Abstract
Background
Global data on durability of first-line antiretroviral therapy (ART) in children with HIV is limited. We assessed time to switch to second-line therapy in 16 European countries and Thailand.
Methods
Children <18-years initiating combination ART (≥2 nucleoside reverse transcriptase inhibitor (NRTI) plus non-NRTI (NNRTI) or boosted-protease inhibitor (PI)) were included. Switch to second-line was defined as: (i) change across drug class (PI to NNRTI or vice versa) or within PI-class plus change of ≥1 NRTI; (ii) change from single to dual PI; or (iii) addition of a new drug class. Cumulative incidence of switch was calculated with death and loss-to-follow-up as competing risks.
Results
Of 3,668 children included, median [IQR] age at ART initiation was 6.1 [1.7,10.5] years. Initial regimens were 32% PI, 34% nevirapine (NVP), 33% efavirenz-based. Median duration of follow-up from ART start was 5.4 [2.9,8.3] years. Cumulative incidence of switch at 5 years was 21% (95% CI 20, 23), with lowest incidence in Russia/Ukraine and highest in UK/Ireland. Median time to switch was 30 [15, 58] months, two-thirds of switches were related to treatment failure. In multivariable analysis, older age, severe immunosuppression and higher viral load at ART start, and NVP-based initial regimens were associated with increased risk of switch. Among those switched, 65% had viral load <400c/mL at 12-months after start of second-line ART.
Conclusions
One in five children switched to second-line by 5 years of ART, with two-thirds failure related. Advanced HIV, older age and NVP-based regimens were associated with increased risk of switch.
Keywords: HIV, children, antiretroviral therapy, second-line, switch
Introduction
Worldwide, an estimated 2.1 million children aged<15 years were living with HIV in 2016, of whom 43% were accessing antiretroviral therapy (ART), with coverage expected to increase further[1]. Sustaining long-term viral suppression on ART throughout childhood and adolescence is a challenge[2]. Observational cohorts in middle and high-income countries with routine viral load monitoring have reported cumulative risk of virological failure in children ranging from 18% to 40% at 3 to 5 years after ART start[3–6]. As children currently require lifelong treatment, subsequent ART options will inevitably be required, and therefore programme planning and forecasting demand for paediatric formulations are needed.
There remain limited and often conflicting estimates on the use of second-line ART in children, with wide variations in both clinical trials and observational cohorts, ranging from 2% to 23% switching at 5 years after ART initiation[6–11]. This reflects variation in initial regimens, monitoring and switching strategies, availability of alternative regimens across studies and settings, and differences in the definitions of ‘switch’ used. Some studies have restricted switch analyses to children with confirmed or unconfirmed virological failure[3,12], which may under-estimate the broader use of second-line treatment due to clinical and/or immunological failure, or major treatment limiting toxicities[6,13].
In this study, we assessed time to switch to second-line ART for any cause and associated factors in the context of routine viral load monitoring, within the European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC), composed of cohorts across 16 European countries and Thailand. These cohorts offer long-term follow-up data to assess incidence of switch in routine-care settings across regions, which may inform other countries moving towards viral load monitoring[14].
Methods
Nineteen paediatric HIV observational cohorts across 17 countries contributed to an individual patient data meta-analysis carried out in December 2014. Routine demographic, clinical, laboratory and treatment related data were pooled electronically using a modified HICDEP protocol (www.hicdep.org). Children were included in this analysis if they were aged <18 years at start of a ‘standard’ combination ART regimen, defined as ≥2 nucleoside reverse-transcriptase inhibitors (NRTI) plus a non-NRTI (NNRTI) or boosted protease inhibitor (PI). Children who participated in clinical trials of switching strategies or treatment interruption were excluded. All cohorts received local ethics approval to transfer anonymised data for this study.
Switch to second-line ART was defined as either (i) change across drug class (from NNRTI to PI or vice versa) or change within PI-class, plus change of ≥1 NRTI; (ii) change from single to dual PI; or (iii) an addition of a new drug class. Switches with documented reasons of simplification, tuberculosis prophylaxis or pregnancy were ignored. This stringent definition of switch was used to reflect World Health Organization (WHO) and European guideline recommendations on the management of treatment failure in children[15–17]. In sensitivity analyses, we (i) ignored any switches during the first 6 months after ART initiation as these were unlikely to be related to treatment failure, (ii) relaxed our switch criteria by not requiring a change of ≥1 NRTI when switching across drug class or within PI-class, if the reason for switch was reported as failure, as some settings may need to preserve NRTIs.
Among patients meeting our definition of switch we described the reasons reported for switching and explored evidence for clinical failure in those with missing reason (defined as (i) viral load>1,000cps/mL; (ii) new CDC B/C event; or (iii) no CD4 gain from ART initiation, within the 6-months prior to switch). We describe the characteristics at time of switch and virological response (<400 c/ml) at 12 and 24-months after switch.
Time to switch was summarised using cumulative incidence, accounting for competing risks of death and loss to follow-up (LTFU). Children were at risk from ART start until the earliest of switch, death, last visit in paediatric care or 21st birthday. Cohorts contributed follow-up data through to December 2013 except for Germany (until April 2012), Portugal (September 2013) and Romania (October 2013). LTFU was defined as children not known to have died or transferred to another clinic, whose last visit was more than two years before the cohort censoring date, or children reported as LTFU by their cohort.
