Abstract
Background
Prior promising results have been reported with deep brain stimulation (DBS) of the anterior limb of the internal capsule in cases with severe obsessive compulsive disorder (OCD) who had exhausted conventional therapies.
Methods
In this pilot study, six adult patients (2 male; 4 female) meeting stringent criteria for severe (minimum Yale-Brown Obsessive Compulsive Scale [Y-BOCS] of 28) and treatment-refractory OCD had DBS electrode arrays placed bilaterally in an area spanning the ventral anterior limb of the internal capsule and adjacent ventral striatum referred to as the ventral capsule/ventral striatum. Using a randomized, staggered-onset design, patients were stimulated at either 30 or 60 days following surgery under blinded conditions.
Results
After 12 months of stimulation, four (66.7%) of six patients met a stringent criterion as “responders” (≥35% improvement in the Y-BOCS and end point Y-BOCS severity ≤16). Patients did not improve during sham stimulation. Depressive symptoms improved significantly in the group as a whole; global functioning improved in the four responders. Adverse events associated with chronic DBS were generally mild and modifiable with setting changes. Stimulation interruption led to rapid but reversible induction of depressive symptoms in two cases.
Conclusions
This pilot study suggests that DBS of the ventral capsule/ventral striatum region is a promising therapy of last resort for carefully selected cases of severe and intractable OCD. Future research should attend to subject selection, lead location, DBS programming, and mechanisms underpinning therapeutic benefits.
Keywords: Arousal, deep brain stimulation, major depression, obsessive compulsive disorder, ventral capsule, ventral striatum
Despite advances in pharmacological and behavioral therapies for obsessive compulsive disorder (OCD), a number of patients fail to improve sufficiently following years of conventional, as well as experimental interventions (1). An option of last resort has been the use of stereotactic neurosurgery (either cingulotomy or anterior capsulotomy) for seriously ill patients with OCD who have exhausted most existing treatments. The available, albeit limited, evidence suggests that ablative procedures may lead to long-term benefits with acceptable levels of risk (2). Although deep brain stimulation (DBS) is also an invasive procedure with potentially serious adverse events, in contrast to ablative approaches, it is an adjustable and partially reversible therapy (3).
Deep brain stimulation has been successfully employed for the treatment of a variety of movement disorders (4). Deep brain stimulation was first reported to be a promising intervention for OCD in a study by Nuttin et al. (5). The specific target in this study, referred to as the ventral capsule/ventral striatum (VC/VS), was chosen in part on positive experiences with gamma knife capsulotomy by the Brown University group (6). These studies, which were staged lesions over two operative sessions over time, demonstrated that adding a more ventral region lesion of the anterior limb of the internal capsule, impinging inferiorly on the ventral striatum, improved outcome (S.A. Rasmussen, unpublished data, 2009). This experience was important to refining and choosing an appropriate target for DBS.
Following the Nuttin et al. (5) publication, a team from the University of Florida was funded by the National Institute of Mental Health to conduct an independent pilot study of DBS in six patients with treatment-refractory OCD who might otherwise have been candidates for ablative neurosurgery. In consultation with National Institute of Mental Health staff, a blinded, staggered-onset design was adopted to enhance objectivity of the behavioral ratings while minimizing withholding of active DBS treatment to a maximum of 2 months following surgery.
Methods and Materials
Patients
This study was conducted at the University of Florida as a collaboration of the departments of Psychiatry, Neurology, and Neurosurgery in consultation with Dr. Benjamin Greenberg of Brown University. Prior to recommending surgery, an independent internal multidisciplinary team (psychiatrist, neurologist, neurosurgeon, and medical ethicist) reviewed all past treatments, evaluations, and procedures to ensure appropriateness of the candidate. Psychiatric diagnoses were based upon administration of the Structured Clinical Interview for DSM-IV (7), review of medical records, and expert clinical interview.
All subjects were adults who met DSM-IV criteria for OCD with a minimum score of 28 on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (8,9). Subjects must have had at least a 5-year history of treatment-refractory OCD symptoms since age 18, and the disorder must have caused substantial suffering as well as a reduction in the subject's functioning. Additional inclusion/exclusion criteria are available in Supplement 1.
Six subjects signed informed consents and were apprised of the risks, possible benefits of, and alternatives to DBS surgery. Partial outcome data from these subjects were included in a recently published report on a worldwide experience with DBS in OCD (10). The first subject was implanted in October 2003; the sixth subject completed 12 months of DBS in January 2008. The two men and four women had a mean age of 36.2 years (range: 27–52) (Table S1 in Supplement 1). Five of the six subjects had childhood onset (i.e., before age 18 years) OCD and the mean duration of illness was 24 years for the cohort (range: 11–35 years). Presurgical mean severity on the Y-BOCS at screening and at baseline was 32.7 and 33.7, respectively. All six subjects had lifetime diagnoses of major depression that were deemed secondary to OCD. One met criteria for a current diagnosis of secondary major depression but most reported depressive symptoms. One subject met criteria for Tourette syndrome. Although tics were present, his obsessive compulsive symptoms caused more subjective distress and dysfunction.
The medications prescribed at baseline were held constant and continued at the same doses as much as possible during chronic DBS. In some cases, the dosages were reduced. Patients were encouraged to apply the cognitive and behavioral skills they had previously learned during Exposure and Response Prevention.
