Key Points
Question
Does the timing of maternal weight gain during pregnancy affect infant birth weight?
Findings
In this preconception cohort study of 1164 women who underwent weight measurement at a median of 19.9 weeks before pregnancy and then over 10 intervals across gestation, only weight gain from the pregravid time to 14 weeks and from 14 to 18 weeks were significantly associated with infant birth weight; subsequent gestational weight gain was not.
Meaning
Maternal weight status in the first half of gestation, rather than the second half, is a determinant of infant birth weight.
Abstract
Importance
Gestational weight gain is a determinant of infant birth weight, but it is unclear whether its timing in pregnancy may hold implications in this regard. Previous studies have yielded conflicting findings on the association of maternal weight gain in early pregnancy with birth weight. However, as these studies have typically recruited women during the first trimester, they are inherently limited by a reliance on self-reported pregravid weight.
Objective
To evaluate the associations of directly measured maternal pregravid weight and the timing of subsequent weight gain across pregnancy with infant birth weight.
Design, Setting, and Participants
In this prospective, preconception, observational cohort study, 1164 newly married women in Liuyang, China, underwent pregravid evaluation at a median of 19.9 weeks before a singleton pregnancy during which they underwent serial weight measurements. The study was conducted from February 1, 2009, to November 4, 2015. Data analysis was performed between September 1, 2016, and May 6, 2017.
Exposure
Maternal weight gain was calculated for the following 10 gestational intervals: from pregravid to less than 14, 14 to 18, 19 to 23, 24 to 28, 29 to 30, 31 to 32, 33 to 34, 35 to 36, 37 to 38, and 39 to 40 weeks.
Main Outcomes and Measures
Associations of pregravid weight and weight gain within each of the 10 gestational intervals with the outcome of infant birth weight.
Results
The mean (SD) age of the 1164 women included in the study was 25.3 (3.1) years. Pregravid weight was consistently associated with infant birth weight. However, among the 10 gestational intervals, only weight gain from pregravid to 14 weeks and from 14 to 18 weeks was associated with birth weight. Birth weight increased by 13.6 g/kg (95% CI, 3.2-24.1 g/kg) of maternal weight gain from pregravid to 14 weeks and by 26.1 g/kg (95% CI, 3.8-48.4 g/kg) of maternal weight gain from 14 to 18 weeks.
Conclusion and Relevance
Maternal weight only in the first half of gestation is a determinant of infant birth weight. Before pregnancy and early gestation may be a critical window for intervention to affect subsequent birth weight.
This observational cohort study examines the association of maternal weight gain, measured from before pregnancy through gestation, with infant birth weight.
Introduction
Maternal weight gain in pregnancy holds implications for both mother and child. Insufficient gestational weight gain has been linked to low birth weight and preterm birth, while excessive weight gain has been associated with infant macrosomia and maternal postpartum weight retention. Recently, a multinational study involving 8 geographically diverse regions (Brazil, China, India, Italy, Kenya, Oman, United Kingdom, and United States) showed that the pattern of weight gain during pregnancy is similar across human populations around the world, despite local differences in ethnicity, culture and beliefs, behaviors, and clinical practices. The apparent conservation of this pattern in the face of genetic and lifestyle differences further underscores the anticipated physiologic importance of the pattern of gestational weight gain. Clinically, however, our understanding of the implications of the timing of weight gain in pregnancy remains limited.
There is currently growing recognition of the potential association of early-life intrauterine exposures with developmental programming of the fetus that may hold implications for the health of the baby both in the short term (eg, birth weight at delivery) and long term (eg, risk of cardiovascular disease in adulthood). In this context, a clinical question that emerges is the effect of maternal weight gain in early pregnancy on infant birth weight. Previous studies have yielded conflicting findings in this regard, with several, but not all, suggesting that first-trimester weight gain is not related to birth weight, but gains in second or third trimester may be associated with this outcome. However, these studies have typically recruited women in the first trimester rather than before pregnancy. Therefore, these studies are inherently limited by a reliance on self-reported pregravid weight, resulting in potential bias and misclassification that may contribute to their conflicting findings. Accordingly, to objectively address the implications of early pregnancy weight gain, the optimal study design is one in which women are evaluated shortly before pregnancy and then longitudinally across the subsequent gestation. In this context, we have established a prospective preconception cohort and directly evaluated the associations of pregravid weight and the timing of weight gain during pregnancy with infant birth weight.