The associations between time to switch and characteristics at ART initiation were investigated using competing risks proportional hazards regression[18]. In univariable analysis, associations with the following factors at the start of ART were explored: age, sex, immunosuppression (WHO 2007 classification severe vs. non severe for age[19]), viral load (VL), CDC stage (C vs N/A/B), initial ART regimen, calendar year (1997-<2004, 2004-<2008, ≥2008), region of cohort (UK & Ireland, Thailand, Russia & Ukraine (Eastern Europe) and remaining countries (Central & Western Europe)). The final multivariable model was selected using backwards elimination (exit probability p=0.05), with baseline hazard stratified by region. Region was not included in the multivariable model as a covariate due to evidence of non-proportional hazards between regions. The functional form of continuous age was explored using regression splines. Differences in the effect of initial regimen on switch by age and year at ART start were explored. A sub-group analysis in children aged <3 years in the UK/Ireland compared NVP+3NRTIs (rarely used in other regions) to other initial regimens. P-values are two-sided, and analyses were carried out using STATA v14.1 (Stata Corporation, College Station, Texas, USA).
Results
Of 3,953 children who initiated ART, 3,668 (93%) met the study inclusion criteria (88 were excluded due to participation in clinical trials, 197 initiated on non-standard regimens). Half of the children were male; 90% were perinatally infected (Table 1).
Table 1. Characteristics of children at ART initiation by initial ART regimen.
| bPI (n=1191) |
EFV (n=1214) |
NVP (n=1263) |
Total (n=3668) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Male | 559 | 47% | 589 | 49% | 599 | 47% | 1747 | 48% |
| Perinatal infection* | 1110 | 93% | 973 | 80% | 1208 | 96% | 3291 | 90% |
| Age (years), median [IQR] | 3.2 | [0.7, 8.4] | 9.5 | [6.1, 12.6] | 3.9 | [0.9, 8.5] | 6.1 | [1.7, 10.5] |
| <1 | 357 | 30% | 4 | 0% | 340 | 27% | 701 | 19% |
| 1 - <3 | 224 | 19% | 67 | 6% | 226 | 18% | 517 | 14% |
| 3 - <6 | 182 | 15% | 220 | 18% | 202 | 16% | 604 | 16% |
| 6 -<11 | 233 | 20% | 474 | 39% | 347 | 27% | 1054 | 29% |
| 11+ | 195 | 16% | 449 | 37% | 148 | 12% | 792 | 22% |
| CDC stage C | 146 | 12% | 155 | 13% | 129 | 10% | 430 | 12% |
| CD4% in those <5yrs (n=1183/1614), median [IQR] | 23 | [16, 32] | 16 | [11, 22] | 22 | [14, 34] | 21 | [14, 32] |
| CD4 count in those≥5yrs (n=1614/2054), median [IQR] | 281 | [134, 462] | 202 | [55, 360] | 170 | [43, 358] | 220 | [63, 388] |
| WHO severely immunocompromised (n=2808) | 436 | 48% | 634 | 63% | 508 | 57% | 1578 | 56% |
| HIV RNA (log10 copies/ml) (n=2518), median [IQR] | 5.2 | [4.5, 5.3] | 5 | [4.4, 5.4] | 5.1 | [4.4, 5.7] | 5 | [4.4, 5.6] |
| Calendar year of ART initiation | ||||||||
| 1997 - <2004 | 127 | 11% | 356 | 29% | 516 | 41% | 999 | 27% |
| 2004 - <2008 | 357 | 30% | 477 | 39% | 498 | 39% | 1332 | 36% |
| ≥2008 | 707 | 59% | 381 | 31% | 249 | 20% | 1337 | 36% |
| Region | ||||||||
| UK/Ireland | 201 | 17% | 438 | 36% | 436 | 35% | 1075 | 29% |
| Eastern Europe | 402 | 34% | 122 | 10% | 108 | 9% | 632 | 17% |
| Central and Western Europe | 541 | 45% | 407 | 34% | 321 | 25% | 1269 | 35% |
| Thailand | 47 | 4% | 247 | 20% | 398 | 32% | 692 | 19% |
bPI: boosted protease inhibitor; EFV: efavirenz; NVP: nevirapine; ART: antiretroviral therapy; IQR: interquartile range. *Non-perinatal route of infection reported as: parenteral (non-injected drug use) (5%), blood products/transfusion (2%), other (1%) or unknown (3%)
The three largest cohorts were from the UK/Ireland (29% of children), Thailand (19%), and Ukraine (13%). Earliest year of ART initiation ranged from 1997 in UK/Ireland to 2002 in Thailand. Approximately one-third of children started ART on efavirenz (EFV), nevirapine (NVP) or PI-based regimens (93% lopinavir/ritonavir). Median age at the start of ART was 6.1 [IQR 1.7,10.5] years, and lower in children who initiated on PI- and NVP-based regimens than EFV-based regimens (Table 1). A larger proportion of children initiating on PI-based regimens started treatment in later calendar years (≥2008) compared to other regimens. Children in the UK/Ireland and Thailand were more likely to initiate on NNRTI-based regimens compared to other regions where more children started on PI-based regimens. The median duration of follow-up after start of ART was 5.4 [2.9,8.3] years. The median gap between VL measurements after ART start varied across regions: 36, 26 and 13-14 weeks in Eastern Europe, Thailand and the rest of Europe, respectively.
