Abstract
The increasing occurrence of tetrabromobisphenol A (TBBPA) in the environment is raising questions about its potential environmental health impacts as it has been shown to cause various deleterious effects in humans. The fact that the highest concentrations of TBBPA have been reported in wastewater sludge is concerning as effluent discharge and biosolids land application are likely a route by which TBBPA can be further disbursed to the environment. Our objectives in this study were to evaluate the effect of biochar (BC) and activated carbon (AC) in promoting the biodegradation of TBBPA, and characterize the response of anaerobic sludge microbial communities following amendments. Both carbonaceous amendments were found to promote the reductive debromination of TBBPA. Nearly complete transformation of TBBPA to BPA was observed in the amended reactors ~20 days earlier than in the control reactors. In particular, the transformation of diBBPA to monoBBPA, which appears to be the rate-limiting step, was accelerated in the presence of either amendment. Overall, microbial taxa responding to the amendments, i.e., ‘sensitive responders’, represented a small proportion of the community (i.e., 7.2%), and responded positively. However, although both amendments had a similar effect on TBBPA degradation, the taxonomic profile of the sensitive responders differed greatly from one amendment to the other. BC had a taxonomically broader and slightly more pronounced effect than AC. This work suggests that BC and AC show great potential to promote the biodegradation of TBBPA in anaerobic sludge, and their integration into wastewater treatment processes may be helpful for removing TBBPA and possibly other emerging hydrophobic contaminants.
Keywords: TBBPA, Flame-retardants, Reductive dehalogenation, Biochar, Activated carbon, Wastewater treatment
1. Introduction
Tetrabromobisphenol A (TBBPA) is currently the most widely used brominated flame-retardant in electrical and electronic equipment. The increasing occurrence of TBBPA in environmental samples, including aquatic sediments, agricultural soils, and wastewater sludge (Liu et al., 2016), has raised some concerns regarding wildlife (Chen et al., 2016), and human health as TBBPA has been shown to disrupt thyroid and estrogen regulative functions, cause liver and kidney damage, and increase risks of uterine cancer in mammals (Dunnick et al., 2015). In wastewater sludge, TBBPA concentrations as high as 732 mg kg−1 dry weight have been recorded (Li et al., 2016), and wastewater effluents discharging into receiving waters still contain measurable levels of TBBPA (up to 18.8 ng L−1; Liu et al., 2016). This suggests that conventional wastewater treatment plants do not efficiently remove TBBPA, hence making wastewater effluent discharge and biosolids land application likely routes by which TBBPA enters the environment (Liu et al., 2016). Although microbial reductive debromination of TBBPA to bisphenol A (BPA) has been reported in sewage sludge, the anaerobic natural microbial attenuation of hydrophobic organic contaminants is generally a slow process (Lefevre et al., 2016). Recently, there has been a growing interest in using carbonaceous sorbent amendments, such as biochar (BC) and activated carbon (AC), for the immobilization and removal of inorganic and organic contaminants from wastewater (Mohan et al., 2014; Inyang and Dickenson, 2015; Huggins et al., 2016). Both carbonaceous amendments are generated through the conversion of waste biomass (e.g., wood, manure, crop residues and municipal waste) under elevated temperatures (350–800 °C) and oxygen-limited conditions (Inyang and Dickenson, 2015). The resulting pyrolytic carbon materials, due to their highly porous structures, large surface areas, and high ion-exchange capabilities, display great potential for the sorption of a wide range of contaminants (Inyang and Dickenson, 2015). However, conversely because of their extraordinary sorption capabilities, there is a concern that organic compounds strongly adsorbed onto BC and AC surfaces will no longer be bioavailable for microbial degradation. Although carbonaceous amendments have been shown to slow down the biodegradation of some herbicides (Muter et al., 2014), and even prevent BDE-47 degradation by a degrading strain of Pseudomonas putida (Xin et al., 2014), most studies have reported an increase in the biodegradability of a wide range of organic pollutants in the presence of BC and AC, including, pentachlorophenol (Tong et al., 2014; Yu et al., 2015), azo dyes (Van Der Zee et al., 2003), phenanthrene (Leglize et al., 2008), 2,6-Dichlorophenol (Agarry et al., 2013), and polychlorinated biphenyls (Kjellerup et al., 2014). These findings suggest that combining both carbonaceous amendment materials and microbial degradation could be a promising strategy to improve the removal of a wide range of contaminants from wastewater and sludge. Although BC and AC have been found to enhance the biodegradation of a wide range of contaminants, their effect on TBBPA biodegradation has yet to be tested. Therefore, our objective in this study was to evaluate the effect of BC and AC on TBBPA microbial reductive debromination in wastewater sludge, and characterize the response of microbial communities to BC and AC amendments. To this end, anaerobic sewage sludge bench-scale bioreactors degrading TBBPA, identical to the ones used in a previous study and for which we had provided an in-depth characterization of the microbial communities (Lefevre et al., 2016), were used. Bioreactors were amended with BC and AC, and over the course of 77 days, the concentration of TBBPA and its degradation by-products, as well as microbial community composition were monitored using liquid chromatography with tandem mass spectrometry (LC-MS/MS), and MiSeq Illumina high-throughput sequencing, respectively.
2. Material and methods
2.1. Chemicals and black carbon amendment materials
Tetrabromobisphenol A (TBBPA, 4,4′-Isopropylidenebis (2,6-dibromophenol), 97% purity, CAS 79-94-7) was purchased from Sigma-Aldrich (St Louis, MO, US). High performance liquid chromatography (HPLC) grade acetone was used to prepare the 0.4 g/L TBBPA stock solution used to spike the anaerobic sludge reactors. For LC-MS/MS analysis, TBBPA for calibration standards was purchased from Wellington Laboratories (Guelph, Canada), and Bisphenol A (BPA; CAS 80-05-7) was purchased from AccuStandard (New Haven, CT, US). Mono, di and tri-bromobisphenol A (CAS 6073-11-6, 29426-78-6, and 6386-73-8, respectively) used for LC-MS/MS analysis were provided by Dr. Goran Marsh, (Stockholm University, SE). 13C12-TBBPA (Wellington Laboratories, Guelph, Canada) served as the internal standard for TBBPA and lesser brominated BPAs, and 13C12-BPA (Wellington Laboratories, Guelph, Canada) served as the internal standard for BPA. D8-BPA (Wellington Laboratories, Guelph, Canada), and 13C12-6-hydroxy 2,2′,4,4′-tetrabromodiphenyl ether (Cambridge Isotope Laboratories, Tewksbury, MA, US) were used as recovery standards to assess recoveries of 13C12-BPA and 13C12-TBBPA, respectively. Solvents used for LC-MS/MS analyses were purchased from Honeywell, Burdick & Jackson Laboratories (St. Muskegon, MI, US). Activated carbon (DARCO, 4–12 mesh particle size, granular, CAS 7440-44-0) was purchased from Sigma-Aldrich and used as received. Biochar (100% hardwood, Cowboy brand) was purchased from a local retail hardware store (ACE, Durham, NC), crushed and sieved to 10–20 mesh prior to use.