Stimulation and Optimization
At 30 days postsurgery, subjects were randomized to either true DBS stimulation or sham stimulation. Half of the patients had DBS turn on at that point. At 60 days postsurgery, the three subjects previously assigned to sham underwent true DBS stimulation. Patients, raters, and the study psychiatrists were kept blind to the manipulations made (true stimulation, sham stimulation, or no change) at the postsurgery 30-day and 60-day visits and assignment was not disclosed until 120 days postimplantation after ratings were obtained. The standardized sham-controlled programming procedure was performed by the study neurologist (M.S.O.) as previously described (11). Patients were informed that they would have the device activated at some point during the first 90 days following the 1-month postoperative visit. Active settings were kept stable for the first 6 months and for at least 30 days before assessments whenever possible.
Details of device implantation and intraoperative testing are provided in Supplement 1.
Outcome Measures
Yale-Brown Obsessive Compulsive Scale severity was assessed categorically at each rating point according to percentage change from baseline. In this study, a responder was defined as both a 35% percentage change and an actual score of 16 or less at the time of assessment. The score of 16 was selected because it corresponded to mild-to-moderate symptoms at the diagnostic threshold for OCD and generally would not qualify a patient for entry into a clinical drug trial. Yale-Brown Obsessive Compulsive Scale scores were also analyzed as a continuous outcome with repeated-measures analysis of variance (ANOVA; two-tailed). The Y-BOCS was administered by expert clinicians, either the principal investigator (W.K.G.), one of the study psychiatrists, or a psychiatric research nurse (N.R.). For the most part, these assessments were conducted face-to-face, but some were completed on the telephone because of long travel distances.
Secondary outcome measures included the Hamilton Rating Scale for Depression (12), the Clinical Global Impressions Severity Scale (13), the Profile of Mood States (POMS) (14), and the SF-36 (15) as a measure of quality of life. Cognitive performance was assessed before implantation and after chronic DBS with a neuropsychological battery that included the Wisconsin Card Sorting Test (WCST) (16), the Karolinska Scales of Personality (17), Controlled Oral Word Association Test (18), Hopkins Verbal Learning Task (19), Grooved Pegboard (20), Tower of London Task (21), and a measurement of working memory capacity (Wechsler Adult Intelligence Scale-Third Edition Digit Span) (22). With respect to the WCST, the computerized version was used (WCST-Computerized Version Three, for Windows) to allow more computation of expected test-retest changes and to compare treatment-associated alterations in performance. In evaluating the degree to which treatment-associated neuropsychological change exceeded that expected by chance, a reliable change score was calculated to reflect the amount of test-retest change expected by chance.
Patients were closely monitored for deterioration in psychiatric status or stimulation-related adverse effects throughout the study. Deep brain stimulation continued until it was interrupted by stimulator battery depletion, at which time the implantable neurostimulators were replaced in outpatient surgery. In one case that required higher voltage settings, the two Soletra models (Medtronic, Minneapolis, Minnesota) were replaced by two larger Kinetra models (Medtronic) to reduce the frequency of replacement surgeries.
Results
DBS Lead Locations and Programming
A summary of the active DBS contacts used for chronic stimulation is provided in Table 1 along with lead locations. Three patients (patients 2, 3, and 5) had sham DBS programming for 1 month and then were subsequently activated at the next study visit under double-blind conditions. All patients were activated in a single contact monopolar setting for the first 6 months. Following the 6-month time point, trials of multiple monopolar stimulation resulted in salvage of one patient (patient 3) but not in the other two nonresponders (patients 2 and 4).
Table 1. DBS Programming and Lead Locations at 12 Months of Chronic Stimulation.
| Patient | DBS Setting | Lateral | AP | Axial |
|---|---|---|---|---|
| 1a | Rt 1-C+, 5 V, 210 μs, 135 Hz | 10.4 | 16.2 | 1.7 |
| Lt 0-C+,4 V, 210 μs, 135 Hz | 6.3 | 13.7 | − 3.8 | |
| 2 | Rt 2-C+, 3.5 V, 210 μs, 135 Hz | 10.5 | 17.3 | 8.4 |
| Lt 2-C+, 3.5 V, 210 μs, 135 Hz | 12.8 | 18.1 | 9.4 | |
| 3a | Rt 0-1-C+, 8.5 V, 150 μs, 130 Hz | 4.8 (0 contact) | 18.0 | − 3.8 |
| Lt 0-1-C+, 7.5 V, 150 μs, 130 Hz | 10.4 (0 contact) | 18.8 | − 3.8 | |
| 4 | Rt 1-C+,6.5 V, 180 μv, 135 Hz | 8.9 | 12.4 | − 2.6 |
| Lt 1-C+,6.5 V, 180 μv, 135 Hz | 13.4 | 16.0 | − 2.3 | |
| 5a | Rt 0-1-C+, 2.5 V, 210 μv, 135 Hz | 9.2 (0 contact) | 12.2 | − 1.7 |
| Lt 1-C+, 2.5 V, 210 μv, 135 Hz | 12.2 (1 contact) | 14.8 | 4.8 | |
| 6a | Rt 1-O+, 3.5 V, 90 μs, 135 Hz | 9.4 | 15.9 | 1.5 |
| Lt 1-O+, 3.3 V, 90 μs, 135 Hz | 11.2 | 15.2 | .9 |
Table shows patients 1 through 6 with chronic DBS settings at the active contact at 12 months of DBS. The DBS settings show right side, left side, volts, pulse width, and rate. The lateral, anteroposterior, and axial coordinates of the center of the active contact relative to the mid-commissural point are provided.
