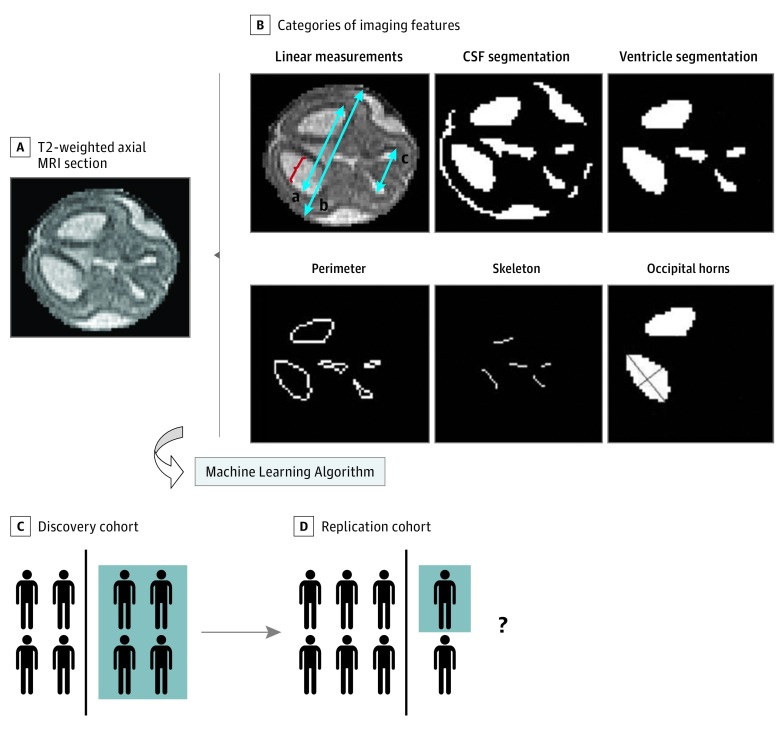
This case-control study assesses the use of fetal magnetic resonance imaging and machine learning to predict the need for postnatal cerebrospinal fluid diversion in patients with fetal ventriculomegaly.
Key Points
Question
Can fetal magnetic resonance imaging be used to predict the need for postnatal cerebrospinal fluid diversion in patients with fetal ventriculomegaly?
Findings
In this case-control study of 50 patients with fetal ventriculomegaly, multiple imaging features were extracted from fetal magnetic resonance imaging and integrated by machine learning to yield a model that correctly classified postnatal cerebrospinal fluid diversion status with 82% accuracy. In an independent replication cohort study, the model achieved 91% accuracy.
Meaning
An image-based predictive model with high accuracy and generalizability may provide prenatal prognostic information and help guide postnatal clinical management in fetal ventriculomegaly.
Abstract
Importance
Which children with fetal ventriculomegaly, or enlargement of the cerebral ventricles in utero, will develop hydrocephalus requiring treatment after birth is unclear.
Objective
To determine whether extraction of multiple imaging features from fetal magnetic resonance imaging (MRI) and integration using machine learning techniques can predict which patients require postnatal cerebrospinal fluid (CSF) diversion after birth.
Design, Setting, and Patients
This retrospective case-control study used an institutional database of 253 patients with fetal ventriculomegaly from January 1, 2008, through December 31, 2014, to generate a predictive model. Data were analyzed from January 1, 2008, through December 31, 2015. All 25 patients who required postnatal CSF diversion were selected and matched by gestational age with 25 patients with fetal ventriculomegaly who did not require CSF diversion (discovery cohort). The model was applied to a sample of 24 consecutive patients with fetal ventriculomegaly who underwent evaluation at a separate institution (replication cohort) from January 1, 1998, through December 31, 2007. Data were analyzed from January 1, 1998, through December 31, 2009.
Exposures
To generate the model, linear measurements, area, volume, and morphologic features were extracted from the fetal MRI, and a machine learning algorithm analyzed multiple features simultaneously to find the combination that was most predictive of the need for postnatal CSF diversion.
Main Outcomes and Measures
Accuracy, sensitivity, and specificity of the model in correctly classifying patients requiring postnatal CSF diversion.
