26 (1.19–1.32) | <0.001 |
| 5 | 1.29 (1.22–1.36) | <0.001 |
| 6 | 1.29 (1.22–1.36) | <0.001 |
| 7 | 1.24 (1.17–1.31) | <0.001 |
| 8 | 1.23 (1.16–1.30) | <0.001 |
| 9 | 1.23 (1.16–1.30) | <0.001 |
| 10 | 1.20 (1.13–1.27) | <0.001 |
| 11 | 1.25 (1.17–1.34) | <0.001 |
| 12 | 1.23 (1.12–1.35) | <0.001 |
95% CI: 95% Confidence Interval. Odds Ratio represents odds of having ≥ 1+ median dipstick proteinuria per month during follow-up using non-AKI group as reference group.
Pre-specified Subgroup Analyses
To examine whether diabetes mellitus explained the risk for worsening proteinuria following AKI, we examined proteinuria severity after AKI, stratified by baseline diabetes status. Patients with diabetes had a higher prevalence of proteinuria (≥1+) at baseline than patients without diabetes (29% versus 16%). However, in patients with or without diabetes, those experiencing AKI had a relative increase in proteinuria in the subsequent 12 months compared to their non-AKI counterparts (Figure 3 a–b).
Figure 3.
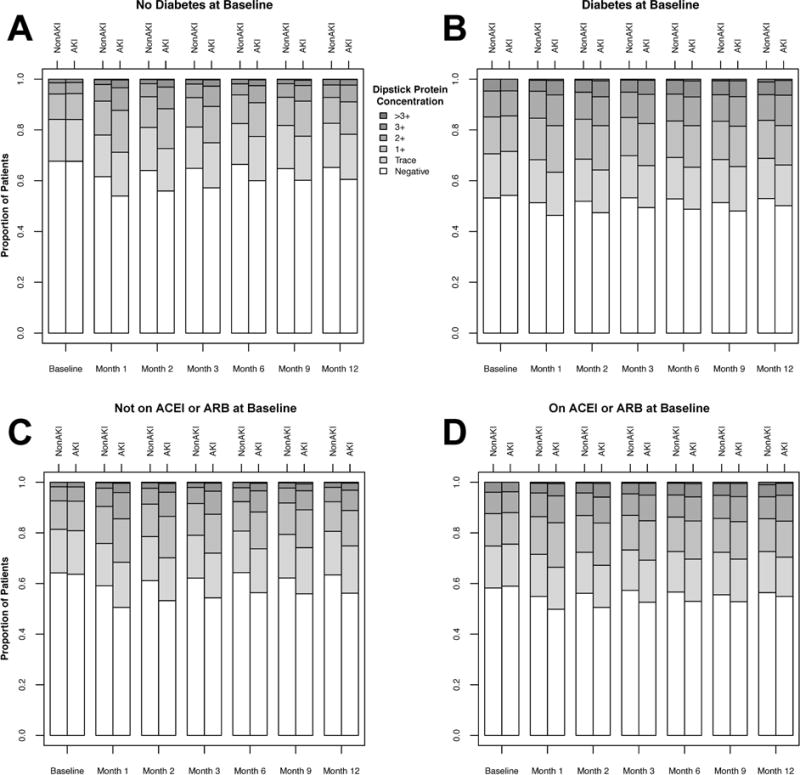
Bar plot depicting degree of proteinuria in patients experiencing versus those not experiencing AKI, stratified by no history of diabetes mellitus (A: top left), or prior diagnosis of diabetes mellitus (B: top right); not on angiotensin converting enzyme inhibitor or angiotensin II receptor blocker (ACEI/ARB) within 90 days prior to admission (C: bottom left), or on ACEI/ARB within 90 days prior to admission (D: bottom right).
In the subgroup analysis stratified by pre-admission ACEI/ARB use, patients on ACEI/ARB had a higher prevalence of proteinuria (≥1+) at baseline than those not on ACEI/ARB (25% versus 19%). However, in patients previously on ACEI/ARB and those not on ACEI/ARB, patients experiencing AKI exhibited increased proteinuria in the 12 months following hospital discharge. (Figure 3 c–d).
