Abstract
This study was designed to investigate the in vitro and in vivo antioxidant activities of inulin. The in vitro assays demonstrated that the antioxidant activities of inulin, including the DPPH radical scavenging activity, ABTS scavenging activity and ferric reducing power, were weak and significantly lower than those of Vitamin C (P < 0.05). The influence of dietary supplementation with inulin on the antioxidant status of laying hens was evaluated with in vivo antioxidant assays. The results indicated that inulin supplementation quadratically improved the egg production rate of the laying hens (P < 0.01). The antioxidant enzyme activities in the serum, including SOD, CAT, and GSH-Px, and the total antioxidant capacity increased quadratically as inulin levels increased (P < 0.001). The levels of MDA in the serum decreased quadratically as inulin levels increased (P < 0.001). These findings suggest that inulin has the potential to improve the antioxidant status of laying hens.
Introduction
Free radicals are part of normal metabolites for many organisms, and a complex system of endogenous and exogenous antioxidant sources in the body are employed to mitigate the potential damage from free radicals [1]. When the body is in a state of aging or stress, these highly reactive chemical species are produced excessively, and structural abnormalities and dysfunction of the cell and mitochondrial membranes can arise [2]. Excessive free radicals affect animal performance, even resulting in the development of diseases [3]. Improving the antioxidant status of living animals is one of the primary methods for improving bird performance in the poultry production industry [4, 5].
Antioxidants are a class of chemical substances that reduce free radicals and inhibit oxidation directly or indirectly. Oxidative stress in the body may be alleviated by exogenous supplementation with antioxidants. However, several synthetic antioxidants have shown potential adverse effects, such as liver injury and carcinogenesis, especially long-term administration of synthetic antioxidants [6]. Thus, exploration of safe and natural antioxidants to resist oxidative stress has become a research hotspot in recent years.
Inulin is a type of fructosan, extracted primarily from plant organs, such as the tubers of dahlia and the roots of elecampane, chicory and Jerusalem artichoke [7, 8]. Inulin is a linear polymer polysaccharide in which D-fructose is joined by the linkage of a -(2→1) glycosidic bond in the molecular structure, and the typical terminal is a D-glucose molecule [9, 10]. Because of the glycosidic bond types, inulin cannot be digested by digestive enzymes of monogastric animals. However, inulin can be utilized by the beneficial bacteria in the large intestine [11]. Currently, because of the biocompatible and biodegradable properties of the human body, inulin and its derivatives have been widely used in food and pharmaceutical industries [12, 13]. Generally, inulin is used as a texture modifier, fat replacer, sugar substitute, and prebiotic in food and pharmaceutical industries [14]. However, few studies have investigated the antioxidant activity of inulin. To further clarify the activities of inulin, the present study was conducted to research the in vitro and in vivo antioxidant activities of inulin. The in vivo antioxidant study was designed to assess the effects of supplementation with inulin on the antioxidant status of laying hens.
Materials and methods
Materials and reagents
Inulin from Jerusalem artichoke root was purchased from Beijing Wede biological Technology Co., Ltd. (Beijing, China). The average polymerization degree of inulin was approximately 20 fructosyl fructose units. The reagents including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, Vitamin C and 2,2-azino-bis-3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) radical were obtained from Sigma-Aldrich (St. Louis, USA). All other chemicals used were analytical grade and bought from local suppliers.
In vitro antioxidant activity assay
DPPH radical scavenging assay
In this study, the DPPH radical scavenging activity of inulin was determined by the colorimetric method as described previously [15] with several modifications. Briefly, 1.0 mL of 0.1 mM DPPH solution in ethanol was prepared and was mixed with 3.0 mL of 0.25–10 mg/mL of inulin. The mixture was placed at room temperature. After incubation for 30 min, the mixture was determined at the wavelength of 517 nm against ethanol. The DPPH radical scavenging activity of inulin was computed using the formula below:
| (1) |
where A0 was the absorbance of the control group (water instead of the sample solution). A1 was the absorbance of the test group. A2 was the absorbance of the sample (ethanol instead of the DPPH solution). Vitamin C was used as the positive control.
