Abstract
Recent studies in tissue engineering have adopted extracellular matrix (ECM) derived scaffolds as natural and cytocompatible microenvironments for tissue regeneration. The dentin matrix, specifically, has been shown to be associated with a host of soluble and insoluble signaling molecules that can promote odontogenesis. Here, we have developed a novel bioink, blending printable alginate (3% w/v) hydrogels with the soluble and insoluble fractions of the dentin matrix. We have optimized the printing parameters and the concentrations of the individual components of the bioink for print accuracy, cell viability and odontogenic potential. We find that, while viscosity, and hence printability of the bioinks, was greater in the formulations containing higher concentrations of alginate, a higher proportion of insoluble dentin matrix proteins significantly improved cell viability; where a 1:1 ratio of alginate and dentin (1:1 Alg-Dent) was most suitable. We further demonstrate high retention of the soluble dentin molecules within the 1:1 Alg-Dent hydrogel blends, evidencing renewed interactions between these molecules and the dentin matrix post crosslinking. Moreover, at concentrations of 100 μg/ml, these soluble dentin molecules significantly enhanced odontogenic differentiation of stem cells from the apical papilla (SCAP) encapsulated in bioprinted hydrogels. In summary, the proposed novel bioinks have demonstrable cytocompatibility and natural odontogenic capacity, which can be a used to reproducibly fabricate scaffolds with complex three-dimensional microarchitectures for regenerative dentistry in the future.
Keywords: Bioprinting, 3D Printing, Hydrogel Bioink, Pulp Regeneration, Regenerative Dentistry, Alginate, Dentin
1. Introduction
Craniofacial and dental tissues are naturally built with intricate 3D features and present geometrically-controlled mechanical and biological functions in the body.[1] Fabrication of functional 3D tissue constructs for regenerative dentistry and craniofacial reconstruction remains a critical challenge in dentistry and medicine.[2, 3] Despite the great need for regenerative strategies that allow for controllable fabrication and mimicry of these complex craniofacial and dental tissues, biomanufacturing methods that are primarily devoted to addressing the regeneration of various dental structures are very limited.[4, 5] For instance, while the field of regenerative endodontics (root canal treatment) has already evolved to the clinical practice,[6–8] current procedures rely exclusively on evoked bleeding of the dental pulp structure and, the body’s own ability to remodel the blood clot in the root canal. [9–11] Although, stem cell based regenerative methods have shown promise in clinical practice[12], the few biofabrication-inspired methods that have been recently proposed are not yet close to being a clinical reality.[3, 5, 13]
3D bioprinting has emerged as an exciting technology that has tremendous potential to address this critical need in regenerative dentistry.[3] Bioprinting allows for precise positioning of cellularized scaffolds on-demand, either embedded in hydrogels or free from scaffold support.[3, 14] Various 3D printing technologies have been utilized for deposition and patterning of cell-laden bioinks, including microextrusion,[15] inkjet,[16] magnetic levitation,[17, 18] as well as laser and light lithography.[19–22] These systems have addressed numerous challenges in regenerative medicine, and many types of tissues have now been successfully bioprinted, including bone,[23] heart,[24] liver[25] and several other examples.[26, 27] Although a wide range of synthetic materials has been used for 3D bioprinting, these materials cannot represent the complexity of natural extracellular matrices (ECMs) in different tissues. The concept of utilizing decellularized extracellular matrix as improved bioinks for 3D bioprinting was reported by Pati et al, where the authors suggest that the ideal bionks would consists of the natural molecules from the parent tissue.[28] Nevertheless, there currently is a lack of bioinks that are primarily devoted to regeneration of dental tissues,[3] and specifically those with odontogenic potential, which may be used in the rapidly evolving field of regenerative endodontics.
To address this knowledge gap, here we describe the synthesis, characterization and bioprinting of a novel bioink consisting of a hydrogel blend that takes advantage of the biological functionalities of the dental extracellular matrix and the printability of alginate. We refer to this bioink as Alg-Dent. We have previously demonstrated that the dentin matrix comprises, a pool of insoluble (mostly collagen) and insoluble non-collagenous components,[28, 29] including proteoglycans, glycosaminoglycans, chemokines and growth factors, which present high osteogenic/odontogenic differentiation potential.[30–32] For instance, acid soluble non-collagenous components, extracted from the demineralized dentin, have been shown to induce differentiation of dental pulp cells in a dose dependent manner, even in the absence of other growth factors and serum.[28] Meanwhile, the enzyme extracted insoluble components of the dentin matrix, comprises mostly collagen, and provides critical cell adhesion and degradation derived cues to support cell proliferation and function. These natural extracellular matrix-derived constituents add to a long list of recent examples which demonstrate how the body’s own matrix can be used alone and in combination with other hydrogels to engineer biomimetic scaffold materials for stem cells[33] with improved regenerative capacity.[34] While we pay particular attention to optimizing the components of the bioinks for desirable printability and cell viability, we hypothesize that the proposed Alg-Dent retains enough soluble odontogenic cues to enhance the differentiation potential of bioprinted stem cells for regenerative dentistry applications.
2. Materials and methods
2.1 Isolation of dentin matrix
The acid insoluble component of the dentin matrix, was extracted according to the protocol described for bone matrix,[35] with slight modifications. For clarity, we refer to these insoluble proteins simply as ‘insoluble dentin matrix’. Briefly, human third molars, that were stored at −20°C after extraction, were sectioned into slices using a slow speed diamond saw (Struers Accutom-5) after which the pulp and enamel were removed. The dentin fragments were then rinsed in PBS containing 0.1% (w/v) penicillin and streptomycin, flash frozen in liquid nitrogen and ground in a coffee mill. The powdered dentin was demineralized under agitation using 0.5 N HCl (25 ml per gram) for 24 hours. The insoluble matrix was filter-separated under vacuum and rinsed under distilled water. Lipids were extracted using a 1:1 (v/v) mixture of chloroform and methanol for 1 hour, after which the demineralized matrix was rinsed thoroughly with methanol and distilled water. The resulting matrix was then flash frozen, lyophilized for 16 hours and immersed in pepsin (1 mg/ml of 0.01 N HCl) for 72 hours at room temperature. The resultant demineralized and digested insoluble dentin matrix was assessed for collagen content using a hydroxyproline assay[36] (Supplementary Figure S1) and stored at −20°C.