2.2. Batch reactor operation and sampling
Six bench scale anaerobic sludge reactors were assembled in 2 L glass media bottles previously treated for the removal of organic residues in a muffle furnace at 550 °C for at least 4 h. Under an anaerobic workstation (operated with a gas mixture of 90% H2, 5% N2, and 5% CO2), reactors were filled with 1.5 L of activated sludge (with 4.3 ± 0.6 g/L of suspended solids) collected the same day at the North Durham wastewater treatment facility (Durham, NC, US). While reactors #1 and 2 did not receive any amendments, reactors #3 and 4 were amended with 70 mg/L of biochar (2.5% by dry sludge weight), and reactors #5 and 6 with 70 mg/L of activated carbon (2.5% by dry sludge weight). Reactors #1, 3, and 5 (i.e., one for each treatment) were selected to represent the abiotic reactor and were autoclaved three times at 121 °C for 45 min. To provide co-metabolic conditions, which have previously been shown to stimulate TBBPA degradation (Lefevre et al., 2016), all reactors received 5 mM (final concentration) of sodium acetate trihydrate. Finally, all reactors were spiked with 12.4 mL of 0.4 g/L TBBPA stock solution for a target final concentration of 6 μM before being hermetically sealed with 45 GL polypropylene screw caps equipped with silicone o-rings and blue butyl rubber stoppers (Bellco Glass Inc., Vineland, NJ, US) to ensure anoxic conditions throughout the experiment. Reactors were kept at room temperature (23 ± 0.7 °C) in the dark, and manually shaken once per day. Over the course of the experiment, pH was evaluated using a pH meter (Fig. S1a), and the volume of gas generated in the reactors was measured (Fig. S1b) by inserting a sterile needle attached to a graduated glass Luer lock™ syringe through the stoppers. For each reactor, on days 0, 2, 16, 29, 43, 63, and 77, 2.5 mL of sludge was subsampled in triplicate in an anaerobic workstation, stored at −20 °C until later analyses of TBBPA and debromination products. Additionally, on days 0, 29, 43, and 63, 5 mL of sludge was collected in triplicate, stored at −80 °C, and later thawed for microbial community analysis. Overall, a total of 18% of the initial volume of the reactors was sampled.
2.3. Physical and chemical characterization of the carbon amendment materials
Amendment characterization included pore volume and distribution (Table S1), specific surface area, total and leachable C and N content, moisture, labile and resilient organic matter, and ash content (Table 1). Surface chemical functional groups were also analyzed (Fig. S2). Zeta potential over pH was measured for both amendments (Fig. S3). Finally, concentration of major inorganic elements was performed (Table S2). The details of the methods used for each analysis can be found as supplemental material.
Table 1.
Physicochemical characteristics of activated carbon (AC) and biochar (BC). From left to right: pH, electrical conductivity (EC), total BET surface area (SBET), micro- (Smicro) and mesopore (Smeso) surface area, total pore (Vt), micropore (Vmicro)and mesopore (Vmeso) volumes, moisture, labile matter, resident matter and ash contents (in weight %), C and N mass content, and leachate carbon and nitrogen content.
| Sample | pH | EC μs/cm | SBET (cm2/g) | Smicro (cm3/g) | Smeso (cm3/g) | Vt (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Moisture w% | Labile OM w% | Resilient OM w% | Ash w% | C w% | N w% | leaching C (μg/g) | leaching N (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | 9.2 | 316.8 | 508 | 239 | 269 | 0.417 | 0.096 | 0.321 | 8.7 ±0.2 | 5.6 ±0.7 | 77.6 ±0.5 | 8.1 ±0.2 | 82.8 | 0.61 | 52.5 ±5.8 | ≤LQ (1 μg/g) |
| BC | 9.9 | 454.6 | 4.85 | 2.34 | 2.51 | 0.0096 | 0.0022 | 0.0074 | 6.0 ±0.1 | 12.9 ±2.6 | 78.4 ±2.6 | 2.7 ±0.1 | 77.5 | 1.02 | 1091.7 ±38.2 | 20.2 ±1.6 |
2.4. TBBPA and degradation by-products analyses
For chemical analysis, 200 μL of thawed sludge sample was transferred to a polypropylene microfuge tube. Each extraction batch included triplicate blanks (200 μL LC-MS/MS-grade water) and a matrix spike consisting of 200 μL LC-MS/MS-grade water and 100 μL of each of the TBBPA, BPA, and mixed mono, di and tri-BBPA calibration stock solutions. Blanks and matrix spikes were processed in microfuge tubes alongside samples. Each sample set included a QA/QC check sample prepared like the matrix spike in a LC-MS vial. All samples, blanks, matrix spike, and QA/QC check received 100 μL each of 13C12-TBBPA and 13C12-BPA internal standards and 400 μL of methanol. For extractions, microfuge tubes were homogenized for 10 s using a vortexer, sonicated 5 min in an ultrasonic water bath, and centrifuged for 1 min at 6000 × g. The supernatant was transferred to a LC-MS vial, and the samples were extracted twice more with 400 μL acetonitrile. Extracts were concentrated to 100 μL under N2, then 800 μL LC-MS grade water and 100 μL of recovery standards were added. TBBPA and lesser brominated by-products were analyzed by LC-MS/MS using a Thermo Accela ultra-high pressure LC and Vantage triple quadrupole mass spectrometer. Analytes were separated on a Thermo Hypersil Gold 100 × 2.1 mm column with a methanol-water gradient (80% methanol 0–0.2 min, to 99% methanol at 1.5 min, held at 99% to 3.5 min; 300 μL/min). Analytes were detected by selected ion monitoring (Lefevre et al., 2016).
