Abstract
Background and Purpose
Stroke is a risk factor for dementia, but the risk of dementia after different stroke types is poorly understood. We examined the long-term risk of dementia among survivors of any first-time stroke and of first-time ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage.
Methods
We conducted a 30-year nationwide population-based cohort study using data from Danish medical databases (1982–2013) covering all Danish hospitals. We identified 84,220 ischemic stroke survivors, 16,723 intracerebral hemorrhage survivors, 9,872 subarachnoid hemorrhage survivors, and 104,303 survivors of unspecified stroke types. Patients were aged ≥18 years and survived for at least three months after diagnosis. We formed a comparison cohort from the general population (1,075,588 patients without stroke, matched to stroke patients by age and sex). We computed absolute risks and hazard ratios of dementia up to 30 years after stroke.
Results
The 30-year absolute risk of dementia among stroke survivors was 11.5% (95% confidence interval, 11.2%–11.7%). Compared with the general population, the hazard ratio (95% confidence interval) for dementia among stroke survivors was 1.80 (1.77–1.84) after any stroke, 1.72 (1.66–1.77) after ischemic stroke, 2.70 (2.53–2.89) after intracerebral hemorrhage, and 2.74 (2.45–3.06) after subarachnoid hemorrhage. Younger patients regardless of stroke type faced higher risks of post-stroke dementia than older patients. The pattern of hazard ratios by stroke type did not change during follow-up and was not altered appreciably by age, sex, or preexisting diagnoses of vascular conditions.
Conclusions
Stroke increases dementia risk. Survivors of intracerebral hemorrhage and subarachnoid hemorrhage are at particularly high long-term risk of post-stroke dementia.
Keywords: Cerebrovascular Disorders, Dementia, Epidemiology, Intracranial Hemorrhages, Stroke, Subject terms: Intracranial Hemorrhage, Ischemic Stroke, Epidemiology, Risk Factors
In aging populations, stroke and dementia are leading contributors to the burden of medical and social disability.1 Stroke is a well-known risk factor for dementia.2,3 However, many aspects of this association that could help guide effective prevention strategies for dementia are poorly understood.3
The risk of dementia in patients surviving one year after stroke is twice that of hospital-based or community-based cohorts without stroke.2,3 The only cohort with follow-up beyond 10 years reported a 25-year risk of dementia of 48% after ischemic stroke between 1960 and 1984.4 The long-term risk of dementia after hemorrhagic stroke—either intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH)—is unknown. Hemorrhagic strokes, which are characterized by greater lesion volume and higher intracranial pressure, generally present with more severe brain injury than ischemic strokes. Another difference is that brain regions affected by ICH and SAH differ from those affected by ischemic stroke. Furthermore, compared with ischemic strokes, treatment strategies after ICH and SAH may contribute to lower capacity for brain tissue recovery.5
To clarify the burden of post-stroke dementia in different stroke types, we examined the long-term risk of dementia among survivors of incident stroke of any type and among survivors of ischemic stroke, ICH, and SAH.
Methods
Source population and data sources
We conducted a population-based matched cohort study using national medical databases. The source population included all Danish citizens with a first-time inpatient hospital diagnosis of stroke between January 1982 and December 2013. Denmark has a universal, tax-supported healthcare system, and its registry databases6 maintain extensive individual-level healthcare information. All Danes have been assigned a unique civil registration number since 1968, or at birth, by the Danish Civil Registration System.6 This identifier permits unambiguous linkage between databases. The Civil Registration System maintains up-to-date information on the civil and vital status of the entire population, allowing for nearly complete follow-up.
It is standard medical practice for all Danish patients with stroke to receive inpatient hospital care.7 The Danish National Patient Registry (DNPR) has registered >99% of all inpatient admissions since 1977.7 One primary discharge diagnosis,8 which represents the main reason for hospitalization, and optional secondary diagnoses are coded according to the International Classification of Diseases, Eighth Revision until 1993 and Tenth Revision since then. Outpatient specialty clinic visits have been included since 1995. Starting in 1999, the DNPR registered computed tomography of the brain and magnetic resonance imaging procedures, according to the Nordic Classification of Surgical Procedures.8 The Danish Psychiatric Central Register (DPCR) includes information on all inpatient psychiatric admissions since 1969 and on all outpatient clinic psychiatric contacts since1995.9 Diagnosis and procedure codes used in this study are provided in Table I in the online-only Data Supplement.