To examine whether there is a dose-response relationship between AKI and subsequent proteinuria, we performed a subgroup analysis stratified by AKI severity. We first compared patients with Stage I AKI to matched non-AKI counterparts (covariate balance shown in Supplementary Table S3). Compared to patients without AKI, those with Stage I AKI had higher odds of having ≥1+ proteinuria during each of the 12 months of follow-up with OR’s ranging 1.18–1.32 (P<0.001 for all). Next, we compared patients with Stage II or III AKI to matched non-AKI counterparts (covariate balance shown in Supplementary Table S4). Compared to non-AKI counterparts, patients with Stage II or III AKI had higher odds of having ≥1+ proteinuria throughout follow-up, with OR’s ranging 1.32–1.81 (P<0.001 for all), shown in Supplementary Table S5 and Supplementary Figure S1.
Evaluation of Potential Confounders
Blood Pressure
Since elevated blood pressure can exacerbate proteinuria, we included preadmission systolic blood pressure as a matching covariate. The median baseline systolic blood pressure was 134 mmHg in the non-AKI group and 135 mmHg in the AKI group. We also evaluated the differences in systolic blood pressure between the AKI and non-AKI groups over the duration of follow-up. During the first 2 months of follow-up, mean systolic blood pressure in the AKI group was 0.2–0.9 mmHg lower than in the non-AKI group (Supplementary Table S6). For the remainder of follow-up months, mean systolic blood pressure was 0.1–0.4 mmHg higher in the AKI group.
Urine Concentration
Because differences in urine concentration between the AKI and non-AKI groups (e.g. diuretic cessation or diluting/concentrating defects in the AKI group) could introduce bias, we evaluated urine specific gravity during the follow-up period. To account for potential ascertainment bias, we used IPSW to determine mean urine specific gravity in each group. The mean urine specific gravity in the AKI group was modestly lower at all months of follow-up than in the non-AKI group (Supplementary Table S7).
Subgroup Analyses Stratified by Degree of Baseline Proteinuria
To examine whether AKI is associated with incident proteinuria, worsening proteinuria, or both, we performed subgroup analyses stratified by degree of baseline proteinuria. In patients with negative or trace baseline proteinuria (N=141,034), the odds for developing 1+ or greater proteinuria during 12-month follow-up were higher in patients experiencing AKI than in matched non-AKI counterparts, with OR’s ranging 1.35–1.65 (P <0.001 for all). This association was strongest at 2 months following hospital discharge, with OR 1.65 (95% CI, 1.54–1.76), but persisted throughout follow-up (full details in Supplementary Table S8). In patients with ≥1+ proteinuria at baseline (N=40,194), we examined the odds of developing worsening proteinuria per month of follow-up. Compared to matched non-AKI counterparts, patients experiencing AKI had increased odds of developing worsening proteinuria during 12-month follow-up, with OR’s ranging 1.03–1.37. This association was strongest in the first month following hospital discharge, with OR 1.37 (95% CI, 1.24–1.52), but persisted throughout follow-up (full details in Supplementary Table S9).
DISCUSSION
In this large, national cohort of U.S. Veterans, we found that patients experiencing AKI were at increased risk for developing new or worsening proteinuria in the 12 months following an AKI episode compared to well-matched non-AKI controls. This effect was most pronounced in the first 2 months post-discharge, but persisted throughout follow-up. We observed a dose-response effect between AKI severity and subsequent proteinuria. Our findings remained consistent regardless of diabetes status or baseline ACEI/ARB use.
Acute kidney injury is increasingly common, complicating up to 20% of hospitalizations and growing in incidence by 10–11% per year.1,5–9,31,32 The traditional belief that AKI is ‘self-limited’ has been challenged by studies showing an association between AKI and progressive kidney disease11,14–17 and cardiovascular events.33–35 The combination of population growth, increasing AKI incidence,32 and improved short-term survival1,36,37 have resulted in an expanding population of AKI survivors at risk for adverse renal and cardiovascular outcomes. Prior studies demonstrating that AKI is associated with an increased risk for developing CKD or proteinuria were limited by lack of appropriate controls and assessment of pre-existing proteinuria.24–28 Since proteinuria is a known risk factor for AKI,29,30,38,39 assessing baseline proteinuria levels is essential in examining the association between AKI and subsequent incident or worsening proteinuria. Our study includes a well-matched non-AKI cohort to address previously unaccounted for confounding and evaluates longitudinal change in proteinuria.