Results
A total of 74 patients (41 girls [55%] and 33 boys [45%]; mean [SD] gestational age, 27.0 [5.6] months) were included from both cohorts. In the discovery cohort, median time to CSF diversion was 6 days (interquartile range [IQR], 2-51 days), and patients with fetal ventriculomegaly who did not develop symptoms were followed up for a median of 29 months (IQR, 9-46 months). The model correctly classified patients who required CSF diversion with 82% accuracy, 80% sensitivity, and 84% specificity. In the replication cohort, the model achieved 91% accuracy, 75% sensitivity, and 95% specificity.
Conclusion and Relevance
Image analysis and machine learning can be applied to fetal MRI findings to predict the need for postnatal CSF diversion. The model provides prognostic information that may guide clinical management and select candidates for potential fetal surgical intervention.
Introduction
Fetal ventriculomegaly refers to the enlargement of the cerebral ventricles in utero and occurs in approximately 1 in 1000 births. Fetal ventriculomegaly is commonly diagnosed on the basis of midtrimester ultrasonographic findings and may be further characterized by fetal magnetic resonance imaging (MRI). Fetal ventriculomegaly is defined on the basis of measurements performed at the level of the atrium of the lateral ventricles. An atrial diameter of 10 mm or greater, or 3 to 4 SDs above the mean, is used as a minimum cutoff to define fetal ventriculomegaly at any stage of pregnancy. Causes of fetal ventriculomegaly are broadly divided into loss of cerebral tissue, obstruction of the ventricular system, or excessive cerebrospinal fluid (CSF) production. Although the presence of fetal ventriculomegaly may be associated with hydrocephalus, the 2 terms are not synonymous. Hydrocephalus is clinically defined by increased intracranial pressure; it is commonly associated with ventricular dilation. However, fetal ventriculomegaly may occur in the absence of hydrocephalus. Patients with fetal ventriculomegaly are monitored closely after birth for signs of increased intracranial pressure, such as decreased activity, feeding intolerance, bulging fontanelle, splayed cranial sutures, and enlarging head circumference.
Not all patients with fetal ventriculomegaly become symptomatic and develop hydrocephalus. Fetal ventriculomegaly stabilizes in approximately 55% of cases and resolves in 30%; however, it will progress in 15% of cases. Such patients are likely to show signs of increased intracranial pressure after birth. If a patient develops hydrocephalus, the treatment involves diversion of CSF from the ventricle through surgical ventricular shunting or endoscopic third ventriculostomy. The ability to prospectively identify which patients with fetal ventriculomegaly will develop postnatal hydrocephalus and require subsequent CSF diversion would provide critical prognostic information to parents and aid in the clinical management in the postnatal period. We hypothesize that image analysis and machine learning techniques may be applied to fetal MRI to generate a model predictive of the need for postnatal CSF diversion in fetal ventriculomegaly. We also assessed the performance and generalizability of the fetal MRI-based model in an independent cohort from another institution.
Methods
Study Design and Patient Population
To generate the predictive model, we performed a retrospective case-control study. All patients with cerebral ventriculomegaly diagnosed based on fetal MRI findings and managed in the postnatal period at the Children’s Hospital of Philadelphia (CHOP) from January 1, 2008, through December 31, 2014, were identified from an institutional database. The study was approved by the institutional review board at CHOP, which waived the need for consent for this retrospective cohort study assessed from medical records.
Data were analyzed from January 1, 2008, through December 31, 2015. We reviewed the medical record of each patient with fetal ventriculomegaly. Patients who developed symptomatic hydrocephalus requiring CSF diversion after birth were identified as case patients. The remaining patients in the database were identified as control individuals. All case patients and an equal number of controls were selected and matched by best obstetric estimate of gestational age at the time of diagnostic fetal MRI. Thus, half of the patients in the discovery (or training) cohort, on which the predictive model is based, required CSF diversion, and half did not. The outcome, whether or not CSF diversion was required, was known for each patient in the discovery cohort. The decision to divert CSF in the postnatal period was standardized by adopting criteria for shunt failure that was used in the Shunt Design Trial. Clinical characteristics were collected for each patient. The SD of the differences between right and left atrial diameter was calculated, and lateral ventricle symmetry was defined as a difference of less than 1 SD between right and left atrial diameter in a single patient. Patients with concurrent myelomeningocele or Chiari malformation type II were excluded because many of these patients are known to require CSF diversion after birth.