Stroke survivor cohort
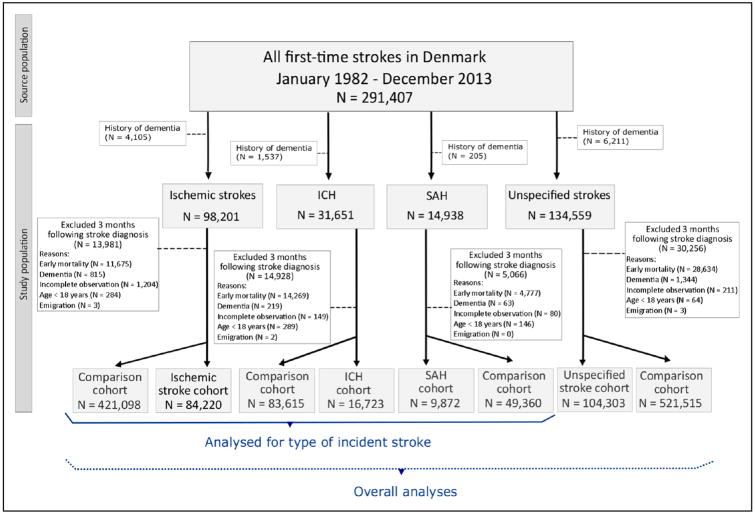
Based on DNPR data, we defined the study population of three-month survivors after an incident primary diagnosis of ischemic stroke, ICH,SAH, or unspecified stroke who were aged ≥18 years and were diagnosed between January 1, 1982, and September 30, 2013 (Figure 1). Compared with medical records, the positive predictive value of an inpatient diagnosis of incident stroke in the DNPR is 81% to 86%.10 We included only patients whose stroke was confirmed by a primary diagnosis, to increase validity of the type of stroke diagnosis. Patients whose stroke was diagnosed solely in an emergency department were excluded, as stroke diagnosis may be inaccurate in this setting.10
Figure 1.
Population of first-time stroke survivors and the general population comparison cohort. ICH indicates intracerebral hemorrhage and SAH, subarachnoid hemorrhage.
The start date of the study was chosen as five years after the establishment of the DNPR in 1977, in an effort to exclude patients with preexisting diagnoses of stroke, other cerebrovascular disease, or hemiplegia (as an indicator of previous stroke12). Based on the data in the DNPR and the DPCR, we excluded patients with diagnoses of dementia and of mild cognitive impairment or an amnestic syndrome, which may represent prodromal dementia, up to the index date. The index date was set three months after the stroke diagnosis date. The delay served to exclude not only patients with early mortality but also any false-positive incident dementia diagnosis in the acute period caused by delirium or by stroke-related language, sensory, or motor barriers to cognitive assessment.2,13,14
General population comparison cohort
We constructed a comparison cohort from the general population using the Civil Registration System, the DNPR, and the DPCR. On his/her index date, each stroke patient was matched by sex and year of birth to up to five individuals from the general population without diagnoses of cerebrovascular diseases, hemiplegia, dementia, mild cognitive impairment, or amnestic syndrome.
Incident dementia
The study outcome was incident all-cause inpatient or outpatient dementia diagnoses recorded in the DNPR or DPCR. The positive predictive value of an International Classification of Diseases-Tenth Revision dementia registration in the two databases is 86%.15 We classified dementia as Alzheimer's disease, vascular dementia, or any other dementia.15
Potential confounders
We used inpatient and outpatient DNPR records from 1977 onward and DPCR records from 1968 onward (except emergency department diagnoses), to identify stroke risk factors that could potentially confound the association between stroke and dementia. As of the index date, we ascertained vascular factors, defined as any preexisting coding of diabetes mellitus, atrial fibrillation or flutter, hypertension, hyperlipidemia (including hypercholesterolemia), myocardial infarction, heart failure, peripheral vascular disease, and smoking.2,17,18 We also ascertained any preexisting diagnosis of traumatic brain injury,19,20 depression,21 or substance abuse.22,23 Details on the ascertainment of smoking and substance abuse are found in the Methods section in the online-only Data Supplement.