Proteinuria is a well-established and potentially modifiable risk factor in both CKD progression19–22,40–46 and cardiovascular events.47–49 Preclinical studies demonstrate that AKI can lead to development of proteinuria, even after apparent recovery.23,50 Prior studies postulate that albuminuria may result from acute changes in glomerular capillary permeability,51 inflammation,52 impaired proximal tubular reclamation,53,54 or impaired myogenic autoregulation,55 any of which could exert maximal effect soon after an AKI event. In our study, systolic blood pressure in the AKI and non-AKI groups were comparable, and were not increased from baseline throughout the follow-up period. However, even in the setting of normal blood pressure, impaired autoregulation may result in unregulated transmission of arterial hydrostatic pressure,55,56 which could in turn cause increased proteinuria/albuminuria. Our results extend upon recent studies suggesting proteinuria may be an early marker of AKI,57–61 by demonstrating that these changes persist well beyond hospital discharge.
The optimal care for AKI survivors is not well-defined. Few patients see a nephrologist or have proteinuria measured following AKI.62–64 We previously demonstrated in a regional VA cohort that only about 1/3 of patients with hospitalized AKI receive any proteinuria surveillance (urine dipstick or quantitative measurement) in the year following hospitalization, and only 12% had a quantitative measurement.65 While the results of our current study indicate that AKI is associated with an increased risk for worsening proteinuria, it would be premature to recommend routine surveillance or intervention among all AKI survivors. Future work to characterize the type and mechanism of this proteinuria is warranted to examine prognostic implications, and to determine whether this represents a potentially modifiable risk factor in the AKI to CKD pathway.
Strengths of this study include the use of a large, well-phenotyped, matched, Veteran population with extensive laboratory data, vital statistics, and pharmacy records within an integrated healthcare system. Our study is not without limitations. Despite rigorous attempts to control for confounding, the observational nature of this study precludes our ability to make causal inferences between AKI and subsequent proteinuria. For example, patients who experience AKI may undergo more frequent follow-up and more aggressive surveillance, leading to ascertainment bias. However, we included frequency of pre-admission urine dipstick measurements in the matching process, and the proportion of patients with at least one dipstick measurement during follow-up was similar between the two groups. Furthermore, we used IPSW to account for potential differential rates of proteinuria testing and death in the AKI and non-AKI groups. Second, since proteinuria is affected by blood pressure and ACEI/ARB use, it is plausible that differential care practices in the AKI and non-AKI groups impacted our results. For example, increased blood pressure or ACEI/ARB discontinuation in the AKI group could have confounded our results. However, our evaluation of post-discharge systolic blood pressure revealed no clinically significant differences between the AKI and non-AKI groups, suggesting that blood pressure alterations following AKI did not confound our findings. Additionally, our findings remained consistent in the subgroup of patients not on ACEI/ARB at baseline, suggesting that our results are unlikely to be explained by differential practice patterns between the AKI and non-AKI groups. Nonetheless, a more comprehensive evaluation of these and other potential mediators using a time-varying study design is an important area of future investigation. Third, we are limited by the semi-quantitative, nonspecific nature of urine dipstick measurements. These results may be affected by timing of collection, and intra- or inter-observer variability, which were not adjusted for in our analysis, but are unlikely to be differentially affected by AKI status. Since urine dipstick protein is a concentration-based assay, hydration status and tubular concentrating or diluting defects could also have impacted our results. However, subjects were matched with respect to baseline diuretic use and we examined post-discharge urine specific gravity. The mean specific gravity was modestly lower at all months of follow-up in the AKI group than in the non-AKI group, which could bias toward the null. Urinary tract infections, urine pH, and/or gross hematuria were not accounted for in our analysis, and could differ between AKI and non-AKI groups. Despite these limitations, we chose to use urine dipstick protein for our primary outcome, because it is a routine laboratory test, making our findings more generalizable. Finally, by requiring both pre- and post-hospitalization measurement of dipstick protein for study inclusion, we may have selected a study cohort that differs from the general population. This does not, however, alter the validity of our findings, as this group of patients is likely at highest risk for adverse outcomes after AKI and stands to benefit from future prospective studies.
In conclusion, while the population of AKI survivors at risk for progression to CKD is growing, optimal care practices for these patients are unclear. We demonstrate that AKI is a risk factor for new-onset or worsening proteinuria, suggesting a potential mechanism linking AKI and future CKD. The type of proteinuria, its mechanism, and clinical significance warrant further study, and represent a potentially modifiable risk factor in the pathway from AKI to CKD.