Methods
Study Population
This prospective, observational, preconception cohort study recruited women at the time of marriage in the Liuyang region of the Hunan province in China. The study protocol has previously been described in detail. Participants underwent baseline cardiometabolic characterization at recruitment and then, whenever they subsequently became pregnant, were followed up across the pregnancy to delivery through their clinical care. Beginning on February 1, 2009, 3375 women were recruited into this cohort, of whom 2482 have completed a singleton pregnancy. Of these, 999 women did not have recorded weight measurements in pregnancy because they did not obtain their antenatal care at the participating hospital. The remaining 1483 women had at least 1 weight measurement in pregnancy. After the exclusion of 31 women who were more than 5 weeks pregnant at their baseline assessment based on back-dating of gestational age at delivery and 288 women in whom infant birth weight was not available (due to delivery elsewhere), the study population for the present analysis consisted of 1164 women (eFigure in the Supplement). The study was completed on November 4, 2015. Data analysis was performed between September 1, 2016 and May 6, 2017. The cohort study was approved by the institutional research ethics boards of Central South University (Changsha, Hunan, China), Ottawa Hospital Research Institute (Ottawa, Canada), and Mount Sinai Hospital (Toronto, Canada), and all participants provided written informed consent; there was no financial compensation.
Recruitment and Study Measurements
As previously described, to recruit a preconception cohort in a cost-efficient manner requires a study design whereby one can identify women who are likely to get pregnant in the near future. For this reason, the Liuyang Maternal and Infant Hospital was selected for this study because women in its catchment area typically attend a premarriage health clinic for assessment at the time of marriage registration. Thus, by recruiting at these clinics, we established this cohort consisting of women who indicated that they were planning to become pregnant in the next 6 months. After recruitment, participants were asked to undergo an overnight fast and then attend a baseline assessment that consisted of interviewer-administered questionnaires (regarding demographics, lifestyle, and medical history), physical examination, and the drawing of venous blood samples for biochemical analyses (including lipid and fasting glucose levels), as previously described. Blood pressure and anthropometric measurements were performed by trained research staff, including weight, height, waist circumference, and calculated body mass index.
Once pregnant, participants received obstetrical care through clinical services at Liuyang Maternal and Infant Hospital. Weight measurements during pregnancy were obtained through this clinical care. Data collected at delivery included length of gestation, infant sex and birth weight, and maternal weight.
Exposures and Outcomes
We first examined the distribution of the timing (weeks’ gestation) of the weight measurements in pregnancy in the study population. Recognizing that clinical visits become more frequent as pregnancy progresses from the first to second to third trimester, we defined the following 10 gestational intervals: less than 14, 14 to 18, 19 to 23, 24 to 28, 29 to 30, 31 to 32, 33 to 34, 35 to 36, 37 to 38, and 39 to 40 weeks. The numbers of weight measurements within these intervals were 541, 884, 828, 885, 512, 604, 593, 742, 882, and 568, respectively. If a woman had more than 1 antenatal visit within an interval, we took the mean of her weight measurements for that interval. Weight gain in the first gestational interval was calculated as the antenatal weight up to less than 14 weeks minus the weight measured at the pregravid assessment; the weight gain in subsequent intervals was calculated as the difference in weight between the indicated interval and the preceding one. Cumulative gestational weight gain in each interval was calculated as the weight in that interval minus the weight measured at the pregravid assessment. The outcome of interest was infant birth weight.
Statistical Analysis
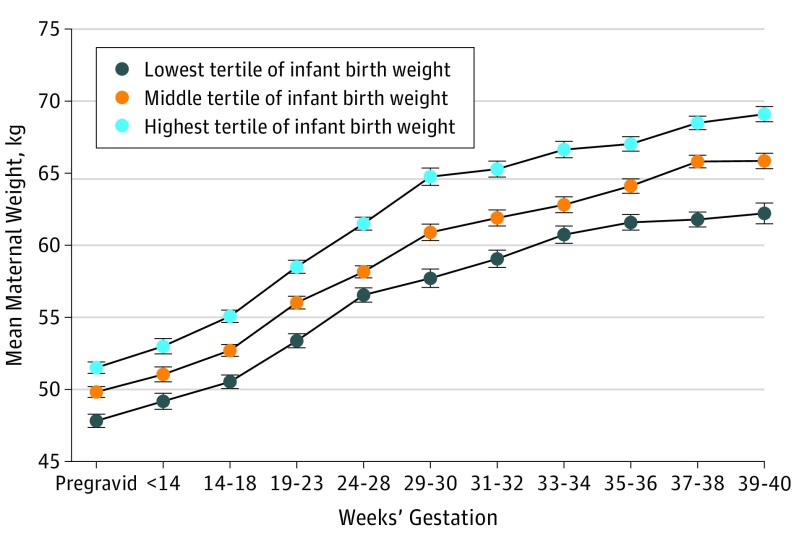
Table 1 reports the characteristics at pregravid assessment, across pregnancy, and at delivery for the study population, stratified into tertiles according to infant birth weight. Continuous variables are presented as mean (SD) if normally distributed, median (interquartile range) if skewed, and categorical variables as numbers and proportions. To illustrate the association between maternal weight gain during pregnancy and infant birth weight, we constructed a mean plot of maternal weight from the pregravid assessment and across the 10 gestational intervals by birth weight tertile (Figure).
