Abstract
Exposure to inorganic arsenic (iAs) in drinking water remains a global issue of concern and is associated with a range of health outcomes, including immune dysfunction. Young children have been identified as a particularly sensitive population, yet mechanisms of adverse health outcomes are understudied. Here we set out to examine the effects of iAs exposure on circulating serum proteins in adolescents. To identify proteins as potential indicators of disease, levels of total urinary arsenic (U-tAs) and its methylated metabolites were determined and serum proteins assessed for differences in expression. The results indicate an enrichment of TNF-regulated immune and inflammatory response proteins that display decreased expression levels in relation to increasing U-tAs. Notably, when analyzed in the context of the arsenical proportions, there was minimal overlap between the protein lists, with the most robust response observed in relation to %MMAs. These data represent the first assessment of protein expression in serum in adolescents exposed to inorganic arsenic.
Keywords: arsenic, arsenic metabolism, proteomics, early childhood exposure
1. Introduction
Exposure to elevated levels of inorganic arsenic (iAs) in drinking water remains a global issue of concern. Over 100 million people worldwide are exposed to levels of iAs in their drinking water that exceed the World Health Organization’s recommended limit of 10 μg/L [1]. While new populations continue to be identified, those at risk of elevated exposure include, but are not limited to, populations in Bangladesh, Mexico, the United States, and China, among others [2].
Chronic exposure to iAs is associated with a range of health outcomes in adults, including diabetes mellitus, impaired cognition and neurological effects, hypertension, immune dysfunction, and skin, lung, bladder, liver, and kidney cancers [3]. Of increasing concern, young children have been identified as a particularly sensitive population [3, 4]. Specifically, early childhood exposure to iAs has been associated with outcomes manifesting during both adolescence and adulthood, including impaired cognitive development, increased mortality due to bladder, laryngeal, and lung cancers, increased non-cancer mortality due to bronchiectasis, myocardial infarction, and increased infection risk [3, 5-7]. The long-lasting impact of iAs exposure during early childhood suggests that early life represents a critical period during which there is heightened sensitivity to the toxic effects of iAs [3, 4].
The mechanisms underlying the health effects of childhood exposure to iAs remain understudied. Previous studies examining immune functioning in iAs-exposed children suggest that iAs can act as an immunosuppressant. Specifically, it has been reported that children with iAs exposure exhibited decreased plasma concentrations of the Th1 cytokines, TNF-α and IL-2, and reduced responsiveness on functional immune tests [8, 9]. In support of these data, there is also evidence that iAs exposure during childhood impairs monocyte functioning and immune-specific reactive oxygen species (ROS) signaling [10, 11]. Paradoxically, there is also substantial evidence that iAs acts as a pro-inflammatory agent in children. For instance, in utero exposure to arsenic is associated with an activation of inflammation, including the NF-kB signaling cascade [12, 13]. Early life exposure has also been linked to iAs-induced chronic inflammation mediated impaired lung function [14]. Taken together, this suggests that iAs can act as an immunomodulatory agent during childhood and development, possibly impacting maturation of the immune system during a critical period of development [10, 15]. Such an effect may play a critical role in the development of the diverse adverse health effects associated with iAs exposure.
Also unknown is the impact that specific arsenic metabolites may have on protein expression during childhood. Arsenic metabolism is a multi-step process, with six major arsenic species that have been identified in human urine. These species include inorganic arsenics (iAsIII and iAsV), which are metabolized to the monomethylated arsenic species (MMAs), monomethylarsenous acid (MMAIII) and monomethylarsonic acid (MMAV). MMAs are methylated again to become dimethylated (DMAs), forming dimethylarsinous acid (DMAIII) and dimethylarsinic acid (DMAV) [16]. The efficiency of these methylation reactions has been identified as an important factor underlying the effects observed following iAs exposure as iAs, MMAs, and DMAs are differentially associated with As-related outcomes such as hypertension, atherosclerosis, cancer, and chromosomal aberrations [16, 17]. Additionally, we have previously demonstrated that urinary iAs is positively associated with lower gestational age and newborn length, while urinary MMAs is associated with lower gestational age and birth weight [18]. Therefore, inter-individual differences in the efficiency of arsenic methylation may play a role in metabolite-specific associated disease risk. The impact that differences in iAs metabolism may have on protein expression during childhood is currently unknown.