2.5. Microbial community analyses
2.5.1. Illumina MiSeq sample preparation
Thawed sludge samples (collected in triplicate from each biotic reactor on during 4 time points for a total of 36 individual samples) were centrifuged at 8000 × g for 15 min and total genomic DNA was extracted from the pelleted biomass using the MoBio Power Soil DNA isolation kit (MoBio, Carlsbad,CA,US) withmodificationsas described in Lefevre et al. (2016). The primers 314F and 805R (underlined; Takahashi et al., 2014) modified with the Illumina overhang adapter sequences (314F: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGA-CAGCCTACGGGNBGCASCAG-3′ and 805R: 5′-GTCTCGTGGGCTCGGA-GATGTGTATAAAGAGACAGGACTACNVGGGTATCTAACC-3′) were used in a first PCR to generate amplicons of the V3-V416S rDNA regions, as described in the “16S Metagenomic Sequencing Library Preparation” workflow outlined by Illumina (Illumina, Inc., San Diego, CA, US). Three PCR reactions per sample were performed in order to account for preparation biases. The three reactions were then pooled together and 25 μL were purified using AMPPure XP beads (Beckman Coulter, Brea, CA, US) according to the manufacturer’s protocol. For each sample, 5 μL of cleaned-up PCR products were dual-indexed in a second PCR using the Nextera XT N7XX and N5XX index sequences according to the “16S Metagenomic Sequencing Library Preparation” workflow outlined by Illumina. Products were then purified using AMPPure XP beads and quantified on a Qubit 2.0 fluorometer. Each of the 36 samples was then normalized to the same concentration, pooled together, and run on the paired-end MiSeq platform using the V3 sequencing technology at the Duke IGSP Genome Sequencing and Analysis Core Facility (Durham, NC, US).
2.5.2. Illumina MiSeq data analyses
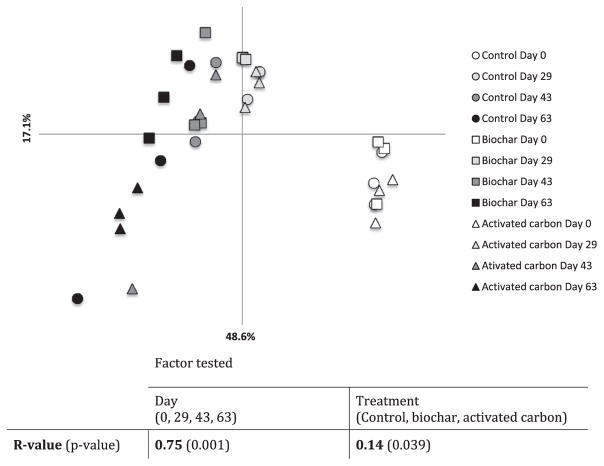
QIIME was used to analyze the generated Illumina MiSeq reads (See workflow Fig. S4). After assembling forward and reverse reads, and filtering the assembled reads based on quality score and length, a total of 9,763,678 sequences were obtained. Sequences were clustered together in operational taxonomic units (OTUs) using Uclust with a similarity cutoff of 97%. Because generation of sequencing errors is a well-documented drawback of next-generation sequencing platforms (Loman et al., 2012), an additional filtering step removing OTUs with a minimum abundance threshold of 0.004% was applied (the justification for the selection of this threshold is presented in Fig. S5). This additional filtering step left 2,051,646 reads that clustered into 1361 OTUs. OTUs taxonomic assignment was performed using the RDP classifier against the Greengenes taxonomy reference released in August 2013, with a confidence threshold of 50%. Microbial community analyses were performed using a Bray-Curtis similarity matrix-based principal coordinate analysis (PCoA). An analysis of similarity (ANOSIM) was performed in order to test for the effect of time (Day 0 vs. Day 29 vs Day 43 vs Day 63), and treatment (biochar vs. activated carbon vs control), on microbial community composition.
3. Results and discussion
3.1. TBBPA degradation
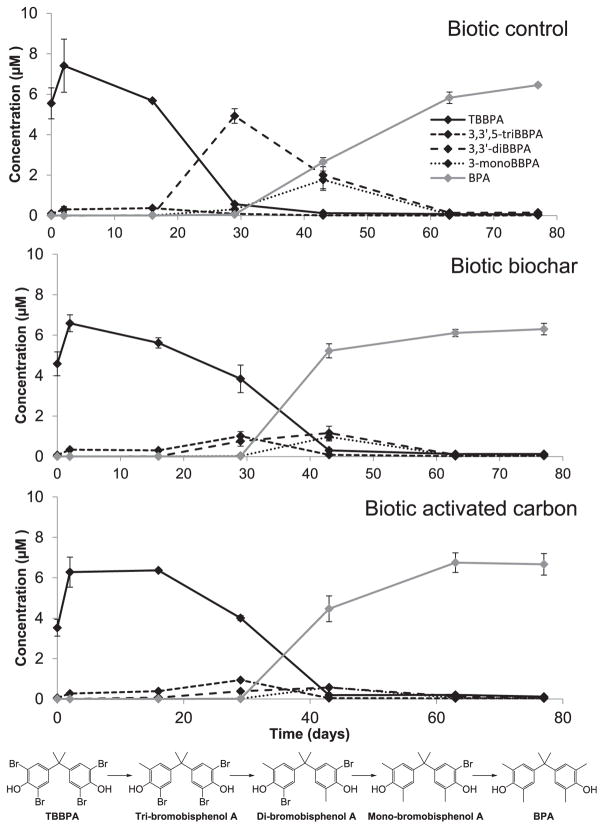
Concentrations of TBBPA and its congeners were monitored in all reactors over 77 days using LC-MS/MS. TBBPA was completely transformed to a stoichiometrically equivalent amount of BPA by Day 63 in the biotic reactors (Fig. 1). In contrast, no TBBPA degradation and no gas production were observed in the abiotic reactors (Fig. S1b and S6), indicating that TBBPA degradation was microbially-mediated. The pH of the abiotic control was consistently lower than that of the abiotic amended reactors (i.e., 6.0 and 6.3 when averaged over time, respectively; Fig. S1a), suggesting that both carbonaceous amendments have a liming effect, as previously observed in soils (Wang et al., 2017). Indeed the calculated pH of stability for BC and AC was 9.9 and 9.2 respectively (Table 1). The biotic reactors, however, whether they had received carbonaceous amendments or not, had a similar and slightly higher pH (i.e., 6.6) than the abiotic reactors (Fig. S1a), suggesting that despite the liming effect of BC and AC, the pH was ultimately controlled by microbial activity. During the course of the experiment, 3,3′,5-tribromobisphenol A (3,3′,5-triBBPA), 3,3′-dibromobisphenol A (3,3′-diBBPA), and 3-monobromobisphenol A (3-monoBBPA) were the only identified intermediary products of TBBPA transformation to BPA, and observed changes in parent and metabolites concentration were mass balanced, suggesting that reductive debromination was the only degradation pathway taking place (Fig. 1). Only one diBBPA isomer (3,3′-diBBPA) was detected, supporting the premise that biological reductive dehalogenation of TBBPA is position-dependent, as previously proposed in Arbeli and Ronen (2003). Indeed, if debromination of the three bromines on 3,3′,5-triBBPA was equally favorable, we would expect the proportion of 3,3′-diBBPA to be 50% of the two dibrominated BPA isomers. No further BPA degradation occurred in the biotic reactors. This finding is not surprising as BPA accumulation has been commonly observed under anoxic conditions (Arbeli and Ronen, 2003; Chang et al., 2012; Liu et al., 2013; Lefevre et al., 2016), and is attributed to the presence of a methylene linker joining the two BPA molecule aromatic rings, preventing their cleavage by anaerobic microorganisms (Chang et al., 2012). Yet, anaerobic biotransformation of TBBPA to BPA ultimately brings the TBBPA degradation process closer to complete mineralization, since subsequent mineralization of BPA can rapidly occur if aerobic conditions are provided, thus making the contaminant well-suited to a two-stage treatment process (Liu et al., 2013).