AP, anteroposterior; DBS, deep brain stimulation; Hz, rate; Lt, left side; μ, pulse width; Rt, right side; V, volts; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Patients who had a clinical response based on Y-BOCS criteria.
OCD Severity
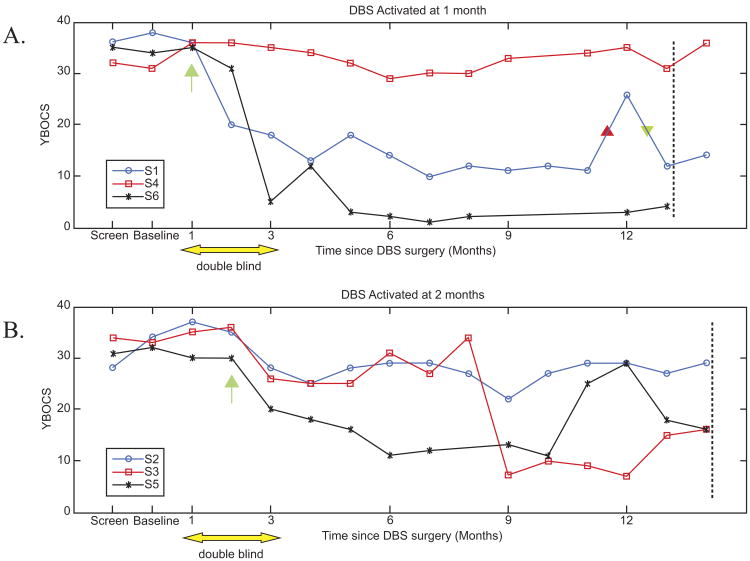
Plots of individual patient Y-BOCS scores over time were grouped by assignment to early (panel A) or delayed (panel B) activation (Figure 1). Visual inspection of these data suggests that little improvement occurred in either group until the device was activated.
Figure 1.
Patients and raters were blind to treatment condition (sham vs. active) for the 90-day period starting with the first postoperative visit as noted by the yellow double arrow. (A) Three patients (S1, S4, and S6) randomized to active DBS at 1 month postimplantation (denoted by green arrow); (B) three patients (S2, S3, and S5) randomized to active at month 2 (denoted by green arrow). In (A) patients received 12 months of active DBS at 13 months postimplantation (marked by dotted vertical line), whereas in (B) patients received 12 months of active DBS at 14 months postimplantation (marked by dotted vertical line). Unbeknownst to subject 1 or the research treatment team, this individual's right-sided battery was depleted between 11 and 12 months (red triangle) following surgery. This event was closely followed by an exacerbation in OCD symptoms that normalized shortly after device replacement (green triangle). Subject 3 did not show a response until after major changes in DBS settings at week 8 status post surgery (see text for details). The reasons for the temporary worsening of subject 5 between months 10 and 12 postimplantation are unclear. Possibilities include impact of significant life events or setting changes at month 10 that improved mood but lowered threshold for panic attacks. These settings were further modified at month 12. DBS, deep brain stimulation; OCD, obsessive compulsive disorder; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
In addition, a mixed-model ANOVA was fitted for the available monthly data of the primary outcome Y-BOCS during the first 12 months of active DBS, using SAS version 9.13 (SAS Institute, Inc., Cary, North Carolina). The model included factors group (activation at month 1 vs. at month 2) and categorical months since DBS activation; a first-order autoregressive covariance structure was used for the repeated measures. We found that there were significant reductions in Y-BOCS scores over time [F(12,55) = 2.02, p = .0392; with decrease of 15.67 ± 11.60 after 12 months of activation] (Table 2); whereas the group effect was not significant [F(1,4) = .02, p = .9040] with Y-BOCS reductions of 5.33 ± 11.67 for early group and –.67 ± 2.52 for late group at month 2.
Table 2. Analyses of Changes in Primary and Secondary Rating Scales During 12 Months of DBS Activation for All Participants (n = 6).