Given evidence that exposure to iAs during childhood leads to abnormal immune functioning [13, 14], we hypothesized that childhood iAs exposure is likely to disrupt expression levels of proteins involved in immune function. Therefore, we conducted a proteomic assessment of serum in iAs-exposed children selected from a cohort of subjects living in Comarca Lagunera, an area in North-Central Mexico. Moreover, in light of the knowledge that different arsenical species yield different health effects, we included an assessment for the relationship of proteins to %iAs, %MMAs and %DMAs. In the present study, we describe differences in protein expression levels in adolescents’ serum associated with concentrations of each class of urinary arsenic metabolites.
2. Materials and methods
2.1 Study Subjects and Sample Collection
The study sample for the present analysis represents a subset of 40 subjects of a cohort reported previously [19]. Participants were children, both male and female, aged 6-12 years living in one of four rural communities in the Comarca Lagunera area, located in north-central Mexico. These communities represent those with the highest arsenic tap water levels (104-360 ppb) detected in the last 20 years in the area. Arsenic is present in the local water supply due to the over-extraction of groundwater. Children included in this study had mothers who remained in these communities for the duration of their pregnancy, and have since remained residents of these same communities.
2.2 Questionnaires
Information was collected through in-person interviews and included socio-demographic variables (education, socioeconomic status), lifetime residential history, lifestyle factors (secondhand smoke), parent’s occupational history, water source types (municipal tap water, bottled, other), current medications, medical history, and diet. Questionnaires were completed by the mothers at their own residing community. Water consumption habits, including source of drinking water, were ascertained through a standardized questionnaire [19].
2.3 Determination of arsenic concentrations in water and in urine
Drinking water samples (well) were collected from each rural community included in the study and analyzed for inorganic arsenic levels. Well water samples from each rural community are representative of the water that participants drank and are provided through the unique local water supply system. Individual exposure was assessed based on U-tAs. A first morning void urine sample was collected in sterile 120-mL screw-topped polypropylene containers. Urine samples were analyzed as described previously [14]. Briefly, samples were analyzed at the Arizona Laboratory for Emerging Contaminants, University of Arizona, Tuscon, Arizona. Urinary AsV, AsIII, MMAV, DMAV, and arsenobetaine were separated by HPLC and concentrations were analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Standard Reference Water, SMR 1640 (NIST, Gaithersburg, MD, USA) and the freeze-dried Urine Reference Material for trace elements (Clinchek-control; RECIPE Chemicals instruments GmbH, Munich, Germany) were used as quality controls for urinary arsenic measurement.
2.4 Assessment of protein expression in serum
Subjects selected for proteomic assessment are representative of the extremes of exposure (median U-tAs high = 399.35 μg/L, median U-tAs low = 26.03 μg/L). As detailed in our prior publication (Bailey, Laine et al. 2014), the relative expression levels of 507 proteins were determined using the Biotin Label-based Human Antibody Array I, L series 507 (RayBiotech, Norcross,GA), which includes cellular signaling proteins such as cytokines, chemokines, growth factors, angiogenic factors, soluble receptors, and soluble adhesion molecules. Protein labeling and hybridization were carried out according to the manufacturer’s instructions using 40 μl of each serum sample. Briefly, primary amines of serum proteins are biotinylated and hybridized to a membrane array containing antibodies specific for each of the 507 protein targets, incubated with a horseradish peroxidase (HRP)-streptavidin conjugate, and detected by chemiluminescence following incubation with an HRP substrate buffer. The protein array contains two types of positive controls: a biotin-labeled protein, independent of the sample, that is spotted on each array in a series of known concentrations enabling signal intensity normalization across arrays, as well as an internal positive control which is an exogenous, nonmammalian protein added to the serum sample prior to biotinylation, serving as a control for the labeling and incubation steps.
2.5 Statistical Analyses
Statistical analyses were performed using Partek Genomics Suite software (version 6.6; Partek, Inc., St Louis, MO). All data were analyzed for their distribution patterns and homogeneity, and filtering was used to remove any non-expressed proteins. Multivariable regression models were used to examine relationships between individual arsenic measures (U-tAs, %iAs, %MMAs, %DMAs) and the normalized, background-subtracted signal intensities of the 393 proteins. Age, sex, and body mass index (BMI) were selected as a priori covariates due to their potential influence on protein expression levels and were controlled for in the models. Statistical significance was defined as p < 0.05, with false discovery (type II error) controlled for using a q-value <0.1.