Switch to second-line
Overall, 820 (22%) children met the definition of switch while 71 (2%) died and 374 (10%) were LTFU before switching. There were significantly fewer patients LTFU in the UK/Ireland, more deaths in Thailand, and fewer patients switching in Eastern Europe (p<0.001) (Figure S1). Among those who switched, the median time from ART start to switch was 30 [16,58] months, the majority (72%) switching from an NNRTI- to a PI-based second-line regimen.
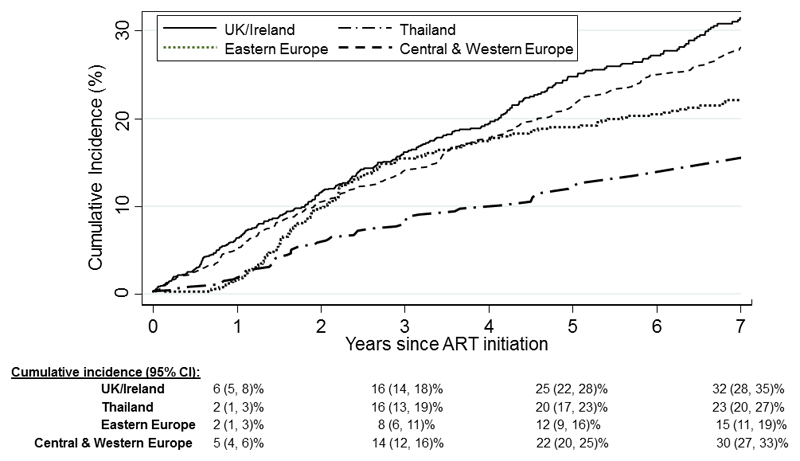
The overall cumulative incidence of switch was 14% (95% CI 13-15%) at 3 years, 21% (20-23%) at 5 years and 27% (26-29%) at 7 years after ART start. The cumulative incidence varied across regions, and these regional differences changed over time (Figure 1). At 1 year after ART start, Thailand and Eastern Europe had lowest cumulative incidence of switch at 2% (1-3%), but it rapidly increased in Thailand to 16% (13-19%) by 3 years, a similar level to Western & Central Europe and UK/Ireland, and plateaued thereafter. At 5 years after ART initiation, the cumulative incidence of switch was lowest in Eastern Europe at 12% (9-16%), and ranged from 20-25% in the other regions.
Figure 1. Cumulative incidence of switch to second-line ART by region.
Reasons for switch
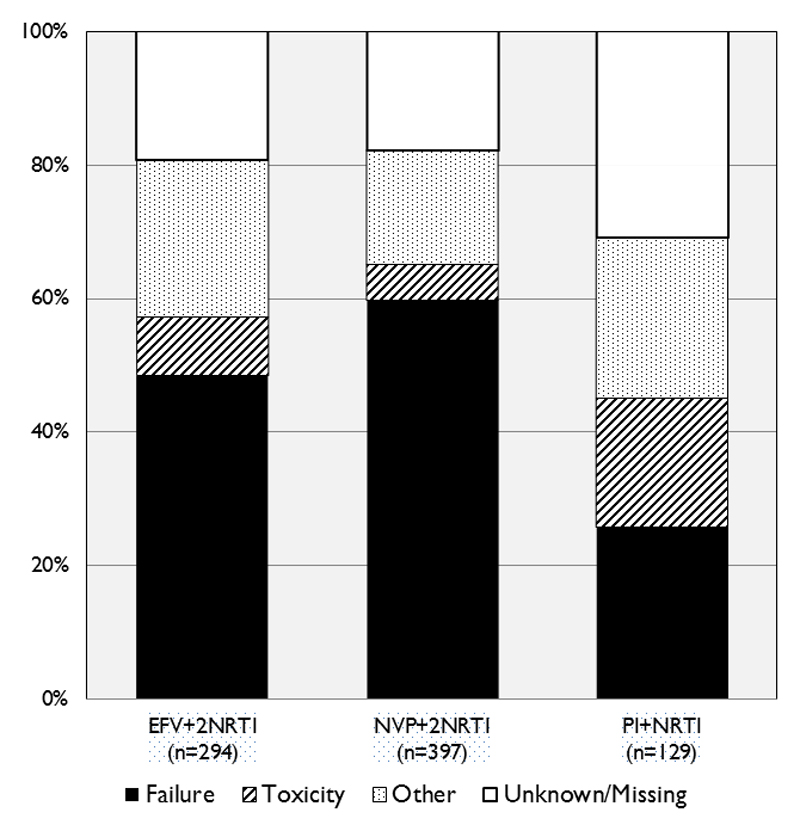
Among those switched to second-line ART, 652 (80%) had a documented reason for switch available, of which 63% were “treatment failure”, 11% “toxicity” and the remainder for “other reasons” including non-compliance. Clinicians were more likely to report toxicity as the reason for switching from a PI-based regimen, whereas NVP-based regimens were more likely to be for failure (p<0.001) (Figure 2). Among the 168 (20%) children with missing reason for switch, 56% were likely to be due to treatment failure based on their clinical, immunological and virological data in the 6 months prior to switch. There was no significant difference in time to switch between those with and without a reason for switch reported (p=0.5).