ABTS radical scavenging assay
The ABTS radical scavenging activity of inulin was evaluated using a previously described procedure [16] with slight modifications. Vitamin C served as the positive reference reagent. The ABTS radical (ABTS+) was produced with the mixture of 5 mL of ABTS solution (7 mM) and 1 mL of potassium persulfate aqueous solution (15 mM) for 24 h in darkness at 20°C. After incubation, the mixture was diluted with deionized water to obtain an absorbance of 0.700±0.002 at a wavelength of 734 nm. Next, 3 mL of the diluted solution was reacted with 0.75 mL of various concentrations (0.25–10 mg/mL) of inulin solutions. The mixture was reacted for 15 min at 20°C before the absorbance at 734 nm was determined against distilled water. The control contained all of the reagents but distilled water instead of the inulin solution, and the absorbance was determined as A0. A1 was the reaction result for inulin. A2 was the result of all the reagents with the ABTS solution used instead of distilled water. The ABTS radical scavenging activity of inulin was calculated by the equation below:
| (2) |
Ferric reducing power
The ferric reducing power of inulin was determined with a reported procedure [17] with minor modifications. Vitamin C served as the positive reference reagent. First, 1.5 mL of the inulin solutions of 0.25 to 4 mg/mL concentrations were mixed with 1.5 mL of sodium phosphate buffer (0.2 M, pH 6.6) and 1.5 mL of potassium ferricyanide (1%, w/v). The mixture was reacted at 50°C for 20 min. After that step, the mixture was cooled quickly. After addition of 1.5 mL of trichloroacetic acid (TCA, 10%, w/v), the mixture was centrifuged at 3000×g for 10 min. After centrifugation, 1.5 mL of the centrifugate was pulled out and was mixed thoroughly with 1.5 mL of deionized water and 0.3 mL of ferric chloride (0.1%, w/v). The absorbance of the mixture was determined at a wavelength of 700 nm against distilled water. The higher the absorbance of the reaction mixture, the stronger the reducing power of the sample. The ferric reducing power of inulin was calculated by the equation below:
| (3) |
where A1 was the absorbance of the inulin sample. A2 was the result of all the reagents with the FeCl3 solution used instead of the distilled water.
In vivo antioxidant activity
Animal design
The experimental procedures used in the feeding study were approved by the Animal Care and Use Committee of Jilin Agricultural University. Three hundred thirty-week-old Hy-Linen brown commercial laying hens were randomly divided into 5 treatment groups, and each group had 6 replicates of 10 birds. All diets were formulated to meet the nutrient requirements for laying hens [18]. Diets had 5, 10, 15, or 20 g/kg of inulin added in crushed form. The feeding experiment lasted 8 weeks. The ingredients of the laying hens’ diet are shown in Table 1. Feed and water were supplied ad libitum. The laying hens were raised individually in wire cages (45×45×50 cm) in a temperature controlled room (22 ± 2°C). Light was provided 16 h per day.
Table 1. Ingredients and nutrient concentrations of the diet (g/kg as fed unless noted).
| Item | Dietary inulin concentration (g/kg) | ||||
|---|---|---|---|---|---|
| 0 (Control) | 5 | 10 | 15 | 20 | |
| Ingredient | |||||
| Corn | 602.0 | 602.0 | 602.0 | 602.0 | 602.0 |
| Soybean meal | 240.0 | 240.0 | 240.0 | 240.0 | 240.0 |
| Soybean oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Fish meal | 22.4 | 22.4 | 22.4 | 22.4 | 22.4 |
| Wheat bran | 31.1 | 26.1 | 21.1 | 16.1 | 11.1 |
| Inulin | 0 | 5.0 | 10.0 | 15.0 | 20.0 |
| Calcium hydrogen phosphate | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Limestone | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| Sodium chloride | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| DL-Methionine | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| L-Lysine | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Premix | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Chemical composition | |||||
| CP | 186.9 | 186.7 | 186.4 | 186.2 | 186.1 |
| Calcium | 33.6 | 33.7 | 33.5 | 33.4 | 33.6 |
| Total phosphorus | 6.0 | 6.0 | 5.9 | 5.9 | 6.0 |
| Lysine | 10.2 | 10.2 | 10.3 | 10.4 | 10.2 |
| Methionine + cysteine | 7.5 | 7.6 | 7.5 | 7.5 | 7.6 |
| ME (MJ/kg) | 11.50 | 11.50 | 11.51 | 11.51 | 11.52 |
The control group was fed the standard diet. The other treatment diets were supplemented with 5, 10, 15, or 20 g of inulin powder/kg of diet by replacing the equivalent amount of wheat bran in the standard diet formulation.
Premix supplied per kilogram of diet: retinyl palmitate, 3.96 mg; cholecalciferol, 0.07 mg; DL-α-tocopheryl acetate, 20 mg; menadione sodium bisulfite, 4 mg; thiamine mononitrate, 1.63 mg; riboflavin, 8 mg; niacin, 30 mg; pantothenic acid, 10 mg; folic acid, 0.5 mg; biotin, 0.2 mg; choline chloride, 250 mg; cyanocobalamin, 0.012 mg; Cu, 8 mg; Fe, 30 mg; I, 0.6 mg; Mn, 50 mg; Se, 0.12 mg; Zn, 40 mg.