2.6 Functional Analyses of Proteins
Proteins that showed statistically significant association with either U-tAs, %iAs, %MMA, or %DMA were analyzed for biological functions, canonical pathways, upstream regulatory molecules, and interacting molecular networks using Ingenuity Pathway Analysis software (Ingenuity Systems, Redwood City, CA). Proteins were also analyzed for KEGG pathway molecular interactions using The Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/), and immunologic signatures using Gene Set Enrichment Analysis (GSEA, http://software.broadinstitute.org/gsea/index.jsp).
3. Results
3.1 Study characteristics
Demographic characteristics of the study sample (n=40), as well as those of the entire cohort (n=358), can be found in Table 1. These subjects were selected as representatives of the extremes of exposure (median U-tAs high = 399.35 μg/L, median U-tAs low = 26.03 μg/L). Anthropometric characteristics recorded include sex, age, (years) weight (kg), and BMI (calculated as weight/height2). The majority of the subjects were male (n=22, 55%), have lived at their current residence their entire lives (n=36, 90%), and were, on average 9.3 years of age (range 7-12). Other possible sources of arsenic exposure, including diet (seafood, rice and others), agrochemicals, fuels, preservatives or other compounds containing arsenicals, were negligible [19].
Table 1.
Demographic and exposure characteristics of study population
| Study Sample | Cohort | |
|---|---|---|
|
| ||
| Mean, Median (Range) or n (n%) | Mean, Median (Range) or n (n%) | |
|
| ||
| Sex | ||
| male | 22 (55%) | 188 (53%) |
| female | 18 (45%) | 170 (47%) |
|
|
||
| age (years) | 9.3, 9.5 (7–12) | 8.9, 9.0 (6–12) |
|
|
||
| height (m) | 1.4, 1.38 (1.18–1.63) | 1.3, 1.3 (1.1–1.7) |
|
|
||
| weight (kg) | 37.0, 34.5 (17.0–82.0) | 34.1, 31.43 (15.9–82.0) |
|
|
||
| BMI* | 18.3, 17.8 (7.3–32.9) | 17.9, 16.7 (7.2–33.8) |
|
|
||
| time current address (yrs) | 9.3, 9.0 (5–12) | 8.4, 8.0 (5–12) |
|
|
||
| U-As Total (μg/L) | 220.0, 135.11 (5.33–664.53) | 141.15, 114.59 (4.72–894.3) |
| Low exposure | 32.94, 26.03 (5.33–109.13) | n/a |
| High exposure | 384.73, 399.35 (159.82–610.51) | n/a |
|
|
||
| %iAs | 21.6, 13.8 (5.44–84.4) | 21.4, 20.58 (3.26–91.38) |
|
|
||
| %MMA | 14.5, 12.7 (7.14–68.42) | 13.23, 4.0 (2.06–42.80) |
|
|
||
| %DMA | 58.8, 68.9 (1.6–81.9) | 61.91, 21.64 (0.99–89.83) |
|
| ||
calculated as weight/height2
Indicators of arsenic exposure examined include total urinary arsenic (U-tAs; μg/L), as well as the percentages of inorganic arsenic (iAs), monomethylated arsenicals (MMAs) and dimethylated arsenicals (DMAs) as indicators of arsenic metabolism. All samples were within detectable limits and U-tAs, defined as the sum of U-iAs, U-MMAs and U-DMAs, ranged from 5.33 μg/L to 664.53 μg/L. The average proportions of U-tAs were 24.4% iAs, 14.9% MMAs, and 60.7% DMAs. Arsenic tap water levels and urinary arsenic levels were highly correlated (R2 = 0.69).
3.2 Identification of proteins associated with total urinary arsenic and arsenic metabolites
Multivariable analyses identified 58 proteins that displayed a statistically significant association (p<0.05, q=0.1) with U-tAs, the vast majority of which are negatively associated (n=56, 96.6%) (Appendix 1). When analyzed in the context of the arsenical proportions, 8 proteins were significantly (p<0.05) associated with %iAs, 18 proteins were associated with %MMAs, and 11 proteins displayed significant association with %DMAs. There were no proteins identified in common across all three methylated metabolites (Figure 1, Appendix 1).
Figure 1.