Table 1. Characteristics of Study Population Stratified According to Tertiles of Infant Birth Weight.
| Characteristic | Infant Birth Weight Tertile | ||
|---|---|---|---|
| Lowest (1800-3060 g) |
Middle (3100-3450 g) |
Highest (3500-6000 g) |
|
| Pregravid Assessment | |||
| Participants, No. | 343 | 434 | 387 |
| Weeks before pregnancy, median (IQR) | 15.4 (2.6 to 46.3) | 20.3 (5.6 to 53) | 22.4 (5.9 to 60.7) |
| Age, mean (SD), y | 25 (3) | 25 (3) | 26 (4) |
| Education, median (IQR), y | 10 (9 to 12) | 9 (9 to 12) | 12 (9 to 12) |
| Smoking, No. (%) | 0 | 1 (0.2) | 5 (1.3) |
| Weight, mean (SD), kg | 47.8 (5.8) | 49.8 (6.8) | 51.5 (7.1) |
| BMI, mean (SD) | 19.5 (2.1) | 20.1 (2.5) | 20.7 (2.8) |
| Waist, mean (SD), m | 69 (8) | 71 (7) | 72 (7) |
| BP, mean (SD), mm Hg | |||
| Systolic | 111 (12) | 112 (12) | 110 (13) |
| Diastolic | 71 (9) | 72 (9) | 71 (9) |
| Cholesterol, mean (SD), mg/dL | |||
| Total | 150.6 (50.2) | 150.6 (38.6) | 150.6 (46.3) |
| LDL | 81.1 (30.9) | 77.2 (27.0) | 81.1 (30.9) |
| HDL | 57.9 (19.3) | 61.8 (15.4) | 57.9 (19.3) |
| Triglycerides, median (IQR), mg/dL | 70.8 (53.1 to 106.2) | 79.6 (53.1 to 115.0) | 79.6 (61.9 to 115.0) |
| Blood glucose, mean (SD), mg/dL | 81.1 (21.6) | 79.3 (21.6) | 82.9 (19.8) |
| During Pregnancy | |||
| Weight gain during gestational intervals, median (IQR), kg | |||
| Pregravid to 14 wk | 1.0 (−1.0 to 4.0) | 0.6 (−1.0 to 3.0) | 1.5 (−0.5 to 4.0) |
| 14-18 wk | 1.5 (0.3 to 2.7) | 1.8 (0.8 to 3.0) | 2.0 (1.0 to 3.5) |
| 19-23 wk | 2.8 (2.0 to 4.0) | 3.0 (2.0 to 4.5) | 3.0 (2.0 to 4.5) |
| 24-28 wk | 3.0 (2.0 to 4.4) | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 4.7) |
| 29-30 wk | 2.5 (1.5 to 4.0) | 2.5 (1.5 to 3.7) | 2.2 (1.2 to 3.5) |
| 31-32 wk | 1.5 (0.5 to 2.0) | 1.0 (0.3 to 2.0) | 1.0 (0.3 to 2.0) |
| 33-34 wk | 1.0 (0 to 2.0) | 1.0 (0.5 to 2.0) | 1.0 (0.5 to 2.0) |
| 35-36 wk | 1.0 (0.5 to 2.0) | 1.3 (0.5 to 2.5) | 1.0 (0.5 to 2.3) |
| 37-38 wk | 1.1 (0.5 to 2.0) | 1.0 (0 to 2.0) | 1.0 (0.5 to 2.0) |
| 39-40 wk | 0.8 (0.3 to 1.5) | 1.0 (0 to 1.5) | 1.0 (0.4 to 2.0) |
| At Delivery | |||
| Length of gestation, mean (SD), wk | 38.9 (1.6) | 39.1 (1.1) | 39.6 (3.0) |
| Preterm delivery, No. (%) | 32 (9.3) | 3 (0.7) | 3 (0.8) |
| Male infant, No. (%)a | 154 (45.2) | 228 (52.8) | 237 (61.4) |
| Gestational diabetes, No. (%) | 3 (0.9) | 6 (1.4) | 13 (3.4) |
| Pre-eclampsia, No. (%) | 16 (4.7) | 10 (2.3) | 4 (1.0) |
| Total maternal weight gain in pregnancy, median (IQR), kg | 14.5 (12.0-18.5) | 16.0 (13.0-19.5) | 17.5 (14.5 to 22.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein.