Laying performance
The egg production, egg weight and feed consumption were recorded daily for each replicate. Egg mass was count by egg weight times egg production. Feed conversion ratio was calculated by dividing feed consumption by egg mass production.
Antioxidant status of laying hens
At the end of the eighth week, 18 laying hens were randomly selected from each treatment (3 hens per replicate), and blood samples were taken from the wing vein into a non-heparinized tube. The blood was allowed to clot at 37°C for 100 min. After that step, the individual blood was separated by centrifugation at 3000×g for 10 min and 4°C to obtain the serum. The serum samples were stored at −20°C until the analysis of antioxidant enzyme activities and concentration of malondialdehyde (MDA). The antioxidant enzymes included superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). The total antioxidant capacity (T-AOC) of serum was also determined. All these indicators were assayed by the colorimetric method and followed the instructions of the kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The antioxidant enzyme activities were expressed as units (U) per milliliter of serum.
Statistical analysis
All in vitro experiments were performed at least in triplicate. Analyses of all samples were run in triplicate and averaged. The resulting values are presented as the mean ± standard deviation (SD). The one-way ANOVA in SPSS 17.0 for Windows (SPSS Inc., Chicago, IL) was used for statistical analysis. Duncan’s post hoc tests were performed when significant differences were found (P < 0.05). The influence of inulin doses on indicators was determined using curve estimation for linear and quadratic terms.
Results and discussion
Antioxidant activity in vitro
DPPH radical scavenging activity
DPPH radical scavenging activity is extensively used to assess the antioxidant capacity of biological samples. It is a stable free radical and shows the strongest absorption at a wavelength of 517 nm. In this method, the antioxidant substances are able to reduce the DPPH radical to the yellow diphenyl-picrylhydrazine [19]. The relationship between the antioxidant ability of the sample and the yellow color compound content in the reaction system is linear and dose-dependent. Therefore, the antioxidant activity of a sample can be expressed as its ability to scavenge the DPPH radical.
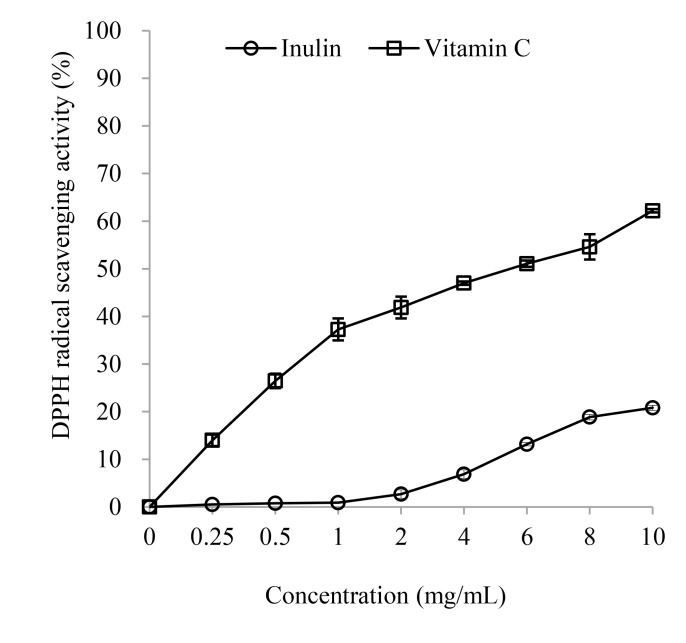
The DPPH radical scavenging ability of inulin is shown in Fig 1. The results indicated that both inulin and Vitamin C increased DPPH radical scavenging activities in a concentration-dependent manner. As the concentration of inulin increased from 0.25 to10 mg/mL, the DPPH radical scavenging activity of inulin increased linearly (R2 = 0.985, P.<0.05). The DPPH radical scavenging activity of Vitamin C increased quadratically (R2 = 0.829, P<0.05) when the Vitamin C levels increased from 0.25 to10 mg/mL. At a dose of 10 mg/ml, the DPPH radical scavenging activity of inulin was 20.81±0.15%. However, the DPPH scavenging ability of inulin was significantly lower than that of Vitamin C in doses ranging from 0.25 to 10 mg/mL (P < 0.05). Therefore, inulin has weaker DPPH radical scavenging activity than Vitamin C in vitro.
Fig 1. DPPH radical scavenging activity of inulin.