2.2 Hydrogel bioink preparation
The resulting dentin matrix component was then neutralized with 0.1 N NaOH and buffered with 10× PBS, and subsequently combined with 3% (w/v) sodium alginate to make hydrogel blends. Briefly, 3% (w/v) sodium alginate was dissolved in appropriate cell medium and combined with the insoluble dentin matrix at a 2:1, 1:1 or 1:2 ratio by volume to produce alginate-dentin (Alg-Dent) hydrogel blends, containing 2, 1.5 and 1% (w/v) alginate respectively. The hydrogel blends were crosslinked with 0.3 M CaCl2, Pure 3% (w/v) alginate was used as a control.
2.3 Rheological properties
To determine how the addition of the insoluble dentin matrix affected the viscosity of the alginate gels, rheological measurements were carried out for various compositions of uncrosslinked Alg-Dent bioinks using a DHR-1 rheometer (TA Instruments). Parallel-plates with a diameter of 8 mm and the measurement gap set at 500 μm were used to determine viscosity of the hydrogel precursors. The shear rate was swept from 0.1 to 100 s−1, with the recordings taken every 10 points per frequency sweep range The temperature was maintained at 25°C throughout the measurement period. Three independent samples were evaluated.
2.4 Compressive modulus
Mechanical properties of the hybrid bioinks were characterized by performing unconstrained compression tests at room temperature using a mechanical testing machine (MTS Criterion Model 42, MTS Systems Corporation, MN, USA) equipped with a 100 N load cell. The samples (5 × 2 mm discs) were prepared in a PDMS mold and cross-linked in a bath of 0.3 M CaCl2 for 5 minutes and then hydrated with PBS. Compression was performed with a cross-head speed of 1.0 mm min−1 on samples centered on the test platen. The resultant stress was plotted as function of strain for representative specimens of each group, and the compressive modulus of the hydrogel was derived from the slope of the linear portion of the curve ranging from 0 to 10% strain (N = 6).
2.5 Hydrogel printability
Alg-Dent bioinks were 3D printed using a customized extrusion printing system (modified Hyrel3D) with a coaxial nozzle attachment consisting of a 26 and 19 gauge inner and outer needle, respectively. The bioprinting strategy involved streaming the bioink mixture through the inner nozzle while calcium chloride solution was pumped into the outer needle, using a syringe pump (NE300, New Era Pump Systems Inc.), at a flow rate of 45 μl/min for all bioink mixtures. Contact between the bioink and the calcium chloride streams at the tip of the nozzle resulted in covalent crosslinking of the bioink and formation of a fiber, as described previously (Figure 1).[37] Print path designs were created using CAD (FreeCAD) and processed with the accompanying 3D printing software. In order to adapt printing parameters for each bioink composition, a multi-layer, 20 mm × 20 mm grid structure was fabricated using printer feed rate parameters ranging from 0.5 to 0.8 units (n=3). The printer feed rate takes into account the crosssectional area of the print path, the speed of movement of the print head, and the number of pulses of the servo-motor required for each 10 nL of bioink being pushed through the nozzle by the plunger. These numbers are reported as arbitrary units ranging from 0–1. The resulting constructs were qualitatively inspected for accuracy of the print job and the most effective value of the feed rate parameter for each bioink was used for subsequent prints. Print fidelity measurements were performed on 4 layer, 15 mm × 15 mm grids printed using the ideal feed rate parameter for each bioink. Accuracy of printed structures was determined as a percentage of concurrence of the dimensions, measured using ImageJ, of each square on the grid with the design specifications, in 12 locations per sample in the X–Y plane and 3 locations along the Z axis. (N=3)
Figure 1.
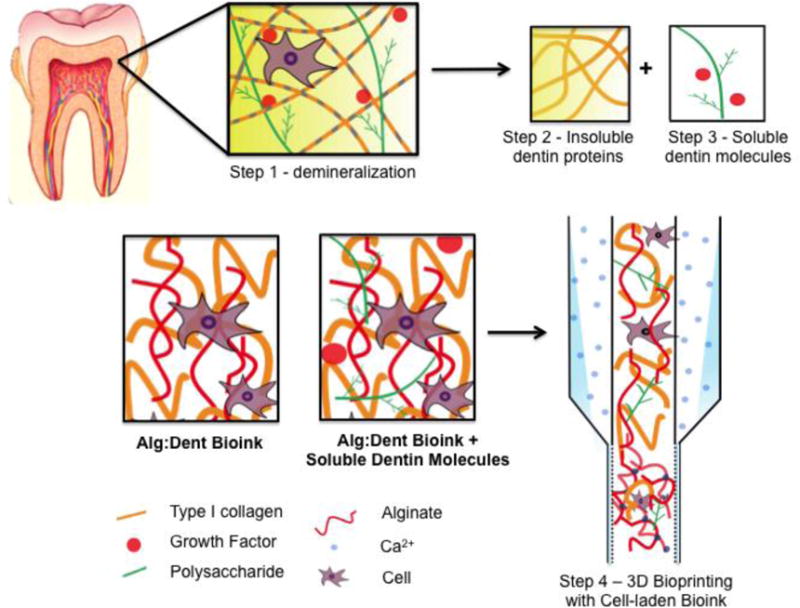
Schematic representation of the steps involved in the isolation of the insoluble dentin proteins and the soluble dentin molecules, formulation of the Alg-Dent hydrogel blends and the extrusion based printing process
2.6 Cell culture
A mouse odontoblast-like cell line (OD21)[38] cultured in DMEM with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin was used for initial cytocompatibility studies. SCAP (passage 6–8) cells, extracted from human third molars according to previously published protocols[39], were grown in alpha MEM containing 0.292 g/l l-glutamine, 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin and used for cell viability and differentiation assays. For gene expression assays in the presence of soluble dentin molecules in 1:1 Alg-Dent hydrogels, SCAP cell culture medium was supplemented with 50 μg/ml ascorbic acid and 5 mM β-glycerophosphate. All cells were maintained at 37°C, 5% CO2 and 95% humidity conditions. OD21 and SCAP were encapsulated in the hybrid gels at a density of 2 × 106 cells ml−1 and 0.8 × 106 cells ml−1, respectively.