SI conversion factors: To convert total, LDL, and HDL cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555; and triglycerides to millimoles per liter, multiply by 0.0113.
Infant sex was missing for 1 woman in the highest tertile and for 2 women in each of the other 2 tertiles.
Figure. Maternal Weight Associated With Weeks’ Gestation in Pregnancies.
Stratified according to tertiles of infant birth weight.
To determine whether pregravid weight and cumulative gestational weight gain up to each of 14, 18, 23, 28, 30, 32, 34, 36, 38, and 40 weeks were associated with infant birth weight, we constructed 10 multiple linear regression models evaluating pregravid weight and weight gain up to each of these points in time, with additional adjustment for infant sex and total length of gestation (Table 2). Similarly, a series of 10 multiple linear regression models was constructed with the same additional covariates to assess whether pregravid weight and discrete weight gain within each of the 10 gestational intervals were associated with infant birth weight (Table 3). All tests were 2-sided and performed at level of significance of P = .05. Since the analyses were designed as exploratory to identify the intervals in pregnancy during which weight gain is associated with birth weight, we did not apply adjustment for multiple testing.
Table 2. Associations of Prepregnancy Weight and Cumulative Gestational Weight Gain With Infant Birth Weight.
| Model | Maternal Weight Variable | Infant Birth Weight, g, β (95% CI)a | |
|---|---|---|---|
| Analyses With Observed Data | Analyses With Multiple Imputation Data | ||
| 1 | Prepregnancy weight | 15.2 (8.7-21.7) | 15.6 (11.5-19.8) |
| Gestational weight gain to 14 wk | 13.6 (3.2-24.1) | 13.2 (5.5-20.9) | |
| 2 | Prepregnancy weight | 15.8 (11.0-20.6) | 16.5 (13.4-20.6) |
| Gestational weight gain to 18 wk | 17.4 (9.5-25.4) | 18.5 (11.9-25.2) | |
| 3 | Prepregnancy weight | 14.9 (9.8- 20.1) | 16.3 (12.3-20.4) |
| Gestational weight gain to 23 wk | 18.9 (10.8-27.0) | 18.8 (12.5-25.0) | |
| 4 | Prepregnancy weight | 15.1 (10.3-20.0) | 16.6 (12.6-20.6) |
| Gestational weight gain to 28 wk | 19.6 (12.5-26.7) | 20.7 (14.8-26.7) | |
| 5 | Prepregnancy weight | 18.3 (12.7-23.9) | 16.6 (12.4-20.4) |
| Gestational weight gain to 30 wk | 21.4 (13.3-29.5) | 20.7 (13.2-24.9) | |
| 6 | Prepregnancy weight | 19.5 (13.9-25.2) | 16.4 (12.3-20.3) |
| Gestational weight gain to 32 wk | 19.8 (12.0-27.7) | 19.1 (14.1-25.3) | |
| 7 | Prepregnancy weight | 18.1 (11.6-24.5) | 16.5 (12.5-20.5) |
| Gestational weight gain to 34 wk | 19.7 (11.6-27.8) | 19.1 (13.7-24.5) | |
| 8 | Prepregnancy weight | 14.6 (9.3-19.9) | 16.0 (12.0-20.0) |
| Gestational weight gain to 36 wk | 18.6 (12.0-25.2) | 17.3 (12.2-22.3) | |
| 9 | Prepregnancy weight | 17.1 (12.7-21.5) | 16.2 (12.2-20.2) |
| Gestational weight gain to 38 wk | 16.5 (11.3-21.8) | 17.6 (12.7-22.6) | |
| 10 | Prepregnancy weight | 19.0 (13.2-24.7) | 15.9 (11.9-19.8) |
| Gestational weight gain to 40 wk | 16.7 (9.5-23.9) | 17.1 (12.1-22.1) | |
β represents an estimate of linear regression coefficient, representing the change in infant birth weight (grams) when the indicated maternal weight variable changes by 1 kg. None of the 95% CI findings crossed 0, indicating that there is a significant association between the indicated maternal weight variable and infant birth weight. All models are adjusted for prepregnancy weight, cumulative gestational weight gain up to the indicated point in pregnancy, total length of gestation, and infant sex.