Image Acquisition and Analysis
Image analysis techniques were applied to T2-weighted, half-Fourier acquisition single-shot turbo-spin-echo MRI performed in all patients. All images underwent preprocessing, which included smoothing and bias correction. Commonly used linear measures were obtained using measurement tools in EasyViz software (Medical Insight). Atrial diameter was measured in the axial plane on a single section at the level of the atria of the lateral ventricles and the glomus of the choroid plexus (Figure 1A). The fronto-occipital horn ratio (FOHR) was calculated using the same axial section used to measure atrial diameter; it is the mean of the maximum frontal and occipital horn diameters divided by the biparietal diameter [(a + c)/2b] (Figure 1A). Next, using the same axial section used to measure atrial diameter, automatic segmentations of the intracranial, ventricle, and subarachnoid spaces were obtained using the level set tool in MIPAV software (version 7.2.0; Medical Image Processing, Analysis, and Visualization) to yield 2-dimensional (2-D) area measurements (Figure 1B). To obtain a volumetric measurement, segmentation of the ventricle and subarachnoid space was additionally performed on sections 3 above and 3 below the axial section used to measure atrial diameter. The areas computed for each section were then summed together to yield ventricle and subarachnoid space 3-D volumes. All segmentations were manually confirmed and corrected as needed using the open-source image analysis software ITK-SNAP (version 3.4.0; http://www.itksnap.org). Although infrequent, as many as 3 of the 7 axial sections were excluded from analysis per patient owing to poor image quality. Two-dimensional area and 3-D volume were obtained as absolute number of voxels (nonnormalized) and as number of voxels divided by intracranial area for each section (normalized). All measurements were also normalized by image resolution.
Figure 1. Imaging Feature Selection and Study Schema.
From the fetal magnetic resonance image (MRI), multiple imaging features were extracted for each patient. All extracted features were assessed by a machine learning algorithm to generate a model (black vertical line) that best differentiates patients who required postnatal cerebrospinal fluid (CSF) diversion from those who did not (blue background) (n = 25 in both groups). The fetal MRI–based model was then applied to a replication cohort consisting of patients different from those in whom the model was trained to assess the model performance (represented by the question mark). B, a indicates occipital horn diameter; b, biparietal diameter; c, bifrontal diameter; bracket, atrial diameter; short blue line, minor axis; and long blue line, major axis.
Fetal MRI Feature Selection
In addition to the linear, area, and volume measurements described above, permutations of the measures and additional morphologic features were extracted from the fetal images and subsequently used to develop a predictive model. Inclusion of such additional features was motivated by prior studies of imaging factors predictive of outcome in patients with fetal ventriculomegaly that involved volumetric analysis and ventricle morphologic analysis. The ratio of the ventricle area to the subarachnoid space area was computed, and the area of the ventricle system to the smaller and larger occipital horns was compared. Morphologic operations on binary images in Matlab software (MathWorks) were used to calculate 2-D features such as the perimeter, the distance between the perimeter and the skull, and the ratio of the area to the perimeter of the ventricle. In addition, features specifically related to the occipital horns of the lateral ventricle were extracted, because these areas are the first to dilate in fetal ventriculomegaly. The ventricle system, including the occipital horns, was then modeled as a skeleton or line diagram (Figure 1B). The thickness, defined as the distance between the skeleton and the boundary of the ventricle, was calculated. Each occipital horn was then modeled as an ellipse, and major and minor axis lengths were measured (Figure 1B). Finally, the angle formed between the 2 major axes of the occipital horns was computed. A complete list of categories of imaging features used to generate the model is provided in eTable 1 in the Supplement.
Machine Learning and Cross Validation
For each patient in the discovery cohort, all features were extracted from the individual fetal MRI and input into a machine learning algorithm that determined the combination of features that were most predictive of whether a patient required postnatal CSF diversion. All features were initially tested for individual predictive value. Features were then sequentially added to the model to best predict outcome until no further improvement in accuracy was achieved. A final set of features was identified that most accurately classified each patient in the cohort as requiring CSF diversion or not. A multidimensional pattern classification method known as support vector machines was used. To determine the accuracy of the fetal MRI-based model in correctly predicting the need for postnatal CSF diversion, leave-one-out cross validation was performed. Accuracy of the predictions was obtained by dividing the sum of the true-positive and true-negative findings by the total number of patients. Sensitivity was calculated as the sum of true-positive findings divided by the sum of the true-positive and false-negative findings. Specificity was calculated as the number of true-negative findings divided by the sum of true-negative and false-positive findings. A receiver operating characteristics analysis was performed, and the area under the curve (AUC) was calculated with 95% CI. A cutoff point was determined for the optimal sensitivity and specificity. In the receiver operating characteristics plot, the cutoff point is the point on the curve closest to (0, 1). Baseline clinical characteristics of patients who did and did not undergo postnatal CSF diversion were compared using the unpaired 2-tailed t test, with the level of significance for a 2-sided comparison set at 5% (P < .05).