| Variable | Num df | Den df | F Value | Pr > F |
|---|---|---|---|---|
| Y-BOCSa | 12 | 55 | 2.02 | .0392a |
| POMS Total | 9 | 32 | 2 | .0723 |
| POMS T | 7 | 20 | 1.23 | .3347 |
| POMS-D | 7 | 20 | 1.75 | .1550 |
| POMS-A | 7 | 20 | 1.49 | .2256 |
| POMS-Va | 7 | 20 | 4.21 | .0053a |
| POMS-Fa | 7 | 20 | 3.08 | .0228a |
| POMS-C | 7 | 20 | 1.15 | .3743 |
| HAM-D17a | 12 | 50 | 2.22 | .0249a |
| SF-36 PF | 9 | 22 | 1.52 | .2025 |
| SF-36 RP | 9 | 22 | .94 | .5143 |
| SF-36 BP | 9 | 21 | 2.27 | .0587 |
| SF-36 GH | 9 | 22 | .98 | .4861 |
| SF-36 Va | 9 | 22 | 3.5 | .0079a |
| SF-36 SF | 9 | 22 | 1.77 | .1326 |
| SF-36 RE | 9 | 22 | 1.63 | .1680 |
| SF-36 MH | 9 | 22 | 1.39 | .2536 |
| SF-36 Total | 9 | 22 | .82 | .6038 |
DBS, deep brain stimulation; HAM-D17, Hamilton Depression 17-Item Rating Scale; POMS-A, Profile of Mood States anger-hostility subscore; POMS-C, Profile of Mood States confusion-bewilderment subscore; POMS-D, Profile of Mood States depression-dejection subscore; POMS-F, Profile of Mood States fatigue-inertia subscore; POMS T, Profile of Mood States tension subscale; POMS Total, Profile of Mood States total score; POMS-V, Profile of Mood States vigor-activity subscore; SF-36 BP, Medical Outcomes Study Short Form Health Survey bodily pain subscale; SF-36 GH, Medical Outcomes Study Short Form Health Survey general health subscale; SF-36 MH, Medical Outcomes Study Short Form Health Survey mental health subscale; SF-36 PF, Medical Outcomes Study Short Form Health Survey physical functioning subscale; SF-36 RE, Medical Outcomes Study Short Form Health Survey role limitations due to emotional problems subscale; SF-36 RP, Medical Outcomes Study Short Form Health Survey role limitations due to personal health problems subscale; SF-36 SF, Medical Outcomes Study Short Form Health Survey social functioning subscale; SF-36 Total, Medical Outcomes Study Short Form Health Survey total score; SF-36 V, Medical Outcomes Study Short Form Health Survey vitality subscale; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Significant difference on analysis of variance for repeated measures obtained during the first 12 months of DBS activation.
Categorical response and number of patients attaining a Y-BOCS score ≤16 are shown in Table 3 for the 12 months of DBS activation. Four (67%) of six patients met criteria for a responder status (Y-BOCS ≥ 35% change from baseline and Y-BOCS ≤16) after 12 months of DBS. Three (S1, S5, and S6 from Figure 1) of four responders reached these criteria after 2 to 3 months of active DBS. The remaining responder (S3 from Figure 1) did not show improvement in either OCD or mood until a second monopolar contact was activated after month 8 and voltage was increased. Within 2 months of these setting changes, Y-BOCS scores decreased from 33 to 10 (at 10 months) and this improvement was sustained.
Table 3. Categorical Responses and End Point Severity During DBS for OCD (n = 6).
| Duration of DBS Activation (months) | Total n | <25% Y-BOCS ↓ (n, %) | 25%–35% Y-BOCS ↓ (n, %) | ≥35% Y-BOCS ↓ (n, %) | Severity Y-BOCS ≤ 16 (n, %) |
|---|---|---|---|---|---|
| 1 | 6 | 4 (67) | 0 (0) | 2 (33) | 0 (0) |
| 2 | 6 | 2 (33) | 1 (17) | 3 (50) | 1 (17) |
| 3 | 6 | 3 (50) | 0 (0) | 3 (50) | 3 (50) |
| 4 | 6 | 3 (50) | 0 (0) | 3 (50) | 2 (33) |
| 5 | 6 | 3 (50) | 0 (0) | 3 (50) | 3 (50) |
| 6 | 5 | 3 (60) | 0 (0) | 2 (40) | 2 (40) |
| 7 | 6 | 1 (17) | 0 (0) | 5 (83) | 4 (67) |
| 8 | 5 | 2 (40) | 0 (0) | 3 (60) | 3 (60) |
| 9 | 4 | 2 (50) | 0 (0) | 2 (50) | 2 (50) |
| 10 | 5 | 3 (60) | 0 (0) | 2 (40) | 2 (40) |
| 11 | 6 | 2 (33) | 0 (0) | 4 (67) | 3 (50) |
| 12 | 6 | 2 (33) | 0 (0) | 4 (67) | 4 (67) |
Categorical response is shown against duration of DBS activation, which is not the same as time since implantation. Number of nonresponse is indicated in the column labeled <25% reduction on the Y-BOCS. Number of responders is shown using two different criteria: 25%-35% reduction on the Y-BOCS and the more stringent definition of ≥35% reduction on the Y-BOCS. The last column shows the number of individuals who had a Y-BOCS score ≤16 at that time point.
DBS, deep brain stimulation; OCD, obsessive compulsive disorder; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Secondary Measures
Based on ANOVAs, Hamilton Rating Scale for Depression scores decreased significantly for the entire group [F(12,50) = 2.22, p = .0249] during the 12 months of DBS (Table 2). Similar mixed models indicated that the POMS-V (vigor-activity) increased and POMS-F (fatigue-inertia) decreased significantly overtime [F(7,20) = 4.21,p = .0053; F(7,20) = 3.08, p = .0228; respectively], while changes in the POMS total score were nonsignificant (p = .0723) (Table 2). The SF-36-V (vitality) score significantly increased (improved) [F(9,22) = 3.50, p = .0079] (Table 2).
As noted in Table S2 in Supplement 1, patients classified as responders after 12 months of DBS showed corresponding improvements in functioning, as reflected in changes on the Clinical Global Impressions Severity Scale or work/school activity on the SF-36. The four responders progressed from global severity ratings of “severe” or “extremely severe” at baseline to scores of “not at all” or “marginal” illness at 12 months of DBS. Unfortunately, the two nonresponders remained “severe” on this measure. Two of the three responders who were taking psychotropic medications at baseline were able to have dosages lowered or the number of different medications reduced during DBS.
Adverse Effects
Potential complications of DBS can be separated into those related to surgical implantation, stimulation, and device failure or interruption. There were no device failures beyond the expected stimulation interruptions owing to implantable neurostimulator battery depletion or inadvertent device shutoffs if the magnetic switch was tripped by a theft detector.