Table 3. Associations of Prepregnancy Weight and Weight Gain Within Each Gestational Interval With Infant Birth Weight.
| Model | Maternal Weight Variable | Infant Birth Weight, g, β (95% CI)a | |
|---|---|---|---|
| Analyses With Observed Data | Analyses With Multiple Imputation Data | ||
| 1 | Prepregnancy weight | 15.2 (8.7 to 21.7)b | 15.6 (11.5 to 19.8)b |
| Weight gain from pregravid to 14 wk | 13.6 (3.2 to 24.1)b | 13.2 (5.5 to 20.9)b | |
| 2 | Prepregnancy weight | 14.4 (8.0 to 21.7)b | 14.4 (10.3 to 18.4)b |
| Weight gain from 14 to 18 wk | 26.1 (3.8 to 48.4)b | 22.0 (5.6 to 38.3)b | |
| 3 | Prepregnancy weight | 13.9 (8.0 to 19.7)b | 14.0 (9.9 to 18.0)b |
| Weight gain from 19 to 23 wk | 11.3 (−8.9 to 31.5) | 15.6 (−5.2 to 27.8) | |
| 4 | Prepregnancy weight | 12.5 (6.4 to 18.6)b | 13.0 (7.0 to 19.1)b |
| Weight gain from 24 to 28 wk | 15.3 (−4.1 to 34.8) | 15.6 (−3.6 to 34.8) | |
| 5 | Prepregnancy weight | 15.3 (9.2 to 21.3)b | 15.3 (9.3 to 21.3)b |
| Weight gain from 29 to 30 wk | −1.4 (−26.6 to 23.8) | −2.3 (−27.2 to 22.5) | |
| 6 | Prepregnancy weight | 24.7 (16.2 to 33.1)b | 25.0 (16.7 to 33.3)b |
| Weight gain from 31 to 32 wk | −1.0 (−39.9 to 37.9) | −2.0 (−40.0 to 35.9) | |
| 7 | Prepregnancy weight | 21.4 (12.8 to 30.2)b | 21.5 (12.8 to 30.1)b |
| Weight gain from 33 to 34 wk | 15.6 (−39.6 to 70.8) | 13.8 (−40.4 to 68.1) | |
| 8 | Prepregnancy weight | 15.7 (8.0 to 23.4)b | 15.5 (7.9 to 23.0)b |
| Weight gain from 35 to 36 wk | 4.6 (−32.9 to 42.0) | 4.8 (−32.0 to 41.5) | |
| 9 | Prepregnancy weight | 17.5 (11.4 to 23.8)b | 14.3 (10.2 to 18.3)b |
| Weight gain from 37 to 38 wk | 7.9 (−18.9 to 34.7) | 16.9 (−8.1 to 41.8) | |
| 10 | Prepregnancy weight | 16.5 (10.3 to 22.7)b | 14.1 (10.0 to 18.2)b |
| Weight gain from 39 to 40 wk | −2.0 (−34.8 to 30.9) | 2.3 (−30.1 to 36.8) | |
β represents an estimate of linear regression coefficient, representing the change in infant birth weight (grams) when the indicated maternal weight variable changes by 1 kg. All models are adjusted for prepregnancy weight, weight gain within each interval, total length of gestation, and infant sex.
The 95% CI did not cross 0, indicating that there is a significant association between the indicated maternal weight variable and infant birth weight.
To evaluate the statistical effect of missing data on the association between covariates and birth weight, the analyses in Tables 2 and 3 were also performed with multiple imputation by the chained equations method. Missing data were considered as resulting from a missing at random mechanism. We included all of the weight measurements in pregnancy, pregravid weight, and the other variables from the multiple linear regression models (infant sex and total length of gestation) in the imputation model. Discriminant function for categorical covariates and linear regression for continuous covariates were used to impute values. Since the maximum amount of missingness among the 10 weight measurements was 58.2%, we generated 100 imputed data sets. The multiple linear regression analyses in Tables 2 and 3 were repeated using each of the augmented data sets, parameter estimates were averaged across the 100 analyses, and their SEs were computed using the Rubin method. All analyses were conducted using SAS, version 9.4 (SAS Institute). Imputation analyses were done by PROC MI, GENMOD, and MIANALYZE.
Results
The study population consisted of 1164 women (mean [SD] age, 25.3 [3.1] years) who underwent pregravid assessment at median 19.9 weeks before a singleton pregnancy and delivered at a mean 39.4 weeks’ gestation. Table 1 reports the characteristics of the study population stratified into tertiles of infant birth weight. The Figure shows the pattern of maternal weight gain over the 10 gestational intervals in these infant birth weight tertiles. Findings in the Figure suggest that maternal weight before and during pregnancy was associated with infant birth weight.
On multiple linear regression analyses, both pregravid maternal weight and cumulative gestational weight gain up to each of 14, 18, 23, 28, 30, 32, 34, 36, 38, and 40 weeks were associated with infant birth weight, after adjustment for infant sex and total length of gestation (Table 2). These findings were unchanged on the multiple imputation analyses (Table 2).