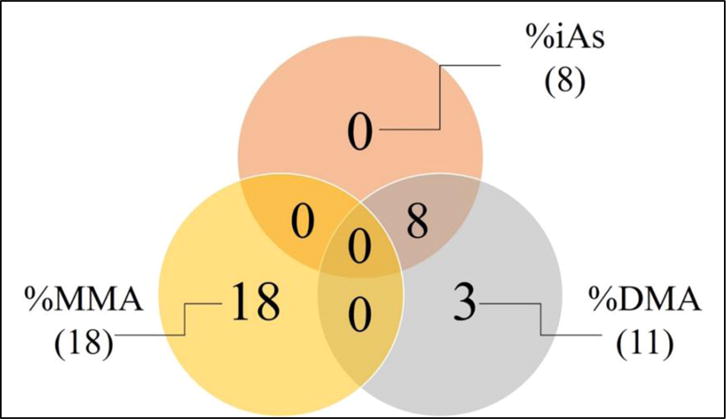
Total number of %iAs, %MMA, and %DMA associated proteins as well as commonality among arsenical associated lists. Venn diagram illustrating the number of serum protein that have a statistically significant association between expression levels and urinary proportions of iAs, MMAs and/or DMAs.
The 58 proteins identified as being significantly associated with U-tAs were analyzed for functional interactions (Appendix 2). Canonical pathway analysis revealed a strong enrichment of cytokine-mediated communication between immune cells (p= 1.12 × 10-2), comprised largely of a family of interleukins, including Interferon, Alpha 13 (IFNA1/IFNA13) and Chemokine (C-X-C Motif) Ligand 8 (CXCL8). Network analysis revealed a protein cluster involved in cellular development, cellular growth and proliferation (p= 1 × 10-34), which interacts with the major histocompatibility complex (MHC), class II (Figure 2).
Figure 2.
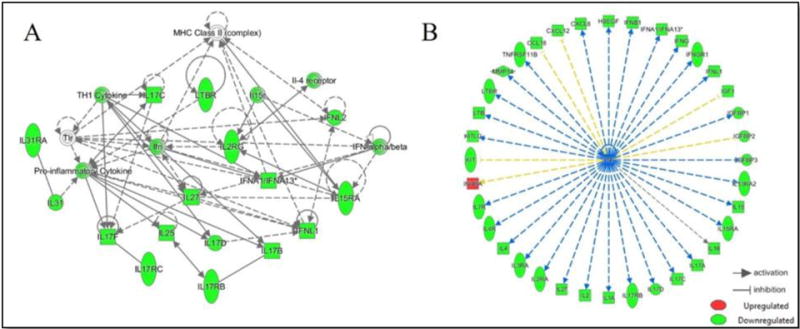
Interacting network of U-tAs associated serum proteins and upstream regulation by TNF. Interacting network of U-tAs-associated serum proteins are identified (A). TNF is predicted to regulate the expression of the majority (n=36, 62%) of the U-tAs associated proteins (B). Proteins are displayed as predicted to be upregulated (red) or downregulated (green).
A similar approach was used to investigate the pathways that were enriched among the proteins associated with the arsenical proportions. In relation to %iAs, canonical pathway analysis revealed several proteins involved in immune surveillance (p= 2.90 × 10-4), and include Platelet-Derived Growth Factor C (PDGFC) and Platelet-Derived Growth Factor D (PDGFD). Network analysis revealed a cellular growth and proliferation component (p=1.00 × 10-24), mediated by ERK 1/2 kinase and containing several members of the PDGF family, which are responsible for cellular proliferation and differentiation.
In relation to %MMAs, altered proteins were associated with an apoptosis signaling pathway (p=1.09 × 10-9), including Tumor Necrosis Factor (Ligand) Superfamily, Member 10 (TNFSF10) and many related TNF receptor superfamily (TNFRSF) members and vascular endothelial growth factors B and C (VEGFB, VEGFC). Network analysis centered on organismal and cardiovascular system development and connective tissue disorders (p= 1 × 10-47), modulated by ERK 1/2 and NFkB.
In relation to %DMAs, proteins involved in immune surveillance, specifically macropinocytosis signaling (p= 3.36×10-8), were altered and include Platelet Derived Growth Factor B (PDGFB), PDGFC, and PDGFD. Network analysis reveals cellular growth and proliferation (p= 1 × 10-33), and includes many members of the PDGF family modulated by NFkB. Additionally, DAVID was used to confirm all pathway analyses and showed Cytokine-cytokine receptor interaction as the common top KEGG pathway for U-tAs (p= 3.76 × 10-21), %iAs (p=0.015), %MMAs (p= 6.80 × 10-09), and %DMAs (p=0.015).