2.7 Cell viability
Viability of encapsulated cells was quantified on days 1, 3 and 5 post printing using a membrane permeability based fluorescent live/dead staining kit (Molecular probes) and the number of live/dead cells was counted with ImageJ Image Analysis Software from 4 images per sample, out of fibers printed on a glass slide without a pre-defined 3D architecture. Cell viability was reported as a percentage of live/total cells (N = 3). Cytocompatibility of the print process was further confirmed in thicker 3D constructs (day 5) in a 4-layer 10 mm × 10 mm grid cell-laden scaffold.
2.8 Extraction of soluble dentin matrix molecules
After preparing Alg-Dent hydrogels and determining the optimal bioink composition based on printability and cell survival, we selected the 1:1 Alg-Dent hydrogel blend to test the odontogenic potential of the soluble dentin matrix molecules when mixed into the Alg:Dent hydrogel blends.[29] To isolate the soluble dentin matrix molecules, powdered dentin was treated with phosphoric acid (pH 1–2) containing 10 mM N-ethylmalemide and 5 mM phenylmethylsuphonyl fluoride (Sigma; Gillingham, Dorset, UK) for 7 days at 4°C and the solution was changed each day. The soluble extracts were then dialyzed against distilled water for 7 days before being lyophilized and stored at 20°C. We then utilized 1:1 Alg-Dent hydrogels for our studies on retention and function of soluble dentin matrix molecules. Printed constructs of 1:1 Alg-Dent hybrid hydrogels containing 1, 10 and 100 μg/ml soluble dentin molecules were incubated in 1.2 ml of DMEM at 37 °C for 7 days. Samples were collected in triplicate from each of the wells on days 0, 3 and 7. To determine the concentration of noncollagenous molecules retained in the hydrogel bioinks over time, we determined the concentration of a representative component that is abundant in the dentin matrix, sulfated glycosaminoglycan (GAG). The leachate was measured using a dimethyl methylene blue based colorimetric assay[40] and quantified (N = 4) by measuring absorbance at 525 nm using a microplate spectrophotometer (Epoch Biotek). The amount of GAGs in the leachate was utilized as a representative measure of the total soluble dentin molecules in solution.
2.9 Alkaline phosphate protein expression and qRT-PCR
SCAP cells encapsulated in 1:1 Alg-Dent hybrid hydrogels containing 1, 10 and 100 μg/ml soluble dentin molecules were cultured for 7 days. The cells were then fixed and stained with ALP staining kit (Stemgent). The cells expressing (dark red) ALP were counted in 4 regions of each sample using ImageJ and ALP expression was reported as a percentage of cells expressing ALP versus total cells in the construct (N=3). For the qRT-PCR studies, total RNA was isolated from cells cultured in differentiation medium for 10 days, using a Quick-RNA MicroPrep kit (Zymo Research). cDNA was synthesized using Superscript III First Strand Synthesis System (Invitrogen) in a SimplyAmp thermal cycler (Applied Biosystems). qRT-PCR was performed using a Step One Plus RT-PCR system (Applied Biosystems) and a Power SYBR green PCR Master mix (Invitrogen). The sequences for the primers for runt-related transcription factor (RUNX2), ALP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (housekeeping gene) are listed in Table 1. The qRT-PCR conditions were as follows: denaturation at 95 °C (30s), primer annealing at 52 °C (30s) and elongation at 72 °C (30s). Gene expression was quantified using 2−ΔΔCt method for N=3.
Table 1.
Primer sequences used in qRT-PCR
| Gene | Sequence |
|---|---|
| GAPDH | for: 5′-AACAGCGACACCCACTCCTC |
| rev: 5′-CATACCAGGAAATGAGCTTGACAA | |
| RUNX2 | for: 5′-AGATGATGACACTGCCACCTCTG |
| rev: 5′-GGGATGAAATGCTTGGGAACT | |
| ALP | for: 5′-ACATTCCCACGTCTTCACATTT |
| rev: 5′-AGACATTCTCTCGTTCACCGCC |
2.10 Statistical analysis
Data was analyzed with One/Two-way ANOVA followed by Tukey post-hoc tests (α = 0.05) with Graphpad Prism.
3. Results and discussion
The ideal biomaterial for extrusion bioprinting of cell-laden hydrogels should retain a combination of desirable physical and biological properties. The physical properties (i.e. viscosity and stiffness) should ensure reproducible scaffold fabrication, while the biological properties (i.e. cell-adhesion binding sites, MMP degradation, addition of growth factors) should result in an extracellular microenvironment that allows for adequate cell-survival and differentiation. Our rationale for utilizing a combination of alginate and dentin as the constituents of our hydrogel bioinks was that while alginate is a biocompatible scaffold that can be easily printed using recently developed methods of extrusion and crosslinking,[37] the dentin matrix proteins retain the natural cell-adhesive (RGD) and MMP binding sites that are important for viability, proliferation, and differentiation.[4] These biological moieties, in turn, are absent in pure alginate.[34] Therefore we hypothesized that a combination of alginate and dentin could result in a simple, yet effective, biocompatible hydrogel bioink that has high printability and desirable biological properties for dentin and dental pulp regeneration by supporting an odontogenic and mineralizing phenotype. Figure 1 is a schematic depiction of the stages involved in the isolation of the individual components from the dentin matrix and formulation of the bioinks and printing strategy employed in this study.