Statistical analysis
Follow-up started on the index date and continued until incident dementia, death, emigration, 30 years after stroke diagnosis, or December 31, 2013, whichever occurred first. We computed absolute risks of dementia, accounting for the competing risk of death,24 and incidence rates per 1000 person-years. We used stratified Cox regression, taking into consideration the matching design, to compute hazard ratios (HRs) and their 95% confidence intervals (CIs) as measures of the relative risk of dementia among stroke survivors. We stratified person-time to calculate incidence rates and HRs during three intervals: 3 to 12 months, >1 to 10 years, and >10 to 30 years after stroke diagnosis. We also stratified our analyses by age and sex. Analyses were performed for patients with any incident stroke—and separately for patients with ischemic stroke, ICH, and SAH—and for matched individuals from the general population (Figure 1). The proportionality assumption was fulfilled for the three follow-up intervals in the pooled data set, as ascertained by inspection of log–log plots.
To compare our relative risks for any stroke with results presented in the literature,4,25-31 we repeated our analyses, restricting our study population to the stroke type, age range, and maximum follow-up periods applied in previous relevant cohort studies.
We adjusted our analyses by potential confounders to examine whether post-stroke dementia risk could be attributed to stroke injury, rather than to the presence of identified risk factors for stroke. We created three adjusted models to separate traditional vascular factors for ischemic stroke32 from other stroke risk factors, as well as to avoid collinearity among vascular conditions (Methods in the online-only Data Supplement).
We performed three sensitivity analyses to assess the robustness of our results. First, we assessed any potentially biased estimates because of stroke type misclassification by restricting the study population to patients with stroke with structural brain imaging (magnetic resonance imaging or computed tomography) performed during the index admission. Second, we assessed bias because of inclusion of possible cases of prevalent dementia by redefining the index date as six months after stroke.25 Finally, we assessed period effects by stratifying analyses in three index periods (1982–1994, 1995–2003, and2004–2013). In this stratified analysis, we restricted follow-up to 10 years, to ensure more homogeneous follow-up among the periods. We selected these periods, first, because the International Classification of Diseases-Tenth Revision codes were introduced in 1994, and outpatient specialist clinic diagnoses became available in 1995. Second, because after 2003, wider adoption of brain imaging procedures to guide thrombolytic therapy increased the proportion of strokes registered with specific diagnoses in the DNPR.33 All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC). This study was approved by the Danish Data Protection Agency (record no. 2014-54-0922).
Results
Study population
We identified 279,349 patients with a first-time stroke and no history of dementia or diagnoses that may represent prodromal dementia. Three months after stroke diagnosis, the stroke cohort included 215,118 eligible patients (median age, 72 years; range, 18–108 years). Twelve percent of ischemic stroke patients, 45% of ICH patients, 32% of SAH patients, and 21% of patients with unspecified stroke did not survive until the index date. The general population comparison cohort included 1,075,588 individuals (Figure 1). Among the stroke survivors, 110,815 had a specific diagnosis. Before 2004, the relative distribution of stroke diagnoses was 30% ischemic stroke, 7% ICH, 5% SAH, and 58% unspecified stroke; from 2004 onward, this distribution was 58% ischemic stroke, 8% ICH, 5% SAH, and 29% unspecified stroke. From 2000 onward, 83% of patients with specific stroke diagnoses underwent brain imaging during their hospital admission. About one third of ischemic stroke survivors, two fifths of ICH survivors, and more than three quarters of SAH survivors were below the age of 65 years. The characteristics of the cohorts at study entry are provided in Table II in the online-only Data Supplement.
Risk of dementia in first-time stroke survivors
During median follow-up of five years (interquartile range [IQR], 2.2–9.6), 17,144 (8.0%) stroke survivors received dementia diagnoses, 57.9% of stroke survivors died, and 0.1% emigrated from Denmark. Mortality and emigration in the general population were 45.9% and 0.2%, respectively. The 30-year absolute dementia risk among stroke survivors was 11.5% (95% CI, 11.2–11.7). Compared with the general population, stroke survivors had a 1.80 (95% CI, 1.77–1.84) increased risk of dementia. The relative risk was higher during the first year after stroke (Table), when the absolute risk was 1.12% (95% CI, 1.08–1.17%). The 10-year risk of dementia was 7.64% (95% CI, 7.51–7.76%). The relative risks compared with those reported in earlier cohort studies are presented in Table III in the online-only Data Supplement.