Replication Cohort
To assess the performance of the predictive model in an independent cohort of participants different from those in which the model was trained, patients with fetal ventriculomegaly were selected from a preexisting database from Columbia University Medical Center from January 1, 1998, through December 31, 2007. Data were analyzed from January 1, 1998, through December 31, 2009. This cohort is known as a replication (or validation) cohort. The classifications predicted by the model for each patient were then compared with the true classification, that is, postnatal CSF diversion or not, which was known based on the medical record. Characteristics of the discovery and replication cohorts were compared using the unpaired 2-tailed t test or χ2 test.
Results
A total of 74 patients (41 girls [55%] and 33 boys [45%]; mean [SD] gestational age, 27.0 [5.6] months) were included from both cohorts. From the fetal database, 253 patients were identified with fetal ventriculomegaly. Of the patients meeting inclusion and exclusion criteria, 25 patients with fetal ventriculomegaly underwent postnatal CSF diversion. A separate 25 patients were selected among the patients who did not undergo CSF diversion after birth; thus, 50 patients were included in the discovery cohort. The 2 groups in the discovery cohort were well matched in terms of baseline characteristics (Table 1).
Table 1. Discovery Cohort Characteristics.
| Characteristic | Treatment Groupa | |
|---|---|---|
| CSF Diversion (n = 25) |
No CSF Diversion (n = 25) |
|
| Female, No. (%) | 15 (60) | 11 (44) |
| Gestational age, mean (SD), wk | 26 (6) | 26 (5) |
| Lateral ventricle symmetry, No. (%) | 17 (68) | 19 (76) |
| Genetic syndrome, No. (%) | 4 (16) | 4 (16) |
| Germinal matrix hemorrhage, No. (%)b | 5 (20) | 11 (44) |
| Cyst, No. (%) | 1 (4) | 0 |
| Infection, No. (%) | 0 | 1 (4) |
| Follow-up time, median (IQR), mo | NA | 29 (9-46) |
| Time to shunt placement, median (IQR), d | 6 (2-51) | NA |
Abbreviations: CSF, cerebrospinal fluid; IQR, interquartile range; NA, not applicable.
Unless otherwise indicated, differences between groups did not reach statistical significance (P > .10 using χ2 test or Fisher exact test in instances with <5 participants).
P = .08.
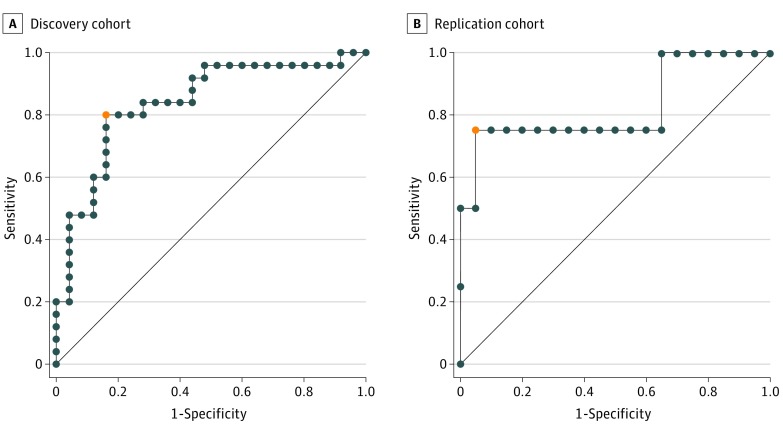
The fetal MRI-based predictive model correctly classified postnatal CSF diversion status with an accuracy of 82% based on leave-one-out cross validation. The sensitivity was 80%, and the specificity was 84%. Among the 25 patients with fetal ventriculomegaly who developed hydrocephalus requiring CSF diversion, 13 (52%) underwent surgical intervention within the first week of life. The replication cohort consisted of 24 patients, of whom 4 (17%) underwent postnatal CSF diversion. The 2 groups were similarly matched in terms of baseline clinical characteristics, although mean (SD) atrial diameter was slightly smaller in the replication cohort (15 [8] mm) vs the discovery cohort (15 [4] mm; P = .03) (Table 2). Among the patients who underwent CSF diversion, the median time to CSF diversion was 13.5 days (interquartile range, 23.5 days); the mean (SD) time, 13.5 (13.9) days. Among patients who did not undergo CSF diversion, the minimum follow-up time was 20.6 months. When applied to the replication cohort, the fetal MRI-based model classified the postnatal CSF diversion status with 91% accuracy (Table 2). A comparison of model performance in the discovery and replication cohorts is shown in Figure 2.
