Abstract
Objective
There has been growing interest in the role that implicit processing of drug cues can play in motivating drug use behavior. However, the extent to which drug cue processing biases relate to the processing biases exhibited to other types of evocative stimuli is largely unknown. The goal of the present study was to determine how the implicit cognitive processing of smoking cues relates to the processing of affective cues using a novel paradigm.
Methods
Smokers (n = 50) and non-smokers (n = 38) completed a picture-viewing task, in which participants were presented with a series of smoking, pleasant, unpleasant and neutral images while engaging in a distractor task designed to direct controlled resources away from conscious processing of image content. Electroencephalogram recordings were obtained throughout the task for extraction of event-related potentials (ERPs).
Results
Smokers exhibited differential processing of smoking cues across three different ERP indices compared to nonsmokers. Comparable effects were found for pleasant cues on two of these indices. Late cognitive processing of smoking and pleasant cues was associated with nicotine dependence and cigarette use.
Conclusions
Results suggest that cognitive biases may extend across classes of stimuli among smokers. This raises important questions about the fundamental meaning of cognitive biases and suggests the need to consider generalized cognitive biases in theories of drug use behavior and interventions based on cognitive bias modification.
Keywords: smoking, nicotine, cognitive bias, event-related potentials, cue reactivity
Introduction
Over the past several decades, there has been a surge of interest in the role that implicit processes play in substance use disorders, spawning several reviews on the topic (e.g. Field, Munafo, & Franken, 2009) and numerous theoretical models (e.g. Franken, 2003). In line with this theoretical shift, there has been a recent increase in the use of neural measures of drug cue processing (Engelmann et al., 2012). Results from these studies have been generally consistent with the broader cognitive bias literature, confirming differential processing of drug cues among addicted individuals (Littel, Euser, Munafo, & Franken, 2012) and identifying several neurobiological substrates of these responses (Chase, Eickhoff, Laird, & Hogarth, 2011). However, fundamental questions about the nature of cognitive bias remain unanswered. The overwhelming majority of studies rely on tasks that involve contrasting drug cues with an emotionally inert control condition being completed by drug users and sometimes (though not always) a non-user or lighter-user control group. Without other comparison conditions, we cannot know the degree to which processing biases in response to drug cues may reflect a general sensitivity that would extend to other classes of salient stimuli.
To date, very few studies have attempted to tackle these issues. We are aware of only one study that has examined the generality of cognitive processing biases in both smokers and non-smokers using behavioral measures (Leventhal et al., 2008). Studies using neural measures have been somewhat more plentiful, but findings have been mixed (Minnix et al., 2013; Versace et al., 2011). Accordingly, the aim of the present study was to assess electrophysiological responses to both smoking and affective cues among both smokers and non-smokers. To achieve this, we modified an established affective processing task (Carretie, Hinojosa, Martin-Loeches, Mercado, & Tapia, 2004) to include smoking stimuli. The task was designed to direct controlled cognitive processing away from the stimuli of interest to better capture automatic (implicit) processing. Given the novel nature of the task, a data-driven exploratory analytic approach was used to assess multiple event-related potential (ERP) indices of cognitive processing, as well as the association of these indices with clinically relevant measures of smoking behavior (e.g. nicotine dependence, cigarettes per day).