Having thus established that cumulative gestational weight gain up to each of the indicated points in pregnancy is associated with birth weight, we next sought to evaluate the impact of weight gain within the 10 discrete intervals that these times identify. On multiple linear regression analyses adjusted for pregravid weight, infant sex, and total length of gestation (Table 3), only weight gain from pregravid to 14 weeks and from 14 to 18 weeks were significantly associated with infant birth weight (as was prepregnancy weight). Birth weight increased by 13.6 g/kg (95% CI, 3.2-24.1 g/kg) of maternal weight gain from the pregravid to 14-week interval and by 26.1 g/kg (95% CI, 3.8-48.4 g/kg) of maternal weight gain from 14 to 18 weeks. Of note, weight gain within each of the 8 intervals after 18 weeks was not associated with birth weight. These findings were unchanged on the multiple imputation analyses (Table 3).
Furthermore, the main findings were unchanged in a series of sensitivity analyses, performed as follows: restriction to women with delivery at 37 or more weeks (n = 1126) (eTable 1 in the Supplement), exclusion of women with preeclampsia (n = 30) (eTable 2 in the Supplement), exclusion of women with gestational diabetes (n = 22) (eTable 3 in the Supplement), exclusion of women who smoke (n = 6) (eTable 4 in the Supplement), additional adjustment for time from pregravid assessment to pregnancy (eTable 5 in the Supplement), exclusion of those in whom time from pregravid assessment to pregnancy exceeded the 90th percentile of the distribution of this variable (eTable 6 in the Supplement), and adjustment for pregravid body mass index in place of pregravid weight (eTable 7 in the Supplement).
Discussion
In this study, we have prospectively measured maternal weight prior to pregnancy and then longitudinally across the subsequent gestation. With this approach, we demonstrate that pregravid maternal weight is consistently associated with infant birth weight. More importantly, only maternal weight gain from prepregnancy to 14 weeks and from 14 to 18 weeks was associated with birth weight, whereas gestational weight changes thereafter were not. Taken together, these data thus identify maternal weight status in the first half of gestation as a key determinant of infant birth weight and highlight the importance of the timing of weight gain in pregnancy.
Pregnancy cohorts typically recruit women in first or second trimester, at which point pregravid weight is generally obtained by self-report. The reliability of self-reported weight may vary at the extremes of body weight, with underestimation in those with higher weight. Underestimation of self-reported weight has been documented in previous studies, including in young women. Such underestimation of pregravid weight potentially could yield misclassification of gestational weight gain that may have contributed to the conflicting findings reported thus far on pregravid weight-gain timing. Some studies have suggested that maternal weight gain in the second trimester is a key determinant of birth weight, while others have implicated weight gain in both the second and third trimesters.
Similarly, whereas several studies have suggested that first-trimester weight gain is not related to birth weight, a study of 651 overweight/obese women found that high gestational weight gain before 20 weeks predicted an increased risk of large-for-gestational-age infants at delivery. Although the potential association between self-reported pregravid weight and these conflicting findings cannot be determined, a prospective preconception cohort would be the ideal study design for objective evaluation of the timing of gestational weight gain and its implications. However, this design has generally not been implemented for practical reasons, owing to the potentially prohibitive cost of characterizing women and then waiting indefinitely for a subsequent pregnancy.
In this context, we selected the Liuyang region of China as a setting in which newly married women could be identified (through attendance at the premarriage health clinics) and were likely to undergo a first pregnancy relatively shortly thereafter (based on societal practices in the region). In establishing a preconception cohort in this setting, we have been able to directly evaluate gestational weight gain in association with pregravid assessment performed at a median of 19.9 weeks before pregnancy and assess its impact in 10 intervals across gestation in over 1000 women. In doing so, we demonstrate that only early pregnancy weight gain before 18 weeks was associated with infant birth weight. A previous study in which 389 women had weight measured within 6 months of pregnancy also found that maternal weight change in the first trimester had a greater influence on newborn weight than did changes in the second and third trimesters. It thus appears that, when assessed in relation to directly measured (rather than self-reported) pregravid weight, the association between early gestational weight gain and birth weight is readily detectable.