It is well documented that the printability of cell-laden hydrogels is directly correlated with the material’s rheological properties, especially prior to crosslinking.[41] This is especially true for extrusion-based 3D bioprinters, where the material is dispensed by the gradual loading of the hydrogel precursor through the downward motion of a piston or plunger. This downward loading is then converted into shear stresses within the hydrogel at the hydrogel-nozzle interface. Therefore, for a biomaterial to have desirable printability, it should also have reasonable pseudoplasticity, or shear-thinning properties.[42] This, in turn, will facilitate extrusion. We therefore first characterized the viscosity of both alginate and the Alg-Dent hydrogel blends at different mixing ratios as a function of shear rate. Figure 2 illustrates the rheological properties of our hydrogel groups, where the 3% alginate hydrogel precursor had the highest viscosity, and all combinations of Alg-Dent precursors had viscosities that were nearly an order of magnitude lower than the alginate alone. Despite the differences in absolute viscosity between the hydrogel precursor groups, the apparent thinning (or decrease in viscosity as a function of shear rate) was nearly the same for pure alginate and the Alg-Dent combinations. Although the viscosity of the bioinks was substantially different, which is known to influence the hydrogel printability, our results suggest that the shear thinning properties of the hydrogel precursors was not detrimentally influenced by the inclusion of dentin matrix proteins.
Figure 2.
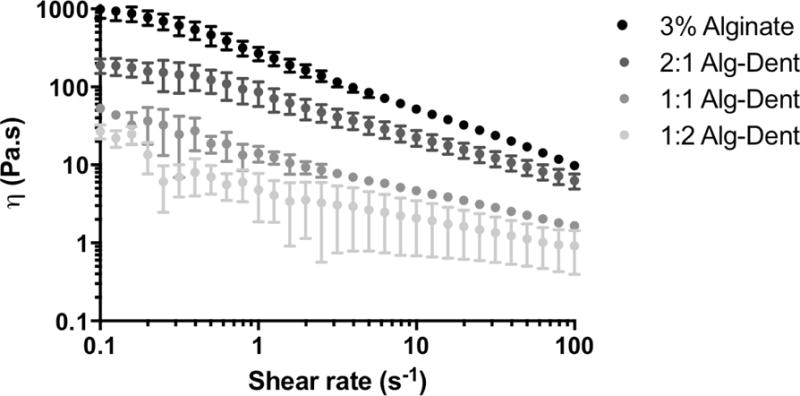
Rheological properties of 3% Alginate and hybrid dentin-derived hydrogels composed of alginate and the insoluble dentin matrix proteins at volume ratios of 2:1, 1:1 and 1:2.
We then characterized the effect of bioink feed rate on the quality of the resulting prints. Effectively, the feed rate parameter in our system accounts for a combination of robot (X and Y) speed and bioink dispense rate. Our qualitative results (Figure 3) suggest that the hydrogels with a higher concentration of alginate required the feed rate parameter to be set to a lower value (0.5 for 3% alginate) than that used for hydrogels containing more dentin (0.6, 0.7 and 0.8, for 2:1, 1:1 and 1:2 Alg-Dent hydrogel, respectively). Our interpretation of these data is that the hydrogels with a higher viscosity, which are consequently dispensed at lower speed, require a slower movement of the print-head/stage for desirable print outcomes. Less viscous bioinks (such as 1:2 Alg-Dent hydrogel precursors), on the other hand, are extruded much more rapidly and should require higher robot speeds for a set dispense rate.
Figure 3.
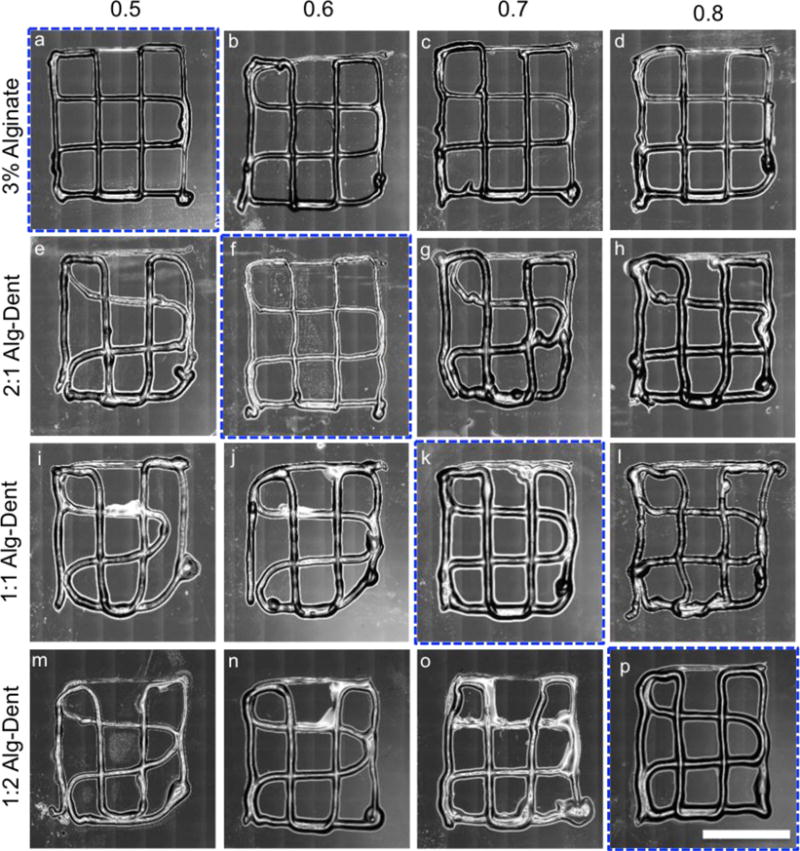
Effect of feed rate (0.5–0.8) on the printability of 3% Alginate, 2:1, 1:1 and 1:2 Alg-Dent hydrogel ratios. The feed rates outlined in blue were selected for subsequent experiments. (10 mm scale bar)
We then 3D printed scaffold constructs with different geometries using the various combinations of Alg-Dent hydrogels and the selected feed rates determined above. Figure 4a–d shows grid-like structures 3D printed using fluorescent particle-laden 3% alginate, 2:1, 1:1 and 1:2 Alg-Dent hydrogel blends. Sharper turns and more regular lines were printed with blends having higher volume ratios of alginate. A comparison of the OHSU symbol 3D printed using 3% alginate (Figure 4e) versus 1:1 Alg-Dent hydrogels (Figure 4f) further confirms this observation. Notably the curved geometry of the OHSU symbol was especially difficult to achieve with the 1:1 Alg-Dent hydrogels, whereas the straight lines of the geometries in Figure 4a–c were more easily attainable. All hydrogel compositions resulted in a comparably high degree of conformation with print specifications both in the X–Y plane (Figure 4g) and in the Z direction (Figure 4h), when printed with the selected feed rate parameters. Supplementary videos 1 and 2 demonstrate printing of fluorescently labeled 3% (w/v) alginate and 1:1 Alg:Dent bioinks in a grid pattern.