Methods
Participants and Procedures
Smokers (n = 50) and non-smokers (n = 38) were recruited from the local community. Smokers were required to report smoking an average of 15 or more cigarettes per day for at least the past year and not be actively attempting to quit. Non-smokers were required to have used cigarettes or other nicotine products no more than 20 times throughout their lifetime, with no use in the prior year. Smokers were instructed to smoke normally prior to their arrival at the session. Smoking status was confirmed via expired air carbon monoxide sampling (Smoker ≥ 8 ppm; Non-Smoker ≤5 ppm). After breath samples were obtained, smokers were allotted 10 minutes to smoke a cigarette of their own brand in an ad lib fashion in order to standardize the time since their last cigarette. Non-smokers delayed for an equivalent period of time. A brief battery of measures was then completed. For smokers, this included a measure of smoking history, as well as the Wisconsin Inventory of Smoking Dependence Motives-68 (WISDM; Piper et al., 2004). All participants were fitted with a 64-channel electroencephalogram (EEG) cap that included the standard 10–20 montage of sintered Ag/AgCl electrodes, along with additional electrodes to monitor eye movements. Signals were amplified using a Neuroscan Synamps2 system with associated software and digitally sampled at a rate of 250 Hz. Participants completed a computerized cue viewing task (described in detail below) while wearing the cap. Smokers were explicitly told they would have the opportunity to smoke later in the session to enhance the salience of smoking cues.
Cue Task, Data Processing & Analysis
The task consisted of 600 trials, divided evenly among 12 blocks. Each block included 34 presentations of a neutral picture (standard-neutral; 68% of trials) and four presentations each of four categories of rare stimuli (neutral, pleasant, unpleasant, and smoking; 8% of trials each). Each stimulus was presented for 300 ms and stimuli were separated by a 1000 ms inter-trial interval. Presentation occurred in a fixed, pseudorandom order, with brief breaks included between each block. All stimuli were surrounded by a two-inch border that was either red or blue. Participants were instructed to count the number of times the border changed color from one trial to the next within each block. They were awarded $0.25 for each block if their answer was within ±1 of the correct count, with $0.05 less for each number removed from those values down to $0.00. These instructions were designed to motivate participants to direct controlled cognitive resources to a distracting task (i.e. counting frames), minimizing explicit processing of image content. ERP data was processed and cleaned using standard procedures. Amplitudes were quantified through the use of a temporal-spatial principal component analysis (PCA). As only the rare trial categories were of interest for the present analyses, standard trials were discarded at this stage and are not discussed further. Primary analyses entailed examination of PCA-generated factor scores using a mixed model framework, with subject as a random effect. To determine group differences in reactivity, separate models were run contrasting each of the different salient cue categories (i.e. pleasant, unpleasant and smoking) with neutral cues. Moderation was examined through the introduction of additional subject-level covariates into the model. Additional methodological details about the study, including inclusion/exclusion criteria, information about task stimuli and additional details about the recording and processing of ERP data are available in the online supplemental materials.
Results
One non-smoker had excessive artifacts throughout the EEG recording, so was excluded from all further analyses. No other participant had more than 7% of trials rejected. Smokers and non-smokers were well-balanced on demographic characteristics, differing only in education level X2 (1) = 11.7, p < .001, with a larger proportion of non-smokers having some post-secondary education. Thus, education was included as a covariate in group comparison analyses. A total of 12 temporal factors were extracted from the data, with anywhere from 2–4 spatial factors per temporal component. The first seven temporal factors exhibited clear peaks within time-windows of interest, with the first spatial factor for each of these temporal factors accounting for a dominant portion of the variance and exhibiting clear topographical mappings consistent with expected patterns of activation. Thus, it was these values that were targeted for further analysis.