Table. Dementia rates and hazard ratios, according to type of incident stroke.
| Stroke type | Cohort | Number | Dementia | |||
|---|---|---|---|---|---|---|
| Person-years | Number of events | Rate* (95% CI) | Hazard Ratios (95% CI) | |||
| 3-12 months after stroke diagnosis | ||||||
| All | Stroke | 215,118 | 151,097 | 2,391 | 15.8 (15.2-16.5) | 2.57 (2.44–2.70) |
| Comparison | 1,075,588 | 786,778 | 4,996 | 6.35 (6.17-6.53) | reference | |
| Ischemic | Stroke | 84,220 | 59,158 | 854 | 14.4 (13.5-15.4) | 2.29 (2.11–2.48) |
| Comparison | 421,098 | 305,561 | 1,973 | 6.46 (6.17-6.74) | reference | |
| ICH | Stroke | 16,723 | 11,663 | 241 | 20.7 (18.1-23.3) | 4.34 (3.64–5.16) |
| Comparison | 83,615 | 61,268 | 300 | 4.90 (4.34-5.45) | reference | |
| SAH | Stroke | 9,872 | 7,161 | 78 | 10.9 (8.47-13.3) | 6.78 (4.82–9.54) |
| Comparison | 49,360 | 36,478 | 62 | 1.70 (1.28-2.12) | reference | |
| > 1-10 years after stroke diagnosis | ||||||
| All | Stroke | 189,094 | 965,398 | 11,797 | 12.2 (12.0-12.4) | 1.73(1.70–1.77) |
| Comparison | 913,584 | 5,616,392 | 44,322 | 7.89 (7.82-7.97) | reference | |
| Ischemic | Stroke | 73,805 | 356,777 | 4,151 | 11.6 (11.3-12.0) | 1.64(1.58–1.71) |
| Comparison | 357,489 | 2,019,490 | 15,440 | 7.65 (7.52-7.77) | reference | |
| ICH | Stroke | 14,507 | 76,128 | 1,032 | 13.6 (12.7-14.4) | 2.60(2.40–2.82) |
| Comparison | 70,662 | 448,899 | 2,802 | 6.24 (6.01-6.47) | reference | |
| SAH | Stroke | 9,242 | 62,684 | 241 | 3.84 (3.36-4.33) | 2.63(2.24–3.10) |
| Comparison | 45,794 | 327,665 | 565 | 1.72 (1.58-1.87) | reference | |
| > 10-30 years after stroke diagnosis | ||||||
| All | Stroke | 49,968 | 258,725 | 2,956 | 11.4 (11.0-11.8) | 1.58(1.50–1.66) |
| Comparison | 188,878 | 1,264,005 | 9,233 | 7.30 (7.16-7.45) | reference | |
| Ischemic | Stroke | 16,703 | 84,607 | 947 | 11.2 (10·5-11.9) | 1.62 (1.48–1.76) |
| Comparison | 63,445 | 416,557 | 3,004 | 7.21 (6.95-7.47) | reference | |
| ICH | Stroke | 4,237 | 24,937 | 267 | 10.7 (9.42-12.0) | 2.10(1.78–2.47) |
| Comparison | 17,075 | 125,648 | 687 | 5.47(5.06-5.88) | reference | |
| SAH | Stroke | 4,902 | 38,296 | 246 | 6.42 (5.62-7.23) | 2.28 (1.92–2.70) |
| Comparison | 22,039 | 191,296 | 560 | 2.93 (2.68-3.17) | reference | |
Per 1,000 person-years
CI indicates confidence interval; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage
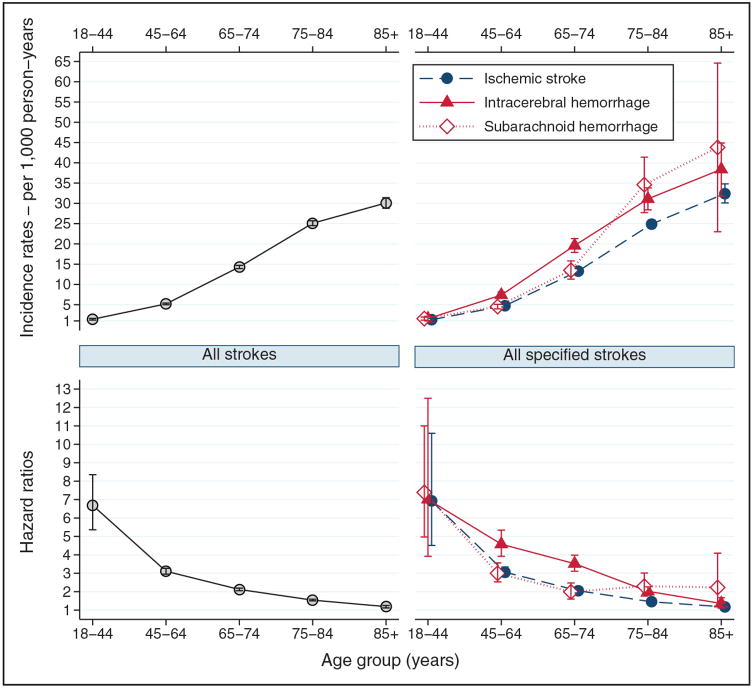
The incidence of dementia increased with increasing age on the index date (Figure 2). Stroke survivors aged ≥65 had higher 10-year absolute risks of dementia (9.74%; 95% CI, 9.58–9.90). In contrast, the relative risk (95% CI) decreased with age: 6.69 (5.36–8.35) among stroke survivors aged <45 years, 3.11 (2.95–3.27) among those aged 45 to 64 years, and1.65 (1.62–1.68) among those aged >64 years. This pattern was stable during the follow-up (Table IV in the online-only Data Supplement). The incidence of post-stroke dementia was somewhat higher for women than for men, but the HR was higher for men than for women (Table V in the online-only Data Supplement).