Table 2. Discovery and Replication Cohorts and Performance Metrics.
| Characteristic | Discovery Cohort (n = 50) |
Replication Cohort (n = 24) |
|---|---|---|
| Baseline | ||
| Gestational age, mean (SD), wk | 26 (5) | 28 (5) |
| Female, No. (%) | 30 (60) | 11 (46) |
| Ventricle symmetry, No (%) | 36 (72) | 15 (63) |
| Atrial diameter, mean (SD), mma | 17 (8) | 15 (4) |
| Performance metrics (95% CI) | ||
| Accuracy | 0.82 (0.64-0.96) | 0.91 (0.77-1.07) |
| AUC | 0.84 (0.73-0.95) | 0.83 (0.56-1.04) |
| Sensitivity | 0.80 (0.64-0.96) | 0.75 (NA) |
| Specificity | 0.84 (0.70-0.98) | 0.95 (0.85-1.04) |
Abbreviations: AUC, area under the curve; NA, not applicable.
P = .03 using the χ2 test.
Figure 2. Receiver Operating Characteristic (ROC) Analysis.
Discovery (Children’s Hospital of Philadelphia) and replication (Columbia University Medical Center) cohort results are shown. The orange dot represents the shortest distance from the top left point (0, 1) to the ROC curve and the optimal threshold. The diagonal line is equivalent to chance.
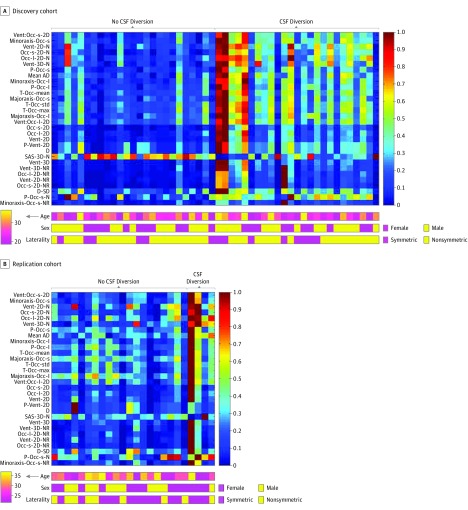
In total, 77 features were selected to serve as input for the machine learning algorithm. The 4 most predictive features identified in the discovery cohort included (1) the ratio of the areas of the smaller occipital horn and lateral ventricles (AUC, 0.86; 95% CI, 0.75-0.96), (2) the minor axis length of the smaller occipital horn of the lateral ventricle (AUC, 0.86; 95% CI, 0.75-0.96), (3) the area of the ventricle normalized to intracranial area (AUC, 0.85; 95% CI, 0.74-0.96), and (4) the area of the smaller occipital horn normalized to intracranial area (AUC, 0.85; 95% CI, 0.74-0.96) (eFigure in the Supplement). The distribution of values for the top 30 imaging features is displayed as a heat map for the discovery and replication cohorts (Figure 3). A list of the top 30 imaging features is provided in eTable 2 in the Supplement.
Figure 3. Heat Maps of the Most Predictive Imaging Features.
Heat maps show the distribution of the top 30 imaging features discriminating between patients who required or did not require cerebrospinal fluid (CSF) diversion among the discovery and replication cohorts. Each vertical bar represents a patient, and each horizontal bar represents an imaging feature. The distributions of age, sex, and lateral ventricle symmetry are plotted below each heat map. AD indictates atrial diameter; D, distance; max, maximum; N, normalized to intracranial size; NR, normalized to resolution; Occ, occipital horn; Occ-l, larger Occ; Occ-s, smaller Occ; P, perimeter; SAS, subarachnoid space; T, thickness; 3D, volume; 2D, area; and Vent, ventricle.