Figure 2. Reasons reported for switch to second-line ART, by initial ART regimen.
Factors at ART initiation associated with switch
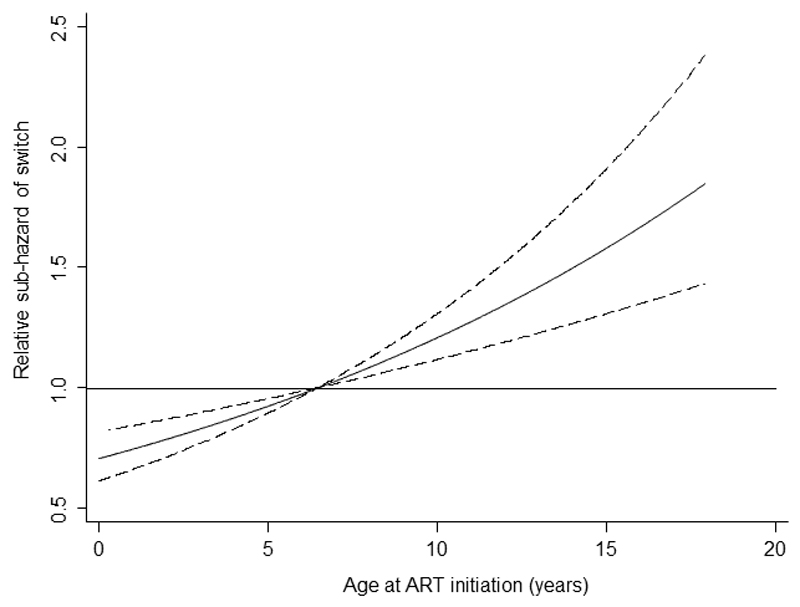
Children who initiated on NVP-based regimens had over two-fold increased risk of switch compared to children starting on PI-based regimens (adjusted sub-hazard ratio (sHR) 2.47, 95% CI 1.86-3.27, p=0.001), while those starting EFV-based regimens had a smaller increased risk (sHR 1.53 (1.16-2.02), p=0.002) (Table 2). In a sub-group analysis of UK/Ireland children aged <3 years, there was no difference in risk of switch between children taking NVP with a 3-NRTI (n=40) versus 2-NRTI (n=41) backbone (sHR 0.99 (0.51, 1.91), p=0.97), with both groups at increased risk of switch compared to those starting a PI-based regimen. The effect of age on hazard rate of switch was linear, with a 5% increase in risk of switch per year increase in age at start of ART (sHR 1.06, 95% CI 1.03-1.08, p<0.0001) (Figure 3). Severe immunosuppression and higher VL at ART start were associated with increased risk of switch. After adjusting for these factors, sex, CDC stage and calendar year at ART initiation were not associated with risk of switch. There was no evidence of an interaction between age at ART start and initial regimen (p=0.3).
Table 2. Cumulative incidence of switch at 5 years after ART initiation and factors associated with switch to second-line ART.
| Cumulative incidence (%) at 5 years (95% CI) | Univariable model | Multivariable model† | |||
|---|---|---|---|---|---|
| SHR (95% CI) | P | aSHR (95% CI) | P | ||
| First-line ART regimen | |||||
| Boosted PI+NRTI | 12 (10 - 15) | 1 | <0.0001 | 1 | <.0001 |
| EFV+2NRT | 23 (20 - 26) | 1.94 (1.58 - 2.39) | 1.53 (1.16 - 2.02) | ||
| NVP+2NRTI | 27 (24 - 29) | 2.23 (1.83 - 2.73) | 2.47 (1.86 - 3.27) | ||
| Age at ART initiation | |||||
| Per 1 year increase | - | 1.04 (1.03 - 1.06) | <0.0001 | 1.06 (1.03 - 1.08) | <0.001 |
| <1 | 19 (16 - 22)) | 0.85 (0.69 - 1.05) | <0.0001 | - | |
| 1 - <3 | 18 (16 - 22) | 0.79 (0.62 - 0.99) | |||
| 3 - <6 | 15 (12 - 19) | 0.79 (0.64 - 0.99) | |||
| 6 - <11 | 21 (18 - 24) | 1 | |||
| ≥ 11 | 32 (28 - 36) | 1.45 (1.21 - 1.74) | |||
| HIV RNA at ART initiation, c/mL | |||||
| <100,000 | 17 (14 - 20) | 0.83 (0.69 - 0.98) | 0.03 | 0.74 (0.61 - 0.90) | 0.003 |
| ≥100,000 | 21, (19 - 24) | 1 | 1 | ||
| WHO severely immunocompromised | |||||
| No | 16 (14 - 19) | 1 | 0.01 | 1 | 0.04 |
| Yes | 23 (21 - 25) | 1.26 (1.06 - 1.49) | 1.23 (1.01 - 1.50) | ||
| CDC stage C diagnosis | |||||
| No | 20 (19 - 22) | 1 | 0.01 | - | |
| Yes | 28 (24 - 33) | 1.27 (1.05 - 1.54) | |||
| Calendar year of ART initiation | |||||
| 1991 - <2004 | 27 (24 - 29) | 1.53 (1.31 - 1.78) | <0.0001 | - | |
| 2004 - <2008 | 20 (18 - 22) | 1 | |||
| ≥2008 | 16 (13 - 20) | 0.79 (0.64 - 0.98) | |||
Proportional hazards regression model accounting for competing risks of death and LTFU.