ABTS radical scavenging activity
The ABTS radical scavenging activity method was pioneered by Miller and Rice-Evans (1993) [20]. Through oxidation by oxygen activity, colorless ABTS is transformed to the stable, blue-green ABTS• +, which is specifically absorbed at a wavelength of 734 nm. When colored ABTS• + is reacted with any antioxidant ingredient, ABTS• + is reduced to the original colorless ABTS. The ABTS radical cation de-coloration assay only needs a short reaction time (approximately 15 min) and has been extensively used to evaluate the antioxidant activity of biological samples [21].
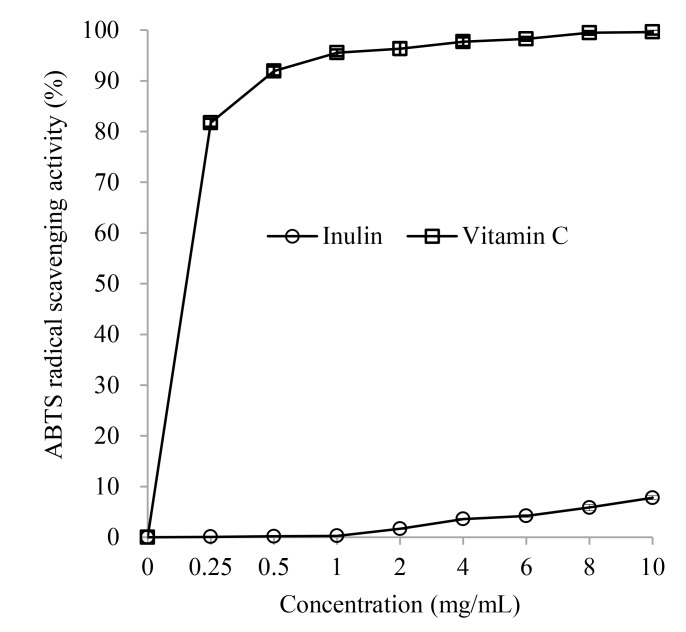
The ABTS radical scavenging ability of inulin is presented in Fig 2. The activity of inulin toward the ABTS radical was related to inulin dosage within the test range (0.25–10 mg/mL). The activity increased linearly (R2 = 0.988, P<0.05) as inulin levels increased. At a dose of 10 mg/mL, the scavenging activity of inulin was only 7.79±0.37%. Furthermore, the activity of inulin toward the ABTS radical was significantly lower than that of Vitamin C at each dosage (P < 0.05). The results of the ABTS radical cation de-coloration assay indicated that inulin has weaker ABTS radical scavenging activity than Vitamin C in vitro.
Fig 2. ABTS radical scavenging activity of inulin.
Ferric reducing power
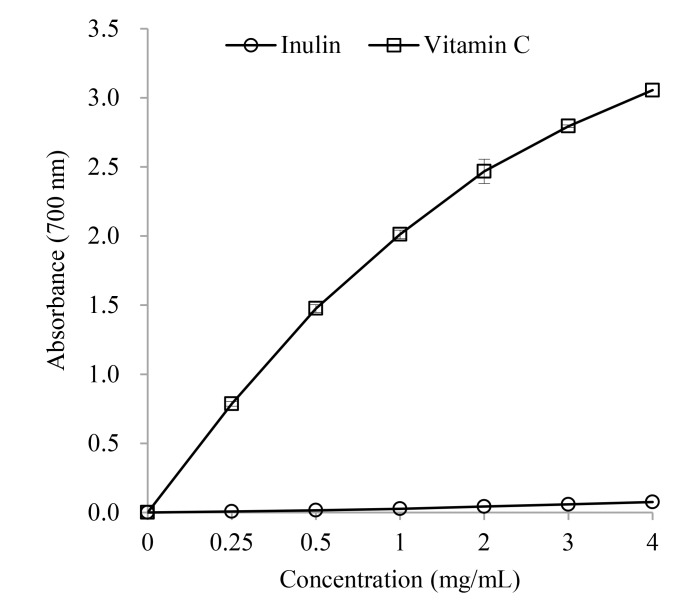
The ferric reducing ability of inulin is presented in Fig 3. A dose-dependent reducing power of inulin was observed. Inulin showed a good linear connection (R2 = 0.989, P < 0.05) to concentration in the range from 0.25 to 4.0 mg/mL. However, inulin showed significantly lower ferric reducing power than Vitamin C at doses of detected concentrations (P < 0.05).
Fig 3. Ferric reducing power of inulin.
