Abstract
The purpose of our article is to assess the current understanding of Indian spice ‘Curcumin’ against amyloid-β (Aβ)-induced toxicity in Alzheimer’s disease (AD) pathogenesis. Natural products, such as ginger, curcumin and gingko biloba have been used as diets and dietary supplements to treat human diseases, including cancer, cardiovascular, respiratory, infectious, diabetes, obesity, metabolic syndromes and neurological disorders. Products derived from plants are known to have protective effects, including anti-inflammatory, anti-oxidant, anti-arthritis, pro-healing and boosting memory cognitive functions. In the last decade, several groups have designed and synthesized curcumin and its derivatives and extensively tested using cell and mouse models of AD. Recent research on amyloid-β and curcumin has revealed that curcumin prevents amyloid-β aggregation and crosses the blood brain barrier (BBB), reach brain cells and protect neurons from various toxic insults of aging and amyloid-β in humans. Recent research has also reported that curcumin ameliorates cognitive decline and improves synaptic functions in mouse models of AD. Further, recent groups have initiated studies on elderly individuals and patients with AD and the outcome of these studies is currently being assessed. This article highlights the beneficial effects of curcumin on AD. This article also critically assesses the current limitations of curcumin’s bioavailability and urgent need for new formulation to increase its brain levels to treat patients with AD.
Keywords: Alzheimer’s disease, Amyloid beta, curcumin, oxidative stress, mitochondria, reactive oxygen species, amyloid precursor protein
1. Introduction
Natural products derived from plants and herbs have been extensively used as diets and dietary supplements to treat human diseases, including cancer, cardiovascular, respiratory, infectious, diabetes, obesity, metabolic syndromes and neurological disorders. In addition, these products have been used to delay aging process. Natural products are the major source of diets that have multiple cell protective effects, including anti-inflammatory, anti-oxidant, anti-arthritis and enhancing memory cognitive functions [1, 2].
Physical exercise and healthy diets have been reported to have implications to delay disease progression of Alzheimer’s disease (AD) in elderly individuals and improved cognitive functions in subjects with mild cognitive impairment and early AD patients [3, 4]. There are a large number of natural products and herbs currently available, including curcumin, green tea and vitamin C, vitamin E, beta carotene, Gingko Biloba, Ginseng, Rosemary, Sage, and many others [1–3]. The purpose of our article is to summarize natural products and their benefits to human diseases. Our article also focuses on beneficial effects of curcumin in AD.
2. Curcumin
Curcumin is the major constituent of the Asian spice, turmeric isolated from the rhizome of Curcuma Longa [5, 6]. Curcumin was isolated in 1815 as a yellow coloring-matter from the rhizomes of Curcuma longa (turmeric) [7] and named it curcumin. Curcumin has been used historically in Ayurvedic medicine (Curcumin is popularly referred as Haldi in India and its chemical name is diferuloylmethane and its molecular formula is C21H20O6 (Figure 1). It molecular mass is 368.37 g/mol. Curcumin is extensively used for medicinal purposes in Asia and other parts of the world. Curcumin is used in foods because of its color and flavor. It is also used as cosmetic product, particularly for skin.
Figure 1.
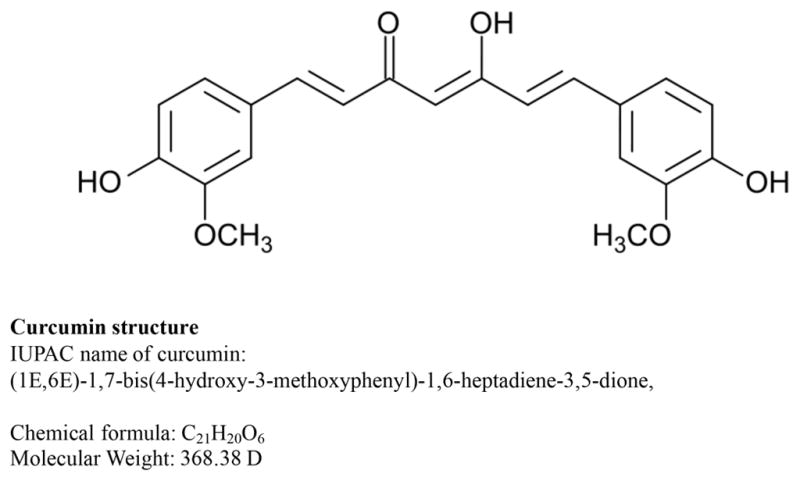
Structure of curcumin
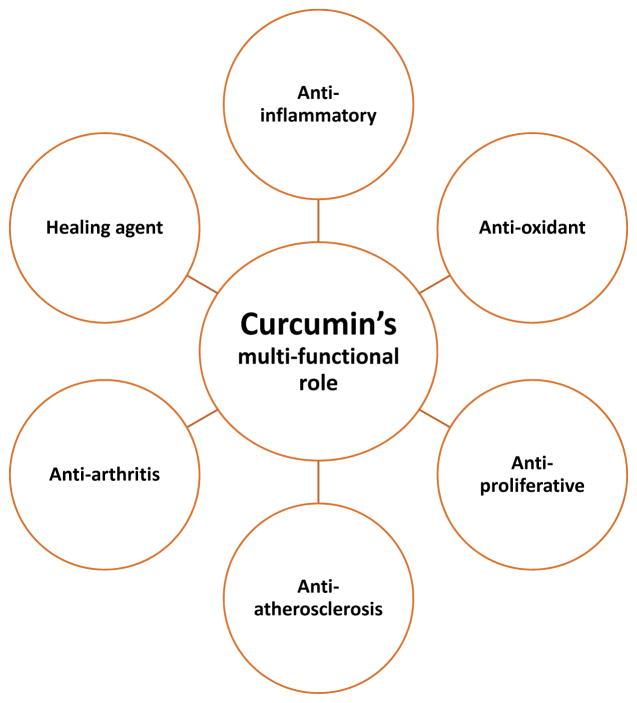
The chemical structure of curcumin is comprised of two aryl rings with ortho-methoxy OH groups linked to beta-diketone moiety [8]. Several years of research revealed that curcumin has several protective and therapeutic properties, including anti-inflammatory [9–12], antioxidant [9, 10, 13], antiproliferative, anti-atherosclerosis, and anti-arthritis [9]. Curcumin is a strong healing agent [14] (Figure 2). A recent study reported that curcumin enhanced the levels of glutathione and antioxidant enzymes, superoxide dismutase and catalase in the brains of lead-poisoned rats and significantly reduced lead-induced damage [15].
Figure 2.
Therapeutic properties of Curcumin in human health and disease
3. Curcumin and Alzheimer’s disease
Alzheimer’s disease (AD) is the most common form of dementia in elderly individuals and is the sixth leading cause of death in United States. AD is an age-dependent and progressive neurodegenerative disease, characterized by the loss of memory, cognitive functions and changes in behavior and personality [16–20]. According to 2015 World Alzheimer Report, it was estimated that 47.5 million people have dementia worldwide, and the numbers are estimated to go up to 75.6 million by 2030 and to 131.5 million by 2050. Dementia has a huge economic impact and the 2015 total estimated healthcare cost is about $818 billion [21].
Causal factors are known for AD for a small proportion (1–2%) of total AD patients and causal factors are still unknown for vast majority of AD cases. Several risk factors have been identified, major being ApoE4 genotype and polymorphisms in several genetic loci, including sortilin related receptor 1, clusterin, complement component receptor 1, CD2AP, EPHA1, and MS4A4/MS4A6E genes are other contributing risk factors [22]. In addition, type 2 diabetes, traumatic brain injury, stroke and diet, environmental factors are other contributing factors. Above all, aging is number ONE risk factor.
Several years of intense research have revealed that AD is associated with multiple cellular changes, including mitochondrial damage, loss of synapses, amyloid beta (Aβ) formation and accumulation, activation of microglia and astrocytes, phosphorylation of tau and neurofibrillary tangles formation and loss of neurons [16–25]. Therapeutic strategies have been developed based on these cellular changes and currently being tested in preclinical (animal models) and human clinical trials. However, we do not have drugs/agents that can delay and/or prevent disease progression of AD. Further, we still do not have early detectable biomarkers that can identify cognitive decline and memory problems in elderly individuals.
4. Natural Products and Antioxidants and Alzheimer’s Disease
Natural products have been used to delay the progression of disease in elderly individuals and AD patients. Several groups studied efficacies of natural products and antioxidants, including vitamin E, curcumin, Ginkgo biloba and melatonin to determine, if antioxidants reduce Aβ and tau pathologies and enhance cognitive functions in mouse models of AD [26–31]. The outcome of these AD mice studies is positive, AD mice treated with antioxidants showed reduced soluble Aβ levels, improved cognitive behavior. Based on encouraging outcome of AD mice studies, several clinical trials were conducted in AD patients and elderly individuals using vitamin E, vitamin C and E together, vitamin E + donepezil, Formula F + donepezil, statins and huperzine A [32–48].
In a recent review article, Tang and Taghiglou (2017) [49] nicely summarized the latest developments in curcumin research on AD. They focused on mechanisms of action of curcumin in AD, including curcumin’s effect on inhibitions of Aβ and tau, copper binding ability, cholesterol lowering ability, anti-inflammatory and modulation of microglia, AChE inhibition, antioxidant properties and modification of insulin signaling pathways. They also covered bioavailability of curcumin, and current challenges of curcumin therapy to AD patients [49].
Overall, the outcome of these natural products and antioxidants are effective and delay disease progression in elderly individuals, but less effective in patients with severe AD.
5. Curcumin and Amyloid Beta
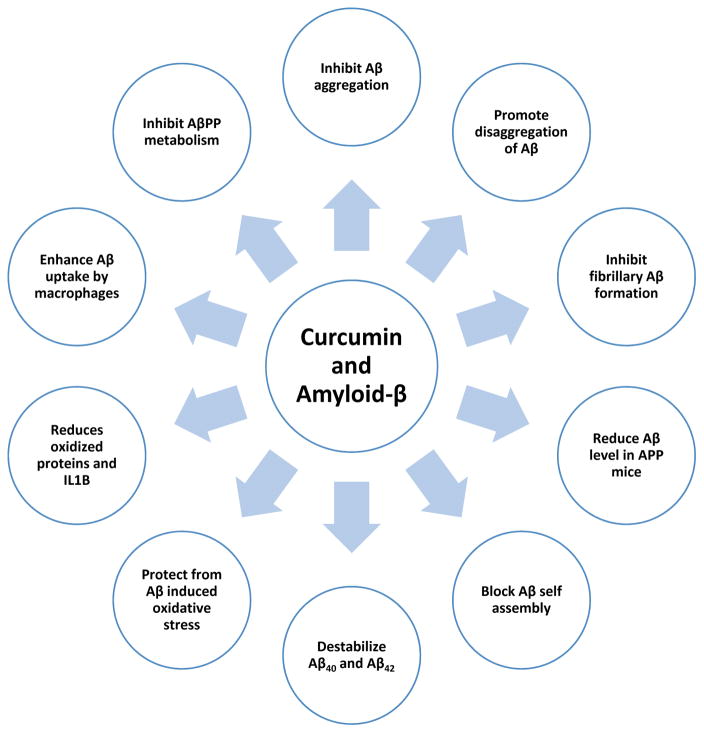
In the last decade, considerable progress has been made on curcumin in AD. Several lines of evidence suggest that curcumin has anti-amyloid properties in AD: 1). Findings from an in vitro study revealed that curcumin inhibits Aβ aggregation as well as disaggregates to form fibrillar Aβ40 [31]. 2) Several in vivo studies revealed that curcumin promote disaggregation of existing amyloid deposits and prevent aggregation of new amyloid deposits, even reduce the size of remaining deposits [31, 50]. 3) Curcumin and its derivatives are reported to inhibit the fibrillar Aβ formation from Aβ monomer and also destabilizes preformed fibrillar Aβ in vitro, indicating that curcumin protective against Aβ toxicity [51]. 4). The levels of Aβ (40%) and Aβ deposits (43%) were reduced in the brains of APP mice treated with low doses of curcumin relative to untreated APP mice. At higher concentration, curcumin binds to amyloid beta and blocks its self assembly [31]. 5) A recent study reported that curcumin destabilizes Aβ40 and Aβ42 [52]. 6) Further, curcumin-derived isoxazoles and pyrazoles bind to the Aβ and inhibit AβPP metabolism [53]. 7) Curcumin protects PC12 cells and normal human embelical endothelial cells from amyloid-β-induced oxidative stress [54]. 8) Curcumin reduced the levels of oxidized proteins and IL1B in the brains of APP mice [55]. 9) Curcumin enhances Aβ uptake by macrophages in AD patients, bone marrow derived dendritic cells may correct immune defects in AD patients and provide immunotherapy approach to AD patients [56]. 10) Curcumin inhibits peroxidase and modulate the cytopathologies in AD patients [57]. 11) Curcumin binds to redox-active metals, iron and copper, it suppresses inflammatory damage by preventing metal induction of Nf-kB [58] (Figure 3).
Figure 3.
Anti-amyloid properties of curcumin. Curcumin regulates Aβ metabolism and inhibits Aβ aggregation several ways.