3.3 Transcription factor regulation of arsenic-associated proteins
Analysis was performed for all arsenic-associated protein lists in order to identify key upstream regulators, including cell signaling molecules and transcription factors (Appendix 2). Tumor necrosis factor (TNF) was found to be the top regulator of the U-tAs-associated proteins (p=1.59 × 10-27), and was predicted to be inhibited in relation to U-tAs. For the individual arsenicals, Dual Specificity Phosphatase 5 (DUSP5) was the top regulator of the %iAs-associated proteins (p= 1.98 × 10-5), pyruvic acid was the top regulator of the %MMAs-associated proteins (p= 1.98 × 10-10), and for %DMAs-associated proteins, Fibroblast Growth Factor 2 (Basic) (FGF2) (p= 1.47 × 10-5) was the most significant regulator. Because of the small number of proteins in each arsenical-associated list, predicted effects (i.e., activation or inhibition) could not be established.
4. Discussion
Early childhood exposure to iAs has been associated with adverse health outcomes manifesting during both adolescence and adulthood [3, 5-7]. Moreover, inter-individual differences in the efficiency of arsenic metabolism has been shown to influence iAs-associated disease, with high urinary proportions of MMAs associated with detrimental outcomes such as cancer of the urinary bladder, skin cancer, and cardiovascular disease [16, 17]. Despite this, the effects of childhood exposure to iAs remain understudied, particularly in terms of effects on cellular and molecular functions. In the present study, we set out to identify biological pathways which may be modulated at the protein level by early life arsenic exposure. Using a subset of 40 subjects from a previously reported cohort [19], we have identified proteins with expression levels associated with inorganic arsenic exposure. While we have previously examined prenatal arsenic-associated differences in the expression in the newborn cord serum proteome [13], to our knowledge this is the first study to examine differences in the expression of proteins in children’s serum associated with total urinary arsenic, as well as proportions of each class of urinary arsenic metabolites. The proteins that showed altered expression largely fell into functional categories of inflammation and immune response. Additionally, TNF was identified as a predicted upstream regulator of the U-tAs proteome, as well as a mediator of metabolite-dependent differences in identified proteins and regulators.
We demonstrate a massive repression of immune-associated cytokines as U-tAs levels increase. Interestingly, TNF was identified as a key regulator of these repressed proteins. TNF has a known roll in immune response, apoptosis, inflammation, and cell migration, as well as having been implicated in numerous diseases [3]. Our prior research highlights TNF as a regulator of the newborn proteome [13] as well as the newborn transcriptome [12]. Here we show that many of the identified suppressed proteins are involved in the major histocompatibility (MCH) complex II, which is responsible for triggering localized inflammation and B cell activation in the adaptive immune response [20], and included many interleukins and interferons. These data highlight altered expression of cytokines known to play a role in adaptive and innate immune signaling and could provide a mechanistic hypothesis for increased risk of infection [21].
When analyzed in relation to inter-individual differences in arsenic metabolism in adolescents, we found minimal overlap between the %iAs, %MMAs and %DMAs-associated proteins. These data suggest a urinary arsenic metabolite-specific response to iAs exposure and reflects the strong inter-individual component to the response. The most robust response in terms of differential protein expression was observed in relation to %MMAs. This finding is interesting as inter-individual differences in iAs metabolism, particularly elevation in %MMAs, have been shown to be associated with disease risk [16-18]. In our prior work, we have shown inter-individual differences in iAs metabolism as it relates to epigenetic signatures of disease [13, 22]. Proteins associated with %MMAs include many TNF superfamily (TNFSF) members. Among these are TNFSF10 and TNFSF15, previously shown to play roles in hepatic and cardiovascular disease [3, 23-27]. In addition to the TNFSF, members of the VEGF family also displayed a positive association with %MMAs. The VEGF family is a known regulator of angiogenesis [28]. VEGFB has been shown to play a role in cancer metastasis and tumor invasion [29] and VEGFB expression has been found to be upregulated in ovarian, colorectal, renal, and prostate cancer [30, 31]. VEGFC also plays a role in metastatic spread of certain tumor types [30]. These data highlight differences in the expression of proteins with known association to arsenic-associated diseases.