Discussion
Our findings suggest that image analysis and machine learning techniques can be applied to fetal MRI to predict the need for postnatal CSF diversion in patients with fetal ventriculomegaly with high accuracy and generalizability. A significant strength of the computational approach is the ability to simultaneously assess multiple imaging features, most of which are not appreciable by visual inspection alone. Rather than examining a single imaging feature at a time, several types of measurements were incorporated into the model, including linear measures, area, volume, and morphologic features. Integration of patterns of ventricle size, shape, and configuration more fully characterized fetal ventriculomegaly and the development of postnatal hydrocephalus compared with any single imaging feature. When applied to the replication cohort, the model achieved a higher accuracy than in the discovery cohort, thereby supporting its generalizability despite a mildly lower mean atrial diameter among the replication cohort and the use of different MRI scanners from different institutions.
The image-based predictive model has several clinical applications. Counseling in the prenatal setting is challenging in fetal ventriculomegaly, and the model can assist clinicians in providing families with prognostic information related to the anticipated need for CSF diversion in the postnatal period. After birth, patients diagnosed in utero with ventriculomegaly are monitored closely in the neonatal intensive care unit for hydrocephalus, including signs of increased intracranial pressure, such as lethargy, vomiting, apnea, upgaze paresis, bulging fontanelle, increasing head circumference, and splayed cranial sutures. Patients deemed to have high risk by the model would warrant closer and possibly an extended duration of monitoring before leaving the hospital, whereas the inpatient observation period may be shortened in low-risk patients. Although the model did not reach 100% sensitivity or specificity, we envision its use as a guide or supplementary clinical tool in addition to clinical judgment and monitoring of the severity and rate of increase of ventriculomegaly in the postnatal period. Furthermore, as fetal surgery techniques continue to advance, a noninvasive predictive model may aid in patient selection for future clinical trials to assess in utero CSF diversion.
In addition to clinical benefit, the application of image analysis and machine learning techniques to create the model yielded novel imaging features associated with postnatal CSF diversion, which in turn provide further insight into the pathophysiologic features of fetal ventriculomegaly. For example, one of the most predictive features generated by the model was the minor axis of the occipital horn of the lateral ventricle at the level of the atria, which was similar to atrial diameter. Clinically, atrial diameter is currently the most commonly reported linear measure for fetal ventriculomegaly. The occipital horn of the lateral ventricle is the first area to dilate, and without constriction from the striatum, the atrium dilates to a greater extent than other ventricle regions, such as the frontal horns. Thus, our findings corroborate the importance of atrial diameter and similar measures in predicting the development of hydrocephalus and need for CSF diversion. Furthermore, the minor axis of the smaller occipital horn (ranked among the top features) outperformed mean atrial diameter (ranked eighth), suggesting that the computer-generated measure may more accurately summarize the ventricle system compared with a single linear measurement obtained by hand. In addition, FOHR, one of the most commonly reported measures in postnatal ventriculomegaly, ranked poorly in the predictive model compared with area and volume measurements of the ventricular system. This finding is consistent with prior reports that atrial diameter is more closely correlated with ventricle size compared with FOHR in fetal ventriculomegaly. The performance of individual imaging features that were examined in building the model offers feedback regarding the relevance of these features in fetal ventriculomegaly.
Other groups have used measurements obtained from fetal MRI to predict the need for postnatal CSF diversion in fetal ventriculomegaly and associated conditions. Gu et al examined biparietal diameter, head circumference, occipital-frontal diameter, and the width of the lateral and third ventricles. Among 45 patients with fetal ventriculomegaly, of whom 30 (66.7%) required CSF diversion within 3 months, the authors demonstrated that a lateral ventricle width of 15 mm or greater on prenatal imaging studies correctly identified patients requiring surgical intervention after birth with an accuracy of 69% (sensitivity, 67%; specificity, 73%). The inclusion of patients with open neural tube defects accounts for the higher rate of surgical intervention compared with the discovery and replication cohorts in our study. In a similar study, Hankinson et al assessed the correlation between atrial diameter and the need for postnatal ventricle shunting. Among 38 patients referred for neurosurgical consultation for fetal ventriculomegaly, 6 of the 8 patients (75%) with an atrial diameter greater than or equal to 20 mm underwent shunting. The authors concluded that an atrial diameter of 20 mm or greater was associated with the need for postnatal shunting. Other studies in patients with myelomeningocele examined similar features, such as the posterior horn of the lateral ventricle and FOHR, to estimate the need for postnatal shunting. In common among all the aforementioned studies is the search for 1 or a few imaging measures or thresholds on which to build a decision rule. Although these thresholds are convenient for clinical practice, in our experience, they are insufficient to capture the complexity of fetal ventriculomegaly and development of hydrocephalus. Instead, our model uses machine learning to combine multiple variables predictive of CSF diversion, which may explain its higher combination of accuracy, sensitivity, and specificity compared with prior related studies.