Fig. 1.
TBBPA degradation and formation of BPA in the biotic reactors. Concentration of TBBPA, BPA, and transformation by-products (i.e., 3,3′,5-triBBPA, 3,3′-diBBPA, 3-monoBBPA) in the control reactor, and reactors amended with biochar and activated carbon over time. Error bars represent standard deviation from the mean. TBBPA reductive debromination pathway is shown below the graphs. No loss of TBBPA was observed in the abiotic controls (See Fig. S4).
In the present study, initial transformation of TBBPA occurred significantly (p < 0.05) faster in the control than in the reactors amended with BC and AC (Fig. 1). Nearly all TBBPA (92.2 ± 1.4%) had been depleted in the control by Day 29 while only 41.9 ± 7.9 and 35.6 ± 7.1% of the spiked TBBPA had been transformed in the BC and AC-amended reactors, respectively. Nevertheless, the complete debromination of TBBPA was significantly (p < 0.05) more rapid (i.e., achieved 20 days earlier) in the amended reactors. By Day 43, 79.4 ± 3.8 and 71.4 ± 9.3% of initial TBBPA had been transformed to BPA in the BC and AC-amended reactors, respectively, whereas only 36.5 ± 7.2% of TBBPA had been completely debrominated in the control reactor. These data suggest that BC and AC amendments modified the kinetics of TBBPA degradation and BPA formation in the anaerobic sludge reactors, and overall, accelerated the complete transformation of TBBPA to BPA. However, there was no significant difference in TBBPA debromination between the AC and BC-amended reactors at any time point, indicating that both amendments had the same effect on TBBPA debromination.
Clear differences in the distribution of the three intermediary products of TBBPA debromination were observed between the amended reactors and the control. In both amended reactors, 3,3′,5-triBBPA, 3,3′-diBBPA, and 3-monoBBPA were never detected at concentrations greater than 1 μM. In contrast, in the control reactor, 3,3′-diBBPA accumulated at Day 29 with a concentration reaching 4.9 ± 0.4 μM, and 3-monoBBPA concentration was nearly twice that measured in the amended reactors (i.e., 1.8 ± 0.4) at Day 43. Our previous study (Lefevre et al., 2016), which used the same reactor settings without the addition of carbonaceous materials, showed a similar distribution of TBBPA by-products to that presently observed in the control reactor. A transient accumulation of the dihalogenated congener during the microbial reductive debromination of TBBPA, or even TCBPA (tetrachlorobisphenol A), has been previously reported in the literature (Arbeli and Ronen, 2003; Chang et al., 2012; Sun et al., 2014). These observations suggest that the transformation of 3,3′-diBBPA to 3-monoBBPA may be the limiting step of TBBPA reductive debromination. A possible explanation for this finding is that the presence of an unequal number of halogens on each phenolic ring creates a stronger positive dipole moment of the tri- and monoBBPA molecules, which makes them more susceptible to undergo nucleophilic attack compared to diBBPA (Arbeli and Ronen, 2003). Thus, mono- and triBBPA are in theory more easily debrominated compared to diBBPA. The fact that no diBBPA accumulated in the amended reactors (Fig. 1) suggests that the presence of BC and AC facilitated the transformation of diBBPA to monoBBPA. Others have also observed this trend in polyhalogenated compounds. For instance, in a study of microbial degradation of Aroclor 1260, Kjellerup et al. (2014) noted that the addition of granulated AC resulted in the formation of more extensively de-chlorinated by-products compared to the amendment-free control. Several other studies have demonstrated that TBBPA microbial debromination could be facilitated by the presence of extracellular electron mediators such as vitamin B12, riboflavin, 2-hydroxy-1,4-naphthoquinone, humic acid (Chang et al., 2012; Wang et al., 2013), or solid amendments containing a relatively high level of organic carbon such as gray chalk, sediments and soil particles (Arbeli and Ronen, 2003). Similarly, BC was previously shown to function as an electron shuttle, promoting electron transfers from Geobacter sulfurreducens cells to adsorbed pentachlorophenol (PCP; Yu et al., 2015), and overall stimulating soil microbial community extracellular electron transfer (Tong et al., 2014), hence accelerating PCP dechlorination. Through the same electron-mediating properties, AC has also been shown to accelerate azo dyes microbial degradation (Van Der Zee et al., 2003). Surface redox-active moieties (RAMs), which can function both as electron donors and acceptors, as well as the presence of conductive polycondensed aromatic structures, are likely responsible for BC and AC electron-mediating properties (Van Der Zee et al., 2003). Our analysis or the functional groups displayed on the surface of AC and BC indicated the presence of a C=O stretching that might correspond to quinone redox-active moieties (Fig. S2) as suggested by Yu et al. (2015). Therefore, it is possible that a similar process takes place with TBBPA whereby the amendments accelerate the microbial extracellular respiration of adsorbed TBBPA and dehalogenation products, and promote the transformation of diBBPA to monoBBPA.