Adverse Effects of Implantation
No unexpected adverse events occurred as a result of implantation. There were no serious adverse events such as seizures or cerebral hemorrhages. All adverse events associated with implantation/anesthesia were anticipated and time-limited. These included discomfort at the surgical site, incision pain, headache, nausea, scalp tingling or numbness, and intubation-related sore throat.
Adverse Effects of Stimulation
Effects of acute and chronic DBS are best distinguished. During intraoperative testing or subsequent optimization, a wide range of settings were surveyed that in some instances led to unwanted or unusual emotional, perceptual, or somatic experiences. All these effects occurred within seconds or minutes of DBS onset and could be reversed, typically within seconds and always within minutes of changing the stimulation parameters. Transient emotional effects included euphoria, giddiness, anxiety, panic attacks (previously reported [23]), or sadness. A contralateral smile accompanied by mirth was induced intraoperatively in five of six patients and was previously reported in one of these cases (24). Ventral stimulation (contact 0) was more likely to induce anxiety/panic (23,25). Transient olfactory and gustatory sensations such as smelling popcorn or tasting metal were experienced with deep contacts only.
Hypomania was observed at some point during chronic DBS in four (including one of the nonresponders, S2) of the six patients. In all cases, the degree of mood elevation abated over time or responded to device adjustment. None of the subjects gave a history of bipolar disorder. Difficulty falling asleep was a common complaint that was dealt with by adding as required sedative-hypnotics or by device adjustment. In one case (S5), DBS-induced insomnia was alleviated by lowering the ongoing dose of escitalopram from 80 mg to 30 mg daily. A serious adverse event (classified as such because hospitalization was required) secondary to a change in DBS settings occurred in S2. In this instance, DBS-induced improvement in mood was accompanied by a reciprocal worsening in OCD. This exacerbation in OCD resolved after adjustment of DBS. After this event, we extended the in-clinic observation period following major changes in DBS parameters.
Effects of DBS Interruption
The clinical effects and time course of DBS interruption were similar to those reported previously by Greenberg et al. (25). Worsening in mood or increased anxiety were typically the first symptoms reported following battery depletion or inadvertent inactivation by metal detectors. Other signs of depression, such as diminished energy or interest, also emerged within days of device interruption but none expressed suicidality. Exacerbation of OCD symptoms generally lagged the emergence of affective or anxiety symptoms. In all cases, restoration of DBS function led to reversal of the transient clinical deterioration. An illustration of these phenomena is shown for S1 (Figure 1, panel A). Other than drainage of battery power over time, there were no device failures or lead fractures.
Neuropsychological Test Performance
The results of fourteen measures from six neuropsychological tests are reported here. All measures were administered at each patient visit. Test order was counterbalanced across visits to reduce order effects, though some measures (e.g., HVLT) with delayed performance trials were typically administered during the first half of testing to allow the appropriate delay interval to transpire. For each measure, 90% confidence intervals for reliable change were computed using established measures, and each patient's results were categorized as representing an improvement, no change, or a decline from baseline. Results from the 6- and 12-month evaluations are presented in Table 4.
Table 4. Reliable Changes in Neuropsychological Performance from Baseline Among Responders and Nonresponders.
| Baseline Mean (SD) | 6 Month | 1 Year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Responders (3) | Nonresponders (3) | Responders (4) | Nonresponders (2) | ||||||||||
|
|
|
|
|
||||||||||
| + | nc | − | + | nc | − | + | nc | − | + | nc | − | ||
| WAIS 3 DS | |||||||||||||
| Forward | 7.00 (1.9) | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 |
| Backward | 5.33 (1.6) | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 |
| WAIS raw | 19.33 (6.0) | 0 | 3 | 0 | 0 | 2 | 1 | 0 | 4 | 0 | 0 | 1 | 1 |
| Verbal Fluency CFL | 44.50 (15.64) | 0 | 3 | 0 | 1 | 2 | 0 | 0 | 3 | 1 | 1 | 1 | 0 |
| Wisconsin Card Sort | |||||||||||||
| Categories | 5.67 (.82) | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 |
| Persev errors | 6.33 (2.58) | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 |
| Persev responses | 6.33 (2.58) | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 4 | 0 | 0 | 2 | 0 |
| Tower of London | |||||||||||||
| Movement count | 44.50 (19.77) | 0 | 3 | 0 | 1 | 2 | 0 | 0 | 4 | 0 | 1 | 1 | 0 |
| Total time | 415.17 (199.36) | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 0 |
| Grooved Pegboard | |||||||||||||
| Dominant | 101.83 (28.62) | 2 | 1 | 0 | 1 | 2 | 0 | 3 | 1 | 0 | 0 | 2 | 0 |
| Nondominant | 124.17 (41.90) | 2 | 1 | 0 | 1 | 1 | 1 | 3 | 1 | 0 | 0 | 1 | 1 |
| Hopkins VLT | |||||||||||||
| Total | 26.33 (3.33) | 0 | 3 | 0 | 2 | 1 | 0 | 1 | 3 | 0 | 0 | 2 | 0 |
| Delayed recall | 8.67 (2.80) | 0 | 3 | 0 | 1 | 2 | 0 | 1 | 3 | 0 | 0 | 2 | 0 |
| Recog discrimination | 11.17 (1.17) | 0 | 2 | 1 | 0 | 2 | 1 | 0 | 3 | 1 | 0 | 1 | 1 |
+, improvement beyond the 90% confidence interval for reliable change; −, decline in performance beyond the 90% confidence interval for reliable change; nc, no change from baseline; Persev, perseveration errors; Recog, recognition; VLT, verbal learning task; WAIS, Wechsler Adult Intelligence Scale; WAIS 3 DS, Wechsler Adult Intelligence Scale version 3 Digit Span.