While we have identified a large TNF-mediated response in relation to U-tAs with metabolite-specific differences, the study is not without its limitations. Despite being one of the largest proteomic studies to date, we have only assessed a fraction of the thousands of circulating serum proteins and due to the small sample size in the present study associations seen should be confirmed in a larger cohort. Additionally, it possible that other environmental exposures and/or potential confounding variables not assessed in the present study may contribute to the observed differences. Moreover, while proteins identified have known associations with arsenic-related adverse outcomes it is unknown if the current cohort will present with such diseases. Future studies could examine both genomic and epigenetic mechanisms that may underlie the observed functional differences in protein expression [32].
4. Conclusion
In summary, the data from the present study suggest that iAs acts as an immunomodulator, and also that it does so in a metabolite-specific manner. The proteomic differences in expression seen here highlight the role of iAs in possibly impacting maturation of the immune system during a critical period of development [10], and as such, has the potential to alter disease risk later in life. Early childhood exposure to iAs has been associated with outcomes manifesting during both adolescence and adulthood, and the long-lasting impact of iAs exposure during early childhood suggests that early life represents a critical period during which there is heightened sensitivity to the toxic effects of iAs [3, 4]. These data inform potential mechanisms by which early life and childhood exposure to iAs may be linked to detrimental health outcomes.
Supplementary Material
Acknowledgments
This research was supported by The National Institute of Environmental Health Sciences (R01ES019315, T32ES007018, P42ES005948 and P42ES04940) and The University of Coahuila (UADEC-204759-14).
Appendix A. Supplementary data
Supplementary data to this article can be found online
Footnotes
Financial Interests
The authors declare they have no actual or potential competing financial interests.
References
- 1.WHO. Guidelines for drinking-water quality. 4th. Vol. 1. WHO Press; 2011. [Google Scholar]
- 2.Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58(1):201–235. [PubMed] [Google Scholar]
- 3.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano J, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental health perspectives. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vahter M. Health Effects of Early Life Exposure to Arsenic. Basic & Clinical Pharmacology & Toxicology. 2008;102(2):204–211. doi: 10.1111/j.1742-7843.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 5.Rahman M, Sohel N, Yunus M, Chowdhury ME, Hore SK, Zaman K, Bhuiya A, Streatfield PK. Increased Childhood Mortality and Arsenic in Drinking Water in Matlab, Bangladesh: A Population-Based Cohort Study. PLoS ONE. 2013;8(1):e55014. doi: 10.1371/journal.pone.0055014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;120(11):1527–1531. doi: 10.1289/ehp.1104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamadani J, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen S, Huda S, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. International Journal of Epidemiology. 2011;40(6):1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed S, Moore SE, Kippler M, Gardner R, Hawlader MDH, Wagatsuma Y, Raqib R, Vahter M. Arsenic Exposure and Cell-Mediated Immunity in Pre-School Children in Rural Bangladesh. Toxicological Sciences. 2014;141(1):166–175. doi: 10.1093/toxsci/kfu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soto-Peña GA, Luna AL, Acosta-Saavedra L, Conde P, López-Carrillo L, Cebrián ME, Bastida M, Calderón-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. The FASEB Journal. 2006;20(6):779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- 10.Luna AL, Acosta-Saavedra LC, Lopez-Carrillo L, Conde P, Vera E, De Vizcaya-Ruiz A, Bastida M, Cebrian ME, Calderon-Aranda ES. Arsenic alters monocyte superoxide anion and nitric oxide production in environmentally exposed children. Toxicology and applied pharmacology. 2010;245(2):244–251. doi: 10.1016/j.taap.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Pineda-Zavaleta AP, García-Vargas G, Borja-Aburto VH, Acosta-Saavedra LC, Vera Aguilar E, Gómez-Muñoz AS, Cebrián ME, Calderón-Aranda ES. Nitric oxide and superoxide anion production in monocytes from children exposed to arsenic and lead in region Lagunera, Mexico. Toxicology and applied pharmacology. 2004;198(3):283–290. doi: 10.1016/j.taap.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, et al. Activation of Inflammation/NF-κB Signaling in Infants Born to Arsenic-Exposed Mothers. PLoS Genet. 