6. Curcumin and its Multifunctional Role in Human Health
Several groups studied curcumin’s functional properties and its physiological relevance to Aβ in AD. Summaries of important studies are given below.
Shen and colleagues (2016) [59] studied the bio-availability of curcumin using biochemical assays. Using experimental and theoretical approaches, they compared curcumin and its degradation products for its biological activities against AD, including the superoxide anion radical (O2(.-)-scavenging activity, Aβ fibrils (fAβ) formation-inhibiting activity, and enzymatic inhibition activity. They showed that compared to the parent compound curcumin, the degradation products mixture possessed higher O2(.-)-scavenging activity and stronger inhibition against fAβ formation. The docking simulations revealed that the bioactive degradation products should make important contribution to the experimentally observed enzymatic inhibition activities of curcumin. Given that curcumin is readily degraded under physiological condition, their findings strongly suggest that the degradation products contribute to the diverse biological activities of curcumin. These findings not only provide novel insights into the complex pharmacology of curcumin due to its poor bioavailability, but also open new avenues for developing therapeutic applications of this natural product [59].
Ghosh et al (2015) [60] critically assessed multi-functional properties of curcumin. They discussed various aspects of curcumin including its antioxidant, hypoglycemic, anti-inflammatory and anti-cancer activities. Apart from these well-known activities, this natural polyphenolic compound also exerts its beneficial effects by modulating different signaling molecules including transcription factors, chemokines, cytokines, tumor suppressor genes, adhesion molecules, and microRNAs. Based on multi-functional properties of curcumin, curcumin, therefore, could be a therapeutic option for the treatment of these diseases, provided limitations in its oral bioavailability can be overcome [60].
Rao et al (2015) [61] studied computational aspects of curcumin and Aβ, a toxic protein found in the brains of AD patients. They constructed computational models of Aβ hexapeptide (16) KLVFFA(21) octamer steric-zipper β-sheet assembly and full-length Aβ fibril β-sheet assembly. Curcumin binding in these models was evaluated by molecular docking and molecular dynamics simulation studies. In both the models, curcumin was oriented in a linear extended conformation parallel to fiber axis and exhibited better stability in the Aβ hexapeptide octamer steric-zipper model (Ebinding = −10.05 kcal/mol) compared to full-length Aβ fibril model (Ebinding = −3.47 kcal/mol). Analysis of molecular dynamics trajectories of curcumin bound to full-length Aβ fibril shows good stability with minimum Cα-atom root mean square deviation shifts. Interestingly, curcumin binding led to marked fluctuations in the 14 HQKLVFFA 21 region that constitute the fibril spine with root mean square functional values ranging from 1.4 to 3.6 Å. These results show that curcumin binding to Aβ shifts the equilibrium in the aggregation pathway by promoting the formation of non-toxic aggregates [61].
Ferrari and colleagues (2014) [62] discussed molecular links between curcuminoids metal chelating agents in relation to Aβ in AD. They examined the metal complexing ability of substituted curcuminoids as new potential AD therapeutic agents. The K2T derivatives originate from the insertion of a -CH2COOC(CH3)3 group on the central atom of the diketonic moiety of curcumin. They retain the diketo-ketoenol tautomerism which is solvent dependent. In aqueous solution the prevalent form is the diketo one but the addition of metal ion (Ga(3+), Cu(2+)) causes the dissociation of the enolic proton creating chelate complexes and shifting the tautomeric equilibrium towards the keto-enol form. The formation of metal complexes is followed by both NMR and UV-vis spectroscopy. The density functional theory calculations on K2T21 complexes with Ga(3+) and Cu(2+) are performed and compared with those on curcumin complexes. Ga(K2T21)2(H2O)2](+) was found more stable than curcumin one. Good agreement is detected between calculated and experimental (1)H and (13)C NMR data. The calculated OH bond dissociation energy and the OH proton dissociation enthalpy, allowed to predict the radical scavenging ability of the metal ion complexed with K2T21, while the calculated electronic affinity and ionization potential represent yardsticks of antioxidant properties. Their theoretical calculations suggest that the proton-transfer-associated superoxide-scavenging activity is enhanced after binding metal ions, and that Ga(3+) complexes display possible superoxide dismutase-like activity [62].
Konno and colleagues (2014) [63] studied the impact of a new non-peptidyl inhibitor of beta-site amyloid precursor protein cleaving enzyme 1 in AD. They focused on the curcumin framework, two phenolic groups combined with an sp2 carbon spacer for low-molecular and high lipophilicity. The structure-activity relationship study of curcumin derivatives is described. Their results indicate that phenolic hydroxyl groups and an alkenyl spacer are important structural factors for the inhibition of beta-site amyloid precursor protein cleaving enzyme 1 and, furthermore, non-competitive inhibition of enzyme activity is anticipated from an inhibitory kinetics experiment and docking simulation [63].
Pandey and colleagues (2008) [64] studied curcumin’s inhibitory properties against alpha synuclein (AS) aggregation. Reasoning that oligomerization kinetics and mechanism of amyloid formation are similar in Parkinson’s disease (PD) and AD, we investigated the effect of curcumin on alpha-synuclein protein aggregation. In vitro model of AS aggregation was developed by treatment of purified alpha-synuclein protein (wild-type) with 1 mM Fe3+ (Fenton reaction). It was observed that the addition of curcumin inhibited aggregation in a dose-dependent manner and increased alpha-synuclein solubility. The aggregation-inhibiting effect of curcumin was next investigated in cell culture utilizing catecholaminergic SH-SY5Y cell line. A model system was developed in which the red fluorescent protein was fused with A53T mutant of alpha-synuclein and its aggregation examined under different concentrations of curcumin. To estimate aggregation in an unbiased manner, a protocol was developed in which the images were captured automatically through a high-throughput cell-based screening microscope. The obtained images were processed automatically for aggregates within a defined dimension of 1–6 microm. Greater than 32% decrease in mutant alpha-synuclein aggregation was observed within 48 h subsequent to curcumin addition. Their data suggest that curcumin inhibits alpha-synuclein oligomerization into higher molecular weight aggregates and therefore should be further explored as a potential therapeutic compound for PD and related disorders [64].
Baum and Ng (2004) [58] studied links between curcumin and copper and iron in AD. Using spectrophotometry, they quantified curcumin affinity for copper, zinc, and iron ions. Zn2+ showed little binding, but each Cu2+ or Fe2+ ion appeared to bind at least two curcumin molecules. The interaction of curcumin with copper reached half-maximum at approximately 3–12 μM copper and exhibited positive cooperativity, with Kd1 approximately 10–60 μM and Kd2 approximately 1.3 μM (for binding of the first and second curcumin molecules, respectively). Curcumin-iron interaction reached half-maximum at approximately 2.5–5 μM iron and exhibited negative cooperativity, with Kd1 approximately 0.5–1.6 μM and Kd2 approximately 50–100 μM. Curcumin and its metabolites can attain these levels in vivo, suggesting physiological relevance. Since curcumin more readily binds the redox-active metals iron and copper than redox-inactive zinc, curcumin might exert a net protective effect against amyloid-β toxicity or might suppress inflammatory damage by preventing metal induction of NF-kappaβ [58].
Overall, finding from these studies indicate that curcumin has multiple beneficial properties, including inhibits the aggregation of mutant proteins such as Aβ and alpha-synuclein and huntingtin, and also showed the properties of metal chelating, anti-inflammatory and anti-oxidant.
7. Curcumin and its Derivatives Design and Synthesis
Several groups have designed and synthesized curcumin and its analogs and tested their properties and beneficial effects using in vitro, in vivo studies.
Lakey-Beitia and colleagues (2017) [65] synthesized new curcumin derivatives and studied anti-Aβ aggregation and anti-inflammatory activities in AD. Nine curcumin derivatives were synthesized by etherification and esterification of the aromatic region. From these derivatives, compound 8 exhibited an anti-inflammatory effect similar to curcumin, while compounds 3, 4, and 10 were more potent. Moreover, when the anti-aggregation activity is considered, compounds 3, 4, 5, 6, and 10 showed biological activity in vitro. Compound 4 exhibited a strong anti-aggregation effect higher than curcumin. Mono-functionalized curcumin derivatives showed better bioactivity than difunctionalized compounds. Moreover, the presence of bulky groups in the chemical structure of curcumin derivatives decreased bioactivity [65].
Okuda and colleagues (2016) [66] designed and synthesized a series of curcumin derivatives and evaluated their inhibitory activities against both tau and Aβ aggregation. They described the development of the more potent aggregation inhibitor 3-[(1E)-2-(1H-indol-6-yl) ethenyl]-5-[(1E)-2-[2-methoxy-4-(2-pyridylmethoxy) phenyl] ethenyl]-1H-pyrazole (compound 4, PE859). They conclude that their compound has a better pharmacokinetic profile and pharmacological efficacy in vivo than curcumin, making it suitable as a drug for AD [66].
By fusing donepezil and curcumin, Yan and colleagues (2017) [67] made a novel series of compounds as multitarget-directed ligands against AD. Among them, compound 11b displayed potent AChE inhibition (IC50=187nM) and the highest BuChE/AChE selectivity (66.3). Compound 11b also inhibited 45.3% Aβ42 self-aggregation at 20μM and displayed remarkable antioxidant effects. The metal-chelating property of compound 11b was elucidated by determining the 1:1 stoichiometry for the 11b-Cu(II) complex. The excellent blood-brain barrier permeability of 11b also indicated the potential for the compound to penetrate the central nervous system [67].
Mishra and colleagues (2017) [68] designed and synthesized a novel series of donepezil based multi-functional agents “(E)-5,6-dimethoxy-2-(4-(4-substituted piperazin-1-yl) benzylidene)-2,3-dihydro-1H-inden-1-ones” as potential anti-Alzheimer’s agents. In-vitro studies revealed that these compounds demonstrated moderate to good AChE and amyloid-β aggregation inhibitory activity. These derivatives are also endowed with admirable antioxidant activity. Among the entire series compounds IP-9, IP-13 and IP-15 appeared as most active multi-functional agents and displayed marked AChE inhibitory, Aβ disaggregation and antioxidant activity. Studies indicate that IP-13 and IP-15 showed better AChE inhibitory activity than the standard drug donepezil and IP-9, IP-13 as well as IP-15 exhibited better Aβ aggregation inhibitory activity than curcumin. These compounds (IP-9, IP-13 and IP-15) successfully diminished H2O2 induced oxidative stress in SH-SY5Y cells and displayed excellent neuroprotective activity against H2O2 as well as Aβ induced toxicity in SH-SY5Y cells in a concentration dependent manner. Moreover, these derivatives did not exert any significant toxicity in SH-SY5Y cells in cytotoxicity assay. To elucidate the plausible binding mode of the compounds IP-9, IP-13 and IP-15, molecular docking studies and molecular dynamics simulation studies were also performed and the results indicate their significant interactions with the active sites of AChE as well as Aβ42 peptide. Based on these findings, they conclude that IP-9, IP-13 and IP-15 are potent multi-functional agents against AD and might serve as promising lead candidates for anti-AD drug development [68].
Awasthi and colleagues (2017) [69] studied two familial Aβ42 mutations, namely A2V (harmful) and A2T (protective) have been analyzed and compared with the WT by performing all-atom molecular dynamics simulations in the absence and presence of curcumin, a well-known inhibitor of Aβ plaque formation. Mutant A2V was found to exhibit highest stability followed by WT and mutant A2T in the absence of curcumin. This stability trend was found to be reversed in the presence of curcumin, suggesting a significant change in the conformational landscape of Aβ42 folding. Due to significant differences in the folding and interaction patterns of the mutants A2V and A2T, curcumin exhibited higher binding affinity for mutant A2T as compared to that of A2V [69].
Li and colleagues (2014) [70] designed and synthesized a series of novel 2-methoxy-phenyl dimethyl-carbamate derivatives as site-activated MTDLs based on rivastigmine and curcumin. Most of them exhibited good to excellent AChE and BuChE inhibitory activities with sub-micromolar IC50 values. Among all the compounds, 6a demonstrated the most potent AChE inhibition with IC50 value of 0.097μM, which is about 20-fold than that of rivastigmine. In addition, the three selected compounds 5a, 6a and 6e demonstrated inhibitory activity against Aβ self-aggregation similar to cucurmin in TEM assay, which is obviously different from the weak activity of rivastigmine. Moreover, the hydrolysate of 6a (compound 7) also showed potent ABTS(+) scavenging and moderate copper ion chelating activity in vitro [70].