Multivariable regression model stratified by region.
SHR: Sub Hazard Ratio, aSHR: adjusted Sub Hazard Ratio, ART: Antiretroviral Therapy, PI: Protease Inhibitor, NRTI: Nucleoside Reverse Transcriptase Inhibitor, EFV: Efavirenz, NVP: Nevirapine
Figure 3. Relative hazard of switch by age at ART initiation.
Footnote: Relative hazard for age predicted from a proportional hazard regression model including ART regimen, WHO immunosuppression status and viral load at ART initiation. Hazard rate is plotted relative to a child of age 6.7 years (the median age of the cohort), dashed lines represent the 95% CI.
Sensitivity analyses
When 63 switches in the first 6-months of ART were excluded, the cumulative proportion of switch was lower, at 19% (95% CI, 18-21%) at 5 years after ART start. Including switches across class or within PI class without a simultaneous change in ≥1NRTI, if the reported reason for switch was failure, increased the number of children switching to second-line by 95 and the cumulative proportion of switch at 5 years to 24% (95% CI, 22-25%). Additional switches were predominately from Thailand (51%), and from an NNRTI to a PI-based regimen (82%). The median time to switch was comparable to that observed in the main analysis. In multivariable analyses, the same factors remained associated with switch, with limited change to the point estimates, apart from the risk of switch for NVP-based regimens was reduced to 2.13 and 2.16 respectively. In addition, risk of switch was increased for children starting ART before 2004 when ignoring switches <6 months (data not shown).
Characteristics at time of switch and response to second-line ART
Among those switched to second-line, median age at switch was 11 [6,15] years, VL (n=671) was 4.1 [3.0,4.9] log10 copies/mL and CD4% (n=682) was 20 [11,29] %. The majority of children (72%) received a PI-based second-line regimen (lopinavir/ritonavir (57%), atazanavir (11%), darunavir (5%)), and <1% (n=7) received an integrase inhibitor (INSTI)-based regimen. The median duration of follow-up after start of the second-line regimen was 3.8 (1.8,6.5) years; among those with a VL measurement at 12 (n=561) and 24 (n=480) months after start of second-line, 65% and 69% were suppressed at <400 copies/mL, respectively.
Discussion
This study of time to switch to second-line ART for any cause in children with routine viral load monitoring is the largest to date. It includes many national cohorts across Europe[5,20], benefits from long duration of follow-up (over 5 years) and low levels of mortality and loss-to-follow-up. There are four key findings from our study. First, approximately 80% of children remained on their first-line regimen at 5 years after ART initiation (allowing for minor drug modifications and treatment simplifications). One-in-five children met our definition of switch at 5 years, although there were significant regional variations in the proportion switching, with lowest estimates of 12% in Eastern Europe and highest of 25% in the UK and Ireland.
Second, older age at start of ART was associated with increased risk of switch, with no evidence that this effect varied by initial regimen. Previous studies have reported increased risk of virological failure among children starting ART at older ages[21]. While our outcome was switch for all-causes, over two-thirds of the switches were failure-related. The higher risk of switch in older children may reflect increased risk of failure as well as greater treatment options and/or willingness to switch adolescents experiencing failure compared to younger children. A recent global meta-analysis (including EPPICC), of approximately 100,000 children on ART, the large majority from sub-Saharan Africa, also reported increased risk of switch to second-line with older age at ART start[22]. This highlights the need to consider novel adherence or support interventions for children initiating treatment at older ages, particularly adolescents[23]. This may include treatment simplification strategies such as the ‘weekends off’ short-cycle therapy. The BREATHER trial randomised adolescents virologically suppressed on EFV-based regimens to continuous treatment versus short treatment cycle of 5 days on and 2 days (weekends) off-ART. The latter group reported high acceptability[24], maintained high levels of viral suppression, with low rates of switch at 48 and 144 weeks of follow-up and reported no difference in inflammation markers [25,26].