Figure 4.
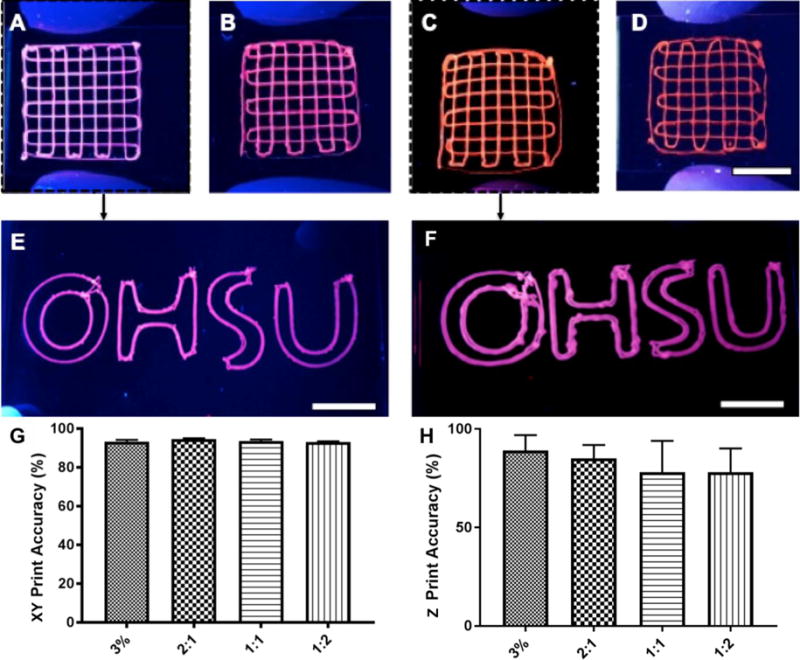
Photographs of (a) 3% Alginate, and (b) 2:1, (c) 1:1 and (d) 1:2 Alg-Dent hydrogels laden with fluorescent microparticles under UV light. The letters OHSU written via bioprinting using (e) 3% Alginate, and the (f) 1:1 Alg-Dent gel. A high degree of print accuracy was shown in relation to design parameters in the (g) X–Y plane for all groups, (h) whereas a trend for increasing printing accuracy in Z direction was found with increasing dentin matrix concentration in the gels. (10 mm scale bars)
We have recently characterized the effect of cell 3D microenvironment on the regenerative capacity of dental pulp-like cells (OD21),[13] and shown that the stiffness of hydrogel scaffolds is an important factor determining the viability, spreading and proliferation of these cells. Since the bioinks we developed here are primarily intended for dental regeneration – more specifically for future studies and applications in regenerative endodontics – we characterized the mechanical properties of each hydrogel bioink composition (Figure 5a–b). Figure 5a shows the stress and strain response of each hydrogel combination, while Figure 5b shows the range of mechanical properties obtained with these materials. Pure alginate had a compressive modulus of approximately 6 kPa, and the addition of 2, 1 and ½ parts of dentin to 1 part of alginate resulted in a significant decrease (p<0.05) in compressive modulus to approximately 1 to 2 kPa. An important limitation of the Alg-Dent bioinks is that the compressive modulus of the scaffolds is not finely tunable,[34] which is an advantage of other bioinks that we have developed previously.[25]
Figure 5.
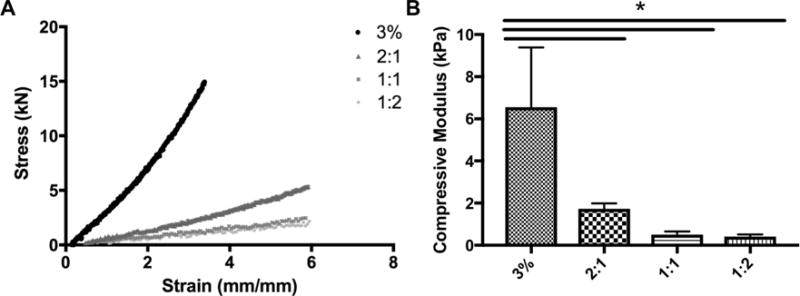
(a) Stress and strain response of hydrogel bioinks measured under compressive loading. (b) 3% Alginate had the highest compressive modulus, and was significantly higher than all hybrid gels.