Overall, results indicated that the clinical effectiveness of DBS in this population was achieved without significant neuropsychological morbidity. At 6 months post-DBS, only 2.1% (1 of 42) of the change comparisons showed a decline in the responders (one patient declined in HVLT recognition), while only 7.1% (3 of 42) of comparisons showed a decline in the non-responders (one showed reduction in HVLT recognition, one showed a decline in non-dominant Pegboard performance, and one showed a reduction in WAIS-III digit span raw score). At 1 year, 5.4% (3 of 56) of the responder comparisons showed a decline, while 10.7% (3 of 28) of the non-responder comparisons declined. In contrast, 14.3% (6 of 42) of the responder comparisons and 21.4% (9 of 42) of the non-responder comparisons showed improvement at 6 months. The corresponding values at one year were 17.9% (10 of 56 responder comparisons) and 10.7% (3 of 28 non-responder comparisons) respectively. Thus, the vast majority (77.4%) of comparisons for both responders and non-responders showed no reliable change in neuropsychological test performance at either the 6- or 12-month postsurgical evaluation.
Discussion
Twelve months of bilateral stimulation of the VC/VS was associated with marked improvement in obsessive compulsive symptoms in four (66.7%) of six subjects with severe and intractable OCD. Even the two individuals who were classified as nonresponders requested that stimulation be continued because they experienced some subjective relief of anxiety, depressive, or tic symptoms. For the six subjects as a whole, both OCD and depressive symptoms improved significantly compared with baseline at the 12-month mark. The procedures were generally well tolerated and the only serious adverse event leading to urgent hospitalization was an exacerbation in OCD symptoms due to stimulation that was reversed by changing the device settings. Both subjects who experienced hypomania as an early stimulation-induced side effect were ultimately clinical responders. Other transient stimulation-induced side effects, including panic, gustatory, and olfactory hallucinations, have been previously described (25). No significant changes in neuropsychological performance were detected at either 6 or 12 months of stimulation.
With a few notable exceptions (26–28), most published reports of DBS in OCD have been uncontrolled and open-label. The study by Abelson et al. (26) of anterior capsular DBS in OCD included a double-blind phase in which subjects received active or sham stimulation in a randomized “on-off” sequence of four 3-week blocks. Only one of four subjects experienced a marked improvement in OCD symptoms during this 3-week blinded period; another subject showed moderate benefit during subsequent, longer term, open-label treatment. Nuttin et al. (27) used longer 3-month treatment blocks in their crossover study of VC/VS DBS in six subjects with OCD. In four of the subjects who showed improvement during stimulation-on conditions, the duration of the stimulation-off phase needed to be abridged or aborted because of marked clinical deteriorations including expressions of suicidality. These within-subject findings support the efficacy of DBS but also illustrate the perils of crossover designs in this population and when using this target. Using a different anatomic target, the subthalamic nucleus (STN), Mallet et al. (28) conducted a double-blind crossover study of DBS in 16 subjects with severe, refractory OCD. Subjects were randomized to either 3 months of active or sham STN stimulation followed by 3 months of the opposing treatment condition separated by a 1-month washout. The findings supported a therapeutic effect of STN stimulation in refractory OCD but raised concerns about the high frequency of serious adverse events. Responders in this study were defined by only a 25% improvement on the Y-BOCS.
The pilot study reported herein employed a design that helped control for expectation bias. Although OCD has a low placebo response rate compared with other psychiatric disorders (29) and this study included only treatment-refractory patients, one cannot completely discount the influence of expectation in a patient (and his/her clinician) who may see neurosurgery as the last viable option. To mitigate this bias, patients were randomized (following a 1-month postsurgical stabilization period) to either active or sham (1-month delayed) DBS in a double-blind fashion. The patient remained blind to the timing of stimulation for 90 days. Variations on this design were considered, such as a longer sham period or crossover from active to sham, but these were rejected for clinical reasons. No statistically significant between-group effects were found for the comparison of the early versus delayed patients, but this is not surprising given the small sample size. Inspection of the Y-BOCS scores (Figure 1) for each subject suggests that clinically significant reductions did not occur during the sham phase. In contrast, among responders, Y-BOCS scores began to show reductions during the first month of active DBS. Three subjects, S1, S6, and S5, exhibited more than a 25% reduction in Y-BOCS scores during the 1-month double-blind assessment period and went on to be identified as responders at 12 months of active treatment.
Finding the optimal settings for an individual subject proved challenging. Given there are two devices with several adjustable variables in each patient (e.g., electrode configuration, voltage, frequency, and pulse width), the number of possible settings is exceedingly large. Additionally, unlike other experiences with DBS, there is not a clear positive symptom (e.g., tremor improvement) to gauge settings. In this study, particularly the double-blind phase, the goal was to select parameters that produced some benefit in mood or anxiety symptoms acutely, with minimal side effects. Further, the goal was to maintain these settings for a month or more without making major changes. Using this conservative approach, only three of six subjects met our criteria for response (>35% reduction in Y-BOCS after 6 months of DBS). It was not until several major changes were made in the device settings that one additional subject (S3) showed a response after the first 6 months of active DBS.