Maternal pregravid weight is likely associated with infant birth weight because it reflects a composite of the shared genetic, environmental, and lifestyle features of mother and child that will influence fetal growth and weight at delivery. More intriguing is the question of how maternal weight gain in early pregnancy may influence this outcome. It is believed that weight gain in the first half of pregnancy primarily reflects the deposition and expansion of maternal tissues (eg, adipose tissue), whereas gestational weight gain thereafter is mainly attributed to fetal and placental growth and fluid expansion. The fetus does not accumulate fat or much lean tissue in the first trimester, but grows rapidly thereafter. In this context, it has been suggested that early pregnancy and midpregnancy weight gain could affect subsequent adiposity of the offspring by increasing the availability of maternal fuels (eg, glucose, free fatty acids, amino acids). The resultant intrauterine environment may program fetal development toward a propensity for the accumulation of fat or lean tissue later in pregnancy. This possibility is supported by a growing body of evidence linking early pregnancy maternal weight gain with both cord blood hormones (eg, insulin) that may affect fetal growth and, most notably, subsequent childhood adiposity. Specifically, a series of studies has shown that maternal weight gain in the first half of pregnancy is associated with body mass index and fat mass of the child between the ages of 7 and 10 years. Collectively, these data suggest that our identification of directly measured early gestational weight gain as associated with infant birth weight may reflect programming that holds implications for future growth trajectories in childhood.
The present findings hold clinical implications. First, these data identify prepregnancy and early gestation as a critical window that may affect subsequent birth weight at delivery. They lend support for suggestions calling for clinical emphasis on optimizing maternal health before pregnancy for the purpose of transgenerational benefit. Second, the present findings suggest that interventions to limit weight gain in the latter half of pregnancy may have a modest association with birth weight. In agreement with this concept, randomized clinical trials, such as the LIMIT and UPBEAT (UK Pregnancies Better Eating and Activity Trial) studies, found that intensive interventions to improve diet and physical activity during pregnancy did not reduce the incidence of large-for-gestational-age infants at delivery. To affect birth weight, it may be necessary for future trials to intervene during the critical window of early gestation or ideally before pregnancy.
Limitations
A limitation of this study is that the population comprised a single ethnicity (Chinese). Another potential limitation is that our study population was generally lean, such that the applicability of these findings to overweight/obese women remains to be established. In addition, there were 288 women for whom birth weight was not available because they delivered elsewhere than at the participating hospital. Furthermore, the size of the study population is not sufficient for rigorous evaluation of the association between maternal weight gain in the indicated intervals and the likelihood of small-for-gestational-age or large-for-gestational-age infants at delivery. Finally, given its observational nature, this study design cannot provide evidence that the modification of early weight gain can affect birth weight. Nevertheless, our data have highlighted prepregnancy/early gestation as a possible window of opportunity during which to evaluate such intervention in future clinical trials.
Conclusions
This prospective, preconception cohort study demonstrates that, when objectively measured prior to pregnancy, pregravid maternal weight is consistently associated with infant birth weight. More importantly, subsequent maternal weight gain from before pregnancy to 14 weeks and from 14 to 18 weeks is associated with birth weight, whereas gestational weight changes thereafter are not. Thus, the timing of gestational weight gain is relevant to infant birth weight, with maternal weight status in the first half of gestation emerging as a key determinant of infant birth weight.
eFigure. Derivation of Study Population of 1164 Women
eTable 1. Multiple Linear Regression Models With Restriction to Women With Delivery at ≥37 weeks
eTable 2. Multiple Linear Regression Models With Exclusion of Women With Pre-Eclampsia
eTable 3. Multiple Linear Regression Models With Exclusion of Women With Gestational Diabetes
eTable 4. Multiple Linear Regression Models With Exclusion of Women Who Smoke
eTable 5. Multiple Linear Regression Models With Additional Adjustment for Time From Pre-Gravid Assessment to Pregnancy
eTable 6. Multiple Linear Regression Models With Exclusion of Those in Whom Time From Pregravid Assessment to Pregnancy Exceeded the 90th Percentile of the Distribution of This Variable
eTable 7. Multiple Linear Regression Models With Adjustment for Pregravid Body Mass Index in Place of Pregravid Weight
References