Tiwari and colleagues (2014) [71] studied the neurogenesis of curcumin Induction of neurogenesis by targeting endogenous neural stem cells (NSC) could be a promising therapeutic approach to such diseases by influencing the brain self-regenerative capacity. Curcumin, a neuroprotective agent, has poor brain bioavailability. Herein, they report that curcumin-encapsulated PLGA nanoparticles (Cur-PLGA-NPs) potently induce NSC proliferation and neuronal differentiation in vitro and in the hippocampus and subventricular zone of adult rats, as compared to uncoated bulk curcumin. Cur-PLGA-NPs induce neurogenesis by internalization into the hippocampal NSC. Cur-PLGA-NPs significantly increase expression of genes involved in cell proliferation (reelin, nestin, and Pax6) and neuronal differentiation (neurogenin, neuroD1, neuregulin, neuroligin, and Stat3). Curcumin nanoparticles increase neuronal differentiation by activating the Wnt/β-catenin pathway, involved in regulation of neurogenesis. These nanoparticles caused enhanced nuclear translocation of β-catenin, decreased GSK3β levels, and increased promoter activity of the TCF/LEF and cyclin-D1. Pharmacological and siRNA-mediated genetic inhibition of the Wnt pathway blocked neurogenesis-stimulating effects of curcumin. These nanoparticles reverse learning and memory impairments in an amyloid beta induced rat model of AD-like phenotypes, by inducing neurogenesis. In silico molecular docking studies suggest that curcumin interacts with Wif-1, Dkk, and GSK3β. These results suggest that curcumin nanoparticles induce adult neurogenesis through activation of the canonical Wnt/β-catenin pathway and may offer a therapeutic approach to treating neurodegenerative diseases such as AD, by enhancing a brain self-repair mechanism [71].
Fang and colleagues (2014) [72] designed and synthesized eight dimethylaminomethyl-substituted curcumin derivatives and tested antioxidant properties of these derivatives [72]. The antioxidant test revealed that the synthesized compounds had higher free radical scavenging activity towards both 2,2-diphenyl-1-picrylhydrazyl free radicals (DPPH) (IC50 1.5–29.9μM) and galvinoxyl radicals (IC50 4.9–41.1μM) than the lead compound curcumin. Besides, compound 3a could effectively inhibit the Aβ self-aggregation in vitro. Investigated in phosphate-buffered solutions (pH=7.4) in the presence or absence of 0.1% FBS 3a showed a good stability while curcumin did not. Furthermore, 3a showed a good lipophilicity (logP=3.48), suggesting a potential ability to penetrate the blood-brain-barrier. The aqueous solubility of the hydrochloride salt of 3a (16.7mg/mL) has also been significantly improved as compared with curcumin (<0.1mg/mL) [72].
These studies have provided important information about the design and synthesis of curcumin and its derivatives. Further these studies also provided physical and protective properties of curcumin and its derivatives.
8. New Formulations and Increased Bioavailability of Curcumin
Shakeri and Sahebkar (2016) [73] critically assessed new formulations of curcumin in their recent patent commentary – It is well documented that curcumin has multiple beneficial effects for human diseases including cancer, cardiovascular, respiratory, infectious, obesity, obesity, metabolic syndromes and neurological diseases. However, bioavailability of curcumin is very limited, leading to a challenge for the disease treatment. The possible reasons for low bioavailability are – 1) low intestinal absorption, 2) rapid metabolism and 3) hydrophobic nature. Hence new formulations of curcumin are urgently needed, mainly to increase curcumin’s water-solubility for AD treatment.
Recently, several researchers modified curcumin’s formulations for human applications (Hu et al 2015 [74], Ma et al 2013 [75] and several patent applications).
Hu and colleagues (2015) [74] suggested a dripping pill formulation with polyethoxylated 40 hydrogenated castor oil, poloxamer 188 and polyethalyne glycol 4000 may improve solubility and bioavailability of curcumin.
In a 2014 patent application, a formulation with turmeric oil containing 45% Ar-turmerone was proposed an effective method to enhance the bioavailability of curcumin (US8859020). If successful, this approach will be useful to deliver curcumin in vivo and also for human clinical trials. Curcumin-turmeric formulation in weight ratios of 10:1 and 12:1 have found to improve cognitive function and reduce oxidative stress [73,75].
In another patent, Lilienfeld and colleagues used iontophoretic approach for transdermal delivery of curcumin and its analogs to treat AD (EP2306824). They used glycinated-based curcumin prodrugs for the delivery of curcumin into the blood stream. These prodrugs have water solubility and their charged nature allows a transdermal flux during iontophoresis [73].
In another patent (WO 2010074971), investigators used nitrogen-containing analogs for AD treatment. Adding additional amino-acid moiety to curcumin stricture increases the water solubility and the transport across the blood brain barrier (BBB) [73].
In a recent patent (US20080075761), investigators used a hybrid of methylene blue and curcumin and introduced intra-nasally, where curcumin absorbed through olfactory mucosa and transported to the brain [73].
These improved formulations are expected to increase curcumin’s water solubility and enhance brain curcumin levels. Once curcumin reach brain sufficiently, it is expected to reduce toxic effects caused by amyloid-β and phosphorylated tau.
9. Curcumin and its Blood Brain Barrier Properties
Curcumin crosses the BBB because of its lipophilic property and binds to amyloid deposits [31]. Adverse effects have not been reported thus far. Therefore, curcumin can be used for human clinical trials. Summaries of important studies of curcumin crossing the BBB are given below.
Barbara and colleagues (2017) [76] investigated the novel strategies for a targeted delivery of curcumin into the brain are highly desired. They encapsulated curcumin as active ingredient in PLGA (polylactide-co-glycolic-acid) nanoparticles (NPs), modified with g7 ligand for BBB crossing. They performed in depth analyses of possible toxicity of these NPs, uptake, and, foremost, their ability to influence Aβ pathology in vitro using primary hippocampal cell cultures. Their results show no apparent toxicity of the formulated NPs, but a significant decrease of Aβ aggregates in response to curcumin loaded NPs. They concluded that brain delivery of curcumin using BBB crossing NPs is a promising future approach in the treatment of AD [76].
Wang and colleagues (2014) [77] studied the autophagy induced effects curcumin in APP/PS1 double transgenic AD mice. Moreover, curcumin induced autophagy in the mice, evidenced by LC3 immunofluorescence analysis and western blot assays on LC3. Furthermore, they found that curcumin significantly decreased the expression of Phosphatidylinositol 3-Kinase (PI3K), phosphorylated Akt and rapamycin (mTOR) at protein levels, respectively. Taken together, their data suggests that curcumin inhibits Aβ generation and induces of autophagy by downregulating PI3K/Akt/mTOR signaling pathway, and further shows a neuroprotective effect. Meanwhile, curcumin might be a candidate neuroprotective agent for the treatment of AD patients by inducing autophagy [77].
These studies suggest that curcumin crosses the BBB and reach brain and protect neurons from Aβ-induced toxicities.
10. Research on Curcumin and Cell Models of Alzheimer’s Disease
Using cell cultures, several groups independently studied the physiological importance of curcumin using cell culture studies. Summaries of important studies are given below.
Reddy and colleagues (2016) [78] investigated the protective effects of a natural product – ‘curcumin’ against Aβ-induced mitochondrial and synaptic toxicities in AD neurons. Using human neuroblastoma (SHSY5Y) cells, curcumin and Aβ peptide, we studied protective effects of curcumin against Aβ. Further, they also studied preventive (curcumin+Aβ) and intervention (Aβ+curcumin) affects of curcumin against Aβ in SHSY5Y cells. Using real-time RT-PCR, immunoblotting, immunofluorescence analysis, they measured mRNA and protein levels mitochondrial dynamics, mitochondrial biogenesis, synaptic genes. They also assessed mitochondrial function by measuring hydrogen peroxide, lipid peroxidation, cytochrome oxidase activity and mitochondrial ATP. Cell viability studied using MTT assay. Amyloid-β was found to impair mitochondrial dynamics, reduced mitochondrial biogenesis and decreased synaptic activity and mitochondrial function. On the other hand, curcumin enhanced mitochondrial fusion activity and reduced fission machinery and increased biogenesis and synaptic proteins. Mitochondrial function and cell viability were elevated in curcumin treated cells. Interestingly, curcumin pre- and post-treated cells incubated with amyloid-β showed reduced mitochondrial dysfunction, maintained cell viability and mitochondrial dynamics, mitochondrial biogenesis and synaptic activity. Further, curcumin protective effects were stronger in pretreated SHSY5Y cells than post-treated cells, indicating that curcumin works better in prevention than treatment in AD neurons. The study findings suggest that curcumin is a promising drug molecule to treat AD patients [78].
Yi and colleagues (2016) [79] studied the inhibitory properties of curcumin in amyloid-β aggregation. By immobilizing the capture oligomeric and fibrils specific antibodies, in separate fluidic channels, a novel surface plasmon resonance biosensor was designed for monitoring the oligomeric and fibrillar species of Aβ42 simultaneously. The influence of curcumin, Cu(2+) and methylene blue on the amount of toxic oligomers and fibrils was evaluated. The half maximal inhibitory concentration (IC50) of curcumin and methylene blue was determined. The formation of amyloid-β fibrils was also validated by the thioflavin T fluorescence assay. The results demonstrate the utility of surface plasmon resonance as an analytical tool for rapid and comprehensive monitoring of amyloid-β aggregation and screening of Aβ modulators [79].
Using human neuroblastoma (SH-SY5Y) cells, Uguz and colleagues (2016) [80] studied anti-inflammatory and anti-oxidant properties. They investigated the effect of curcumin on Ca(2+) signaling, oxidative stress parameters, mitochondrial depolarization levels and caspase-3 and -9 activities that are induced by the H2O2 model of oxidative stress in SH-SY5Y cells. SH-SY5Y cells were divided into four groups namely, the control, curcumin, H2O2, and curcumin + H2O2 groups. The dose and duration of curcumin and H2O2 were determined from published data. The cells in the curcumin, H2O2, and curcumin + H2O2 groups were incubated for 24 h with 5 μM curcumin and 100 μM H2O2. Lipid peroxidation and cytosolic free Ca(2+) concentrations were higher in the H2O2 group than in the control group; however, their levels were lower in the curcumin and curcumin + H2O2 groups than in the H2O2 group alone. Reduced glutathione (GSH) and glutathione peroxidase (GSH-Px) values were lower in the H2O2 group although they were higher in the curcumin and curcumin + H2O2 groups than in the H2O2 group. Caspase-3 activity was lower in the curcumin group than in the H2O2 group. They concluded that curcumin strongly induced modulator effects on oxidative stress, intracellular Ca(2+) levels, and the caspase-3 and -9 values in an experimental oxidative stress model in SH-SY5Y cells [80].
Chandra and colleagues (2017) [81] studied the effects of curcumin in different domains of amyloid-β peptide. There are three specific regions in the amyloid-β peptide sequence where variations cause enhanced toxicity in AD: the N-terminus, the central salt bridge, and the C-terminus. They investigated, if there is a close conformational connection between these three regions, which may suggest a concerted mechanism of toxicity. They measure the effects of Zn2+ and curcumin on Aβ40 and compare these with their previously reported effects on Aβ42. Aβ42 and Aβ40 differ only near the C-terminus, where curcumin interacts, while Zn2+ interacts near the N-terminus. Therefore, this comparison should help us differentiate the effect of modulating the C- and the N-termini. They find that curcumin allows fibril-like structures containing the salt bridge to emerge in the mature Aβ40 aggregates, but not in Aβ42. In contrast, they find no difference in the effects of Zn+2 on Aβ40 and Aβ42. In the presence of Zn+2, both of these fail to form proper fibrils, and the salt bridge remains disrupted. These results indicate that modulations of the Aβ termini can determine the fate of a salt bridge far away in the sequence, and this has significant consequences for Aβ toxicity. They also infer that small molecules can alter oligomer-induced toxicity by modulating the aggregation pathway, without substantially changing the final product of aggregation [81].
Liu and colleagues (2016) [82] developed a neuroprotective potential algorithm (NPA) by evaluating twenty-three standardized and chemically characterized Ayurvedic medicinal plant extracts in a panel of bioassays targeting oxidative stress, carbonyl stress, protein glycation, Aβ fibrillation, acetylcholinesterase (AChE) inhibition, and neuroinflammation. The twenty-three herbal extracts were initially evaluated for: 1) total polyphenol content (Folin-Ciocalteu assay), 2) free radical scavenging capacity (DPPH assay), 3) ferric reducing antioxidant power (FRAP assay), 4) reactive carbonyl species scavenging capacity (methylglyoxal trapping assay), 5) anti-glycative effects (BSA-fructose, and BSA-methylglyoxal assays) and, 6) anti-Aβ fibrillation effects (thioflavin-T assay). Based on assigned index scores from the initial screening, twelve extracts with a cumulative NPA score ≥40 were selected for further evaluation for their: 1) inhibitory effects on AChE activity, 2) in vitro anti-inflammatory effects on murine BV-2 microglial cells, and 3) in vivo neuroprotective effects on Caenorhabditis elegans post induction of Aβ42 induced neurotoxicity and paralysis. Among these, four extracts had a cumulative NPA score ≥60 including phyllanthus emblica (amla; Indian gooseberry), mucuna pruriens (velvet bean), punica granatum (pomegranate) and curcuma longa (turmeric; curcumin). These extracts also showed protective effects on H2O2 induced cytotoxicity in differentiated cholinergic human neuronal SH-SY5Y and murine BV-2 microglial cells and reduced tau protein levels in the SH-SY5Y cells [82].
Ngo and colleagues (2016) [83] studied the inhibitory properties of curcumin against amyloid-β. They screened out four compounds that have chemical and structural similarity with curcumin more than 80% from all FDA-approved oral drugs. Using all-atom molecular dynamics simulation and the free energy perturbation method they showed that among predicted compounds anti-arrhythmic medication propafenone shows the best anti-amyloidogenic activity. The in vitro experiment further revealed that it can inhibit amyloid-β aggregation and protect cells against amyloid-β induced cytotoxicity to almost the same extent as curcumin [83].