Thirdly, children initiating a NVP-based regimen had increased risk of switch, as did those starting EFV (although to a lesser degree), compared to those starting PI-based regimens. This is consistent with findings from previous studies showing increased risk of switch to second-line in NVP- versus PI-based regimens[3,5,27]. This could partly reflect the reluctance of clinicians to switch children failing a PI-based regimen due to the difficulty in deciding what to switch to, and recommendations to first address adherence issues due to the high resistance barrier[17]. However, studies have also shown increased risk of virological failure for NVP- versus PI-based regimens[5,28], and a higher proportion of the switches from NVP-based regimens in our cohort were reported as failure-related. These findings support PENTA and US guideline recommendations to consider PI-based first-line regimens in all children (aged>14 days) and adolescents[17,29]. Nonetheless NVP remains a widely used, low-cost, essential drug option for children in resource-limited settings with poor access to PIs[22,30]. It is important to note that the majority of children who initiated NVP in our cohort remained on it at 5 years (73%), suggesting those who tolerated and responded to NVP did achieve long durability on this first-line regimen.
Outside of Eastern Europe, our estimates of switch at 5 years were remarkably similar across regions, despite wide variations in the initial regimens used, and age and immune status at start of ART. Since, severe immunosuppression at ART initiation was confirmed as a risk factor for switch in our study[11], one may have expected higher switch rates in Thailand which had both the highest proportion of children starting NNRTI-based regimens (93%) and who were severely immunocompromised. The lower levels of switch observed in Thailand most likely reflect differences in frequency of viral load testing, as well as availability/readiness to switch to second-line regimens.
Comparison of unadjusted cumulative incidence estimates of switch across studies and contexts is challenging as the distributions of risk factors in heterogeneous populations is not taken into account. Notwithstanding this limitation, our overall estimate of switch to second-line of 21% at 5 years is comparable to recent findings from the Asia Pacific cohort, which reported 23% switch at 5 years among children with routine viral load monitoring[11]. Importantly the authors report that children without viral load monitoring had a 53% lower incidence of switch compared to children with viral load monitoring. As more countries shift towards routine or targeted viral load monitoring[30], the use of second-line ART, which is currently very low (≤3%) in settings without viral load monitoring[22], is expected to increase following improved detection of treatment failure and efforts to improve availability of PI-based and INSTI-based regimens in paediatric formulations[31]. The global use of second-line ART may then reach similar levels to that observed in our cohort.
The clinical implication of the shift to routine viral load testing remains unclear. The PENPACT-1 trial reported no difference in clinical outcomes when switching children early or late, at high (30,000 copies/ml) or low (1000 copies/ml) viral load levels, although the trial was conducted mainly in high-income countries. However, earlier switch did minimise the accumulation of drug resistance mutations in those initiating on NNRTI-based regimens[7,32]. Similarly, recent studies of adult patients in sub-Saharan African (the large majority initiated on NNRTI-based regimens) have reported that delayed switch to second-line after prolonged virological failure was associated with accumulation of resistance mutations which limited the NRTI options for second-line ART[33], as well as increased risk of failure, morbidity and mortality on second-line[12,34,35]. These findings are likely to be generalisable to children in such settings. More information on accumulation of drug resistance mutations whilst on failing PI-based regimens is needed.
Fourthly, over two-thirds of children in our cohort achieved viral suppression at 12 and 24 months after switch to second-line. This is broadly consistent with the prevalence of suppression at 1 year after switch reported in other paediatric cohorts [36–38]. However one-third of patients experienced viremia. It is unclear if this is due to poor adherence or resistant virus. Further studies on the clinical outcomes on second and third-line ART in children and adolescents are warranted.
There are some important limitations to this study. First, 20% of children switched to second-line had no reported reason for switch. Although for these children we used data on clinical status, CD4 and VL in the 6 months before switch to assess likelihood of the switch being failure-related; this may not reflect the true reason for switch. Second, there are unmeasured potential confounders including exposure to maternal/infant antiretroviral prophylaxis, adherence, resistance profile and availability of alternative regimens, all of which may influence the probability of switch.
In summary, in our cohort of children with routine viral load monitoring, a fifth had switched to second-line while the large majority remained on their first-line regimen at 5 years of ART. These estimates provide an insight on the expected use of second-line regimen as the global paediatric HIV population matures and access to viral load monitoring expands. A commitment to the availability of affordable paediatric drugs with high resistance barriers[39, 40] and low pill burden will be essential to ensure these needs are met[31].
Supplementary Material
Summary.
One in five children in this European and Thai paediatric HIV cohort collaboration switched to second-line ART by 5 years of therapy. Two-thirds of switches were due to treatment failure. Nevirapine (NVP)-based regimens were associated with increased risk of switch.
Acknowledgements
We thank all the patients for their participation in these cohorts, and the staff members who cared for them.
Funding: European Union Seventh Framework Programme for research, technological development and demonstration under EuroCoord grant agreement n° 260694; Medical Research Council programme MC_UU_12023/26.
Footnotes
Conflict of interest: No conflict of interest.
Author contributions:
All members of the Project team participated in discussions about the study design, choice of statistical analyses, and interpretation of the findings, and were involved in the preparation and review of the final manuscript. Additionally, Jeannie Collins and Ruth Goodall drafted the manuscript, and Ruth Goodall performed all statistical analyses.
All members of the Writing group were involved in the collection of data and interpretation of the findings.