Limitations
Despite the strength of the image analysis and machine learning techniques applied, our study has limitations. First, data were collected retrospectively from the medical record for patients in the discovery and replication cohorts. Variables such as gestational age, age in days at CSF diversion, and imaging features, however, were documented and not subject to recall bias. Second, because it is not feasible to follow up all patients with fetal ventriculomegaly in the study indefinitely, some patients labeled as not requiring CSF diversion after birth may have had surgery at outside hospitals subsequent to the time of the most recent follow-up at CHOP. However, more than half of patients with fetal ventriculomegaly underwent CSF diversion in the first week of life, and those labeled as not requiring CSF diversion were followed up for more than 2 years, suggesting that few patients were likely to be misclassified in terms of intervention. Third, despite evidence of external validity, widespread adoption of the model may be limited by access to image analysis tools to extract imaging features on which the model is based. Consequently, we are developing software for use by clinicians to extract relevant imaging features and apply the predictive model in the prenatal setting to yield a single numeric output that describes the percentage of likelihood of postnatal CSF diversion.
Conclusions
The integration of multiple fetal MRI features through image analysis and machine learning methods was used to predict with approximately 80% accuracy the need for postnatal CSF diversion in patients with fetal ventriculomegaly. The predictive model generalized well to an independent cohort. The minor axis of the smaller occipital horn,which is similar to atrial diameter, was among the most predictive features. A prenatal image-based predictive model may change clinical practice in the care of patients with fetal ventriculomegaly by providing prognostic information to patients and families and aiding in clinical management after birth. With a renewing interest in fetal surgery, the predictive model may be used to select patients with the highest likelihood of requiring postnatal CSF diversion as candidates for in utero shunting. Work is currently under way to incorporate the fetal MRI-based predictive model into user-friendly software, and future studies will investigate the integration of the software into clinical workflow and prospectively assess model accuracy.
eTable 1. List of Extracted Imaging Features
eTable 2. Descriptions of Abbreviations for Top 30 Features
eFigure. Box Plot of the Top 4 Most Predictive Imaging Features
References
- 1.Edwards JH. Congenital malformations of the central nervous system in Scotland. Br J Prev Soc Med. 1958;12(3):115-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laurence KM, Carter CO, David PA. Major central nervous system malformations in South Wales, I: incidence, local variations and geographical factors. Br J Prev Soc Med. 1968;22(3):146-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoen L, De Catte L, Demaerel P, et al. . The role of magnetic resonance imaging in the diagnostic work-up of fetal ventriculomegaly. Facts Views Vis Obgyn. 2011;3(3):159-163. [PMC free article] [PubMed] [Google Scholar]
- 4.Goynumer G, Yayla M, Arisoy R, Turkmen O. The criterion value of fetal cerebral lateral ventricular atrium width for diagnosis of ventriculomegaly. Clin Exp Obstet Gynecol. 2014;41(1):67-71. [PubMed] [Google Scholar]
- 5.McKechnie L, Vasudevan C, Levene M. Neonatal outcome of congenital ventriculomegaly. Semin Fetal Neonatal Med. 2012;17(5):301-307. doi: 10.1016/j.siny.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Kelly EN, Allen VM, Seaward G, Windrim R, Ryan G. Mild ventriculomegaly in the fetus, natural history, associated findings and outcome of isolated mild ventriculomegaly: a literature review. Prenat Diagn. 2001;21(8):697-700. [DOI] [PubMed] [Google Scholar]