Xiao and colleagues (2014) [84] used Aβ42 (10 μg/ml) to establish a damaged cell model, and curcumin and Cur1 were used in treatment groups. They measured cell survival and cell growth, intracellular oxidative stress and hTERT expression. After RNA interference, the effects of curcumin and Cur1 on cells were verified. Exposure to Aβ42 resulted in significant oxidative stress and cell toxicity, and the expression of hTERT was significantly decreased. Curcumin and Cur1 both protected SK-N-SH cells from Aβ42 and up-regulated the expression of hTERT. Furthermore, Cur1 demonstrated stronger protective effects than curcumin. However, when telomerase was inhibited by TERT siRNA, the neuroprotection by curcumin and Cur1 were ceased. Our study indicated that the neuroprotective effects of curcumin and Cur1 depend on telomerase, and thus telomerase may be a target for therapeutic effects of curcumin and Cur1 [84].
Deng and colleagues (2014) [85] using bisulfite-sequencing PCR (BSP) assay, they demonstrated that the CpG sites in NEP gene were hypermethylated both in wild-type mouse neuroblastoma N2a cells (N2a/wt) and N2a cells stably expressing human Swedish mutant amyloid precursor protein (N2a/APPswe) associated with familial early onset AD. CUR treatment induced restoration of NEP gene via CpG demethylation. This CUR-mediated upregulation of NEP expression was also concomitant with the inhibition of AKT, subsequent suppression of nuclear transcription factor-κβ (NF-κβ) and its downstream pro-inflammatory targets including COX-2, iNOS in N2a/APPswe cells. This study represents the first evidence on a link between CpG demethylation effect on NEP and anti-inflammation ability of CUR that may provide a novel mechanistic insight into the anti-inflammatory actions of CUR as well as new basis for using CUR as a therapeutic intervention for AD [85].
Sun and colleagues (2014) [86] studied the neuroprotective effect of curcumin against Aβ25-35-induced cell death in cultured cortical neurons. They found that pretreatment of curcumin prevented the cultured cortical neurons from Aβ25-35-induced cell toxicity. In addition, curcumin improved mitochondrial membrane potential, decreased ROS generation and inhibited apoptotic cell death in Aβ25-35 treated neurons. Furthermore, they found that application of curcumin activated the expression of Sirt1 and subsequently decreased the expression of Bax in the presence of Aβ25-35. The protective effect of curcumin was blocked by Sirt1 siRNA. Taken together, our results suggest that activation of Sirt1 is involved in the neuroprotective action of curcumin [86].
Huang and colleagues (2014) [87] studied the signaling effects of curcumin against amyloid-β and hyperphosphorylated tau using human neuroblastoma SH-SY5Y cells. The results indicated that curcumin inhibits amyloid-β-induced tau phosphorylation at Thr231 and Ser396, over-expression of HDAC6, and decrease in phosphorylation of glycogen synthase kinase 3β (GSK3β) at Ser9. However, the protective effect of curcumin on dephosphorylation of GSK3β induced by Aβ is not directly related to cellular oxidative stress. Curcumin depresses Aβ-induced down-regulation of phosphorylations of Akt at Thr308 and Ser473 and 3-phosphoinositide-dependent protein kinase 1 at Ser241, implying that second message PIP3 involves curcumin-protective cell signaling. Furthermore, insulin receptor/phosphatidyl inositol 3-kinase pathway, as a regulatory signaling of second message PIP3, does not participate in amyloid-β-induced deactivation of Akt (dephosphorylation at Thr308 and Ser473). However, Aβ results in over-expression of Phosphatase and tensin homolog (PTEN), a negative regulator of PIP3. Curcumin depresses amyloid-β-induced up-regulation of PTEN induced by Aβ. These results imply that curcumin inhibits amyloid-β-induced tau hyperphosphorylation involving PTEN/Akt/GSK3β pathway [87].
Using APPswe plasmid, Yin and colleagues (2012) [88] established a cell model of AD and studied the beneficial effects of curcumin. They found that curcumin significantly upregulates phosphatidylinositol 3-kinase (PI3K), Akt, nuclear factor E2-related factor-2 (Nrf2), hemeoxygenase 1 and ferritin expression, and that it significantly downregulates heme oxygenase 2, reactive oxygen species and Aβ40/42 expression. These effects of curcumin on PI3K, Akt and Nrf2 were blocked by LY294002 (PI3k inhibitor) and NF-E2-related factor-2 siRNA. The results indicate that the cytoprotection conferred by curcumin on APPswe transfected SH-SY5Y cells is mediated by its ability to regulate the balance between heme oxygenase 1 and 2 via the PI3K/Akt/Nrf2 intracellular signaling pathway [88].
Overall, findings from these cell culture studies strongly suggest that curcumin has multiple beneficial effects against Aβ-induced cascade of cellular changes in AD progression and AD pathogenesis.
11. Research on Curcumin in Mouse models of Alzheimer’s Disease
Using AD mouse mouse models, several investigators reported that curcumin has beneficial effects against amyloid beta aggregation and amyloid-beta induced toxicities in AD. Summaries of these studies are given below.
Zhang and colleagues (2015) [89] studied the working memory and spatial reference memory in rats that received a ventricular injection of Aβ42, representing a rodent model of AD. The rats treated with Aβ42 exhibited obvious cognitive deficits in behavioral tasks. Chronic (seven consecutive days, once per day) but not acute (once a day) curcumin treatments (50, 100, and 200 mg/kg) improved the cognitive functions in a dose-dependent manner. In addition, the beneficial effect of curcumin is accompanied by increased BDNF levels and elevated levels of phosphorylated ERK in the hippocampus. Furthermore, the cognition enhancement effect of curcumin could be mimicked by the overexpression of BDNF in the hippocampus and blocked by either bilateral hippocampal injections with lentiviruses that express BDNF shRNA or a microinjection of ERK inhibitor. These findings suggest that chronic curcumin ameliorates AD-related cognitive deficits and that upregulated BDNF-ERK signaling in the hippocampus may underlie the cognitive improvement produced by curcumin [89].
Elmegeed and colleagues (2015) [90] studied the effects of curcumin in the regression of AD induced in adult female albino rats. The results revealed that treatment of AD groups with compounds 3, 5, 8c or rivastigmin experienced significant increase in brain Ach, GSH, paraoxenase and BCL2 levels with respect to untreated group associated with significant decrease in brain AchE activity, urinary 8-OHG level, serum Caspase-3 level and brain P53 level relative to the untreated group. Immunohistochemical investigation revealed that the selected treatments caused marked increase in ChAT positive cells. These findings were documented by the histological investigation of the brain tissue. The activity of tested compounds showed gradual increase from compound b followed by compound 8c then compound 5. The anti-cholinesterase potential, anti-oxidant properties and anti-apoptotic activity are responsible for the anti-AD potential of these compounds [90].
Lim and colleagues (2001) [55] studied the synergic effects of ginger and curcumin in double mutant mice of AD. In an in vitro thioflavin T fluorescence assay, 100 μg/ml OCGP inhibited amyloid-β accumulation to the same extent as did 10 μM curcumin. Furthermore, AβPP/PS1 double-transgenic mice treated with OCGP (50 or 100 mg/kg/day given orally for 14 weeks) exhibited reduced Aβ plaque accumulation in the hippocampus and lower levels of glial fibrillary acid protein and cyclooxygease-2 expression compared with vehicle-treated controls. These results suggest that OCGP may prevent memory impairment in AD by inhibiting Aβ accumulation and inflammation in the brain [55].
Samy and colleagues (2016) [91] compared the beneficial effects of erythropoietin and/or curcumin in intracerebro-ventricular (ICV) streptozotocin-induced Alzheimer’s like disease in rats. Rats received ICV injection of either saline (control, n=8 rats), or streptozotocin. Three weeks following surgery, streptozotocin-injected rats were assigned into 4 groups (8 rats each); vehicle, curcumin (80mg/kg/day, orally), erythropoietin (500 IU/kg every other day, intraperitoneally) and combined (curcumin and erythropoietin)-treated groups. After 3 months of treatment, rats were subjected to neurobehavioral testing, and then killed for biochemical and histological assessment of hippocampus. Fas ligand protein and caspase-8 activity as mediators of extrinsic apoptotic pathway, oxidative stress markers (malondialdehyde and reduced glutathione) and Aβ40 and Aβ42 peptides were measured. The results showed that administration of erythropoietin suppressed extrinsic apoptosis better thancurcumin, while curcumin was more effective in combating oxidative stress in ICV-streptozotocin injected rats. Both erythropoietin and curcumin treatments (individually or combined) equally reduced the hippocampal amyloid-Aβ accumulation and improved cognitive impairment in Morris water maze and passive avoidance tasks. The combined treatment was the most effective in ameliorating apoptosis and oxidative stress rather than behavioral responses or β-amyloid burden. In conclusion, ICV-streptozotocin-induced Alzheimer’s dementia activates hippocampal Fas ligand-mediated apoptosis, which could be reduced by erythropoietin and/or curcumin treatment. Curcumin supplementation alone could ameliorate cognitive deficits and reverse biochemical alterations in ICV-streptozotocin Alzheimer’s rat model without the hazardous polycythemic effect of long-term erythropoietin injection [91].
Using the APPswe/PS1dE9 double transgenic mice, Wang et al (2014) [92] investigated the effects and mechanisms of curcumin in the prevention and treatment of AD. The water maze test indicated that curcumin can improve spatial learning and memory ability in mice. Immunohistochemical staining and Western blot analysis were used to test major proteins in amyloid-β aggregation, amyloid-β production, and amyloid-β clearance. Data showed that, 3 months after administration, curcumin treatment reduced Aβ40, Aβ42 and aggregation of amyloid-β-derived diffusible ligands in the mouse hippocampal CA1 area; reduced the expression of the γ-secretase component presenilin-2; and increased the expression of amyloid-β degrading enzymes, including insulin-degrading enzymes and neprilysin. This evidence suggests that curcumin, as a potential AD therapeutic method, can reduce amyloid-β pathological aggregation, possibly through mechanisms that prevent its production by inhibiting presenilin-2 and/or by accelerating its clearance by increasing degrading enzymes such as insulin-degrading enzyme and neprilysin [92]
Wang et al (2017) [93] found that curcumin reduced Aβ40, Aβ42 and Aβ-derived diffusible ligands in the mouse hippocampus, and improved learning and memory. However, the mechanisms underlying this biological effect are only partially known. There is considerable evidence in brain metabolism studies indicating that AD might be a brain-specific type of diabetes with progressive impairment of glucose utilization and insulin signaling. They hypothesized that curcumin might target both the glucose metabolism and insulin signaling pathways. They monitored brain glucose metabolism in living APPswe/PS1dE9 double transgenic mice using a micro-positron emission tomography technique. The study showed an improvement in cerebral glucose uptake in AD mice. For a more in-depth study, they used immunohistochemical staining and western blot techniques to examine key factors in both glucose metabolism and brain insulin signaling pathways. The results showed that curcumin ameliorated the defective insulin signaling pathway by upregulating insulin-like growth factor (IGF)-1R, IRS-2, PI3K, p-PI3K, Akt and p-Akt protein expression while downregulating IR and IRS-1. Their study found that curcumin improved spatial learning and memory, at least in part, by increasing glucose metabolism and ameliorating the impaired insulin signaling pathways in the brain [93].
Using 5XAD mice, Maiti and colleagues (2016) [94] studied the protective effects of dietary curcumin and nanocurcumin (NC) provide more sensitivity for labeling and imaging of Aβ plaques in brain tissues from the 5×-familial AD (5×FAD) mice than the classical amyloid-β-binding dyes, such as Congo red and Thioflavin-S. These comparisons were made in postmortem brain tissues from the 5×FAD mice. They observed that Cur and NC labeled amyloid-β plaques to the same degree as amyloid-β-specific antibody and to a greater extent than those of the classical amyloid-binding dyes. Cur and NC also labeled amyloid-β plaques in 5×FAD brain tissues when injected intraperitoneally. Nanomolar concentrations of Cur or NC are sufficient for labeling and imaging of Aβ plaques in 5×FAD brain tissue. Cur and NC also labeled different types of amyloid-β plaques, including core, neuritic, diffuse, and burned-out, to a greater degree than other amyloid-binding dyes. Therefore, Cur and or NC can be used as an alternative to amyloid-β-specific antibody for labeling and imaging of amyloid-β plaques ex vivo and in vivo. It can provide an easy and inexpensive means of detecting amyloid-β-plaque load in postmortem brain tissue of animal models of AD after anti-amyloid therapy [94].