3.2. Microbial community response
Sequencing of DNA extracted from the sludge samples generated a total of 2,051,646 high quality reads that clustered into 1361 OTUs. Chao1 individual-based rarefaction curves (Fig. S7) and Good’s coverage values (>99%; Table S3) indicated that the microbial communities were well sampled, allowing for diversity comparisons between samples. Shannon diversity indexes varied from 8.4 to 8.9 (Table S3), which is in the range of what previous studies on similar systems have found (Lefevre et al., 2016). The most represented phyla were Proteobacteria, Bacteroidetes, Actinobacteria, Chloroflexi, Verrucomicrobia, Planctomycetes, and Firmicutes, which accounted for 26.5, 16.3, 12.0, 8.4, 6.8, 5.3 and 5.2% of the total community (averaged across samples; Fig. S8). Although the PCR primers used in this study (i.e., 314F and 805R; Takahashi et al., 2014) were designed to target both Bacteria and Archaea, only two archaeal OTUs, whose combined relative abundance remained below 0.1% throughout the experiment, were detected. This is much lower than previously reported on similar anaerobic sludge systems (i.e., ~6%; Guo et al., 2015), and is likely a result of the preferential amplification of bacterial over archaeal taxa by the primers used in this study (Takahashi et al., 2014). Over the course of the experiment, whether they had received carbonaceous amendments or not, the reactors shared between 94.0 and 99.5% of their OTUs (Fig. S9). Although the kinetics of TBBPA transformation to BPA differed between amended and control reactors (Fig. 1), in terms of overall microbial community composition, no marked differences could be seen at the phylum level (Fig. S10). All reactors displayed similar microbial population dynamics, characterized by a slight increase of Bacteroidetes and Actinobacteria, and a slight decrease of Verrucomicrobia relative abundances over time (Fig. S10). Bray-Curtis similarity matrix-based PCoA analysis (Fig. 2) revealed that samples clearly clustered by sampling dates, following a nearly identical pattern to that observed in our previous study on similar TBBPA-spiked anaerobic reactors (Lefevre et al., 2016). When an analysis of similarity (ANOSIM) testing for the factor time was performed, an R-value of 0.75 (p < 0.001) was calculated, indicating that time was the main factoring controlling microbial community composition dynamics, which is to be expected during the start-up phase of anaerobic reactors as the composition of microbial communities shifts during the settlement of anoxic conditions (Goux et al., 2016). More surprisingly, the R-value calculated when the factor treatment was tested also revealed an effect of the carbonaceous amendments on microbial community composition at the OTU-level. Though much weaker than that of time (i.e., R-value = 0.13; p = 0.039; Fig. 2), carbonaceous amendments also exerted an effect on microbial community composition dynamics.
Fig. 2.
Bray-Curtis matrix-based Principal Coordinate Analysis (PCoA) and Analysis of similarity (ANOSIM) results. The percentage of variation explained by the two axes is indicated on the graph. The table presents the results of the ANOSIM, with the null hypothesis (H0) stating that the community composition does not differ between days or treatments. H0 is rejected if p ≥ 0.05. The closer the R-value is to 1, the more difference between the groups tested in terms of community composition.
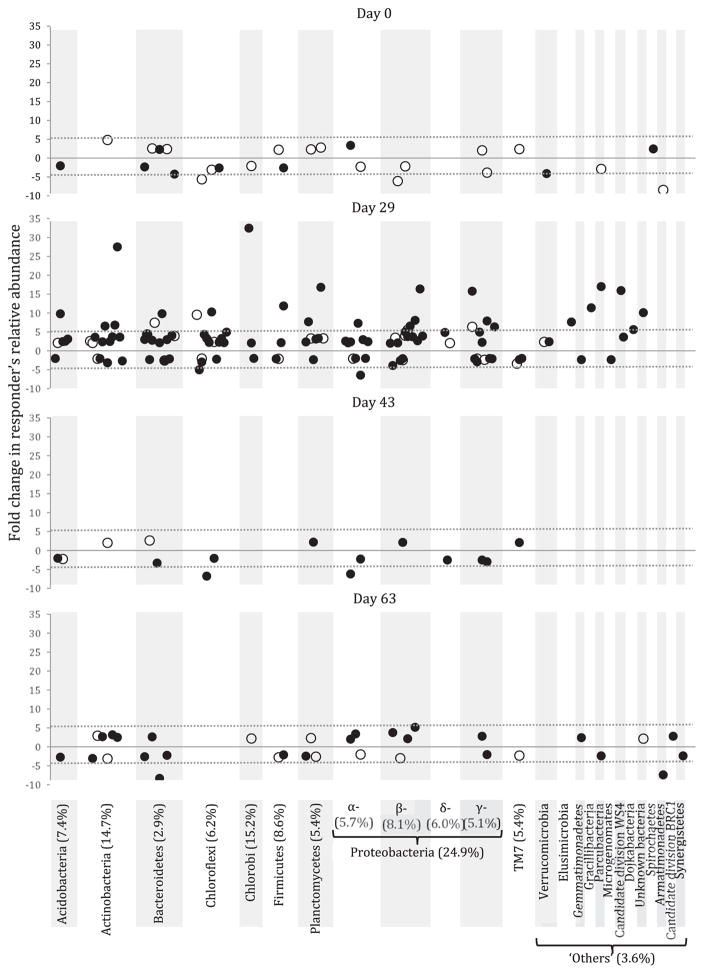
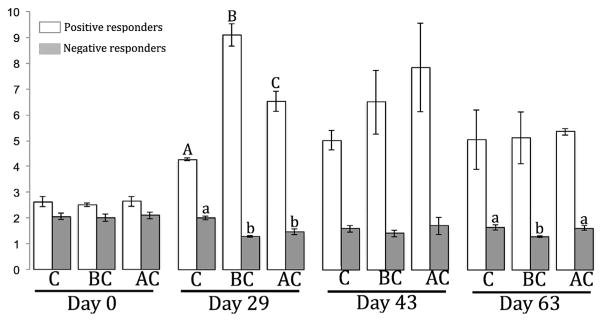
In order to further explore this effect, ‘sensitive responders’ to carbonaceous amendments were identified for each post amendment dates. Following the definition of Dai et al. (2016), we qualified a taxon (i.e., OTU) as ‘sensitive responder’ if its relative abundance significantly increased (positive responder) or decreased (negative responder) at least by a factor of two in the BC- or AC-amended reactors relative to the control. According to this definition, 153 out of the 1361 OTUs detected in this study (i.e., 11.2%) were identified as sensitive responders (Fig. 3), which is in the range of what Dai et al. (2016) found in their study on the effect of BC on soil microbial communities. Although they were distributed among 22 phyla, two thirds of the sensitive responders affiliated to 4 phyla (i.e., Actinobacteria, Bacteroidetes, Chloroflexi, Proteobacteria; Figs. 3 and S11). Overall, sensitive responders represented 7.2% of the total community relative abundance, indicating that in terms of composition dynamics, only a few taxa were affected by BC and AC, while the major part of the community was not affected by the carbonaceous amendments. With the exception of two OTUs, identified responders always displayed the same type of response (i.e., positive or negative) to an amendment. While 95 OTUs were found to be consistently positive and represented overall 5.6% in terms of relative abundance, the 56 OTUs identified as negative responders only represented 1.3% of the community, suggesting that carbonaceous amendments had more of a positive effect on the growth of the responding taxa. Despite the fact that all responding OTUs were detected in both BC and AC-amended reactors, only 8 were found to respond similarly to both amendments (Table S4). Among the other responders, 98 were found to respond only to BC, and 47 only to AC, suggesting that each amendment affected a different fraction of the microbial community, but also that BC had a broader effect compared to AC. As clearly shown in Fig. 3, the large majority of positive responders were detected in the BC-amended reactor at Day 29. This sampling time was also the only one for which we detected responders whose relative abundance increased more than 5 times compared to that of the control (Fig. 3, Table S5), and these ‘highly sensitive responders’ were mostly found in the BC-amended reactor. The relative abundance of the positive responders in the BC reactor was twice that of the control at day 29 (Fig. 4). Positive responders to AC also saw their proportion significantly increase (p < 0.05) compared to the control, but to a lesser extent (Fig. 4), suggesting that BC had a more important effect than AC. This observed effect was the most pronounced at Day 29. While at Day 43, the effect of both amendments had already diminished, at Day 63 it became negligible, altogether suggesting that the response to the community to the black carbon was temporary. The non-carbonized fraction of both BC and AC have been shown to provide nutrients and labile carbon readily utilizable by microorganisms, hence increasing their biomass and activity (Tong et al., 2014; Inyang and Dickenson, 2015). Carbonaceous amendments’ macroporous structures have also been shown to be able to support the growth of microbial biofilms, as well as provide a protecting habitat against grazers (Leglize et al., 2008; Frankel et al., 2016). Thus, around Days 29 and 43, the