The viability of dental pulp-like cells (OD21) bioprinted in the grid-like geometries is shown in Figure 6. These are representative live/dead images of cell-laden 3% alginate, 2:1, 1:1, and 1:2 Alg-Dent hydrogels (Figure 6a–d) after 5 days in culture. Our results suggest that the printing process had negligible cytotoxic effects in all bioink conditions, although 1:2 Alg-Dent hydrogels had significantly lower cell viability than pure alginate (p<0.05) and 2:1 Alg-Dent (p<0.01) on day 1 (Figure 6e). OD21 cells encapsulated in alginate hydrogels showed a consistent trend of reduced viability over time, reaching approximately 65% cell survival after 5 days in culture. These results can be partially attributed to the lack of cell specific (RGD) adhesion binding sites in these gels. Cells encapsulated in the hybrid hydrogels, on the other hand, showed an upward trajectory in cell survival over time. Accordingly, 2:1 Alg-Dent hydrogels had significantly higher cell viability than pure alginate hydrogels after 5 days (p<0.001). Both 1:1 and 1:2 Alg-Dent hydrogels resulted in even higher percentages of viability, being consistently above 90% cell survival at least 5 days after the bioprinting process. 1:1 and 1:2 Alg-Dent hydrogel bioinks had significantly higher viability than both 2:1 Alg-Dent and pure alginate hydrogels (p<0.0001).
Figure 6.
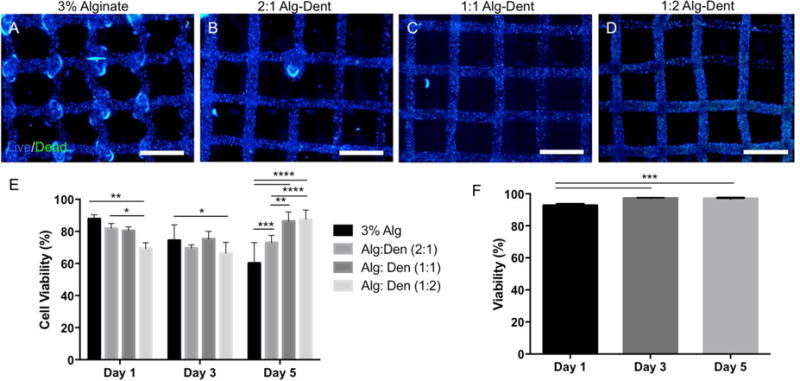
(a–d) Cell viability of 3D bioprinted hydrogel bioinks encapsulated with odontoblast-like cells (OD21s) at day 5 as determined by a live-dead assay kit. (e) Pure alginate hydrogels showed a consistent drop in cell viability over time, whereas hybrid alg-dent gels, especially at ratios of 1:1 and 1:2 had consistently higher cell viability. (f) Viability of human dental stem cells from the apical papilla (SCAPs) printed using a 1:1 alg-dent hybrid hydrogel bioink had viability consistently above 90% for at least 5 days. 1:1 alg-dent hybrid hydrogel bioink was used for all subsequent experiments. (2 mm scale bar)
Since the survival of cell-lines can be higher than that of primary human cells, which tend to be more sensitive to in-vitro cell culture protocols and synthetic biomaterials processing, we also tested the viability of human stem cells from the apical papilla (SCAPs) encapsulated in the developed bioinks. These cells are more representative of the cell types that would be used for clinical applications, especially for dental pulp tissue engineering[6, 7] (Figure 6f). For these latter experiments we chose the 1:1 Alg-Dent hydrogel bioink. The choice for this composition was primarily because these gels showed the best combination of reproducible bioprinting (Figure 4) and high cell viability over time (Figure 6e). SCAP cells cultured for at least 5 days encapsulated in the 1:1 Alg-Dent hydrogels showed consistently high survival rates, with the percentage of viable cells being higher than 90% in all days tested for at least 5 days of cell culture.
The dentin organic matrix can be divided into two main components: (1) proteins of large molecular weight that are insoluble to acidic conditions and represent the highest organic volume ratio in the tissue, which is the case of collagen type I [4, 43]; and (2) smaller non-collagenous proteins, proteoglycans (PGs) with attached GAGs,[44] and growth factors, which can be easily solubilized in acids.[28, 32] We refer to these two pools of proteins as (1) insoluble dentin matrix proteins and (2) soluble dentin molecules. It is believed that the soluble dentin molecules are both “fossilized” in the mineralized dentin matrix, and also bound to the larger collagen molecules via specific amino acid recognition motifs; a process that has been well documented for PGs and GAGs.[45] We have previously demonstrated that the soluble dentin matrix protein content can be extracted and isolated in a controllable manner as a function of the pH of the extracting solution.[28, 29] These proteins when added to OD21 cells plated in 2D have demonstrated a potent and dose-dependent differentiation potential.[28, 29] Accordingly, both cell proliferation and mineral secretion by OD21 cells is obtained after 14 days in culture with as little as 1 μg/mL of soluble dentin molecules; a phenomenon that increased significantly when 10 μg/mL was used. We initially hypothesized that the addition of similar concentrations of soluble dentin molecules to our cell-laden hydrogel bioinks could trigger similar effects. In the 3D hydrogels, however, the retention of the soluble dentin molecules could be achieved by ionic interaction of the negatively charged proteins with the positive charges of the alginate gels, or via amino acid recognition on the dentin-derived collagen surface. To test that we selected the 1:1 Alg-Dent hybrid hydrogels and added varying concentrations of solid dentin molecules to the cell-laden hydrogel precursors prior to bioprinting.