METHODS
Setting and Study Population
This retrospective cohort study included admissions to all Veterans Affairs (VA) Health Administration hospitals with at least 100 admissions per year (N=116) from January 1, 2004 through December 31, 2012. Patients were eligible for study inclusion if they were ≥18 years of age; had ≥1 annual encounter in the VA system for the 2 years prior to hospitalization; had no AKI hospitalizations during a 2-year “look back” period; ≥1 outpatient serum creatinine and urine dipstick measurement 7 to 365 days prior to hospitalization (to establish baseline renal function and proteinuria); ≥1 outpatient dipstick proteinuria measurement in the 12 months following hospital discharge; and hospital length of stay ≤30 days (to avoid enriching for patients receiving long-term/chronic skilled care) (Figure 1). Patients were excluded from the study based upon end stage renal disease (ESRD); history of solid organ transplant; hospice referral during the hospitalization; known primary glomerular disease (e.g. membranous glomerulonephritis, minimal change disease); nephrectomy during the index hospitalization; and pre-hospitalization urine dipstick proteinuria of ≥1000 mg/dL (>3+), as further increase in proteinuria is not detectable by semi-quantitative dipstick measurement. Among patients with more than one qualifying hospitalization, the first hospitalization was selected.
The study was approved by the Institutional Review Board and the Research and Development committee of the Tennessee Valley Healthcare System VA.
Data Sources
The primary data sources used in this study were the Veterans Health Administration Corporate Data Warehouse (CDW) and the Medical SAS files.66–68 Demographics, vital signs, laboratory data, pharmacy information, and inpatient and outpatient International Classification of Diseases, 9th edition (ICD-9) diagnoses were obtained using the CDW. Inpatient Current Procedural Terminology (CPT) codes were obtained using the Medical SAS files. Dialysis was ascertained using ICD-9 and CPT codes, with linkage to the United States Renal Data System (USRDS) records. Codes used to identify comorbidities, procedures, and inpatient exposures are listed in Supplementary Table 10. Medication data was obtained from VA pharmacy fill records and the inpatient Bar Code Medication Administration record. Contrast dye exposure was ascertained using ICD-9 and CPT codes, and text string processing of radiologic procedure names.
Definitions
Baseline kidney function was defined using the Modification of Diet in Renal Disease formula to calculate estimated glomerular filtration rate (eGFR).69 Based on our prior publication, mean outpatient serum creatinine between 7 and 365 days prior to hospitalization was used to calculate baseline eGFR.70 Using the peak creatinine occurring at any time during hospital admission, AKI was staged with modified Kidney Disease Improving Global Outcomes (KDIGO) creatinine-based consensus criteria as follows: Stage I, ≥0.3mg/dL creatinine increase from baseline or creatinine 1.5–1.9 times baseline; Stage II, creatinine 2.0–2.9 times baseline; and Stage III, creatinine 3.0 times baseline or initiation of renal replacement therapy (dialysis).71 To improve AKI definition specificity and because we had baseline creatinine available,72 we did not apply a rolling 48 hour window (Stage I) or the criterion of creatinine ≥4.0mg/dL (Stage III). Urine output data was not available. Pre-admission CKD was defined as an outpatient baseline eGFR <60 ml/min/1.73 m2. ESRD was defined by a baseline eGFR <15 ml/min/1.73 m2, receipt of dialysis, or history of renal transplantation. Cross-linkage with USRDS was used to confirm non-eGFR based ESRD diagnoses (i.e. chronic dialysis, renal transplantation).
For the primary outcome measure, proteinuria was captured using urine dipstick, based on outpatient measurements obtained 7–365 days prior to the index hospitalization, and at 1–365 days post-discharge. Using standard semi-quantitative urine dipstick thresholds, proteinuria was categorized as 0 = negative, trace = 10–29 mg/dL, 1+ = 30–99 mg/dL, 2+ = 100–299 mg/dL, 3+ = 300–999 mg/dL, >3+ = ≥1000 mg/dL. In patients with multiple pre-admission proteinuria measurements, the median value was used to calculate baseline.73 In patients with multiple dipstick proteinuria measurements in individual months of the follow-up period, the median dipstick proteinuria concentration for the month was used for analysis.