Liu and colleagues (2016) [95] studied the curcumin’s effects against neuroinflammation in both APP/PS1 transgenic mice and beta-amyloid-induced neuroinflammation in mixed neuronal/glial cultures. They showed that curcumin significantly alleviated spatial memory deficits in APP/PS1 mice and promoted cholinergic neuronal function in vivo and in vitro. Curcumin also reduced the activation of microglia and astrocytes, as well as cytokine production and inhibited nuclear factor kappa B (NF-κB) signaling pathway, suggesting the beneficial effects of curcumin on AD are attributable to the suppression of neuroinflammation. Attenuation of these beneficial effects occurred when co-administrated with PPARγ antagonist GW9662 or silence of PPARγ gene expression, indicating that PPARγ might be involved in anti-inflammatory effects. Circular dichroism and co-immunoprecipitation analysis showed that curcumin directly bound to PPARγ and increased the transcriptional activity and protein levels of PPARγ. Taking together, these data suggested that PPARγ might be a potential target of curcumin, acting to alleviate neuroinflammation and improve neuronal function in AD [95].
Jia and colleagues (2016) [96] studied curcumin-loaded polymersomes ameliorated cognitive dysfunction in intrahippocampal Aβ42 injected mice. Due to the impermeability of the blood-brain barrier and the nonselective distribution of drugs in the brain, the therapeutic access to intractable neurological disorders is challenging. In this study, dual brain-targeting polymersomes (POs) functionalized by transferrin and Tet-1 peptide (Tf/Tet-1-POs) promoted the transportation of curcumin into the brain and provided neuroprotection. The modification of the ligands that bind to the surface of POs was revealed by X-ray photoelectron spectroscopy analysis. The cell uptake of a coculture model of mouse brain capillary endothelial cells with neurons showed that the Tf/Tet-1-POs had significant transportation properties and possessed affinity for neurons. The pharmacokinetic analysis showed that the BBB permeability-surface efficiency of the Tf/Tet-1-POs was 0.28 mL/h/g and that the brain tissue uptake rate (% ID/g) was 0.08, which were significant compared with the controls. The curcumin-encapsulated Tf/Tet-1-POs provided neuroprotection and ameliorated cognitive dysfunction in intra-hippocampal Aβ42 injected mice. These results suggest that the dual brain-targeting POs are more capable of drug delivery to the brain that can be exploited as a multiple noninvasive vehicle for targeting therapeutics [96].
Feng and colleagues (2016) [97] the insulin signal transduction pathway in APPswe/PS1dE9 double transgenic mice. Immunohistochemical staining and a western blot analysis were used to test the major proteins in the insulin signal transduction pathway. After the administration of curcumin for 6 months, the results showed that the expression of an insulin receptor and insulin receptor substrate-1 decreased in the hippocampal CA1 area of the APPswe/PS1dE9 double transgenic mice, while the expression of phosphatidylinositol-3 kinase, phosphorylated PI3K, serine-threonine kinase and phosphorylated AKT increased. Among the curcumin groups, the medium-dose group was the most effective one. Thus, they believe that curcumin may be a potential therapeutic agent that can regulate the critical molecules in brain insulin signaling pathways. Furthermore, curcumin could be adopted as one of the AD treatments to improve a patient’s learning and memory ability [97].
Using, a sensitive fluorescence-based amyloid-β digestion assay, Chen and colleagues (2016) [98] screened 25 compounds for their ability to upregulate neprilysin (NEP) activity. To our surprise, four compounds, dihydroxylated curcumin, monohydroxylated demethoxycurcumin, and mono- and di-hydroxylated bisdemethoxycurcumin, increased NEP activity, while curcumin did not. The ability of these polyhydroxycurcuminoids to upregulate NEP was further confirmed by mRNA and protein expression levels in the cell and mouse models. Finally, feeding monohydroxylated demethoxycurcumin (also named demethylcurcumin) or dihydroxylated bisdemethoxycurcumin (also named bisdemethylcurcumin) to APPswe/PS1dE9 double transgenic mice upregulated NEP levels in the brain and reduced Aβ accumulation in the hippocampus and cortex. These polyhydroxy curcuminoids offer hope in the prevention of AD [98].
He and colleagues (2016) [99] investigated the effects of curcumin on synapses of APPswe/PS1dE9 double transgenic mice, and the ultra-structures of synapses and synapse-associated proteins. Six months after administration, few abnormal synapses were observed upon electron microscopy in the hippocampal CA1 areas of the APPswe/PS1dE9 double transgenic mice. The treatment of the mice with curcuminresulted in improvements in the quantity and structure of the synapses. Immunohistochemistry and western blot analyses revealed that the expressions of PSD95 and Shank1 were reduced in the hippocampal CA1 areas of the APPswe/PS1dE9 double transgenic mice, but curcumin treatment increased the expressions of these proteins. Our findings suggest that curcumin improved the structure and function of the synapses by regulating the synapse-related proteins PSD95 and Shank1 [99].
Lim and colleagues (2001) [55] studied beneficial effects of curcumin in APPSw mouse model (Tg2576 line). They tested a low (160 ppm) and a high dose of dietary curcumin (5000 ppm) on inflammation, oxidative damage, and plaque pathology. Low and high doses of curcumin significantly lowered oxidized proteins and interleukin-1beta, a proinflammatory cytokine elevated in the brains of these mice. With low-dose but not high-dose curcumin treatment, the astrocytic marker GFAP was reduced, and insoluble amyloid-β, soluble amyloid-β, and plaque burden were significantly decreased by 43–50%. However, levels of APP in the membrane fraction were not reduced. Microgliosis was also suppressed in neuronal layers but not adjacent to plaques. In view of its efficacy and apparent low toxicity, curcumin shows promise for the prevention of AD [55].
Using 5XAD mice, McClure and colleagues (2015) [100] studied a curcumin derivative, FMeC1, and facilitate its safe delivery to the brain. Aside from the translational applicability of this approach, a study in the 5XFAD mouse model suggested that inhalation exposure to an aerosolized FMeC1 modestly improved the distribution of the compound in the brain. Additionally, immunohistochemistry data confirms that following aerosol delivery, FMeC1 binds amyloid plaques expressed in the hippocampal areas and cortex [100].
In a previous study, Zeng and colleagues (2014) [101] demonstrated the grape seed extract (GSE) could reduce brain amyloid-β burden and microglia activation, but which polyphenol plays a major role in these events is not known. They tested pharmacological effects of (−) epicatechin, one principle polyphenol compound in GSE, on transgenic AD mice. APP/PS1 transgenic mice were fed with (−) epicatechin diet (40 mg/kg/day) and curcumin diet (47 mg/kg/day) at 3 months of age for 9 months, the function of liver, amyloid-β levels in the brain and serum, AD-type neuropathology, plasma levels of inflammatory cytokines were measured. Toward the end of the experiment, they found long-term feeding of (−) epicatechin diet was well tolerated without fatality, changes in food consumption, body weight, or liver function. (−) Epicatechin significantly reduced total amyloid-β in brain and serum by 39 and 40%, respectively, compared with control diet. Microgliosis and astrocytosis in the brain of Alzheimer’s mice were also reduced by 38 and 35%, respectively. The (−) epicatechin diet did not alter learning and memory behaviors in AD mice. This study has provided evidence on the beneficial role of (−) epicatechin in ameliorating amyloid-induced AD-like pathology in AD mice, but the impact of (−) epicatechin on tau pathology is not clear, also the mechanism needs further research [101].
Yi and colleagues (2016) [79] studied effects of the co-receptor (Nogo receptor; NgR) of three axonal growth-inhibitory proteins was examined, and effects of curcumin on spatial learning and memory abilities and hippocampal axonal growth were investigated in Aβ40-induced AD rats. Results showed that the expression of NgR in the AD group significantly increased and the number of axonal protein-positive fibers significantly reduced. The spatial learning and memory abilities of AD rats were significantly improved in the curcumin group. Furthermore, hippocampal expressions of NgR mRNA and protein decreased, and the expression of axonal protein significantly increased. There was a negative correlation between the expression of NgR and axonal growth. Together, these results suggested that curcumin could improve the spatial learning and memory abilities of AD rats. The mechanism might be related with its lowering of hippocampal NgR expression and promoting axonal regeneration [79].
Rokka and colleagues (2014) [102] aimed to synthesize an (18)F-labeled curcumin derivate ([(18)F]4) and to characterize its positron emission tomography (PET) tracer-binding properties to amyloid-β plaques in a transgenic APP23 mouse model of AD. They utilized facile one-pot synthesis of [(18)F]4 using nucleophilic (18)F-fluorination and click chemistry. Binding of [(18)F]4 to amyloid-β plaques in the transgenic APP23 mouse brain cryosections was studied in vitro using heterologous competitive binding against PIB. [(18)F]4 uptake was studied ex vivo in rodents and in vivo using PET/computed tomography of transgenic APP23 and wild-type control mice. The radiochemical yield of [(18)F]4 was 21 ± 11%, the specific activity exceeded 1TBq/μmol, and the radiochemical purity exceeded 99.3% at the end of synthesis. In vitro studies of [(18)F]4 with the transgenic APP23 mouse revealed high amyloid-β plaque binding. In vivo and ex vivo studies demonstrated that [(18)F]4 has fast clearance from the blood, moderate metabolism but low blood-brain barrier (BBB) penetration. [(18)F]4 was synthesized in high yield and excellent quality. In vitro studies, metabolite profile, and fast clearance from the blood indicated a promising tracer for amyloid-β imaging [102].
Chin and colleagues (2014) [103] studied ATP levels in the brains of ApoE3 and E4 mice. They found that in the brain of 16-month-old ApoE4-targeted replacement mice, ATP concentrations were significantly lower than in ApoE3 mice. A 3-month dietary supplementation of 0.2 % curcumin numerically increased ATP concentrations in ApoE3 and significantly in ApoE4 mice compared to the respective controls. Curcumin significantly induced the transcription of peroxisome proliferator-activated receptor (PPAR) γ and mitochondrial transcription factor A in ApoE3, but not in ApoE4 mice. Moreover, PPARγ coactivator (PGC)-1α and guanine-adenine repeat binding protein α mRNA was only increased in ApoE3 mice. Consistent with these observations, protein expression of mitochondrial respiratory complexes, especially of complex IV, also appeared to be increased in ApoE3 mice. They concluded that curcumin affects mitochondrial function and gene and protein expression in the murine brain despite its low bioavailability and carriers of the AD-risk genotype ApoE4 may be less responsive to dietary curcumin than ApoE3 carriers [103].
Wang and colleagues (2014) [104] studied anti-BACE-1 and behavioral activities of curcuminoids from rhizomes of Curcuma longa (Zingiberaceae), diarylalkyls curcumin (CCN), demethoxycurcumin (DMCCN), and bisdemethoxycurcumin (BDMCCN) against AD Drosophila melanogaster models. Neuro-protective ability of the curcuminoids was assessed using Drosophila melanogaster model system overexpressing BACE-1 and its substrate APP in compound eyes and entire neurons. Feeding and climbing activity, lifespan, and morphostructural changes in fly eyes also were evaluated. BDMCCN has the strongest inhibitory activity toward BACE-1 with 17 μM IC50, which was 20 and 13 times lower than those of CCN and DMCCN respectively. Overexpression of APP/BACE-1 resulted in the progressive and measurable defects in morphology of eyes and locomotion. Remarkably, supplementing diet with either 1 mM BDMCCN or 1 mM CCN rescued APP/BACE1-expressing flies and kept them from developing both morphological and behavioral defects. Their results suggest that structural characteristics, such as degrees of saturation, types of carbon skeleton and functional group, and hydrophobicity appear to play a role in determining inhibitory potency of curcuminoids on BACE-1 [104].
Hoppe and colleagues (2013) [105] studied mechanisms by which Aβ affects neuronal excitability and curcumin ameliorates synaptic transmission in the hippocampus. Organotypic hippocampal slice cultures exposed to Aβ1-42 were used to study the neuroprotective effects of curcumin through a spectral analysis of multi-electrode array (MEA) recordings of spontaneous neuronal activity. Curcumin counteracted both deleterious effects of amyloid-β; the initial synaptic dysfunction and the later neuronal death. The analysis of MEA recordings of spontaneous neuronal activity showed an attenuation of signal propagation induced by amyloid-β before cell death and curcumin-induced alterations to local field potential (LFP) phase coherence. Curcumin-mediated attenuation of amyloid-β-induced synaptic dysfunction involved regulation of synaptic proteins, namely phospho-CaMKII and phospho-synapsin I. Taken together, these results expand the neuroprotective role of curcumin to a synaptic level. The identification of these mechanisms underlying the effects of curcumin may lead to new targets for future therapies for AD [105].
Tian and colleagues (2013) [106] studied the effects of curcumin on the cholesterol level in brain, vascular cognitive impairment and explored whether the mechanisms for those effects are through activating LXR-β/RXR-α and ABCA1 expression and apoA-I. With a Morris water test, they found that curcumin treatment could attenuate cognitive impairment. With H&E and Nissl stainings, they found that curcumin could significantly ameliorate the abnormal changes of pyramidal neurons. Meanwhile, the expression of LXR-β, RXR-α, ABCA1 and apoA-I mRNA and protein were increased in a dose-dependent manner after curcumin treatment. Interestingly, both serum HDL cholesterol and total cholesterol levels were statistically higher in the curcumin treatment group than those other groups. They concluded that curcumin has the ability to activate permissive LXR-β/RXR-α signaling and thereby modulate ABCA1 and apoA-I-mediated cholesterol transmembrane transportation, which is a new preventive and therapeutic strategy for cerebrovascular diseases [106].