Writing Group (consisting of Project Team first (ordered alphabetically by name except for the first and last author), and also other Writing Group members (ordered alphabetically by cohort name):
Project team: Intira Jeannie Collins (EPPICC epidemiologist), Luminita Ene (“Victor Babes” Hospital Cohort, Romania), Caroline Foster (Collaborative HIV Paediatric Study (CHIPS), UK and Ireland), Christian Kahlert (Swiss Mother and Child HIV Cohort Study, Switzerland), Colette Smit (ATHENA paediatric cohort, Netherlands) Ruth Goodall (EPPICC senior statistician).
Other Writing Group members: Laura Marques (Centro Hospitalar do Porto, Portugal); Ali Judd, Diana M. Gibb (Collaborative HIV Paediatric Study (CHIPS) and National Study of HIV in Pregnancy and Childhood (NSHPC), UK & Ireland); Antoni Noguera-Julian (CoRISPE-cat, Catalonia, Spain); Sara Guillen, Pablo Rojo Conejo (CoRISPE-1, rest of Spain cohort, Spain); Josiane Warszawski (French Perinatal Cohort Study, France); Chris Koenigs (German Pediatric and Adolescent HIV cohort, Germany); Vana Spoulou (Greece Cohort, Greece); Filipa Prata (Hospital de Santa Maria/CHLN, Lisbon, Portugal); Tessa Goetghebuer (Hospital St Pierre Cohort, Brussels, Belgium); Maurizio de Martino, Clara Gabiano (Italian Register for HIV infection in children, Italy); Lars Naver (Karolinska University Hospital, Stockholm, Sweden); Carlo Giaquinto, Claire Thorne (Paediatric European Network for the Treatment of AIDS (PENTA), Italy); Magdalena Marczynska (Polish paediatric cohort, Poland); Liubov Okhonskaia (Republican Hospital of Infectious Diseases, St Petersburg, Russia); Gonzague Jourdain, Narong Lertpienthum , Achara Puangsombat (Program for HIV Prevention and Treatment (PHPT) Study Group, Thailand); Heather Bailey, Ruslan Malyuta, Alla Volokha (Ukraine Paediatric HIV Cohort Study, Odessa, Ukraine); Roxana Radoi (“Victor Babes” Hospital Cohort, Romania).
References
- 1.UNAIDS. Global HIV Statistics: Fact Sheet July, 2017. 2017. [Google Scholar]
- 2.Kim SH, Gerver SM, Fidler S, Ward H. Adherence to antiretroviral therapy in adolescents living with HIV: systematic review and meta-analysis. AIDS. 2014;28(13):1945–56. doi: 10.1097/QAD.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–8. doi: 10.1097/QAI.0b013e3182060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowenthal ED, Ellenberg JH, Machine E, et al. Association between efavirenz-based compared with nevirapine-based antiretroviral regimens and virological failure in hiv-infected children. JAMA. 2013;309(17):1803–9. doi: 10.1001/jama.2013.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duong T, Judd A, Collins I, et al. Long-term virological outcome in children on antiretroviral therapy in the UK and Ireland. AIDS. 2014;28(16):2395–405. doi: 10.1097/QAD.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins I, Cairns J, Le Coeur S, et al. Five-year trends in antiretroviral usage and drug costs in HIV-infected children in Thailand. J Acquir Immune Defic Syndr. 2013;64(1):95–102. doi: 10.1097/QAI.0b013e318298a309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babiker A, Castro nee Green H, Compagnucci A, et al. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. The Lancet infectious diseases. 2011;11(4):273–83. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARROW Trial team. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. The Lancet. 2013 doi: 10.1016/S0140-6736(12)62198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. The Lancet. 2013 doi: 10.1016/S0140-6736(13)61409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judd A. European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) study group in EuroCoord. Early antiretroviral therapy in HIV-1-infected infants, 1996-2008: treatment response and duration of first-line regimens. AIDS. 2011;25(18):2279–87. doi: 10.1097/QAD.0b013e32834d614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohamed TJ, Teeraananchai S, Kerr S, et al. Impact of Viral Load Use on Treatment Switch in Perinatally HIV-Infected Children in Asia. AIDS research and human retroviruses. 2016 doi: 10.1089/aid.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS (London, England) 2014;28(14):2097–107. doi: 10.1097/QAD.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmonde S, Eboua FT, Malateste K, et al. Determinants of durability of first-line antiretroviral therapy regimen and time from first-line failure to second-line antiretroviral therapy initiation. AIDS. 2015;29(12):1527–36. doi: 10.1097/QAD.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lecher S, Williams J, Fonjungo PN, et al. Progress with Scale-Up of HIV Viral Load Monitoring - Seven Sub-Saharan African Countries, January 2015-June 2016. MMWR Morb Mortal Wkly Rep. 2016;65(47):1332–5. doi: 10.15585/mmwr.mm6547a2. [DOI] [PubMed] [Google Scholar]
- 15.Welch S, Sharland M, Lyall EG, et al. PENTA 2009 guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV medicine. 2009;10(10):591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. [Accessed 30 Sept 2015];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1.