Figure 2.
Incidence rates and hazard ratios for dementia according to age at study entry, and type of incident stroke.
Type of incident stroke and dementia risk
During median follow-up of five years (IQR, 2.0–8.8 years), 5,952 ischemic stroke survivors received a dementia diagnosis. During the same median follow-up (IQR, 2.1–10 years), 1,540 ICH survivors received a dementia diagnosis. During median follow-up of 10 years (IQR, 4.4–17 years), 565 SAH survivors received this diagnosis. The 30-year risk (95% CI) of dementia was 11.8% (11.4%–12.2%) after ischemic stroke, 13.3% (12.5%–14.1%) after ICH, and 11.6% (10.3%–13.0%) after SAH. The 10-year risk (95% CI) was 7.65% (7.44%–7.86%) after ischemic stroke, 8.89% (8.43%–9.37%) after ICH and, reflecting the younger age distribution, 3.70% (3.31%–4.11%) after SAH. The median age (IQR) at dementia diagnosis was 81 years (75–86 years) for ischemic stroke, 79 years (72–84 years) for ICH, and 74 years (64–82 years) for SAH.
Hemorrhagic stroke survivors had higher relative risks (95% CIs) of dementia than ischemic stroke survivors. The unadjusted HRs were 1.72 (1.66–1.77) after ischemic stroke, 2.70 (2.53–2.89) after ICH, and 2.74 (2.45–3.06) after SAH. The pattern of HRs by stroke type did not change during follow-up. However, for all three stroke types, the relative risk was higher during the first year after diagnosis, particularly among SAH survivors (Table). After the first year, the increased risk of dementia persisted for all types of stroke, but at a lower level (Table). The risks for ischemic stroke survivors were about 60% to 64% higher relative to the comparison cohort, whereas risks were about 2.1 to 2.6 times higher for survivors of ICH and SAH than for the comparison cohort (Table).
The sex and age pattern of incidence and HR of dementia for each stroke type was similar to the pattern found for all strokes combined (Figure 2; Table V in the online-only Data Supplement). During the first year after stroke diagnosis, the particularly high HR for SAH was attenuated after age stratification (Table IV in the online-only Data Supplement). However, the difference in HRs between hemorrhagic and ischemic strokes still was observed after age or sex stratification (Figure 2; Table V in the online-only Data Supplement) and after adjusting for underlying vascular conditions and other potential confounders (Figure 3).
Figure 3.
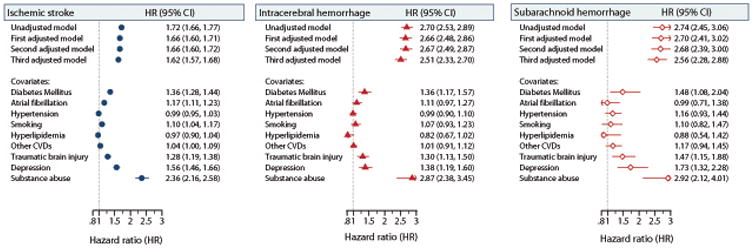
Unadjusted and adjusted hazard ratios (HR; 95% confidence intervals [CIs]) for dementia according to type of incident stroke as assessed by the first, second and third models. Covariate estimates are shown according to the relevant model. Other cardiovascular diseases (CVDs) indicates the presence of myocardial infarction, heart failure or peripheral vascular disease.