Event-Related Potential Components
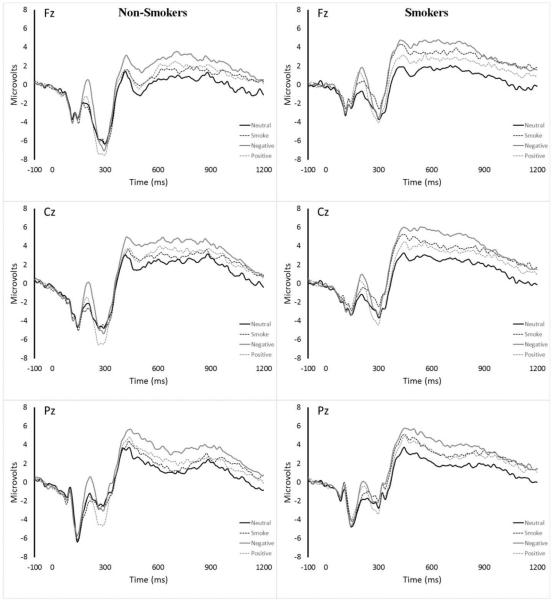
As shown in Table 1, robust differences were observed between both affective cue types and neutral cues across the majority of temporal factors. Smokers exhibited an enhanced P2 response, suppressed N2 response and enhanced P3 response to smoking cues relative to non-smokers. However, only the enhanced P3 response was specific to smoking cues, with a similar pattern of findings for pleasant cues for the P2 and N2 responses. Raw waveforms are presented in Figure 1. Additional details about relevant components are presented in the accompanying online supplement.
Table 1.
Model information
| Unpleasant Cues | Pleasant Cues | Smoking Cues | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cue Type | Smoking Status x | Cue Type | Smoking Status x | Cue Type | Smoking Status x | |||||||
| Cue Type | Cue Type | Cue Type | ||||||||||
|
| ||||||||||||
| F | p | F | p | F | p | F | p | F | p | F | p | |
| P1 (TF5) | 6.72 | .011 | 1.27 | .263 | 0.82 | .369 | 1.45 | .232 | 0.16 | .693 | 3.13 | .081 |
| N1 (TF7) | 1.63 | .205 | 1.74 | .191 | 0.35 | .557 | 0.00 | .948 | 5.33 | .023 | 0.04 | .850 |
| P2 (TF6) | 102.01 | < .001 | 0.31 | .583 | 35.22 | < .001 | 6.43 | .013 | 0.00 | .947 | 8.25 | .005 |
| N2 (TF4) | 0.37 | .542 | 0.49 | .487 | 22.24 | < .001 | 3.45 | .067 | 1.84 | .178 | 5.03 | .028 |
| P3 (TF2) | 57.42 | < .001 | 0.64 | .425 | 12.45 | .001 | 0.31 | .576 | 22.07 | < .001 | 5.82 | .018 |
| LPP (TF3) | 61.79 | < .001 | 1.13 | .292 | 11.47 | .001 | 1.02 | .315 | 4.02 | .048 | 2.30 | .133 |
| Slow Wave (TF1) | 18.75 | < .001 | 0.13 | .721 | 6.71 | .011 | 0.20 | .657 | 8.42 | .005 | 1.25 | .266 |
Note. Education (centered) was included as a covariate in each analysis. Main effects of smoking status were included in the model but are not presented. Significant effects (p < .05) are bolded.
Figure 1.
Midline waveforms for non-smokers and smokers
Associations with Cigarette Use and Dependence
The only consistent associations of ERP amplitude with clinically relevant variables were found for the slow wave. Higher levels of nicotine dependence (WISDM total score) were associated with reduced reactivity to both smoking (r = −.343, p = .015) and pleasant (r = −.410, p = .003) cues relative to neutral cues, whereas the relationship with reactivity to unpleasant cues did not reach significance (p = .144). Similarly, greater self-reported number of cigarettes smoked per day was associated with reduced reactivity to smoking [r = −.403, p = .004] and pleasant [r = −.344, p = .014] cues relative to neutral cues, while the relationship with unpleasant cue reactivity did not reach significance (p = .114).
Discussion
Overall, results of the present study suggest the need for a more nuanced view of cognitive biases, indicating that biased processing of smoking stimuli among smokers may extend to other pleasant stimuli. Our results were consistent with prior research showing that smokers exhibit suppression of the N2 component and enhancement of the P3 component (Littel et al., 2012) when viewing smoking cues. Research reporting the effects of smoking stimuli on the N2 component have generally interpreted similar findings to reflect an internally-generated “prime” or “preparedness” for smoking stimuli among active smokers. That a comparable effect was observed for pleasant cues in the present study suggests that smokers may be primed for positively valenced stimuli more broadly.