An interesting and unexpected observation was improvement on subscales of the POMS and SF-36 that mapped specifically onto constructs of vigor-activity, fatigue-inertia, and vitality. Each of these subscales showed significant changes for the group of six subjects. It should be noted that no corrections were made for multiple comparisons and that these findings may have been due to chance. However, each of these constructs appears to relate to aspects of arousal and motivational state. Interestingly, DBS at this same target has shown promise in relieving symptoms of major depression in highly treatment-refractory patients (30). If replicated across populations, these findings raise the intriguing possibility that DBS of the VC/VS exerts effects on brain motivational systems that could, at least in part, account for benefit in either depressive or OCD symptoms. Field modeling of the active contacts at the VC/VS target in combination with what is known about the axonal trajectories of longitudinal fiber tracts that run through these areas in rodents and primates suggest two possible alternatives. First, increased vitality and arousal may be a direct effect of stimulation on dopaminergic fibers from the medial substantia nigra zona compacta (A9) and adjacent ventral teg-mental area of Tsai (A10) running to the ventral striatum. Recent studies of the effect of DBS in this location on raclopride binding in humans suggest that DBS results in dopamine release in the accumbens (D. Denys, written communication, 2009) alleviates anhedonia in major depression (31). Second, increased vitality and arousal may be due to activation of thalamostriatal and thalamocortical fibers running from midline and intralaminar thalamic nuclei that have been implicated in processes of arousal and awareness (32,33). Behavioral changes that might be associated with elevated levels of vigor and vitality and reduced fatigue could include reduction in social withdrawal and isolation and, possibly, improved ability to engage in cognitive-behavior therapies, either of which would advance therapeutic change.
Limitations of this study include the small sample size and the relatively short (60-day) double-blind period comparison between active and sham stimulation. Improvement in OCD symptoms after open VC/VS DBS was found to continue over 3 months of continuous stimulation and then essentially plateau (10). Although the staggered-onset design may have helped protect blinding of stimulation onset, determination of response was still based on open-label data at the end point. In one case (S1), however, a relapse precipitated unexpectedly by battery depletion (essentially an unintended “triple-blind” experiment) authenticated the contribution of active stimulation to the observed therapeutic response. The ability to reduce the number of psychotropic medications or their dosages in several subjects is a further indication that DBS was the active ingredient in alleviating symptoms.
The effectiveness of the blinding procedure may have been contaminated by earlier intraoperative testing. Some subjects may have been able to distinguish active versus sham conditions because of their prior experience during active testing. The advantages of intraoperative testing include ensuring proper functioning of the devices and placement of the electrodes (as might be indicated, for example, if intraoperative macrostimulation produced adverse effects such as marked acute anxiety). Another potential advantage was to investigate the predictive value of intraoperative testing for long-term outcome. From the standpoint of preserving the blind, however, it may be best to carefully limit intensities used in intraoperative testing in future experiments to protect the blind.
Lead location is an important factor influencing DBS outcome (34,35). Our study benefited from the combined data of the DBS for OCD collaborative research group, which after several initial cases settled on a target more medial and posterior with the tip placed within 1 mm to 2 mm of a plane defined by the posterior border of the anterior commissure (10). The early published cases from this group required multiple monopolar or bipolar contacts to be simultaneously activated, shortening battery life. However, stimulation intensities were substantially reduced in subsequent patient groups receiving DBS at this target, and the most commonly activated contacts were number 0 (in the dorsal part of ventral striatum) or number 1 (in the ventral-most part) (10). Multiple contact activations in movement disorder patients usually are an indication that a lead location is far from an optimal target, but this point in OCD remains to be investigated. Our case S3 was targeted slightly more laterally than the other cases, and that may have been a potential explanation as to why turning on multiple contacts (multiple monopolar stimulation) was necessary to convert this subject to a responder. Alternatively, rather than lead location providing the explanation, the uniqueness of the DBS lead used in this study (large 4-mm spacing between contacts) could have contributed to the need for multiple contact locations to be activated. A lead with smaller electrode contacts could potentially be more efficiently utilized for future OCD DBS studies. Such a device would have the potential to use less current and for that reason might be less likely to induce side effects due to current spread outside of the area required for therapeutic effects. This possibility should be tested in future research.
Conclusions
In agreement with prior open-label reports, this National Institute of Mental Health funded pilot study using a blinded, staggered-onset design suggested that VC/VS region DBS was a promising therapy for carefully selected cases of severe and intractable OCD. Initial signs of improvement were temporally associated with true stimulation, and within the limited window of time (60 days double-blind), no obvious responses were observed during sham. Although larger scale replication will be required, DBS has features conferring notable advantages to ablative neurosurgeries that can neither be adjusted nor reversed. However, DBS entails the risk of surgical and device-related adverse effects and burdens, which can be significant for patients. Future research should attend to subject selection, lead location, DBS programming, and mechanisms underpinning the therapeutic benefits for OCD patients. The energizing and motivational effects of the VC/VS region DBS should also be more carefully studied in both clinical and translational research.
Supplementary Material
Acknowledgments
This study was funded by R21MH064161 (WKG Principal Investigator) and in part by the University of Florida Grant M01 RR000082 National Center for Research Resources/National Institutes of Health. Devices and leads were donated by Medtronic, Minneapolis, Minnesota.