- 1.Caughey AB. Gestational weight gain and outcomes for mothers and infants. JAMA. 2017;317(21):2175-2176. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RF, Abell SK, Ranasinha S, et al. . Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheikh Ismail L, Bishop DC, Pang R, et al. . Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. BMJ. 2016;352:i555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J. Maternal pre-pregnancy BMI, gestational weight gain, and infant birth weight. Econ Hum Biol. 2015;18:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A, Tilling K, Macdonald-Wallis C, et al. . Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy. Am J Clin Nutr. 2011;93(6):1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catov JM, Abatemarco D, Althouse A, Davis EM, Hubel C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity (Silver Spring). 2015;23(5):1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(suppl 1):3-6. [PubMed] [Google Scholar]
- 9.Ruchat SM, Allard C, Doyon M, et al. . Timing of excessive weight gain during pregnancy modulates newborn anthropometry. J Obstet Gynaecol Can. 2016;38(2):108-117. [DOI] [PubMed] [Google Scholar]
- 10.Karachaliou M, Georgiou V, Roumeliotaki T, et al. . Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol. 2015;212(4):502.e1-502.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr. 1999;129(5):988-993. [DOI] [PubMed] [Google Scholar]
- 12.Hickey CA, Cliver SP, McNeal SF, Hoffman HJ, Goldenberg RL. Prenatal weight gain patterns and birth weight among nonobese black and white women. Obstet Gynecol. 1996;88(4, pt 1):490-496. [DOI] [PubMed] [Google Scholar]
- 13.Brown JE, Murtaugh MA, Jacobs DR Jr, Margellos HC. Variation in newborn size according to pregnancy weight change by trimester. Am J Clin Nutr. 2002;76(1):205-209. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar SB, Xu F, Hedderson MM. Trimester-specific gestational weight gain and infant size for gestational age. PLoS One. 2016;11(7):e0159500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams B, Selvin S. Maternal weight gain pattern and birth weight. Obstet Gynecol. 1995;86(2):163-169. [DOI] [PubMed] [Google Scholar]
- 16.Scholl TO, Hediger ML, Ances IG, Belsky DH, Salmon RW. Weight gain during pregnancy in adolescence. Obstet Gynecol. 1990;75(6):948-953. [PubMed] [Google Scholar]
- 17.Retnakaran R, Wen SW, Tan H, et al. . Maternal blood pressure before pregnancy and sex of the baby. Am J Hypertens. 2017;30(4):382-388. [DOI] [PubMed] [Google Scholar]
- 18.Retnakaran R, Wen SW, Tan H, et al. . Maternal pre-gravid cardiometabolic health and infant birthweight. Nutr Metab Cardiovasc Dis. 2017;27(8):723-730. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581-592. [Google Scholar]
- 20.Villanueva EV. The validity of self-reported weight in US adults. BMC Public Health. 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J. 2007;11(2):137-144. [DOI] [PubMed] [Google Scholar]
- 22.Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol. 1992;2(5):715-721. [DOI] [PubMed] [Google Scholar]
- 23.Hivert MF, Rifas-Shiman SL, Gillman MW, Oken E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity (Silver Spring). 2016;24(7):1546-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neufeld LM, Haas JD, Grajéda R, Martorell R. Changes in maternal weight from the first to second trimester of pregnancy are associated with fetal growth and infant length at birth. Am J Clin Nutr. 2004;79(4):646-652. [DOI] [PubMed] [Google Scholar]
- 25.Starling AP, Brinton JT, Glueck DH, et al. . Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101(2):302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifas-Shiman SL, Fleisch A, Hivert MF, Mantzoros C, Gillman MW, Oken E. First and second trimester gestational weight gains are most strongly associated with cord blood levels of hormones at delivery important for glycemic control and somatic growth. Metabolism. 2017;69:112-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen CS, Gamborg M, Sørensen TI, Nohr EA. Weight gain in different periods of pregnancy and offspring’s body mass index at 7 years of age. Int J Pediatr Obes. 2011;6(2-2):e179-e186. [DOI] [PubMed] [Google Scholar]
- 28.Fraser A, Tilling K, Macdonald-Wallis C, et al. . Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. 2010;121(23):2557-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullins E, Murphy O, Davies SC. Pre-conception public health to address maternal obesity. BJOG. 2016;123(2):159-160. [DOI] [PubMed] [Google Scholar]
- 30.Zylke JW, DeAngelis CD. Health promotion and disease prevention in children: it’s never too early. JAMA. 2009;301(21):2270-2271. [DOI] [PubMed] [Google Scholar]
- 31.Dodd JM, Turnbull D, McPhee AJ, et al. ; LIMIT Randomised Trial Group . Antenatal lifestyle advice for women who are overweight or obese. BMJ. 2014;348:g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poston L, Bell R, Croker H, et al. ; UPBEAT Trial Consortium . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study). Lancet Diabetes Endocrinol. 2015;3(10):767-777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Derivation of Study Population of 1164 Women
eTable 1. Multiple Linear Regression Models With Restriction to Women With Delivery at ≥37 weeks
eTable 2. Multiple Linear Regression Models With Exclusion of Women With Pre-Eclampsia
eTable 3. Multiple Linear Regression Models With Exclusion of Women With Gestational Diabetes
eTable 4. Multiple Linear Regression Models With Exclusion of Women Who Smoke
eTable 5. Multiple Linear Regression Models With Additional Adjustment for Time From Pre-Gravid Assessment to Pregnancy
eTable 6. Multiple Linear Regression Models With Exclusion of Those in Whom Time From Pregravid Assessment to Pregnancy Exceeded the 90th Percentile of the Distribution of This Variable
eTable 7. Multiple Linear Regression Models With Adjustment for Pregravid Body Mass Index in Place of Pregravid Weight