Dementia
About half (52%) of dementia diagnoses were classified as other dementia; in this group, the most commonly coded diagnosis was unspecified dementia (International Classification of Diseases-Tenth Revision: F03.9, 92%). As expected, stroke survivors were more likely to receive a specific diagnosis of vascular dementia than Alzheimer's disease (Table VI in the online-only Data Supplement).
Sensitivity analyses
The analyses restricted to patients with stroke who underwent brain imaging corroborated the disparity in post-stroke dementia risk between hemorrhagic and ischemic strokes, with a slightly lower HR in ischemic stroke survivors and a slightly higher HR in ICH and SAH survivors (Table VII in the online-only Data Supplement). Redefining the index date as six months after stroke diagnosis yielded consistent results by type of stroke within the first year after stroke (Table VIII in the online-only Data Supplement). From 1995 onward, dementia incidence has increased. Among ischemic stroke survivors, the HR declined, thereby decreasing the HR for all strokes combined. However, the difference in HRs by stroke type was consistent across all three index periods (Table IX in the online-only Data Supplement).
Discussion
In this nationwide cohort of stroke survivors aged ≥18, 11.5% received dementia diagnoses up to 30 years after their stroke. This risk was increased about two-fold compared with the general population. The risks for post-stroke dementia were substantially higher for hemorrhagic forms of stroke than for ischemic stroke and for stroke occurring at younger ages than at older ages. Risks among survivors were persistently high even after 10 years. The increased risk of post-stroke dementia was not altered appreciably by vascular factors or by other measured factors associated with both stroke and dementia risk.
Our findings extend those of earlier cohort studies based on first-time ischemic strokes or any type of stroke.4,25–31 Most were restricted to older individuals and to shorter follow-up periods.27-31 In the present study, relative risks for any stroke were comparable to the two-fold increased risk with in 10 years of follow-up reported for the Framingham25 and Rotterdam26 cohorts. These investigators also excluded patients with previous stroke or with dementia in the early post-stroke period. Our study is the first to include patients with stroke from 2002 onward, an era of improved therapies and outcomes for acute ischemic stroke.12 This may in part explain why the relative risk for stroke was lower in our study than earlier studies. Risk of dementia among patients with specific types of stroke is available only from a five-year follow-up Taiwanese cohort,5 where the pattern of relative risk by stroke type was similar to that of our study, but of much greater magnitude. Differences in effect size may be explained, in part, by different age distributions, by different methods used to exclude patients with early stroke-related mortality, and different approaches to exclude false-positive incident dementia.
Age is a strong risk factor for dementia and, as expected, the incidence of dementia increased with increasing age at the index date for strokes of all types. The higher relative risk of dementia in younger survivors of all stroke types also suggested a higher impact of the stroke injury on dementia risk in the absence of other competing neurodegenerative pathologies.35 The increased relative risk for dementia after hemorrhagic stroke compared with ischemic stroke persisted after age and sex stratification and after adjusting our analysis for underlying vascular conditions and other measured stroke risk factors. Compared with ischemic stroke, brain injury is often more severe, and therapies are often more invasive and less effective after hemorrhagic stroke.36 Brain injury differences may include higher intracranial pressure and more extensive acute and chronic inflammatory changes associated with hemorrhagic stroke.5,37 SAH and ICH with ventricular or subarachnoid extension are risk factors for communicating hydrocephalus.36 Differences in genetic susceptibility38 may also play a role.
The validity of our findings is enhanced by the use of a nationwide cohort with universal healthcare coverage, standard practices for treating stroke, and virtually complete follow-up. The ascertainment of stroke is high in the DNPR, but hemorrhagic stroke is more often misclassified than ischemic stroke.3 Such differential misclassification likely did not affect the validity of our findings, as HR differences by stroke type were slightly higher in patients known to undergo brain imaging. Ischemic stroke was more often registered as unspecified stroke in the DNPR before brain imaging was widely implemented in 2004,10,11 but HR differences by stroke type were consistent in index periods before and after 2004.