observed temporary increase in positive responders’ population could result from the fact that these taxa might have colonized the porous structure of BC and AC, hence have had a facilitated access to the labile carbon and nutrients leaching from the amendment surfaces. In the present study, the more important stimulating effect of BC over AC could be attributed to the fact that, although both amendments displayed similar C and N contents, labile organic matter of BC was twice that of AC, and leachable C and N were ~20 times more important in BC than in AC (Table 1). In addition, AC typically possesses a higher proportion of micropores, unaccessible to most microbial cells, as compared to BC (Inyang and Dickenson, 2015; Huggins et al., 2016), and as measured in the present study (i.e., 70% of micropores are in the 2–10 nm size range for AC, while 74% are in the 20–80 nm size range for BC, Table S1). Finally, differences in amendments’ surface functional groups (Fig S2) might also have contributed to the differential growth of the microbial communities between AC and BC-amended reactors, as BC might display more redox-active moieties than AC, hence promoting bacterial growth (Yu et al., 2015). Although microbial toxicological studies on black carbon amendments are quite limited (Jonker et al., 2009), the significant decrease of the negative responders at Day 29 suggests that carbonaceous amendments might exert a slight toxicity towards some microbial taxa, as inorganic mineral constituents potentially toxic to microbial cells can also be released from their surface (Inyang and Dickenson, 2015). The elemental composition analysis of the inorganic constituents of BC and AC (Table S2) revealed that AC contained higher concentrations of Al, Cr, Mn, Fe, Co, Ni, Cu, and As than did BC, some of which can be toxic to microbial species. However, further analyses would be required in order to assess the solubility, hence toxicity of these elements towards microbial taxa.
Fig. 3.
Fold change in responding OTUs’ relative abundance between control and amended reactors for each post-amendment sampling date. Responders are defined as OTUs whose relative abundance significantly (p < 0.05) increases or decreases more than twice in the amended reactors relative to the control. Each black and white circle represents a responding OTU detected in the biochar - and activated carbon-amended reactors, respectively. Dash lines on each graph are visual references for a fold change of 5. Phylum-level taxonomic affiliation is indicated at the bottom, and the number of responding OTU in each taxonomic group is indicated between parentheses.
Fig. 4.
Relative abundance of positive and negative responders in each reactor for each sampling day. Error bars represent the standard deviation from the mean. For each sampling day and category of responders, significant differences between treatments (t-test; p < 0.05), when found, are indicated by a different letter (upper case for the positive responders, and lower case for the negative responders). C: control; BC: biochar; AC: activated carbon.
Based on the present characterization of the microbial community response to carbonaceous amendments, two hypotheses can be proposed to link the effect of BC and AC to TBBPA microbial degradation. First, it could be hypothesized that carbonaceous amendments directly affected the growth of the TBBPA-degrading taxa (i.e., the positive responders) by providing them with a source of nutrients and readily usable carbon for their growth, as suggested by Agarry et al. (2013). In addition, BC and AC could also have been used as a physical support for the formation of a TBBPA-degrading microbial biofilm. Leglize et al. (2008) showed that, by preferentially supporting the growth of phenanthrene-degrading bacterial strains that had the ability to form biofilms, AC addition promoted the degradation of phenanthrene. Frankel et al. (2016) also showed that BC-associated biofilms considerably increased naphtenic acids biodegradation in the treatment of oil sand process water. Among the ‘highly sensitive positive responders’ identified in this study, the most affected taxa affiliated to the genus Rhodoferax, from which species able to degrade a wide range of chlorinated compounds have been isolated (Ehrig et al., 1997). Additionally, many responders affiliated to the β-proteobacteria order, Burkholderiales (Table S5, Fig. S11), for which an extensive genomic analysis has been performed, revealing the presence of an unexpectedly high number of genes involved in aromatics biodegradation (Perez-Pantoja et al., 2012). Although the taxonomic composition of the positive responders greatly differed from one amendment to the other, many taxonomically distant bacterial strains are known to possess the ability to degrade TBBPA (An et al., 2011; Wang et al., 2013; Peng et al., 2014). In fact, although the reactors used in our previous study harbored taxonomically different microbial communities compared to that of the reactors used in this study (See Fig. S6 and S8, and Fig. S5 and S6 from Lefevre et al., 2016), TBBPA degradation kinetics were very similar between both studies (see Fig. 1 and 1 from Lefevre et al., 2016). Therefore although taxonomically different, both populations positively affected by BC and AC could have had similar TBBPA degrading capabilities, supporting that TBBPA degradation can be equally carried out by communities presenting distinct taxonomic profiles. However, based solely on the taxonomic community composition presented in this study, and the absence of evidence for the formation of AC- and BC-associated biofilms, it is difficult to assert that the TBBPA degradation was a biofilm-mediated process. Alternatively, it is also possible that the positive responders identified in this study were not directly responsible for the observed TBBPA degradation. Rather, BC and AC might have stimulated the overall activity of TBBPA degrading taxa, without necessarily increasing their biomass. TBBPA anaerobic respiration could also have been promoted through the electron-mediating properties of AC and BC as suggested by others (Van Der Zee et al., 2003; Tong et al., 2014; Yu et al., 2015). These alternative mechanisms could not have been detected by the DNA-based sequencing employed in this study. Additional approaches, such as RNA-based molecular, enzymatic, or gene expression assays should be conducted to have a better understanding of the mechanisms behind AC and BC-promoted TBBPA degradation. Nevertheless, the present study shows that charred carbonaceous amendment could accelerate the debromination of TBBPA in wastewater anaerobic sludge, bringing the TBBPA degradation process a step closer to complete mineralization. As BPA has been shown to mineralize under oxic condition, future work could also be conducted to evaluate the effect of BC and AC on the microbial degradation of BPA under aerobic conditions.