Cohort Selection via Optimal Matching
Because unbalanced baseline characteristics and exposures that increase AKI risk would result in confounding if patients were simply classified by the presence of AKI, we generated a matched cohort of patients such that the distributions of those characteristics were similar in the AKI and non-AKI groups. To ensure perfect balance of preadmission dipstick proteinuria and to facilitate optimal matching in this large cohort, patients were matched on important risk factors for AKI and/or predictors of our outcome within the following strata: 1. median pre-admission dipstick proteinuria 7–365 days prior to admission, 2. baseline eGFR, 3. baseline diabetes, 4. age, and 5. mean outpatient systolic blood pressure in the year prior to admission. Optimal Mahalanobis distance matching74 was used to select patients within each stratum, matching on all baseline factors not perfectly balanced via initial stratification, including demographics, baseline characteristics, pre-morbid diagnoses, hospitalization variables, inpatient exposures, and inpatient diagnoses (full covariate list shown in Supplementary Table S11). Mahalanobis distance matching compares patients directly on their covariates, allowing patients who are similar but not exactly the same to match. Excepting baseline protein dipstick, missing values were permitted and potentially informative missingness was handled using indicator variables.75 Most but not all patients with AKI had a good non-AKI match; thus, we selected the set of 80% best matches for the matched cohort. We assessed the absolute standardized difference among matching variables using a threshold of <10% (0.10) to define adequate covariate balance.76 All design choices were made prior to the analysis of any outcomes.
Statistical Analyses
Baseline cohort characteristics for categorical variables were expressed as proportions and continuous variables were described using median and interquartile ranges (IQR). To account for potential surveillance bias and the anticipated higher rate of death in the AKI group during the follow-up period, we used inverse probability sampling weights (IPSW).77 At each month of follow up, the patients with observed proteinuria measurements were weighted so that the distribution of baseline covariates in the observed patients always mirrored the baseline distribution. Thus having controlled for baseline confounding and ascertainment/survival bias, we presented the comparative distributions of dipstick values over time. We assessed the odds of patients having ≥1+ (30mg/dL) proteinuria during each month of follow-up in the AKI and non-AKI groups using logistic regression with IPSW as sampling weights. We chose a cut-off of ≥1+ proteinuria, which has been previously defined as clinically apparent proteinuria (macroalbuminuria) and strongly associates with kidney disease outcomes.78
Pre-specified Subgroup Analyses
We performed several pre-specified subgroup analyses. First, because diabetes mellitus is associated with proteinuria79 and may modify the relationship between AKI and subsequent degree of proteinuria, we performed a subgroup analysis to assess change from baseline proteinuria during the follow-up period according to diabetic status. Because proteinuria levels are affected by ACEI/ARB,43,80 which may be discontinued during the peri-AKI period, we performed a subgroup analysis stratified by whether patients were on ACEI/ARB at baseline (pharmacy fill within 90 days prior to admission). This was intended to ensure that observed changes in proteinuria in the AKI group were not solely attributable to changes in ACEI/ARB use.
In a third subgroup analysis, we evaluated the relationship between AKI severity and subsequent worsening proteinuria. We compared Stage I AKI with matched non-AKI counterparts, and Stage II or III AKI with matched non-AKI counterparts. As in our main analysis, we performed IPSW logistic regression using AKI as the exposure variable, and assessed the odds of patients having ≥1+ (30mg/dL) proteinuria during each month of follow-up.
Evaluating Potential Confounders
We examined whether differences in systolic blood pressure or urine specific gravity during the follow-up period impacted the results of the primary analysis. To account for potential ascertainment bias during the follow-up period, we performed IPSW for each of these factors. Systolic blood pressure and urine specific gravity are reported as mean ± standard deviation per month of follow-up.
Subgroup Analyses Stratified by Degree of Baseline Proteinuria
To examine whether the association between AKI and subsequent proteinuria is modified by degree of baseline proteinuria, we conducted two additional subgroup analyses. First, in patients with negative or trace baseline proteinuria, we assessed the odds of patients developing ≥1+ proteinuria during each month of follow-up in the AKI and non-AKI groups using logistic regression with IPSW. Second, in patients with ≥1+ proteinuria at baseline, we assessed the odds of patients developing a higher degree of proteinuria during each month of follow-up in the AKI and non-AKI groups using logistic regression with IPSW.
Statistical analyses were performed using R, version 3.1.2.81
Supplementary Material
Acknowledgments
S.K.P. was supported by the Department of Veterans Affairs, Office of Academic Affiliations, Advanced Fellowship Program in Medical Informatics; the National Institutes of Health (NIH) National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) Training Grant K24-DK62849; and the Vanderbilt Center for Kidney Disease (VCKD).