The results of the antioxidant activity in vitro assays indicated that the antioxidant activities of inulin, such as the DPPH radical scavenging activity, ABTS scavenging activity and ferric reducing power were weak and significantly lower than those of Vitamin C (P < 0.05). The reason might be due to the molecular characteristics of inulin. The in vitro antioxidant activities of polysaccharides were connected to the reductive hydroxyl group terminals, which could accept and eliminate the free radicals [22]. However, the amount of reductive hydroxyl group terminals on inulin is limited. Therefore, the in vitro antioxidant activities of inulin are weak.
Antioxidant activity in vivo
Laying performance
The effects of dietary inulin on laying performance are summarized in Table 2. Dietary supplementation with inulin significantly improved egg production and feed consumption of laying hens quadratically (P<0.01). However, there were no linear or quadratic differences in egg mass, egg weight, or feed conversion ratio among inulin treatments.
Table 2. Effects of dietary supplementation with inulin powder on performance of laying hens during the 8-wk period of the feeding experiment.
| Item | Dietary inulin concentration (g/kg) | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | Linear | Quadratic | ||
| Egg production (%) | 94.6b | 95.0ab | 95.5a | 95.6a | 95.2ab | 0.09 | 0.008 | 0.001 |
| Egg weight (g) | 62.8 | 63.1 | 63.1 | 63.1 | 62.5 | 0.21 | 0.670 | 0.537 |
| Egg mass (g/hen per d) | 59.4 | 60.0 | 60.3 | 60.3 | 59.7 | 0.22 | 0.579 | 0.325 |
|
Feed consumption (g/hen per d) |
114.3b | 115.5ab | 116.2a | 116.4a | 115.2ab | 0.23 | 0.112 | 0.005 |
| Feed conversion ratio | 1.93 | 1.93 | 1.92 | 1.93 | 1.91 | 0.02 | 0.655 | 0.726 |
Means within a row with different superscripts differ significantly (P<0.05).
Data are means for 6 replicates of 60 laying hens.
Dose response (linear or quadratic) to different levels of inulin supplementation.
Prebiotics are defined as non-digestible food ingredients that beneficially improve host health by combating undesired bacteria and selectively stimulate the growth and/or activity of healthful bacteria in the intestinal tract [23]. Inulin is one of the most commonly used prebiotics in feed for livestock and poultry [24]. Generally, inulin cannot be hydrolyzed by digestive enzymes in the proximal intestinal tract of humans and monogastric animals because of the glucosidic bond of -(2→1)-linkages [25]. Instead, they are fermented by beneficial bifidobacteria and other lactic acid producing bacteria in the large intestine or colon, thereby enhancing their relative populations [26]. It was reported that gut microflora have a significant influence on host health, nutrition, and growth performance by interacting with the nutrient utilization and gut system development of the host [27]. Reduced numbers of coliforms and increased numbers of Bifidobacteria spp. suggest a more favorable intestinal microflora that may improve gastrointestinal function, feed digestibility and animal performance [28]. Inulin use as a dietary supplement in animal feed has been shown to enhance immune parameters, modulate intestinal microbiota, and improve growth performance in poultry and swine [29, 30]. Our previous study showed that the inclusion of inulin in laying hens’ diet conferred increasing Bifidobacteria count and decreased coliform bacteria count in the cecum [31]. Therefore, the increased egg production in this study might be due to the prebiotic properties of inulin.
Antioxidant status of laying hens
Only measuring the in vitro antioxidant ability of inulin was not enough to estimate its antioxidant effects in biological systems. Antioxidant assays in vivo could reflect the related biological implications of dietary consumption, such as effects on antioxidant enzymes and oxidation-related metabolic pathways. The effects of dietary supplementation with inulin on antioxidant enzyme activities and MDA content in the serum are presented in Table 3. The activities of SOD, CAT, GSH-Px, and T-AOC in the serum of laying hens increased quadratically (P<0.001) with the increased in dietary inulin. Meanwhile, the MDA content in the serum of laying hens was quadratically (P<0.001) decreased as the dosage of inulin increased from 0 to 20 g/kg of diet. Among the levels of inulin supplemented in the laying hens’ diet, 15 g/kg of inulin supplementation appeared to produce the highest antioxidant enzyme activities but the lowest MDA content in the serum of laying hens.