Findings for the slow wave in the present study indicated robust main effects of cue type for all three stimulus conditions (unpleasant, pleasant and smoking), but no interaction with smoking status. Despite the absence of a smoking status effect, the response to smoking cues was inversely related to dependence and use among smokers. As both the acute effects of nicotine withdrawal (Evans et al., 2013) and the chronic effects of smoking (Anokhin et al., 2000) have been associated with reductions in P3 amplitude, it is plausible this negative association is due to broader deficits in cognitive control among smokers. The fact that a significant negative association was also observed for slow wave response to pleasant cues, with a similar but non-significant finding for unpleasant cues provides some support for this assertion.
Comparison of results for smoking and affective cues in the present study highlights important limitations of the existing body of research. If smoking stimuli had been examined independently, numerous effects would suggest the presence of a smoking-specific cognitive bias. However, in most cases a similar pattern of effects was observed for other salient stimuli. Clinical research has paid increasing attention to the benefits inherent to comparison with active control conditions, including the ability to produce a more refined understanding of the relevant mechanisms. We argue the time has come for cognitive bias researchers to similarly embrace this notion. Many common indices of cognitive bias are associated with motivational relevance in general and so by limiting our comparisons, we limit what we can conclude from the research. That an addicted individual attaches more value to their drug-of-choice than they do to a rock should not be terribly surprising, nor is it clinically informative. Of far greater interest is that they may attach more value to it than to food, money, loved ones or their own life. The inclusion of active control conditions not only allows for these comparisons to be made, but opens the door for novel paradigms that can provide additional insight into the responsible mechanisms (see Oliver & Drobes, 2015). Although the present study focuses on smoking, the need for a broader context for comparison extends across all health behaviors. Ultimately, it is our hope that the present study serves to motivate implicit process researchers to include multiple comparison stimuli in their work.
Supplementary Material
References
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O'Connor SJ, Kuperman S, Rohrbaugh JW. The P300 brain potential is reduced in smokers. Psychopharmacology. 2000;149(4):409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Carretie L, Hinojosa JA, Martin-Loeches M, Mercado F, Tapia M. Automatic attention to emotional stimuli: neural correlates. Human brain mapping. 2004;22(4):290–299. doi: 10.1002/hbm.20037. doi: 10.1002/hbm.20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biological psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage. 2012;60(1):252–262. doi: 10.1016/j.neuroimage.2011.12.024. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology. 2013;38(12):2525–2531. doi: 10.1038/npp.2013.159. doi: 10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological bulletin. 2009;135(4):589–607. doi: 10.1037/a0015843. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27(4):563–579. doi: 10.1016/S0278-5846(03)00081-2. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Breitmeyer BG, Miller EK, Tapia E, Li Y. Subliminal processing of smoking-related and affective stimuli in tobacco addiction. Experimental and clinical psychopharmacology. 2008;16(4):301–312. doi: 10.1037/a0012640. doi: 10.1037/a0012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafo MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neuroscience and biobehavioral reviews. 2012;36(8):1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, Cinciripini PM. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: a content comparison. International journal of psychophysiology. 2013;89(1):18–25. doi: 10.1016/j.ijpsycho.2013.04.019. doi: 10.1016/j.ijpsycho.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, Drobes DJ. Cognitive manifestations of drinking-smoking associations: Preliminary findings with a cross-primed Stroop task. Drug and alcohol dependence. 2015;147:81–88. doi: 10.1016/j.drugalcdep.2014.12.010. doi: 10.1016/j.drugalcdep.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of consulting and clinical psychology. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Jackson EF, Costa VD, Robinson JD, Lam CY, Cinciripini PM. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. The European journal of neuroscience. 2011;34(12):2054–2063. doi: 10.1111/j.1460-9568.2011.07915.x. doi: 10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.