M.E.M. was supported by Veterans Health Administration Health Services Research & Development (HSR&D) Career Development Award CDA 08-020 and Investigator Initiated Research (IIR 11-292).
K.A was supported by National Institutes of Health (NIH) National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) K23-DK090304.
G.C. was supported by National Institutes of Health (NIH) Office of the Director (OD) U2COD023196.
A.M.H. was supported by Veterans Health Administration Clinical Science Research and Development (CSR&D) Merit Award 1I01CX000982-01A1
T.A.I. was supported by National Institutes of Health (NIH) National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) K24-DK62849 and Veterans Health Administration Clinical Science Research and Development (CSR&D) Merit Award 1I01CX000414
E.D.S. was supported by Veterans Health Administration Health Services Research & Development (HSR&D) Investigator Initiated Research IIR 13-073; National Institutes of Health (NIH) National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) K24-DK62849; and the Vanderbilt Center for Kidney Disease (VCKD).
MEM receives research funding from AstraZeneca (unrelated to AKI or proteinuria).
The project [publication or poster] described was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All the other authors declared no competing interests.
Disclaimer: The views expressed herein do not necessarily represent those of the Veterans Administration or Vanderbilt University Medical Center.
Previous Presentations: Preliminary findings were presented at the Annual Meeting of the American Society of Nephrology in Chicago, Illinois on November 18, 2016.
References
- 1.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 2.Pannu N, James M, Hemmelgarn B, et al. Association between AKI, Recovery of Renal Function, and Long-Term Outcomes after Hospital Discharge. Clin J Am Soc Nephrol. 2013;8(2):194–202. doi: 10.2215/CJN.06480612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 4.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 6.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CY, McCulloch CE, Fan D, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr SK, Siew ED. Delayed Consequences of Acute Kidney Injury. Adv Chronic Kidney Dis. 2016;23(3):186–194. doi: 10.1053/j.ackd.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2011;81(5):442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 13.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. doi: 10.1001/archinternmed.2010.514. [DOI] [PubMed] [Google Scholar]
- 14.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakar CV, Christianson A, Himmelfarb J, et al. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol. 2011;6(11):2567–2572. doi: 10.2215/CJN.01120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 19.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 20.Iseki K, Iseki C, Ikemiya Y, et al. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int. 1996;49(3):800–805. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 21.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51(6):1908–1919. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 22.Lea J, Greene T, Hebert L, et al. The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med. 2005;165(8):947–953. doi: 10.1001/archinte.165.8.947. [DOI] [PubMed] [Google Scholar]
- 23.Basile DP, Donohoe D, Roethe K, et al. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 24.Askenazi DJ, Feig DI, Graham NM, et al. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69(1):184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 25.Garg AX, Salvadori M, Okell JM, et al. Albuminuria and estimated GFR 5 years after Escherichia coli O157 hemolytic uremic syndrome: an update. Am J Kidney Dis. 2008;51(3):435–444. doi: 10.1053/j.ajkd.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Siegler RL, Pavia AT, Christofferson RD, et al. A 20-year population-based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics. 1994;94(1):35–40. [PubMed] [Google Scholar]
- 27.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher M, Cass A, Bellomo R, et al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med. 2014;11(2):e1001601. doi: 10.1371/journal.pmed.1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu CY, Ordonez JD, Chertow GM, et al. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James MT, Hemmelgarn BR, Wiebe N, et al. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 31.Challiner R, Ritchie JP, Fullwood C, et al. Incidence and consequence of acute kidney injury in unselected emergency admissions to a large acute UK hospital trust. BMC Nephrol. 2014;15:84. doi: 10.1186/1471-2369-15-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46–61. doi: 10.1038/ki.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chawla LS, Amdur RL, Shaw AD, et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9(3):448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg A, Kogan E, Hammerman H, et al. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009;76(8):900–906. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 35.Wu VC, Wu CH, Huang TM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waikar SS, Curhan GC, Wald R, et al. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 37.Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870–877. doi: 10.1053/j.ajkd.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Grams ME, Astor BC, Bash LD, et al. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21(10):1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TM, Wu VC, Young GH, et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22(1):156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med. 1996;334(15):939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 41.Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 42.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 43.Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 44.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 45.Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 46.Kramer BK, Schweda F. Ramipril in non-diabetic renal failure (REIN study). Ramipril Efficiency in Nephropathy study. Lancet. 1997;350(9079):736. doi: 10.1016/s0140-6736(97)26036-8. author reply 736–737. [DOI] [PubMed] [Google Scholar]