Results pertaining to dementia subtypes should be interpreted cautiously. First, a specific diagnosis is not provided for over half of dementia diagnoses using the DNPR and the DPCR. Second, the ascertainment of all-cause dementia in these databases is higher than for specific dementia subtypes.15,16 Third, because stroke is a diagnostic criterion for vascular dementia, the stroke exposure status could potentially bias the direction of associations found for dementia subtypes.1 Another study limitation was the lack of data on education level. However, residual confounding by education is expected to be small because we were able to adjust our analysis for extensive morbidity data associated with education, such as those related to vascular factors and alcohol abuse.40
Our results have public health relevance. Although it is commonly accepted that reducing stroke incidence reduces dementia risk, risk factors and preventive strategies for ICH and SAH differ from those for ischemic stroke.32,41,42 Effective interventions to reduce the incidence of hemorrhagic stroke and effective strategies to reduce the extent of brain injury after hemorrhagic stroke may also help prevent dementia. Even so, 74% of dementia diagnosed among stroke survivors with a specific stroke diagnosis was attributable to ischemic stroke. Thus, advances in ischemic stroke prevention and management are expected to have higher impact on the total burden of dementia.
ICH and SAH survivors were younger than ischemic stroke survivors, and relative risks for dementia were higher in the young. Before the age of 65 years, 40% of dementia diagnosed among stroke survivors with a specific stroke diagnosis were given to those with hemorrhagic stroke, either ICH or SAH. Early-onset dementia, which often occurs during parenting and employment years, is associated with higher societal burden because of loss of productivity and prolongeddisability.43 Thus, younger survivors of ICH and SAH also represent important targets for planning dementia prevention strategies in the future.
Supplementary Material
Acknowledgments
Source of funding: Supported by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation, and the Aarhus University Research Foundation. VWH received support from National Institutes of Health grant P50AG047366.
Footnotes
Disclosures: None
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 3.Savva GM, Stephan BC Alzheimer's Society Vascular Dementia Systematic Review Group. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–e46. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 4.Kokmen E, Whisnant JP, O'Fallon WM, Chu CP, Beard CM. Dementia after ischemic stroke: a population-based study in Rochester, Minnesota (1960-1984) Neurology. 1996;46:154–159. doi: 10.1212/wnl.46.1.154. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk BJ, Vergouwen MD, Kelfkens MM, Rinkel GJ, Hol EM. Glial cell response after aneurysmal subarachnoid hemorrhage - Functional consequences and clinical implications. Biochim Biophys Acta. 2016;1862:492–505. doi: 10.1016/j.bbadis.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Erlangsen A, Fedyszyn I. Danish nationwide registers for public health and health-related research. Scand J Public Health. 2015;43:333–339. doi: 10.1177/1403494815575193. [DOI] [PubMed] [Google Scholar]
- 7.Thorvaldsen P, Davidsen M, Brønnum-Hansen H, Schroll M. Stable stroke occurrence despite incidence reduction in an aging population: stroke trends in the Danish monitoring trends and determinants in cardiovascular disease (MONICA) population. Stroke. 1999;30:2529–2534. doi: 10.1161/01.str.30.12.2529. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7 suppl):54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 10.Krarup LH, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28:150–154. doi: 10.1159/000102143. [DOI] [PubMed] [Google Scholar]
- 11.Johnsen SP, Overvad K, Sørensen HT, Tjønneland A, Husted SE. Predictive value of stroke and transient ischemic attack discharge diagnoses in The Danish National Registry of Patients. J Clin Epidemiol. 2002;55:602–607. doi: 10.1016/s0895-4356(02)00391-8. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Jacobsen JB, Johnsen SP, Bøtker HE, Sørensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology. 2014;82:340–350. doi: 10.1212/WNL.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 14.Pendlebury ST, Klaus SP, Thomson RJ, Mehta Z, Wharton RM, Rothwell PM Oxford Vascular Study. Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (III) applicability of cognitive tests. Stroke. 2015;46:3067–3073. doi: 10.1161/STROKEAHA.115.010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phung TK, Andersen BB, Høgh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220–228. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 16.Phung TK, Andersen BB, Kessing LV, Mortensen PB, Waldemar G. Diagnostic evaluation of dementia in the secondary health care sector. Dement Geriatr Cogn Disord. 2009;27:534–542. doi: 10.1159/000223664. [DOI] [PubMed] [Google Scholar]