We utilized sulfated GAGs as a representative marker of the pool of soluble molecules extracted from dentin. Figure 7a shows the concentration of GAGs released over time when gels containing 1, 10 and 100 μg/mL initial concentration of soluble dentin molecules were maintained in solution for at least 7 days. Our results show a similar release profile for GAGs when a concentration of either 1 or 10 μg/mL were added to the mixture, which may be indicative of the interactions between the added molecules and the recognition motifs in the insoluble dentin matrix in our hydrogel blends. A much greater release of GAGs occurred when a concentration of 100 μg/mL soluble dentin molecules were added, which could have occurred due to saturation of the protein-retaining sites within the hydrogel network. Still, the amount of GAGs released after 7 days is a minute fraction of the initial quantity added to the hydrogel mixture (Supplementary figure S2). It is noteworthy, however, that in contrast to drug release studies where controlled release of soluble dentin molecules is desired, our goal with these cell-laden bioinks is to achieve efficient retention, since the cells of interest are encapsulated in the hydrogel matrix itself. Differentiation assays were performed on cells encapsulated within 1:1 Alg-Dent hydrogel blends supplemented with 1, 10 and 100 μg/mL of soluble dentin matrix molecules to probe their odontogenic potential. We found an increase in ALP activity (p<0.05) at the protein level in cells encapsulated in bioinks containing 100 μg/mL over cells in control 1:1 Alg-Dent hydrogels, even without addition of odontogenic factors in the medium. Lower concentrations of the soluble factors did not result in any significant increase in ALP protein expression. When bioinks where cultured in odontogenic medium, gene expression studies using qRT-PCR showed an upregulation of both ALP and RUNX2, which are osteo/odontogenic lineage specifying markers, after 10 days of culture. Figure 7c shows 16 (p<0.01) and 26-fold (p<0.0001) increase in ALP gene expression in hydrogels with 10 and 100 μg/mL soluble factors respectively, over those cultured in 1:1 Alg-Dent bioink in the absence of soluble factors. In figure 7d, SCAP cells encapsulated in bioprinted hydrogels had a significantly higher (26-fold) RUNX2 gene expression when cultured within the bioinks containing at least 100 μg/mL (p<0.0001). Importantly, this is an order of magnitude higher than the concentration used for our recent studies that achieved high cell-mineralization with as little as 10 μg/mL. [28] The difference is likely due to the fact that in the previous study, cells were cultured in flat plastic substrates and supplemented with 10 μg/mL of soluble dentin molecules every media change, whereas in the current study, the soluble dentin molecules were only added once, at the start, and not supplemented overtime. This may explain the non-significant difference in ALP and RUNX2 gene expression for groups utilizing 1 μg/mL soluble dentin molecules and the mild increase in ALP activity in the 100 μg/mL group. However, taken together, the results show proof of the enhanced odontogenic potential of our hydrogel blends.
Figure 7.
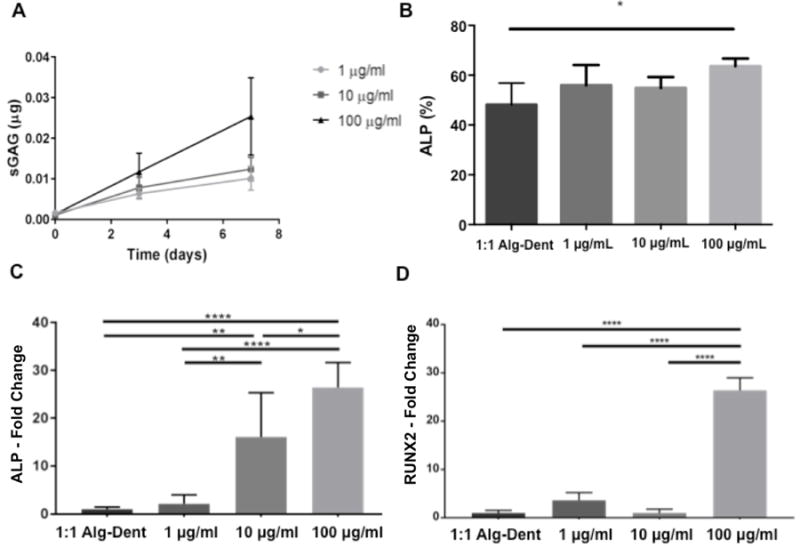
1:1 Alg-Dent hybrid hydrogel bioinks were further embedded with dentin matrix non-collagenous proteins (sulfated glycosaminoglycans shown (sGAG) solubilized from powder dentin after demineralization with phosphoric acid (pH 2). (a) Soluble dentin molecules were retained with greater efficiency up to 10 μg/ml. Gene expression studies differentiation of SCAP cells cultured in 3D bioprinted 1:1 alg-dent bioinks in combination with dentin matrix non-collagenous proteins showed a dose-dependent upregulation in expression of ALP at the (b) protein and (c) gene levels. (d) Expression of RUNX2 was also significantly higher the after 10 days when 100 μg/mL of dentin matrix molecules was mixed in bionk. Bar connects significant differences between groups where * is p<0.05, ** is p<0.01, *** is p<0.001 and **** is p<0.0001.
The precise strategy to 3D bioprint dental pulp tissues for clinical applications remains elusive at the moment. One would have to fabricate the dental pulp tissue construct and implant it as an entire tissue construct in the root canal, which is certainly not consistent with the current standards of clinical practice. Nevertheless, we argue that further developments of biomaterials that facilitate the 3D biofabrication of dental and dental pulp tissues should provide impetus for novel clinical methods of regeneration and dental tissue engineering, which are widely acknowledged as highly necessary in the dental and craniofacial community. Furthermore, the use of these bioinks enables fabrication of scaffolds with precise, reproducible microarchitectures to study the effect of geometry and spatial organization on cell behavior and function in vitro and we believe that studies built on such in vitro models can serve to improve regenerative endodontic techniques in the future.
4. Conclusion
3D bioprinting has emerged as an ideal strategy to fabricate 3D tissue constructs with complex tissue geometries, internal architectures and desirable biological response. Nevertheless 3D printable bioinks for dental regeneration have remained elusive. Here we engineer and characterize novel dentin-derived ECM hybrid cell-laden hydrogel bioinks, comprised of alginate and dentin matrix proteins. The proposed bioinks show high printability and cell survival at various concentrations. Moreover, we demonstrate that these hybrid hydrogels can be embedded with acid-soluble dentin molecules, which enhance odontogenic differentiation of stem cells from the apical papilla. All of these properties of these novel bioinks confer the ability to precisely control the spatial presentation of signaling factors as well as heterotypic cell interactions to effectively engineer the pulp dentin complex. Thus, these biomaterials may represent an important tool for future craniofacial and dental regenerative applications.