- 7.Vergani P, Locatelli A, Strobelt N, et al. . Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol. 1998;178(2):218-222. [DOI] [PubMed] [Google Scholar]
- 8.Parilla BV, Endres LK, Dinsmoor MJ, Curran L. In utero progression of mild fetal ventriculomegaly. Int J Gynaecol Obstet. 2006;93(2):106-109. doi: 10.1016/j.ijgo.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 9.Drake JM, Kestle J; Pediatric Hydrocephalus Treatment Evaluation Group . Rationale and methodology of the multicenter Pediatric Cerebrospinal Fluid Shunt Design Trial. Childs Nerv Syst. 1996;12(8):434-447. [DOI] [PubMed] [Google Scholar]
- 10.Drake JM, Kestle JR, Milner R, et al. . Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998;43(2):294-303. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson TC, Vanaman M, Kan P, et al. . Correlation between ventriculomegaly on prenatal magnetic resonance imaging and the need for postnatal ventricular shunt placement. J Neurosurg Pediatr. 2009;3(5):365-370. doi: 10.3171/2009.1.PEDS08328 [DOI] [PubMed] [Google Scholar]
- 12.Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans Med Imaging. 2008;27(4):425-441. doi: 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 14.Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal ventriculomegaly with a single measurement: the width of the lateral ventricular atrium. Radiology. 1988;169(3):711-714. doi: 10.1148/radiology.169.3.3055034 [DOI] [PubMed] [Google Scholar]
- 15.Pisapia JM, Rozycki M, Akbari H, et al. . Correlations of atrial diameter and frontooccipital horn ratio with ventricle size in fetal ventriculomegaly. J Neurosurg Pediatr. 2017;19(3):300-306. doi: 10.3171/2016.9.PEDS16210 [DOI] [PubMed] [Google Scholar]
- 16.Gezer NS, Güleryüz H, Gezer C, et al. . The prognostic role of prenatal MRI volumetric assessment in fetuses with isolated ventriculomegaly. Turk J Pediatr. 2015;57(3):266-271. [PubMed] [Google Scholar]
- 17.Haratz KK, Nardozza LMM, de Oliveira PS, et al. . Morphological evaluation of lateral ventricles of fetuses with ventriculomegaly by three-dimensional ultrasonography and magnetic resonance imaging: correlation with etiology. Arch Gynecol Obstet. 2011;284(2):331-336. doi: 10.1007/s00404-010-1666-z [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Ding X, Sun X, Tsang S-Y, Xue H. SAMSVM: A tool for misalignment filtration of SAM-format sequences with support vector machine. J Bioinform Comput Biol. 2015;13(6):1550025. doi: 10.1142/S0219720015500250 [DOI] [PubMed] [Google Scholar]
- 19.Lee CS, Hong SH, Wang K-C, et al. . Fetal ventriculomegaly: prognosis in cases in which prenatal neurosurgical consultation was sought. J Neurosurg. 2006;105(4)(suppl):265-270. doi: 10.3171/ped.2006.105.4.265 [DOI] [PubMed] [Google Scholar]
- 20.Wang K-C, Lee JY, Kim S-K, Phi JH, Cho B-K. Fetal ventriculomegaly: postnatal management. Childs Nerv Syst. 2011;27(10):1571-1573. doi: 10.1007/s00381-011-1556-0 [DOI] [PubMed] [Google Scholar]
- 21.Saadai P, Runyon T, Farmer DL. Fetal neurosurgery: current state of the art. Future Neurol. 2011;6(2):165-171. doi: 10.2217/fnl.11.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu JL, Johnson A, Kerr M, et al. . Correlating prenatal imaging findings of fetal ventriculomegaly with the need for surgical intervention in the first 3 months after birth. Pediatr Neurosurg. 2017;52(1):20-25. doi: 10.1159/000449003 [DOI] [PubMed] [Google Scholar]
- 23.Khalil A, Caric V, Papageorghiou A, Bhide A, Akolekar R, Thilaganathan B. Prenatal prediction of need for ventriculoperitoneal shunt in open spina bifida. Ultrasound Obstet Gynecol. 2014;43(2):159-164. doi: 10.1002/uog.13202 [DOI] [PubMed] [Google Scholar]
- 24.Phillips BC, Gelsomino M, Pownall AL, et al. . Predictors of the need for cerebrospinal fluid diversion in patients with myelomeningocele. J Neurosurg Pediatr. 2014;14(2):167-172. doi: 10.3171/2014.4.PEDS13470 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of Extracted Imaging Features
eTable 2. Descriptions of Abbreviations for Top 30 Features
eFigure. Box Plot of the Top 4 Most Predictive Imaging Features