Table 3. Effects of dietary supplementation of inulin powder on serum antioxidant status of laying hens during the 8-wk period of the feeding experiment.
| Dietary inulin concentration (g/kg) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 5 | 10 | 15 | 20 | SEM | Linear | Quadratic |
| SOD (U/mL) | 170.7d | 172.7d | 192.0c | 214.4a | 203.8b | 3.28 | 0.001 | <0.001 |
| CAT (U/mL) | 4.83d | 5.33c | 5.74b | 6.45a | 6.31a | 0.12 | 0.002 | <0.001 |
| GSH-Px (U/mL) | 1373d | 1502c | 1593b | 1716a | 1597b | 22.3 | <0.001 | <0.001 |
| T-AOC (U/mL) | 6.02d | 6.14d | 6.46c | 7.76a | 7.20b | 0.13 | 0.001 | <0.001 |
| MDA (nmol/mL) | 9.18a | 8.52b | 8.32b | 7.09d | 7.89c | 0.13 | 0.001 | <0.001 |
Means within a row with different superscripts differ significantly (P<0.05).
Data are means for 6 replicates of 18 laying hens.
SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; T-AOC, total antioxidant capacity; MDA, malondialdehyde.
Dose response (linear or quadratic) to different levels of inulin supplementation.
SOD, CAT and GSH-Px are three important endogenous antioxidant enzymes in the antioxidants of the cellular enzymatic system, and they play an important role in antioxidant protection processes [32]. MDA is one of the end products of lipid peroxidation, and the level of MDA can indicate the degree of lipid peroxidation in the body. Liver lipogenesis is significantly improved by estrogens in order to meet the demand for egg production in laying hens. Therefore, ameliorating lipid metabolism, improving antioxidant status, and preventing lipid peroxidation are important for laying hens [33]. Dietary supplementation with inulin increased the activities of SOD, CAT, and GSH-Px and decreased the MDA concentration in the serum, which indicated that inulin could enhance the antioxidant status of laying hens.
The antioxidant process in biological systems involves many aspects of antioxidants, such as digestibility, bioavailability and metabolism [2]. The total antioxidant capacity is recommended for studying antioxidant activity in organisms. T-AOC level indicates the status of the non-enzymatic reactive oxygen species (ROS) defense system. The results of the present study indicated that dietary supplementation with inulin (10, 15 and 20 g/kg) significantly enhanced (P<0.05) T-AOC activity in the serum of laying hens (Table 3). This result indicated inulin also had antioxidant capacity related to the non-enzymatic ROS defense system.
The exact mechanisms of the antioxidant abilities of inulin in vivo need further research. However, inulin may scavenge ROS indirectly through the generation of antioxidant enzymes and short-chain fatty acids (SCFAs) [34]. One of the possible reasons for inulin’s antioxidant abilities in vivo is the prebiotic activity of inulin modifying microflora in the gastrointestinal tract of laying hens. Inulin reaches the large intestine undigested, and it can be fermented by Bifidobacterium spp. and other lactic acid-producing bacteria [35]. Our previous study showed that the addition of inulin in the diet conferred increasing Bifidobacteria and Lactobacilli counts in the cecum of laying hens [31]. It was reported that lactic acid bacteria have SOD, and in vitro research has shown that lactic acid itself has the ability to scavenge free radicals. The fermentation of fructooligosaccharide by Bifidobacteria leads to the scavenging of free radicals [36]. Additionally, Lactobacilli resident in the gut may help the body to decrease the MDA level by releasing its intracellular antioxidant constituents [37]. Another possible reason for inulin’s antioxidant abilities in vivo may be due to SCFAs, the anaerobic fermentation products of inulin in the large intestine [38]. It was reported that increased content of butyrate in colonic cells resulted in decreased colonic myeloperoxidase activity and restored glutathione concentration, and butyrate controls the increase in ROS levels [39].
Conclusions
The present study investigated the in vitro and in vivo antioxidant activities of inulin. In vitro studies showed that inulin had weaker DPPH radical scavenging activity, ABTS radical scavenging activity and ferric reducing power than Vitamin C. In vivo studies of laying hens showed that dietary supplementation with inulin significantly improved the antioxidant status of laying hens. The T-AOC and antioxidant enzyme activities of SOD, CAT, and GSH-Px increased, and the MDA concentration in the serum of laying hens decreased. On the basis of the above findings, inulin might have a favorable effect on maintaining or improving antioxidant systems and could be considered a potential antioxidant for the poultry industry. Further research investigating the antioxidant activity mechanisms and utilization of inulin is warranted.
Supporting information
(XLS)
(XLS)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31601972) and the Project funded by China Postdoctoral Science Foundation (No. 2017M621224). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31601972 to HMS) and Project funded by China Postdoctoral Science Foundation (2017M621224 to HMS).