- 17.Bamford A, Turkova A, Lyall H, et al. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV medicine. 2015 doi: 10.1111/hiv.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 19.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: 2007. [Google Scholar]
- 20.Chiappini E, Galli L, Tovo PA, et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS. 2007;21(12):1607–15. doi: 10.1097/QAD.0b013e32823ecf5b. [DOI] [PubMed] [Google Scholar]
- 21.Judd A, Lodwick R, Noguera-Julian A, et al. Higher rates of triple-class virological failure in perinatally HIV-infected teenagers compared with heterosexually infected young adults in Europe. HIV medicine. 2016 doi: 10.1111/hiv.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wools-Kaloustian K, Smith C, Goodall R, et al. Predictors of switch to second-line ART in HIV-positive children: A global analysis (poster 815) CROI Seattle USA. 2017 [Google Scholar]
- 23.Ferrand RA, Briggs D, Ferguson J, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Tropical Medicine & International Health. 2016;21(3):325–33. doi: 10.1111/tmi.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernays S, Paparini S, Seeley J, Namukwaya Kihika S, Gibb D, Rhodes T. Qualitative study of the BREATHER trial (Short Cycle antiretroviral therapy): is it acceptable to young people living with HIV? BMJ Open. 2017;7(2):e012934. doi: 10.1136/bmjopen-2016-012934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler K, Inshaw J, Ford D, et al. BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) in young people with chronic human immunodeficiency virus infection: an open, randomised, parallel-group Phase II/III trial. Health Technol Assess. 2016;20(49):1–108. doi: 10.3310/hta20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turkova A, Butler k, Moore C, et al. Long-term effects of weekends off ART in HIV-1 infected young people on EFV+2NRTIs (Poster #813). Conference on Retroviruses and Opportunistic Infections (CROI) Seattle USA. 2017 [Google Scholar]
- 27.Barlow-Mosha L, Angelidou K, Lindsey J, et al. Nevirapine- Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy in HIV-Infected Infants and Young Children: Long-term Follow-up of the IMPAACT P1060 Randomized Trial. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2016;63(8):1113–21. doi: 10.1093/cid/ciw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barlow-Mosha L, Angelidou K, Lindsey J, et al. Nevirapine- Versus Lopinavir/Ritonavir-Based Antiretroviral Therapy in HIV-Infected Infants and Young Children: Long-term Follow-up of the IMPAACT P1060 Randomized Trial. Clinical Infectious Diseases. 2016;63(8):1113–21. doi: 10.1093/cid/ciw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Chlidren. 2012. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. [Google Scholar]
- 30.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Accessed 7 Dec 2016]; Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?ua=1.
- 31.Penazzato M, Palladino C, Sugandhi N. participants obotPADOM. Prioritizing the most needed formulations to accelerate paediatric antiretroviral therapy scale-up. Current Opinion in HIV and AIDS. 2017;12(4):369–76. doi: 10.1097/COH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 32.Harrison L, Melvin A, Fiscus S, et al. HIV-1 Drug Resistance and Second-line Treatment in Children Randomized to Switch at Low versus Higher RNA Thresholds. Journal of acquired immune deficiency syndromes. 2015;70(1):42–53. doi: 10.1097/QAI.0000000000000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boender TS, Kityo CM, Boerma RS, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016;71(10):2918–27. doi: 10.1093/jac/dkw218. [DOI] [PubMed] [Google Scholar]
- 34.Rohr JK, Ive P, Horsburgh CR, et al. Marginal Structural Models to Assess Delays in Second-Line HIV Treatment Initiation in South Africa. PLOS ONE. 2016;11(8):e0161469. doi: 10.1371/journal.pone.0161469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramadhani HO, Bartlett JA, Thielman NM, et al. The Effect of Switching to Second-Line Antiretroviral Therapy on the Risk of Opportunistic Infections Among Patients Infected With Human Immunodeficiency Virus in Northern Tanzania. Open Forum Infectious Diseases. 2016;3(1):ofw018. doi: 10.1093/ofid/ofw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanoni BC, Sunpath H, Feeney ME. Pediatric Response to Second-Line Antiretroviral Therapy in South Africa. PLoS One. 2012;7(11):e49591. doi: 10.1371/journal.pone.0049591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musiime V, Kaudha E, Kayiwa J, et al. Antiretroviral drug resistance profiles and response to second-line therapy among HIV type 1-infected Ugandan children. AIDS research and human retroviruses. 2013;29(3):449–55. doi: 10.1089/aid.2012.0283. [DOI] [PubMed] [Google Scholar]
- 38.Prasitsuebsai W, Teeraananchai S, Singtoroj T, et al. Treatment Outcomes and Resistance Patterns of Children and Adolescents on Second-Line Antiretroviral Therapy in Asia. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;72(4):380–6. doi: 10.1097/QAI.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viani RM, Alvero C, Fenton T, et al. Safety, Pharmacokinetics and Efficacy of Dolutegravir in Treatment-experienced HIV-1 Infected Adolescents: Forty-eight-week Results from IMPAACT P1093. Pediatr Infect Dis J. 2015;34(11):1207–13. doi: 10.1097/INF.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruel T, Acosta E, Singh T, et al. Dolutegravir pharmacokinetics, safety and efficacy in HIV+ children 2 to <6 years old (poster #806). Conference on Retroviruses and Opportunistic Infections; Boston USA: 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.