Lin and colleagues (2013) [107] evaluated the effect of curcumin on amyloid fibrillation of prion protein on mouse prion protein (mPrP) in a cell-free system. Curcumin reduced the prion fibril formation significantly. Furthermore, they monitored the change in apoptosis and ROS level upon curcumin treatment in N2a cells. Curcumin effectively rescues the cells from apoptosis and decreases the ROS level caused by subsequent co-incubation with prion amyloid fibrils. The assays in cell-free mPrP and in N2a cells of this work verified the promising effect of curcumin on the prevention of transmissible neurodegenerative diseases [107].
Cheng and colleagues (2013) [108] developed a stable curcumin nanoparticle formulation to test in vitro and in AD model, Tg2576 mice. Flash nanoprecipitation of curcumin, polyethylene glycol-polylactic acid co-block polymer, and polyvinylpyrrolidone in a multi-inlet vortex mixer, followed by freeze drying with β-cyclodextrin, produced dry nanocurcumin with mean particle size <80 nm. Nanocurcumin powder, unformulated curcumin, or placebo was orally administered to Tg2576 mice for 3 months. Before and after treatment, memory was measured by radial arm maze and contextual fear conditioning tests. Nanocurcumin produced significantly better cue memory in the contextual fear conditioning test than placebo and tendencies toward better working memory in the radial arm maze test than ordinary curcumin (p=0.14) or placebo. Amyloid plaque density, pharmacokinetics, and Madin-Darby canine kidney cell monolayer penetration were measured to further understand in vivo and in vitro mechanisms. Nanocurcumin produced significantly higher curcumin concentration in plasma and six times higher area under the curve and mean residence time in brain than ordinary curcumin. The P(app) of curcumin and tetrahydro curcumin were 1.8×10(−6) and 1.6×10(−5)cm/s, respectively, for nanocurcumin. The novel nanocurcumin formulation produced highly stabilized nanoparticles with positive treatment effects in Tg2576 mice [108].
Narasingappa and colleagues (2012) [109] described new curcumin-based modified compounds as α-secretase activators. They established that the amino acid conjugates curcumin-isoleucine, curcumin-phenylalanine and curcumin-valine promote the constitutive α-secretase activity and increase ADAM10 immunoreactivity. Strikingly, experiments carried out under conditions mimicking the PKC/muscarinic receptor-regulated pathway display different patterns of activation by these compounds. Overall, their data identified new lead natural compounds for the future development of powerful and stable α-secretase activators and established that some of these molecules are able to discriminate between the constitutive and regulated α-secretase pathways [109].
Hickey and colleagues (2012) [110] studied CAG140 mice, a knock-in (KI) mouse model of HD, display abnormal aggregates of mutant huntingtin and striatal transcriptional deficits, as well as early motor, cognitive and affective abnormalities, many months prior to exhibiting spontaneous gait deficits, decreased striatal volume, and neuronal loss. They studied the ability of life-long dietary curcumin to improve the early pathological phenotype of CAG140 mice. KI mice fed a curcumin-containing diet since conception showed decreased huntingtin aggregates and increased striatal DARPP-32 and D1 receptor mRNAs, as well as an amelioration of rearing deficits. However, similar to other antioxidants, curcumin impaired rotarod behavior in both WT and KI mice and climbing in WT mice. These behavioral effects were also noted in WT mice exposed to the same curcumin regime as adults. However, neither locomotor function, behavioral despair, muscle strength or food utilization were affected by curcumin in this latter study. The clinical significance of curcumin’s impairment of motor performance in mice remains unclear because curcumin has an excellent blood chemistry and adverse event safety profile, even in the elderly and in patients with AD. Together with the clinical experience, the improvement in several transgene-dependent parameters by curcumin supports a net beneficial effect of dietary curcumin in HD [110].
Sundaram and colleagues (2017) [111] studied the effects of early intervention with a potent natural anti-inflammatory agent, curcumin, on Cdk5 dependent p25-mediated neuroinflammation and the progression of neurodegeneration in p25 transgenic mice. They found that curcumin effectively counteracted the p25-mediated glial activation and pro-inflammatory chemokines/cytokines production in p25Tg mice. Moreover, curcumin-mediated suppression of neuroinflammation reduced the progression of p25-induced tau/amyloid pathology and in turn ameliorated the p25-induced cognitive impairments. It is widely acknowledged that to treat AD, one must target the early-stage of pathological changes to protect neurons from irreversible damage. In line with this, their results demonstrated that early intervention of inflammation could reduce the progression of AD-like pathological outcomes. Moreover, their data provide a rationale for the potential use of curcuminoids in the treatment of inflammation associated neurodegenerative diseases [111].
Cashman and colleagues (2012) [112] the effects of curcumin inhibitory properties of inflammatory processes of PBMCs from ALS patients. They developed an in vitro system using human monocytes from patients and monocytic cell lines (i.e. U-937, THP-1) for evaluating curcuminoid potency of innate immune cell stimulation. Bisdemethoxycurcumin and certain analogs potentiated MGAT3, VDR and TLR gene expression 3- to 300-fold in U-937 cells. The effect of curcumins on inflammation in monocytes from patients with ALS was examined. Recursive medicinal chemistry was applied to identify compounds that stimulate the innate immune system for use in the clearance of amyloid-β in AD and the reversal of neuroinflammation and defective SOD-1 accumulation in ALS [112].
Yang and colleagues (2011) [113] evaluated the bone microarchitecture and bone mineral density (BMD) of the proximal tibia in APP/PS1 transgenic mice by micro-computed tomography (micro-CT), and to search for evidence that curcumin can be used to reduce bone mineral losses and treat osteoporosis after senile dementia in these transgenic mice. Three-month-old female mice were divided into the following groups: wild-type mice; APP/PS1 transgenic mice (APP group); and APP/PS1 transgenic mice with curcumin treatment (APP+Cur group). Between 9 and 12 months of age, the APP+Cur group were administered curcumin orally (600ppm). CT scans of the proximal tibia were taken at 6, 9 and 12 months. At 6 months, there were little differences in the structural parameters. At 9 months, the APP groups displayed loss of bone volume ratio (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and connectivity density (Conn.D) and increases in trabecular separation (Tb.Sp) and geometric degree of anisotropy, with significant changes in the BMD parameters. At 12 months, curcumin treatment led to constant increases in the trabecular bone mass of the metaphysis and clearly improved the BMD. By the same time, they measured the TNF-α and IL-6 in the serum among the different groups at 6, 9 and 12 months by ELISA. These results suggest that APP/PS1 transgenic mice are susceptible to osteoporosis, and that curcumin can prevent further deterioration of the bone structure and produce beneficial changes in bone turnover. This study suggests that the changes of inflammation cytokines, including TNF-α and IL-6, may play an important role in the mechanisms of action of curcumin [113].
Zhang and colleagues (2010) [114] investigated the effects of curcumin on amyloid-β levels and APP processing in various cell lines and mouse primary cortical neurons. They show that curcumin potently lowers amyloid-β levels by attenuating the maturation of APP in the secretory pathway. These data provide a mechanism of action for the ability of curcumin to attenuate amyloid-β pathology [114].
Ahmed and colleagues (2010) [115] investigated the effects of curcuminoid mixture and individual constituents on spatial learning and memory in an amyloid-β peptide-infused rat model of AD and on the expression of PSD-95, synaptophysin and camkIV. Curcuminoid mixture showed a memory-enhancing effect in rats displaying AD-like neuronal loss only at 30 mg/kg, whereas individual components were effective at 3–30 mg/kg. A shorter duration treatment with test compounds showed that the curcuminoid mixture and bisdemethoxycurcumin increased PSD-95 expression in the hippocampus at 3–30 mg/kg, with maximum effect at a lower dose (3 mg/kg) with respective values of 470.5 and 587.9%. However, after a longer duration treatment, two other compounds (demethoxycurcumin and curcumin) also increased PSD-95 to 331.7 and 226.2% respectively at 30 mg/kg. When studied for their effect on synaptophysin in the hippocampus after the longer duration treatment, the curcuminoid mixture and all three individual constituents increased synaptophysin expression. Of these, demethoxycurcumin was the most effective showing a 350.1% increase at 30 mg/kg compared to the neurotoxin group. When studied for their effect on camkIV expression after longer treatment in the hippocampus, only demethoxycurcumin at 30 mg/kg increased levels to 421.2%. These compounds salvaged PSD-95, synaptophysin and camkIV expression levels in the hippocampus in the rat AD model, which suggests multiple target sites with the potential of curcuminoids in spatial memory enhancing and disease modifying in AD [115].
Ahmed and Gilani (2009) [116] evaluated if curcuminoids possess AChE inhibitory and memory enhancing activities. The in vitro and ex vivo models of AChE inhibitory activity were used along with Morris water maze test to study the effect on memory in rats. Curcuminoids inhibited AChE in the in vitro assay with IC (50) value of 19.67, bisdemethoxycurcumin 16.84, demethoxycurcumin 33.14 and curcumin 67.69 M. In the ex-vivo AChE assay, curcuminoids and its individμual components except curcumin showed dose-dependent (3–10 mg/kg) inhibition in frontal cortex and hippocampus. When studied for their effect on memory at a fixed dose (10 mg/kg), all compounds showed significant and comparable effect in scopolamine-induced amnesia. These data indicate that curcuminoids and all individual components except curcumin possess pronounced AChE inhibitory activity. Curcumin was relatively weak in the in vitro assay and without effect in the ex-vivo AChE model, while equally effective in memory enhancing effect, suggestive of additional mechanism(s) involved. Thus, curcuminoids mixture might possess better therapeutic profile than curcumin for its medicinal use in AD [116].
Pan and colleagues (2008) [117] studied the effects of memory improving properties of curcumin mice and investigated the neuroprotective effect of curcumin in vitro and in vivo. The mice were given AlCl (3) orally and injections of D-galactose intraperitoneally for 90 days to establish the AD animal model. From day 45, the curcumin group was treated with curcumin for 45 days. Subsequently, the step-through test, neuropathological changes in the hippocampus and the expression of Bax and Bcl-2 were carried out to evaluate the effect of curcumin on the AD model mice. In cultured PC12 cells, AlCl (3) exposure induced apoptosis. The MTT assay was used to measure cell viabilities; flow cytometric analysis to survey the rate of cell apoptosis; DNA-binding fluorochrome Hoechst 33258 to observe nuclei changes in apoptotic cells and Western blot analysis of Bax, Bcl-2 to investigate the mechanisms by which curcumin protects cells from toxicity. Curcumin significantly improved the memory ability of AD mice in the step-through test, as indicated by the reduced number of step-through errors and prolonged step-through latency. Curcumin also attenuated the neuropathological changes in the hippocampus and inhibited apoptosis accompanied by an increase in Bcl-2 level, but the activity of Bax did not change. AlCl (3) significantly reduced the viability of PC12 cells. Curcumin increased cell viability in the presence of AlCl (3). The rate of apoptosis decreased significantly in the curcumin group when measured by flow cytometric analysis. Curcumin protected cells by increasing Bcl-2 level, but the level of Bax did not change. This study demonstrates that curcumin improves the memory ability of AD mice and inhibits apoptosis in cultured PC12 cells induced by AlCl (3). They conclude that the mechanism may involve enhancing the level of Bcl-2 [117].
Using in vivo multiphoton microscopy (MPM), Garcia-Alloza and colleagues (2007) [50] demonstrate that curcumin crosses the blood-brain barrier and labels senile plaques and cerebrovascular amyloid angiopathy (CAA) in APPswe/PS1dE9 mice. Moreover, systemic treatment of mice with curcumin for 7 days clears and reduces existing plaques, as monitored with longitudinal imaging, suggesting a potent disaggregation effect. Curcumin also led to a limited, but significant reversal of structural changes in dystrophic dendrites, including abnormal curvature and dystrophy size. Together, these data suggest that curcumin reverses existing amyloid pathology and associated neurotoxicity in a mouse model of AD. This approach could lead to more effective clinical therapies for the prevention of oxidative stress, inflammation and neurotoxicity associated with AD [50].
Yang and colleagues (2005) [31] studied aggregation inhibitory properties of curcumin in AD. Under aggregating conditions in vitro, curcumin inhibited aggregation as well as disaggregated fibrillar Aβ42 IC, indicating favorable stoichiometry for inhibition. Curcumin was a better Aβ40 aggregation inhibitor than ibuprofen and naproxen, and prevented Aβ42 oligomer formation and toxicity between 0.1 and 1.0 μM. Under EM, curcumin decreased dose dependently amyloid-β fibril formation beginning with 0.125 μM. The effects of curcumin did not depend on amyloid-β sequence but on fibril-related conformation. AD and APP mice (Tg2576 line) brain sections incubated with curcumin revealed preferential labeling of amyloid plaques. In vivo studies showed that curcumin injected peripherally into aged Tg mice crossed the blood-brain barrier and bound plaques. When fed to aged APP mice with advanced amyloid-β accumulation, curcumin labeled plaques and reduced amyloid-β levels and plaque burden. Hence, curcumin directly binds small amyloid-β species to block aggregation and fibril formation in vitro and in vivo. These data suggest that low dose curcumin effectively disaggregates amyloid-β as well as prevents fibril and oligomer formation, supporting the rationale for curcumin use in clinical trials preventing or treating AD [31].