References
- 1.Liu W, Wang JJ, Zhang ZZ, Xu JN, Xie ZH, Slavin M, et al. In vitro and in vivo antioxidant activity of a fructan from the roots of Arctium lappa L. Int J Biol Macromol. 2014; 65: 446–453. doi: 10.1016/j.ijbiomac.2014.01.062 [DOI] [PubMed] [Google Scholar]
- 2.Liu HN, Liu Y, Hu LL, Suo YL, Zhang L, Jin F, et al. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poultry Sci. 2014; 93: 347–353. doi: 10.3382/ps.2013-03225 [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39: 44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 4.Mujahid A, Akiba Y, Toyomizu M. Acute heat stress induces oxidative stress and decreases adaptation in young White Leghorn cockerels by downregulation of avian uncoupling protein. Poultry Sci. 2007; 86: 364–371. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X,Yang ZB,Yang WR,Wang Y, Jiang SZ, Zhang GG. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poultry Sci. 2011; 90: 1720–1727. doi: 10.3382/ps.2010-01280 [DOI] [PubMed] [Google Scholar]
- 6.Muriel P, Rivera-Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol. 2008; 28: 93–103. doi: 10.1002/jat.1310 [DOI] [PubMed] [Google Scholar]
- 7.Petkova NT, Ognyanov M, Todorova M, Denev P. Ultrasound-assisted extraction and characterisation of inulin-type fructan from roots of elecampane (Inula helenium L.). Acta Scientifica Naturalis. 2015; 1: 225–235. [Google Scholar]
- 8.Hu YX, Zhang J, Yu CW, Li Q, Dong F, Wang G, et al. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int J Biol Macromol. 2014; 70: 44–49. doi: 10.1016/j.ijbiomac.2014.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Apolinário AC, de Lima Damasceno BPG, de Macêdo Beltrão NE, Pessoa A, Converti A, da Silva JA. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology, Carbohyd Polym. 2014; 101: 368–378. doi: 10.1016/j.carbpol.2013.09.081 [DOI] [PubMed] [Google Scholar]
- 10.Petrovsky N, Cooper PD. AdvaxTM, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015; 33: 5920–5926. doi: 10.1016/j.vaccine.2015.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudannayake DC, Wimalasiri KMS, Silva KFST, Ajlouni S. Comparison of properties of new sources of partially purified inulin to those of commercially pure chicory inulin. J Food Sci. 2015; 80: C950–960. doi: 10.1111/1750-3841.12857 [DOI] [PubMed] [Google Scholar]
- 12.Vassilev D, Petkova N, Koleva M, Denev P. Ultrasound-Assisted Synthesis of Sucrose and Fructooligosaccharides Esters as Bio-Plasticizers. J Renewe Mate. 2016; 4: 24–30. doi: 10.7569/JRM.2015.634125 [Google Scholar]
- 13.Liu J, Lu JF, Wen XY, Kan J, Jin CH. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int J Biol Macromol. 2015; 72: 1479–1484. doi: 10.1016/j.ijbiomac.2014.09.066 [DOI] [PubMed] [Google Scholar]
- 14.Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, et al. Inulin: properties, health benefits and food applications. Carbohyd Polym. 2016; 147: 444–454. doi: 10.1016/j.carbpol.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 15.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agr Food Chem. 1992; 40: 945–948. doi: 10.1021/jf00018a005 [Google Scholar]
- 16.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Bio Med. 1999; 26: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 17.Jing YS, Zhu JH, Liu T, Bi SX, Hu XJ, Chen ZY, et al. Structural characterization and biological activities of a novel polysaccharide from cultured Cordycep smilitaris and its sulfated derivative. J Agr Food Chem. 2015; 63: 3464–3471. doi: 10.1021/jf505915t [DOI] [PubMed] [Google Scholar]
- 18.National Reasearch Council, 1994. Nutrient requirements of poultry. 9th ed. National Academy Press, Washington, DC, USA. [Google Scholar]
- 19.Luo AX, Ge ZF, Fan YJ, Luo AS, Chun Z, He XJ. In Vitro and In vivo antioxidant activity of a water-soluble polysaccharide from Dendrobium enneanum. Molecules. 2011; 16: 1579–1592. doi: 10.3390/molecules16021579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller NJ, Rice-Evans CA. Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci. 1993; 84: 407–412. [DOI] [PubMed] [Google Scholar]
- 21.Wu LC, Hsu HW, Chen YC, Chiu CC, Lin YI, Ho JA. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006; 95: 319–327. doi: 10.1016/j.foodchem.2005.01.002 [Google Scholar]