- 47.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 48.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balamuthusamy S, Srinivasan L, Verma M, et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am Heart J. 2008;155(5):791–805. doi: 10.1016/j.ahj.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 50.Barrera-Chimal J, Perez-Villalva R, Ortega JA, et al. Mild ischemic injury leads to long-term alterations in the kidney: amelioration by spironolactone administration. Int J Biol Sci. 2015;11(8):892–900. doi: 10.7150/ijbs.11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosling P, Brudney S, McGrath L, et al. Mortality prediction at admission to intensive care: a comparison of microalbuminuria with acute physiology scores after 24 hours. Crit Care Med. 2003;31(1):98–103. doi: 10.1097/00003246-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Molnar Z, Szakmany T, Heigl P. Microalbuminuria does not reflect increased systemic capillary permeability in septic shock. Intensive Care Med. 2003;29(3):391–395. doi: 10.1007/s00134-003-1651-0. [DOI] [PubMed] [Google Scholar]
- 53.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71(6):504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 54.Wagner MC, Campos-Bilderback SB, Chowdhury M, et al. Proximal Tubules Have the Capacity to Regulate Uptake of Albumin. J Am Soc Nephrol. 2016;27(2):482–494. doi: 10.1681/ASN.2014111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffin KA, Picken MM, Bidani AK. Blood pressure lability and glomerulosclerosis after normotensive 5/6 renal mass reduction in the rat. Kidney Int. 2004;65(1):209–218. doi: 10.1111/j.1523-1755.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 56.Bidani AK, Griffin KA, Williamson G, et al. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54(2):393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol. 2011;300(3):F628–638. doi: 10.1152/ajprenal.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molnar AO, Parikh CR, Sint K, et al. Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin J Am Soc Nephrol. 2012;7(11):1749–1760. doi: 10.2215/CJN.13421211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tziakas D, Chalikias G, Kareli D, et al. Spot urine albumin to creatinine ratio outperforms novel acute kidney injury biomarkers in patients with acute myocardial infarction. Int J Cardiol. 2015;197:48–55. doi: 10.1016/j.ijcard.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 60.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devarajan P, Krawczeski CD, Nguyen MT, et al. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis. 2010;56(4):632–642. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harel Z, Wald R, Bargman JM, et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83(5):901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 63.Siew ED, Peterson JF, Eden SK, et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23(2):305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matheny ME, Peterson JF, Eden SK, et al. Laboratory test surveillance following acute kidney injury. PLoS One. 2014;9:e103746. doi: 10.1371/journal.pone.0103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.VIReC. VIReC Factbook: Corporate Data Warehouse (CDW) Consult 2.1 Domain. Hines IL: U.S. Department of Veterans Affairs, Health Services Research & Developement Service, VA Information Resource Center; 2014. [Google Scholar]
- 67.VA Information Resource Center. Medical SAS® Inpatient Dataset FY2009: VIReC Research User Guide. Hines, IL: U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2011. [Google Scholar]
- 68.VA Information Resource Center. VHA Medical SAS® Outpatient Datasets and Inpatient Encounters Dataset FY2009: VIReC Research User Guide. Hines, IL: U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2011. [Google Scholar]
- 69.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 70.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int. 2012;2(1):1–138. [Google Scholar]
- 72.Lin J, Fernandez H, Shashaty MG, et al. False-Positive Rate of AKI Using Consensus Creatinine-Based Criteria. Clin J Am Soc Nephrol. 2015;10(10):1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 74.Rosenbaum PR. Optimal Matching for Observational Studies. J Amer Statist Assoc. 1989;84(408):1024–1032. [Google Scholar]
- 75.D’Agostino RB, Rubin DB. Estimating and Using Propensity Scores with Partially Missing Data. J Amer Statist Assoc. 2000;95(451):749–759. [Google Scholar]
- 76.Rosenbaum PR. Design of Observational Studies. 1. New York, NY: Springer-Verlag; 2010. [Google Scholar]
- 77.Korn EL, Graubard BI. Analysis of health surveys. New York, NY: Wiley; 1999. [Google Scholar]
- 78.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 79.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 80.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 81.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2015. http://www.R-project.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