We thank John Greist, M.D., and Bruce Hyman, Ph.D., for their help in reviewing de-identified medical records of potential subjects.
Dr. Goodman received an honorarium for a speaking engagement in September 2009 from Medtronic, Inc., the manufacturer of the deep brain stimulation device used in this study. Dr. Greenberg has received research funding, honoraria, and consultant fees from Medtronic, Inc. Drs. Okun and Foote have consulted for, received fellowship grants, and received honoraria from Medtronic, Inc. Dr. Okun has consulted for the National Parkinson Foundation and Ask the Doctor. Dr. Okun has received honoraria in the past for a TEVA Pharmaceutical Industries sponsored movement disorders course and has received royalties from Demos publishing. Dr. Okun also holds royalty interest in COMPRESS/Neurotrax.
Footnotes
All other authors reported no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.gov: Deep Brain Stimulation for Treatment-Resistant Obsessive Compulsive Disorder; http://www.clinicaltrials.gov/; NCT00057603.
Supplementary material cited in this article is available online.
References
- 1.Goodman WK, Ward HE, Kablinger AS, Murphy TK. Biological approaches to treatment-resistant obsessive compulsive disorder. In: Goodman WK, Rudorfer MV, Maser J, editors. Obsessive Compulsive Disorder: Contemporary Issues in Treatment. New York: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 2.Baer L, Rauch SL, Ballantine HT, Jr, Martuza R, Cosgrove R, Cassem E, et al. Cingulotomy for intractable obsessive-compulsive disorder. Prospective long-term follow-up of 18 patients. Arch Gen Psychiatry. 1995;52:384–392. doi: 10.1001/archpsyc.1995.03950170058008. [DOI] [PubMed] [Google Scholar]
- 3.Goodman WK, Insel TR. Deep brain stimulation in psychiatry: Concentrating on the road ahead. Biol Psychiatry. 2009;65:263–266. doi: 10.1016/j.biopsych.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Rezai AR, Machado AG, Deogaonkar M, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurgery. 2008;62(suppl 2):809–838. doi: 10.1227/01.neu.0000316285.52865.53. [DOI] [PubMed] [Google Scholar]
- 5.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg BD, Price LH, Rauch SL, Friehs G, Noren G, Malone D, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: Critical issues. Neurosurg Clin North Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 7.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM IV Axis I Disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Publishing; 1997. [Google Scholar]
- 8.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS). Part II. Validity. Arch Gen Psychiatry. 1989;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 9.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive Compulsive Scale (Y-BOCS). Part I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg BD, Gabriels LA, Malone DA, Jr, Rezai AR, Friehs GM, Okun MS, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: Worldwide experience. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.55. published online ahead of print May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: Responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–314. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare. DHEW Publication (ADM); 1976. pp. 76–338. [Google Scholar]
- 14.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. North Tonawanda, NY: Multi-Health Systems; 1971. [Google Scholar]
- 15.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston: New England Medical Center, The Health Institute; 1993. [Google Scholar]
- 16.Heaton RK, Chelune GJ, Talley JL, Kay CG, Curtis G. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Resources; 1993. [Google Scholar]
- 17.Ortet G, Ibáñez MI, Llerena A, Torrubia R. The underlying traits of the Karolinska Scales of Personality (KSP) Eur J Psychol Assess. 2002;18:139–148. [Google Scholar]
- 18.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd. Iowa City: AJA Associates; 1994. [Google Scholar]
- 19.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol. 1999;13:348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 20.Matthews CG, Klöve H. Instruction Manual for the Adult Neuropsychological Test Battery. Madison, WI: University of Wisconsin; 1964. [Google Scholar]
- 21.Anderson PA, Anderson VB, Lajoie GA. The tower of London test: Validation and standardization for pediatric populations. Clin Neuropsychol. 1996;10:54–65. [Google Scholar]
- 22.Wechsler D. WAIS-III: Administration and Scoring Manual:Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 23.Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, et al. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR, et al. What'sina “smile?” Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 26.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. discussion:1272–1274. [DOI] [PubMed] [Google Scholar]
- 28.Mallet L, Polosan M, Jaafari N, Baup N, Welter ML, Fontaine D, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359:2121–2134. doi: 10.1056/NEJMoa0708514. [DOI] [PubMed] [Google Scholar]
- 29.Khan A, Kolts RL, Rapaport MH, Krishnan KR, Brodhead AE, Browns WA. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med. 2005;35:743–749. doi: 10.1017/s0033291704003873. [DOI] [PubMed] [Google Scholar]
- 30.Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 32.Hsu DT, Price JL. Paraventricular thalamic nucleus: Subcortical connections and innervation by serotonin, orexin, and corticotropinreleasing hormone in macaque monkeys. J Comp Neurol. 2009;512:825–848. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.Richardson RM, Ostrem JL, Starr PA. Surgical repositioning of misplaced subthalamic electrodes in Parkinson's disease: Location of effective and ineffective leads. Stereotact Funct Neurosurg. 2009;87:297–303. doi: 10.1159/000230692. [DOI] [PubMed] [Google Scholar]
- 35.Ellis TM, Foote KD, Fernandez HH, Sudhyadhom A, Rodriguez RL, Zeilman P, et al. Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery. 2008;63:754–761. doi: 10.1227/01.NEU.0000325492.58799.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.