- 17.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurin D, Masaki KH, White LR, Launer LJ. Ankle-to-brachial index and dementia: the Honolulu-Asia Aging Study. Circulation. 2007;116:2269–2274. doi: 10.1161/CIRCULATIONAHA.106.686477. [DOI] [PubMed] [Google Scholar]
- 19.Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke. 2011;42:2733–2739. doi: 10.1161/STROKEAHA.111.620112. [DOI] [PubMed] [Google Scholar]
- 20.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8:e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köhler S, Buntinx F, Palmer K, van den Akker M. Depression, vascular factors, and risk of dementia in primary care: a retrospective cohort study. J Am Geriatr Soc. 2015;63:692–698. doi: 10.1111/jgs.13357. [DOI] [PubMed] [Google Scholar]
- 22.Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry. 2007;64:495–502. doi: 10.1001/archpsyc.64.4.495. [DOI] [PubMed] [Google Scholar]
- 23.Nordström P, Nordström A, Eriksson M, Wahlund LO, Gustafson Y. Risk factors in late adolescence for young-onset dementia in men: a nationwide cohort study. JAMA Intern Med. 2013;173:1612–1618. doi: 10.1001/jamainternmed.2013.9079. [DOI] [PubMed] [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, et al. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 26.Reitz C, Bos MJ, Hofman A, Koudstaal PJ, Breteler MM. Prestroke cognitive performance, incident stroke, and risk of dementia: the Rotterdam Study. Stroke. 2008;39:36–41. doi: 10.1161/STROKEAHA.107.490334. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Fratiglioni L, Guo Z, Basun H, Corder EH, Winblad B, et al. Incidence of dementia in relation to stroke and the apolipoprotein E epsilon4 allele in the very old. Findings from a population-based longitudinal study. Stroke. 2000;31:53–60. doi: 10.1161/01.str.31.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Rastas S, Pirttilä T, Mattila K, Verkkoniemi A, Juva K, Niinistö L, et al. Vascular risk factors and dementia in the general population aged >85 years: prospective population-based study. Neurobiol Aging. 2010;31:1–7. doi: 10.1016/j.neurobiolaging.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O'Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67:1363–1369. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 30.Jin YP, Di Legge S, Ostbye T, Feightner JW, Hachinski V. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement. 2006;2:171–178. doi: 10.1016/j.jalz.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Liebetrau M, Steen B, Skoog I. Stroke in 85-year-olds: prevalence, incidence, risk factors, and relation to mortality and dementia. Stroke. 2003;34:2617–2622. doi: 10.1161/01.STR.0000094420.80781.A9. [DOI] [PubMed] [Google Scholar]
- 32.Bang OY, Ovbiagele B, Kim JS. Nontraditional risk factors for ischemic stroke: an update. Stroke. 2015;46:3571–3578. doi: 10.1161/STROKEAHA.115.010954. [DOI] [PubMed] [Google Scholar]
- 33.Demant MN, Andersson C, Ahlehoff O, Charlot M, Olesen JB, Gjesing A, et al. Temporal trends in stroke admissions in Denmark 1997-2009. BMC Neurol. 2013;13:156. doi: 10.1186/1471-2377-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang CY, Li YC, Wang HK, Sung PS, Wang LC, Sun YT, et al. Stroke suggests increased risk of dementia. Curr Alzheimer Res. 2015;12:287–295. doi: 10.2174/1567205012666150302155536. [DOI] [PubMed] [Google Scholar]
- 35.Cholerton B, Larson EB, Baker LD, Craft S, Crane PK, Millard SP, et al. Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis. 2013;36:699–709. doi: 10.3233/JAD-130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 37.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haffner C, Malik R, Dichgans M. Genetic factors in cerebral small vessel disease and their impact on stroke and dementia. J Cereb Blood Flow Metab. 2016;36:158–171. doi: 10.1038/jcbfm.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristensen P. Bias from nondifferential but dependent misclassification of exposure and outcome. Epidemiology. 1992;3:210–215. doi: 10.1097/00001648-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Nordahl H, Lange T, Osler M, Diderichsen F, Andersen I, Prescott E, et al. Education and cause-specific mortality: the mediating role of differential exposure and vulnerability to behavioral risk factors. Epidemiology. 2014;25:389–396. doi: 10.1097/EDE.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 41.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 42.Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36:2773–2780. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 43.Levine DA. Young-onset dementia: unanswered questions and unmet needs. JAMA Intern Med. 2013;173:1619–1620. doi: 10.1001/jamainternmed.2013.8090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.