Overall, findings of these strongly suggest that curcumin and its derivatives have multiple beneficial effects against amyloid-β-induced cognitive impairments and inhibit amyloid-β aggregations and enhances synaptic activities in mouse models of AD.
12. Research on Curcumin in Human Studies
Based on positive and beneficial findings from cell and animal models, several groups conducted clinical studies on humans using curcumin and its derivatives. Summaries of these studies are given below:
Brondino and colleagues (2014) [118] assessed curcumin in clinical trials in AD patients. They evaluated the state-of-the-art of clinical trials of curcumin in AD. They retrieved three published studies, while there are several ongoing clinical trials. To date there is insufficient evidence to suggest the use of curcumin in dementia patients. Of note, short-term use of curcumin appears to be safe. Several reasons could be responsible for the discrepancy between in vitro and in vivo findings and human trials, such as low bioavailability and poor study design [118].
Rainey-Smith and colleagues (2016) [119] conducted for a 12-month period, randomized, placebo-controlled and double-blinded a population of community-dwelling older adults for cognitive function. They tested cognitive function on individuals (n=96) ingested either placebo or 1500 mg/d BiocurcumaxTM for 12 months. The results of a 12-month, revealed that individuals (n=96) ingested either placebo or 1500 mg/d BiocurcumaxTM for 12 months. A battery of clinical and cognitive measures was administered at baseline and at the 6-month and 12-month follow-up assessments. A significant time×treatment group interaction was observed for the Montreal Cognitive Assessment (repeated-measures analysis; time×treatment; F=3·85, P<0·05). Subsequent analysis revealed that this association was driven by a decline in function of the placebo group at 6 months that was not observed in the curcumin treatment group. No differences were observed between the groups for all other clinical and cognitive measures. These findings suggest that further longitudinal assessment is required to investigate changes in cognitive outcome measures, ideally in conjunction with biological markers of neurodegeneration [119].
Veldman and colleagues (2016) [120] studied the efficacies of binding of curcumin analogues, curcuminoids, to amyloid-β containing plaques in postmortem tissue from AD patients. In vitro autoradiography was utilized to explore affinity and displacement of the curcuminoids; curcumin, demethoxycurcumin (DMC), bisdemethoxycurcumin (BDMC) and dimethoxycurcumin (DIMC). They found that BDMC had the highest affinity for amyloid-β containing plaques in cortical AD brain tissue in comparison to other curcuminoids. Subsequently, [(3)H]BDMC showed significantly higher specific binding in cortical AD brain tissue compared to control subjects. These findings suggest that curcumin analogues, especially BDMC, may serve as potential radioligands for amyloid-β plaque neuroimaging [120].
These studies have provided compelling evidence indicating that curcumin has beneficial effects in human studies. Further human clinical studies are needed to confirm beneficial effects of curcumin in humans with AD and subjects with mild cognitive impairment.
13. Conclusions and Future Directions
Natural products have been used as diets and dietary supplements to treat human diseases, including cancer, cardiovascular, respiratory, infectious, diabetes, obesity, metabolic syndromes and neurological disorders. Plant and herbal products are known to have multiple protective effects, including anti-inflammatory, anti-oxidant, anti-arthritis and boosting memory cognitive functions in humans. Recent curcumin research revealed that curcumin boosts cognitive functions in humans. Several groups have designed and synthesized curcumin and its derivatives and extensively tested using cell and mouse models of AD and reported that curcumin has strong anti-amyloid beta aggregation property. Using mouse models of AD, recently several groups studied the BBB crossing properties of curcumin and provided compelling evidence indicating that curcumin crosses BBB. In addition, several groups reported that curcumin ameliorates cognitive decline and improves synaptic functions in mouse models of AD. Further, studies on AD patients and elderly individuals have been initiated and the outcome of these studies is currently being assessed. However, we do not know the answers to the following questions: 1) It is unclear, whether curcumin can improve and/or maintain mitochondrial function in the presence of mutant proteins such as phosphorylated tau and Aβ in AD. 2) Further, we do not have complete molecular understanding of how curcumin protects AD neurons. 3) Currently, we do not know, whether curcumin delay and/or prevent disease progression of AD in humans. Further research is still needed to answer these important questions.
Acknowledgments
Work presented in this article is supported by NIH grants – AG042178, AG47812 and NS105473, the Garrison Family Foundation, and Sex and Gender Alzheimer’s Association (SAGA) grant (to PHR). Present work is also supported by Alzheimer’s Association New Investigator Research Grant 2016-NIRG-39787 and Center of Excellence for Translational Neuroscience and Therapeutics grant number PN-CTNT20115-AR and Sex and Gender Alzheimer’s Association (SAGA) grant (to APR).
Abbreviations
- Aβ
Amyloid beta
- APP
Amyloid precursor protein
- NFTs
Neurofibrillary tangles
- PS1
Preseniin 1
- PS2
Presenilin 2
- PET
Positron emission tomography
- ApoE4
Apolipoprotein epsilon 4 genotype
- ROS
Reactive oxygen species
- ATP
Adenosine triphosphate
- BBB
blood brain barrier
References
- 1.Mohd Sairazi NS, Sirajudeen KN, Asari MA, Muzaimi M, Mummedy S, Sulaiman SA. Kainic Acid-Induced Excitotoxicity Experimental Model: Protective Merits of Natural Products and Plant Extracts. Evid Based Complement Alternat Med. 2015;2015:972623. doi: 10.1155/2015/972623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solanki I, Parihar P, Mansuri ML, Parihar MS. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv Nutr. 2015;6:64–72. doi: 10.3945/an.114.007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6:154–168. doi: 10.3945/an.114.007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spagnuolo C, Napolitano M, Tedesco I, Moccia S, Milito A, Russo GL. Neuroprotective Role of Natural Polyphenols. Curr Top Med Chem. 2016;16:1943–1950. doi: 10.2174/1568026616666160204122449. [DOI] [PubMed] [Google Scholar]
- 5.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11:338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine. 2000;7:303–308. doi: 10.1016/S0944-7113(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 10.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 2010;10:605–610. doi: 10.1016/j.intimp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thuringer A, Leski K, Fickert P, Karpen SJ, Trauner M. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 14.Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Shukla PK, Khanna VK, Khan MY, Srimal RC. Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol. 2003;22:653–658. doi: 10.1191/0960327103ht411oa. [DOI] [PubMed] [Google Scholar]
- 16.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 19.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis 20 Suppl. 2010;2:S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Alzheimer Report. 2015. [Google Scholar]
- 22.Mao P, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer’s disease: implications for early intervention and therapeutics. Biochim Biophys Acta. 2011;1812:1359–1370. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valasani KR, Sun Q, Fang D, Zhang Z, Yu Q, Guo Y, Li J, Roy A, Yan SS. Identification of a small molecule Cyclophilin D inhibitor for rescuing Aβ-mediated mitochondrial dysfunction. ACS Med Chem Lett. 2016;7:294–299. doi: 10.1021/acsmedchemlett.5b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valasani KR, Vangavaragu JR, Day VW, Yan SS. Structure based design, synthesis, pharmacophore modeling, virtual screening, and molecular docking studies for identification of novel cyclophilin D inhibitors. J Chem Inf Model. 2014;54:902–912. doi: 10.1021/ci5000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valasani KR, Sun Q, Hu G, Li J, Du F, Guo Y, Carlson EA, Gan X, Yan SS. Identification of Human ABAD Inhibitors for Rescuing Abeta-Mediated Mitochondrial Dysfunction. Current Alzheimer Res. 2014;11:128–136. doi: 10.2174/1567205011666140130150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima H, Ishihara T, Yokota O, Terada S, Trojanowski JQ, Lee VM, Kuroda S. Effects of alpha-tocopherol on an animal model of tauopathies. Free Radic Biol Med. 2004;37:176–186. doi: 10.1016/j.freeradbiomed.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, Trojanowski JQ, Lee VM, McIntosh TK, Pratico D. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 28.Sung S, Yao Y, Uryu K, Yang H, Lee VM, Trojanowski JQ, Pratico D. Early vitamin E supplementation in young but not aged mice reduces Abeta levels and amyloid deposition in a transgenic model of Alzheimer’s disease. FASEB J. 2004;18:323–325. doi: 10.1096/fj.03-0961fje. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara E, Bryant-Thomas T, Pacheco Quinto J, Henry TL, Poeggeler B, Herbert D, Cruz-Sanchez F, Chyan YJ, Smith MA, Perry G, Shoji M, Abe K, Leone A, Grundke-Ikbal I, Wilson GL, Ghiso J, Williams C, Refolo LM, Pappolla MA, Chain DG, Neria E. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer’s disease. J Neurochem. 2003;85:1101–1108. doi: 10.1046/j.1471-4159.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 30.Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer’s disease by chronic Ginkgo biloba treatment. Exp Neurol. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- 31.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 32.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS, Aggarwal NT, Scherr PA. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 34.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ Alzheimer’s Disease Cooperative S. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 35.Cornelli U. Treatment of Alzheimer’s disease with a cholinesterase inhibitor combined with antioxidants. Neurodegener Dis. 2010;7:193–202. doi: 10.1159/000295663. [DOI] [PubMed] [Google Scholar]
- 36.Schneider LS, Raman R, Schmitt FA, Doody RS, Insel P, Clark CM, Morris JC, Reisberg B, Petersen RC, Ferris SH. Characteristics and performance of a modified version of the ADCS-CGIC CIBIC+ for mild cognitive impairment clinical trials. Alzheimer Dis Assoc Disord. 2009;23:260–267. doi: 10.1097/WAD.0b013e31819cb760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL Alzheimer’s Disease Cooperative Study G. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology. 2009;72:2115–2121. doi: 10.1212/WNL.0b013e3181aa52d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloret A, Badia MC, Mora NJ, Pallardo FV, Alonso MD, Vina J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. J Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 39.Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 40.Isaac MG, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2008:CD002854. doi: 10.1002/14651858.CD002854.pub2. [DOI] [PubMed] [Google Scholar]
- 41.DeCarli C, Frisoni GB, Clark CM, Harvey D, Grundman M, Petersen RC, Thal LJ, Jin S, Jack CR, Jr, Scheltens P Alzheimer’s Disease Cooperative Study G. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 42.Burns A, O’Brien J, Auriacombe S, Ballard C, Broich K, Bullock R, Feldman H, Ford G, Knapp M, McCaddon A, Iliffe S, Jacova C, Jones R, Lennon S, McKeith I, Orgogozo JM, Purandare N, Richardson M, Ritchie C, Thomas A, Warner J, Wilcock G, Wilkinson D British Association for P, group BAPDC. Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J Psychopharmacol. 2006;20:732–755. doi: 10.1177/0269881106068299. [DOI] [PubMed] [Google Scholar]
- 43.Sparks DL, Sabbagh M, Connor D, Soares H, Lopez J, Stankovic G, Johnson-Traver S, Ziolkowski C, Browne P. Statin therapy in Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:78–86. doi: 10.1111/j.1600-0404.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 44.Ciabattoni G, Porreca E, Di Febbo C, Di Iorio A, Paganelli R, Bucciarelli T, Pescara L, Del Re L, Giusti C, Falco A, Sau A, Patrono C, Davi G. Determinants of platelet activation in Alzheimer’s disease. Neurobiol Aging. 2007;28:336–342. doi: 10.1016/j.neurobiolaging.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Pham DQ, Plakogiannis R. Vitamin E supplementation in Alzheimer’s disease, Parkinson’s disease, tardive dyskinesia, and cataract: Part 2. Ann Pharmacother. 2005;39:2065–2072. doi: 10.1345/aph.1G271. [DOI] [PubMed] [Google Scholar]
- 46.Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer’s disease. Ann Pharmacother. 2005;39:2073–2080. doi: 10.1345/aph.1E495. [DOI] [PubMed] [Google Scholar]
- 47.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ Alzheimer’s Disease Cooperative Study G. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 48.Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA, Field TS, Evans DA. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Tang M, Taghibiglou C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J Alzheimers Dis. 2017;58:1003–1016. doi: 10.3233/JAD-170188. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 51.Park SY, Kim DS. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer’s disease. J Nat Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- 52.Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 53.Narlawar R, Pickhardt M, Leuchtenberger S, Baumann K, Krause S, Dyrks T, Weggen S, Mandelkow E, Schmidt B. Curcumin-derived pyrazoles and isoxazoles: Swiss army knives or blunt tools for Alzheimer’s disease? ChemMedChem. 2008;3:165–172. doi: 10.1002/cmdc.200700218. [DOI] [PubMed] [Google Scholar]