4. Conclusions
For the first time, biochar and activated carbon were showed to accelerate the reductive debromination of TBBPA to BPA, a crucial step in complete decomposition of TBBPA, as subsequent mineralization of BPA can rapidly occur if aerobic conditions are provided. Particularly, the transformation of diBBPA to monoBBPA, which appears to be the most limiting step, was stimulated in the presence of either carbonaceous amendment. Only a small portion of the microbial community responded to BC and AC amendments, suggesting that the addition of carbonaceous material for waste-water treatment would likely not alter the bulk of the microbial community, responsible for other essential microbially-mediated wastewater treatment processes. Although TBBPA degradation kinetics were similar in both BC and AC-amended reactors, each amendment affected distinct taxa within the microbial community. Overall, BC displayed a broader and more pronounced effect than AC on the microbial community. These differences are likely reflective of the physicochemical differences existing between the two amendments. Although more work needs to be conducted in order to unravel the mechanisms underlying BC- and AC-promoted TBBPA degradation, it is possible that carbonaceous amendment may have promoted the extracellular electron transfers involved in TBBPA reductive debromination, or stimulated the growth and activity of TBBPA-degrading taxa by providing readily usable carbon and nutrient, or a physical support for the formation of a TBBPA-degrading microbial biofilm. Nevertheless, this study suggests for the first time that BC and AC present great potential for the microbial degradation of TBBPA. Therefore, their integration to wastewater treatments, but also to soils and sediments decontamination strategies, may be promising to promote the removal of TBBPA and possibly other emerging hydrophobic contaminants.
Supplementary Material
Acknowledgments
The authors would like to thank Goran Marsh for synthesizing and providing the mono, di and triBBPA used for the LC-MS/MS analysis, Nicolas Devos and Olivier Fedrigo for their help with Illumina Miseq sequencing, Dwina Martin and the staff of the North Durham wastewater treatment facility for their help in collecting activated sludge, and Andrew Matsumoto for his valuable assistance in operating and sampling the bioreactors, and compiling TBBPA and debromination products data. The authors would also like to thank Erich Pinzón Fuchs for his comments and critical reading of the manuscript.
Funding
Funding for this work was gratefully provided by the NIEHS-supported Duke University Superfund Research Center (NIEHS grant P42-ES010356). The funding institution has no involvement in design, data collection, analysis, interpretation report redaction, and decision to submit the article for publication.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.watres.2017.09.047.
Footnotes
Conflict of interest
The authors declare they have no conflict of interest.
Author contributions
Experimental design: EL, GEG, HH-K, CKG. Amendment material characterization: NB. Bioreactors operation and samples collection: GEG. LC-MS/MS optimization and analyses: GEG, EC, HMS. MiSeq Illumina library preparation and data analyses: EL, CMG. Manuscript redaction: EL, CKG.
References
- Agarry SE, Aremu MO, Aworanti OA. Biodegradation of 2, 6-dichlorophenol wastewater in soil column reactor in the presence of pineapple peels-derived activated carbon, palm kernel oil and inorganic fertilizer. J Environ Prot. 2013;4(6):537–547. [Google Scholar]
- An T, Zu L, Li G, Wan S, Mai B, Wong PK. One-step process for debromination and aerobic mineralization of tetrabromobisphenol-A by a novel Ochrobactrum sp T isolated from an e-waste recycling site. Bioresour Technol. 2011;102(102):9148–9154. doi: 10.1016/j.biortech.2011.06.080. [DOI] [PubMed] [Google Scholar]
- Arbeli Z, Ronen Z. Enrichment of a microbial culture capable of reductive debromination of the flame retardant tetrabromobisphenol-A, and identification of the intermediate metabolites produced in the process. Biodegradation. 2003;14(6):385–395. doi: 10.1023/a:1027304222436. [DOI] [PubMed] [Google Scholar]
- Chang BV, Yuan SY, Ren YL. Anaerobic degradation of tetrabromobisphenol-A in river sediment. Ecol Eng. 2012;49:73–76. doi: 10.1016/j.chemosphere.2011.12.057. [DOI] [PubMed] [Google Scholar]
- Chen J, Tanguay RL, Simonich M, Nie S, Zhao Y, Li L, Bai C, Dong Q, Huang C, Lin K. TBBPA chronic exposure produces sex-specific neurobehavioral and social interaction changes in adult zebrafish. Neurotoxicol Teratol. 2016;56:9–15. doi: 10.1016/j.ntt.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Dai Z, Hu J, Xu X, Zhang L, Brookes PC, He Y, Xu J. Sensitive responders among bacterial and fungal microbiome to pyrogenic organic matter (biochar) addition differed greatly between rhizosphere and bulk soils. Sci Rep. 2016;6:36101. doi: 10.1038/srep36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J, Sanders J, Kissling G, Johnson C, Boyle M, Elmore S. Environmental chemical exposure may contribute to uterine cancer development studies with tetrabromobisphenol a. Toxicol Pathol. 2015;43(4):464–473. doi: 10.1177/0192623314557335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrig A, Müller R, Babel W. Isolation of phenoxy herbicide-degrading Rhodoferax species from contaminated building material. Eng Life Sci. 1997;17(4):351–356. [Google Scholar]
- Frankel ML, Bhuiyan TI, Veksha A, Demeter MA, Layzell DB, Helleur RJ, Hill JM, Turner RJ. Removal and biodegradation of naphthenic acids by biochar and attached environmental biofilms in the presence of co-contaminating metals. Bioresour Technol. 2016;216:352–361. doi: 10.1016/j.biortech.2016.05.084. [DOI] [PubMed] [Google Scholar]