Supplementary Material
Acknowledgments
The authors acknowledge funding from the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institutes of Health (NIH) (R01DE026170), and the Medical Research Foundation of Oregon (MRF). We also thank Dr Anibal Diogenes for kindly providing the SCAP cells used in this study.
References
- 1.Gartner LP. Essentials of oral histology and embryology. 3rd. Baltimore, Md.: Jen House Pub. Co; 1999. p. 163. [Google Scholar]
- 2.Bertassoni LE, Coelho PG. Preface: engineering mineralized and load-bearing tissues: progress and challenges. Adv Exp Med Biol. 2015;881:v–vii. [PubMed] [Google Scholar]
- 3.Obregon F, et al. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J Dent Res. 2015;94(9 Suppl):143S–52S. doi: 10.1177/0022034515588885. [DOI] [PubMed] [Google Scholar]
- 4.Bertassoni LE. Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent Mater. 2017 doi: 10.1016/j.dental.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacking SA, Khademhosseini A. Applications of microscale technologies for regenerative dentistry. J Dent Res. 2009;88(5):409–21. doi: 10.1177/0022034509334774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diogenes A, et al. Regenerative endodontics: A way forward. J Am Dent Assoc. 2016;147(5):372–80. doi: 10.1016/j.adaj.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Albuquerque MT, et al. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93(12):1222–31. doi: 10.1177/0022034514549809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon SR, Tomson PL, Berdal A. Regenerative endodontics: regeneration or repair? J Endod. 2014;40(4 Suppl):S70–5. doi: 10.1016/j.joen.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Galler KM. Clinical procedures for revitalization: current knowledge and considerations. Int Endod J. 2016;49(10):926–36. doi: 10.1111/iej.12606. [DOI] [PubMed] [Google Scholar]
- 10.Mao JJ, et al. Regenerative endodontics: barriers and strategies for clinical translation. Dent Clin North Am. 2012;56(3):639–49. doi: 10.1016/j.cden.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrino JA, et al. Challenges in regenerative endodontics: a case series. J Endod. 2010;36(3):536–41. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima M, et al. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 2017;8(1):61. doi: 10.1186/s13287-017-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athirasala A, et al. A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs. Scientific Reports. doi: 10.1038/s41598-017-02532-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 15.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 16.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Tseng H, et al. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 2016;6:30640. doi: 10.1038/srep30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng H, et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci Rep. 2015;5:13987. doi: 10.1038/srep13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch L, et al. Laser assisted cell printing. Curr Pharm Biotechnol. 2013;14(1):91–7. [PubMed] [Google Scholar]
- 20.Guillotin B, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–6. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Wei Z, Harris BT, Zhang LG. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:4185–4188. doi: 10.1109/EMBC.2016.7591649. [DOI] [PubMed] [Google Scholar]
- 22.Bajaj P, et al. Patterned three-dimensional encapsulation of embryonic stem cells using dielectrophoresis and stereolithography. Adv Healthc Mater. 2013;2(3):450–8. doi: 10.1002/adhm.201200318. [DOI] [PubMed] [Google Scholar]
- 23.Kang HW, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YS, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertassoni LE, et al. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6(2):024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Homan KA, et al. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cubo N, et al. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. 2016;9(1):015006. doi: 10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 28.Salehi S, et al. Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization. Dent Mater. 2016;32(3):334–42. doi: 10.1016/j.dental.2015.11.004. [DOI] [PubMed] [Google Scholar]; 28 Pati F, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;2(5):3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferracane JL, Cooper PR, Smith AJ. Dentin matrix component solubilization by solutions of pH relevant to self-etching dental adhesives. J Adhes Dent. 2013;15(5):407–12. doi: 10.3290/j.jad.a29536. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, et al. Angiogenic activity of dentin matrix components. J Endod. 2011;37(1):26–30. doi: 10.1016/j.joen.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 31.Baker SM, et al. TGF-beta/extracellular matrix interactions in dentin matrix: a role in regulating sequestration and protection of bioactivity. Calcif Tissue Int. 2009;85(1):66–74. doi: 10.1007/s00223-009-9248-4. [DOI] [PubMed] [Google Scholar]
- 32.Lesot H, et al. Cell-matrix interactions: influence of noncollagenous proteins from dentin on cultured dental cells. J Embryol Exp Morphol. 1986;96:195–209. [PubMed] [Google Scholar]
- 33.Smith JG, et al. Dental Pulp Cell Behavior in Biomimetic Environments. J Dent Res. 2015;94(11):1552–9. doi: 10.1177/0022034515599767. [DOI] [PubMed] [Google Scholar]
- 34.Annabi N, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26(1):85–123. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawkins MJ, et al. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 2013;9(8):7865–73. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29(3):225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 37.Colosi C, et al. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28(4):677–84. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanks CT, et al. Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res. 1998;37(3–4):233–49. doi: 10.3109/03008209809002442. [DOI] [PubMed] [Google Scholar]
- 39.Ruparel NB, et al. Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod. 2013;39(3):357–63. doi: 10.1016/j.joen.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 40.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 41.He Y, et al. Research on the printability of hydrogels in 3D bioprinting. Sci Rep. 2016;6:29977. doi: 10.1038/srep29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holzl K, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3):032002. doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 43.Bertassoni LE, et al. The dentin organic matrix – limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8(7):2419–33. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertassoni LE, Swain MV. The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater. 2014;38:91–104. doi: 10.1016/j.jmbbm.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Orgel JP, et al. Decorin core protein (decoron) shape complements collagen fibril surface structure and mediates its binding. PLoS One. 2009;4(9):e7028. doi: 10.1371/journal.pone.0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