2007;3(11):e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey KA, Laine J, Rager JE, Sebastian E, Olshan A, Smeester L, Drobna Z, Styblo M, Rubio-Andrade M, Garcia-Vargas G, et al. Prenatal arsenic exposure and shifts in the newborn proteome: interindividual differences in tumor necrosis factor (TNF)-responsive signaling. Toxicological sciences : an official journal of the Society of Toxicology. 2014;139(2):328–337. doi: 10.1093/toxsci/kfu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivas-Calderon E, Recio-Vega R, Gandolfi AJ, Lantz RC, Gonzalez-Cortes T, Gonzalez-De Alba C, Froines JR, Espinosa-Fematt JA. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicology and applied pharmacology. 2015;287(2):161–167. doi: 10.1016/j.taap.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environmental health : a global access science source. 2013;12(1):73. doi: 10.1186/1476-069X-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng C-H. Arsenic Methylation, Urinary Arsenic Metabolites and Human Diseases: Current Perspective. Journal of Environmental Science and Health, Part C. 2007;25(1):1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y-K, Tseng C-H, Huang Y-L, Yang M-H, Chen C-J, Hsueh Y-M. Arsenic methylation capability and hypertension risk in subjects living in arseniasis-hyperendemic areas in southwestern Taiwan. Toxicology and applied pharmacology. 2007;218(2):135–142. doi: 10.1016/j.taap.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobná Z, Herring AH, Stýblo M, García-Vargas GG, Fry RC. Maternal Arsenic Exposure, Arsenic Methylation Efficiency, and Birth Outcomes in the Biomarkers of Exposure to ARsenic (BEAR) Pregnancy Cohort in Mexico. Environmental Health Perspectives. 2015;123(2):186–192. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Gonzalez-De Alba C. In utero and early childhood exposure to arsenic decreases lung function in children. Journal of applied toxicology : JAT. 2014;35(4):358–366. doi: 10.1002/jat.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-Water Arsenic Exposure Modulates Gene Expression in Human Lymphocytes from a U.S. Population. Environmental Health Perspectives. 2008;116(4):524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, Baker E, Karagas MR. Infant Infections and Respiratory Symptoms in Relation to in Utero Arsenic Exposure in a U.S. Cohort. Environ Health Perspect. 2016;124(6):840–847. doi: 10.1289/ehp.1409282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobna Z, Currier J, Douillet C, Olshan AF, et al. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environmental and molecular mutagenesis. 2014;55(3):196–208. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul KS, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PM. Arsenic and human health effects: A review. Environmental toxicology and pharmacology. 2015;40(3):828–846. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, Van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver international : official journal of the International Association for the Study of the Liver. 2011;31(2):146–162. doi: 10.1111/j.1478-3231.2010.02369.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicology and applied pharmacology. 2011;251(1):59–69. doi: 10.1016/j.taap.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazumder DN. Effect of chronic intake of arsenic-contaminated water on liver. Toxicology and applied pharmacology. 2005;206(2):169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Straub AC, Stolz DB, Vin H, Ross MA, Soucy NV, Klei LR, Barchowsky A. Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice. Toxicology and applied pharmacology. 2007;222(3):327–336. doi: 10.1016/j.taap.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, Ribatti D, Genovese A, Triggiani M, Marone G. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. The Journal of allergy and clinical immunology. 2009;123(5):1142–1149. 1149 e1141–1145. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Zhang Y, Hosaka K, Andersson P, Wang J, Tholander F, Cao Z, Morikawa H, Tegner J, Yang Y, et al. VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(22):E2900–2909. doi: 10.1073/pnas.1503500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunningham SP, Currie MJ, Han C, Turner K, Scott PA, Robinson BA, Harris AL, Fox SB. Vascular endothelial growth factor-B and vascular endothelial growth factor-C expression in renal cell carcinomas: regulation by the von Hippel-Lindau gene and hypoxia. Cancer research. 2001;61(7):3206–3211. [PubMed] [Google Scholar]
- 31.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. The Journal of pathology. 2003;200(2):183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 32.Bailey KA, Smith AH, Tokar EJ, Graziano JH, Kim KW, Navasumrit P, Ruchirawat M, Thiantanawat A, Suk WA, Fry RC. Mechanisms Underlying Latent Disease Risk Associated with Early-Life Arsenic Exposure: Current Research Trends and Scientific Gaps. Environ Health Perspect. 2016;124(2):170–175. doi: 10.1289/ehp.1409360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