- Goux X, Calusinska M, Fossépré M, Benizri E, Delfosse P. Start-up phase of an anaerobic full-scale farm reactor–appearance of mesophilic anaerobic conditions and establishment of the methanogenic microbial community. Bio-resour Technol. 2016;212:217–226. doi: 10.1016/j.biortech.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Guo J, Peng Y, Ni BJ, Han X, Fan L, Yuan Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb Cell Fact. 2015;14:33. doi: 10.1186/s12934-015-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins TM, Haeger A, Biffinger JC, Ren ZJ. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016;94:225–232. doi: 10.1016/j.watres.2016.02.059. [DOI] [PubMed] [Google Scholar]
- Inyang M, Dickenson E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: a review. Chemosphere. 2015;134:232–240. doi: 10.1016/j.chemosphere.2015.03.072. [DOI] [PubMed] [Google Scholar]
- Jonker MT, Suijkerbuijk MP, Schmitt H, Sinnige TL. Ecotoxicological effects of activated carbon addition to sediments. Environ Sci Technol. 2009;43(15):5959–5966. doi: 10.1021/es900541p. [DOI] [PubMed] [Google Scholar]
- Kjellerup B, Naff C, Edwards S, Ghosh U, Baker J, Sowers K. Effects of activated carbon on reductive dechlorination of PCBs by organohalide respiring bacteria indigenous to sediments. Water Res. 2014;52:1–10. doi: 10.1016/j.watres.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Lefevre E, Cooper E, Stapleton HM, Gunsch CK. Characterization and adaptation of anaerobic sludge microbial communities exposed to tetra-bromobisphenol a. PloS one. 2016;11(7):e0157622. doi: 10.1371/journal.pone.0157622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leglize P, Alain S, Jacques B, Corinne L. Adsorption of phenanthrene on activated carbon increases mineralization rate by specific bacteria. J Hazard Mater. 2008;151(2):339–347. doi: 10.1016/j.jhazmat.2007.05.089. [DOI] [PubMed] [Google Scholar]
- Li F, Jiang B, Nastold P, Kolvenbach BA, Chen J, Wang L, Guo H, Corvini PFX, Ji R. Enhanced transformation of tetrabromobisphenol A by nitrifiers in nitrifying activated sludge. Environ Sci Technol. 2016;49(7):4283–4292. doi: 10.1021/es5059007. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Y, Jiang B, Wang L, Chen J, Guo H, Ji R. Degradation, metabolism, and bound-residue formation and release of tetrabromobisphenol A in soil during sequential anoxic–oxic incubation. Environ Sci Technol. 2013;47(15):8348–8354. doi: 10.1021/es4014322. [DOI] [PubMed] [Google Scholar]
- Liu K, Li J, Yan S, Zhang W, Li Y, Han D. A review of status of tetra-bromobisphenol A (TBBPA) in China. Chemosphere. 2016;148:8–20. doi: 10.1016/j.chemosphere.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Loman NJ, Misra RV, Dallman TJ, Constantinidou C, Gharbia SE, Wain J, Pallen MJ. Performance comparison of benchtop high-throughput sequencing platforms. Nat Biotechnol. 2012;30(5):434–439. doi: 10.1038/nbt.2198. [DOI] [PubMed] [Google Scholar]
- Mohan D, Sarswat A, Ok YS, Pittman CU. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–a critical review. Bioresour Technol. 2014;160:191–202. doi: 10.1016/j.biortech.2014.01.120. [DOI] [PubMed] [Google Scholar]
- Muter O, Berzins A, Strikauska S, Pugajeva I, Bartkevics V, Dobele G, Truu J, Truu M, Steiner C. The effects of woodchip-and straw-derived biochars on the persistence of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) in soils. Ecotox Environ Safe. 2014;109:93–100. doi: 10.1016/j.ecoenv.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Peng X, Qu X, Luo W, Jia X. Co-metabolic degradation of tetrabromobisphenol A by novel strains of Pseudomonas sp and Streptococcus sp. Bioresour Technol. 2014;169:271–276. doi: 10.1016/j.biortech.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Pérez-Pantoja D, Donoso R, Agulló L, Córdova M, Seeger M, Pieper DH, González B. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ Microbiol. 2012;14(5):1091–1117. doi: 10.1111/j.1462-2920.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- Sun F, Kolvenbach BA, Nastold P, Jiang B, Ji R, Corvini PFX. Degradation and metabolism of tetrabromobisphenol A (TBBPA) in submerged soil and soil-plants systems. Environ Sci Technol. 2014;48:14291–14299. doi: 10.1021/es503383h. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PloS one. 2014;9(8):e105592. doi: 10.1371/journal.pone.0105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Hu M, Li F, Liu C, Chen M. Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil. Soil Biol Biochem. 2014;70:142–150. [Google Scholar]
- Van Der Zee FP, Bisschops IA, Lettinga G, Field JA. Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environ Sci Technol. 2003;37(2):402–408. doi: 10.1021/es025885o. [DOI] [PubMed] [Google Scholar]
- Wang J, Fu Z, Liu G, Guo N, Lu H, Zhan Y. Mediators-assisted reductive biotransformation of tetrabromobisphenol-A by Shewanella sp XB. Bioresour Technol. 2013;142:192–197. doi: 10.1016/j.biortech.2013.04.062. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Cerdà A, Cao M, Zhang Y, Yin J, Jiang Y, Chen L. Changes in soil chemical properties as affected by pyrogenic organic matter amendment with different intensity and frequency. Geoderma. 2017;289:161–168. [Google Scholar]
- Xin J, Liu X, Liu W, Zheng X. Effects of biochar–BDE-47 interactions on BDE-47 bioaccessibility and biodegradation by Pseudomonas putida TZ-1. Ecotox Environ Safe. 2014;106:27–32. doi: 10.1016/j.ecoenv.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Yu L, Yuan Y, Tang J, Wang Y, Zhou S. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens. Sci Rep. 2015;5:16221. doi: 10.1038/srep16221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.