- 22.Wang JQ, Hu SZ, Nie SP, Yu Q, Xie MY. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid Med Cell Longev. 2016; 64: 1–13. doi: 10.1155/2016/5692852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995; 125: 1401–1412. [DOI] [PubMed] [Google Scholar]
- 24.Tiengtam N, Khempaka S, Paengkoum P, Boonanuntanasarn S. Effects of inulin and Jerusalem artichoke (Helianthus tuberosus) as prebiotic ingredients in the diet of juvenile Nile tilapia (Oreochromis niloticus). Anim Feed Sci Tech. 2015; 207: 120–129. doi: 10.1016/j.anifeedsci.2015.05.008 [Google Scholar]
- 25.Pool-Zobel BL, Van Loo J, Rowland I, Roberfroid MB. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Brit J Nutr. 2002; 87: S273–S281. doi: 10.1079/BJNBJN/2002548 [DOI] [PubMed] [Google Scholar]
- 26.Roberfroid MB. Functional foods: concepts and application to inulin and oligofructose. Brit J Nutr. 2002; 87: S139–S143. doi: 10.1079/BJNBJN/2002529 [DOI] [PubMed] [Google Scholar]
- 27.Giannenas I, Tontis D, Tsalie E, Chronis EF, Doukas D, Kyriazakis I. Influence of dietary mushrooms Agaricus bisporus on intestinal morphology and microflora composition in broiler chickens. Res Vet Sci. 2010; 89: 78–84. doi: 10.1016/j.rvsc.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 28.Shang HM, Song H, Xing YL, Niu SL, Ding GD, Jiang YY, et al. Effects of dietary fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on growth performance, digestibility, and intestinal microbiology and morphology in broiler chickens. J Sci Food Agr. 2016; 96: 215–222. doi: 10.1002/jsfa.7084 [DOI] [PubMed] [Google Scholar]
- 29.He G, Baidoo SK, Yang Q, Golz D, Tungland B. Evaluation of chicory inulin extracts as feed additive for early-weaned pigs. J Anim Sci. 2002; 80: 81. [Google Scholar]
- 30.Nabizadeh A. The effect of inulin on broiler chicken intestinal microflora, gut morphology, and performance. J Anim Feed Sci. 2012; 21: 725–734. doi: 10.22358/jafs/66144/2012 [Google Scholar]
- 31.Shang HM, Hu TM, Lu YJ, Wu HX. Effects of inulin on performance, egg quality, gut microflora and serum and yolk cholesterol in laying hens. Br Poult Sci. 2010; 51: 791–796. doi: 10.1080/00071668.2010.531005 [DOI] [PubMed] [Google Scholar]
- 32.Zuo ZY, Wang WR, Wang Y, Yang ZB, Jiang SZ, Zhang GG. Effects of Astragalus membranaceus on laying performance and antioxidant status of laying hens. J Appl Poultry Res. 2012; 21: 243–250. doi: 10.3382/japr.2011-00351 [Google Scholar]
- 33.Qi XL, Wu SG, Zhang HJ, Yue HY, Xu SH, Ji F, et al. Effects of dietary conjugated linoleic acids on lipid metabolism and antioxidant capacity in laying hens. Arch Anim Nutr. 2011; 65: 354–365. doi: 10.1080/1745039X.2011.617546 [DOI] [PubMed] [Google Scholar]
- 34.Gargari BP, Dehghan P, Aliasgharzadeh A, Jafar-abadi MA. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with Type 2 diabetes. Diabetes Metab. 2013; 37: 140–148. doi: 10.4093/dmj.2013.37.2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasqualetti V, Altomare A, Guarino MPL, Locato V, Cocca S, Cimini S, et al. Antioxidant activity of inulin and its role in the prevention of human colonic muscle cell impairment induced by lipopolysaccharide mucosal exposure. Plos One. 2014; 9: e98031 doi: 10.1371/journal.pone.0098031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Du RT, Wang LF, Zhang HP. The antioxidative effects of probiotic Lactobacillus casei Zhang on the hyperlipidemic rats. Eur Food Res Techn. 2010; 231: 151–158. doi: 10.1007/s00217-010-1255-1 [Google Scholar]
- 37.Wang J, Cao YP, Wang CT, Sun BG. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohyd Polym. 2011; 86: 1192–1197. doi: 10.1016/j.carbpol.2011.06.014 [Google Scholar]
- 38.Munjal U, Glei M, Pool-Zobel BL, Scharlau D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Brit J Nutr. 2009; 102: 663–671. doi: 10.1017/S0007114509274770 [DOI] [PubMed] [Google Scholar]
- 39.Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn’s mucosa through modulation of antioxidant defense machinery. Plos One. 2012; 7: e32841 doi: 10.1371/journal.pone.0032841 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.