- 54.Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1–42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]
- 55.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:3381–3386. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004;6:367–377. doi: 10.3233/jad-2004-6403. discussion 443–369. [DOI] [PubMed] [Google Scholar]
- 59.Shen L, Liu CC, An CY, Ji HF. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci Rep. 2016;6:20872. doi: 10.1038/srep20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem Toxicol. 2015;83:111–124. doi: 10.1016/j.fct.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 61.Rao PP, Mohamed T, Teckwani K, Tin G. Curcumin Binding to Beta Amyloid: A Computational Study. Chem Biol Drug Des. 2015;86:813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari E, Benassi R, Sacchi S, Pignedoli F, Asti M, Saladini M. Curcumin derivatives as metal-chelating agents with potential multifunctional activity for pharmaceutical applications. J Inorg Biochem. 2014;139:38–48. doi: 10.1016/j.jinorgbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Konno H, Endo H, Ise S, Miyazaki K, Aoki H, Sanjoh A, Kobayashi K, Hattori Y, Akaji K. Synthesis and evaluation of curcumin derivatives toward an inhibitor of beta-site amyloid precursor protein cleaving enzyme 1. Bioorg Med Chem Lett. 2014;24:685–690. doi: 10.1016/j.bmcl.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 64.Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of alpha-synuclein. Acta Neuropathol. 2008;115:479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- 65.Lakey-Beitia J, Gonzalez Y, Doens D, Stephens DE, Santamaria R, Murillo E, Gutierrez M, Fernandez PL, Rao KS, Larionov OV, Durant-Archibold AA. Assessment of Novel Curcumin Derivatives as Potent Inhibitors of Inflammation and Amyloid-beta Aggregation in Alzheimer’s Disease. J Alzheimers Dis. 2017 doi: 10.3233/JAD-170071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okuda M, Hijikuro I, Fujita Y, Teruya T, Kawakami H, Takahashi T, Sugimoto H. Design and synthesis of curcumin derivatives as tau and amyloid beta dual aggregation inhibitors. Bioorg Med Chem Lett. 2016;26:5024–5028. doi: 10.1016/j.bmcl.2016.08.092. [DOI] [PubMed] [Google Scholar]
- 67.Yan J, Hu J, Liu A, He L, Li X, Wei H. Design, synthesis, and evaluation of multitarget-directed ligands against Alzheimer’s disease based on the fusion of donepezil and curcumin. Bioorg Med Chem. 2017;25:2946–2955. doi: 10.1016/j.bmc.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 68.Mishra CB, Kumari S, Manral A, Prakash A, Saini V, Lynn AM, Tiwari M. Design, synthesis, in-silico and biological evaluation of novel donepezil derivatives as multi-target-directed ligands for the treatment of Alzheimer’s disease. Eur J Med Chem. 2017;125:736–750. doi: 10.1016/j.ejmech.2016.09.057. [DOI] [PubMed] [Google Scholar]
- 69.Awasthi M, Singh S, Pandey VP, Dwivedi UN. Modulation in the conformational and stability attributes of the Alzheimer’s disease associated amyloid-beta mutants and their favorable stabilization by curcumin: molecular dynamics simulation analysis. J Biomol Struct Dyn. 2017:1–16. doi: 10.1080/07391102.2017.1279078. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Peng P, Tang L, Hu Y, Hu Y, Sheng R. Design, synthesis and evaluation of rivastigmine and curcumin hybrids as site-activated multitarget-directed ligands for Alzheimer’s disease therapy. Bioorg Med Chem. 2014;22:4717–4725. doi: 10.1016/j.bmc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, Karmakar M, Kumari M, Chauhan LK, Patel DK, Srivastava V, Singh D, Gupta SK, Tripathi A, Chaturvedi RK, Gupta KC. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano. 2014;8:76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 72.Fang L, Gou S, Liu X, Cao F, Cheng L. Design, synthesis and anti-Alzheimer properties of dimethylaminomethyl-substituted curcumin derivatives. Bioorg Med Chem Lett. 2014;24:40–43. doi: 10.1016/j.bmcl.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 73.Shakeri A, Sahebkar A. Optimized curcumin formulations for the treatment of Alzheimer’s disease: A patent evaluation. J Neurosci Res. 2016;94:111–113. doi: 10.1002/jnr.23696. [DOI] [PubMed] [Google Scholar]
- 74.Hu L, Shi Y, Li JH, Gao N, Ji J, Niu F, Chen Q, Yang X, Wang S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSci Tech. 2015;16:1327–1334. doi: 10.1208/s12249-014-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Teng E, Hu S, Chen PP, Maiti P, Teter B, Cole GM, Frautschy SA. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J Biol Chem. 2013;288:4056–4065. doi: 10.1074/jbc.M112.393751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, Ballestrazzi A, Vandelli MA, Tosi G, Grabrucker AM. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017;526:413–424. doi: 10.1016/j.ijpharm.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi: 10.1016/j.ejphar.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 78.Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Kandimalla R, Kuruva CS. Protective effects of a natural product, curcumin, against amyloid beta induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J Investig Med. 2016;64:1220–1234. doi: 10.1136/jim-2016-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yi X, Feng C, Hu S, Li H, Wang J. Surface plasmon resonance biosensors for simultaneous monitoring of amyloid-beta oligomers and fibrils and screening of select modulators. Analyst. 2016;141:331–336. doi: 10.1039/c5an01864a. [DOI] [PubMed] [Google Scholar]
- 80.Uguz AC, Oz A, Naziroglu M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J Recept Signal Transduct Res. 2016;36:395–401. doi: 10.3109/10799893.2015.1108337. [DOI] [PubMed] [Google Scholar]
- 81.Chandra B, Mithu VS, Bhowmik D, Das AK, Sahoo B, Maiti S, Madhu PK. Curcumin Dictates Divergent Fates for the Central Salt Bridges in Amyloid-beta40 and Amyloid-beta42. Biophys J. 2017;112:1597–1608. doi: 10.1016/j.bpj.2017.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu W, Ma H, DaSilva NA, Rose KN, Johnson SL, Zhang L, Wan C, Dain JA, Seeram NP. Development of a neuroprotective potential algorithm for medicinal plants. Neurochem Int. 2016;100:164–177. doi: 10.1016/j.neuint.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ngo ST, Fang ST, Huang SH, Chou CL, Huy PD, Li MS, Chen YC. Anti-arrhythmic Medication Propafenone a Potential Drug for Alzheimer’s Disease Inhibiting Aggregation of Abeta: In Silico and in Vitro Studies. J Chem Inf Model. 2016;56:1344–1356. doi: 10.1021/acs.jcim.6b00029. [DOI] [PubMed] [Google Scholar]
- 84.Xiao Z, Zhang A, Lin J, Zheng Z, Shi X, Di W, Qi W, Zhu Y, Zhou G, Fang Y. Telomerase: a target for therapeutic effects of curcumin and a curcumin derivative in Abeta1–42 insult in vitro. PLoS One. 2014;9:e101251. doi: 10.1371/journal.pone.0101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng Y, Lu X, Wang L, Li T, Ding Y, Cao H, Zhang Y, Guo X, Yu G. Curcumin inhibits the AKT/NF-kappaB signaling via CpG demethylation of the promoter and restoration of NEP in the N2a cell line. AAPS J. 2014;16:649–657. doi: 10.1208/s12248-014-9605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Q, Jia N, Wang W, Jin H, Xu J, Hu H. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-beta25–35 in rat cortical neurons. Biochem Biophys Res Commun. 2014;448:89–94. doi: 10.1016/j.bbrc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 87.Huang HC, Tang D, Xu K, Jiang ZF. Curcumin attenuates amyloid-beta-induced tau hyperphosphorylation in human neuroblastoma SH-SY5Y cells involving PTEN/Akt/GSK-3beta signaling pathway. J Recept Signal Transduct Res. 2014;34:26–37. doi: 10.3109/10799893.2013.848891. [DOI] [PubMed] [Google Scholar]
- 88.Yin W, Zhang X, Li Y. Protective effects of curcumin in APPswe transfected SH-SY5Y cells. Neural Regen Res. 2012;7:405–412. doi: 10.3969/j.issn.1673-5374.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L, Fang Y, Xu Y, Lian Y, Xie N, Wu T, Zhang H, Sun L, Zhang R, Wang Z. Curcumin Improves Amyloid beta-Peptide (1–42) Induced Spatial Memory Deficits through BDNF-ERK Signaling Pathway. PLoS One. 2015;10:e0131525. doi: 10.1371/journal.pone.0131525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elmegeed GA, Ahmed HH, Hashash MA, Abd-Elhalim MM, El-kady DS. Synthesis of novel steroidal curcumin derivatives as anti-Alzheimer’s disease candidates: Evidences-based on in vivo study. Steroids. 2015;101:78–89. doi: 10.1016/j.steroids.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 91.Samy DM, Ismail CA, Nassra RA, Zeitoun TM, Nomair AM. Downstream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer’s dementia in rats: Erythropoietin versus curcumin. Eur J Pharmacol. 2016;770:52–60. doi: 10.1016/j.ejphar.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 92.Wang P, Su C, Li R, Wang H, Ren Y, Sun H, Yang J, Sun J, Shi J, Tian J, Jiang S. Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J Neurosci Res. 2014;92:218–231. doi: 10.1002/jnr.23322. [DOI] [PubMed] [Google Scholar]
- 93.Wang P, Su C, Feng H, Chen X, Dong Y, Rao Y, Ren Y, Yang J, Shi J, Tian J, Jiang S. Curcumin regulates insulin pathways and glucose metabolism in the brains of APPswe/PS1dE9 mice. Int J Immunopathol Pharmacol. 2017;30:25–43. doi: 10.1177/0394632016688025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maiti P, Hall TC, Paladugu L, Kolli N, Learman C, Rossignol J, Dunbar GL. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5x-familial Alzheimer’s disease mice. Histochem Cell Biol. 2016;146:609–625. doi: 10.1007/s00418-016-1464-1. [DOI] [PubMed] [Google Scholar]
- 95.Liu ZJ, Li ZH, Liu L, Tang WX, Wang Y, Dong MR, Xiao C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front Pharmacol. 2016;7:261. doi: 10.3389/fphar.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jia T, Sun Z, Lu Y, Gao J, Zou H, Xie F, Zhang G, Xu H, Sun D, Yu Y, Zhong Y. A dual brain-targeting curcumin-loaded polymersomes ameliorated cognitive dysfunction in intrahippocampal amyloid-beta1-42-injected mice. Int J Nanomedicine. 2016;11:3765–3775. doi: 10.2147/IJN.S94622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng HL, Dang HZ, Fan H, Chen XP, Rao YX, Ren Y, Yang JD, Shi J, Wang PW, Tian JZ. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int J Immunopathol Pharmacol. 2016;29:734–741. doi: 10.1177/0394632016659494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen PT, Chen ZT, Hou WC, Yu LC, Chen RP. Polyhydroxycurcuminoids but not curcumin upregulate neprilysin and can be applied to the prevention of Alzheimer’s disease. Sci Rep. 2016;6:29760. doi: 10.1038/srep29760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He Y, Wang P, Wei P, Feng H, Ren Y, Yang J, Rao Y, Shi J, Tian J. Effects of curcumin on synapses in APPswe/PS1dE9 mice. Int J Immunopathol Pharmacol. 2016;29:217–225. doi: 10.1177/0394632016638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McClure R, Yanagisawa D, Stec D, Abdollahian D, Koktysh D, Xhillari D, Jaeger R, Stanwood G, Chekmenev E, Tooyama I, Gore JC, Pham W. Inhalable curcumin: offering the potential for translation to imaging and treatment of Alzheimer’s disease. J Alzheimers Dis. 2015;44:283–295. doi: 10.3233/JAD-140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zeng YQ, Wang YJ, Zhou XF. Effects of (−)Epicatechin on the Pathology of APP/PS1 Transgenic Mice. Front Neurol. 2014;5:69. doi: 10.3389/fneur.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rokka J, Snellman A, Zona C, La Ferla B, Nicotra F, Salmona M, Forloni G, Haaparanta-Solin M, Rinne JO, Solin O. Synthesis and evaluation of a (18)F-curcumin derivate for beta-amyloid plaque imaging. Bioorg Med Chem. 2014;22:2753–2762. doi: 10.1016/j.bmc.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 103.Chin D, Hagl S, Hoehn A, Huebbe P, Pallauf K, Grune T, Frank J, Eckert GP, Rimbach G. Adenosine triphosphate concentrations are higher in the brain of APOE3-compared to APOE4-targeted replacement mice and can be modulated by curcumin. Genes Nutr. 2014;9:397. doi: 10.1007/s12263-014-0397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Kim JR, Lee SB, Kim YJ, Jung MY, Kwon HW, Ahn YJ. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complement Altern Med. 2014;14:88. doi: 10.1186/1472-6882-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoppe JB, Haag M, Whalley BJ, Salbego CG, Cimarosti H. Curcumin protects organotypic hippocampal slice cultures from Abeta1-42-induced synaptic toxicity. Toxicol In Vitro. 2013;27:2325–2330. doi: 10.1016/j.tiv.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Tian M, Zhang X, Wang L, Li Y. Curcumin induces ABCA1 expression and apolipoprotein A-I-mediated cholesterol transmembrane in the chronic cerebral hypoperfusion aging rats. Am J Chin Med. 2013;41:1027–1042. doi: 10.1142/S0192415X13500699. [DOI] [PubMed] [Google Scholar]
- 107.Lin CF, Yu KH, Jheng CP, Chung R, Lee CI. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens. 2013;2:506–519. doi: 10.3390/pathogens2030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15:324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Narasingappa RB, Javagal MR, Pullabhatla S, Htoo HH, Rao JK, Hernandez JF, Govitrapong P, Vincent B. Activation of alpha-secretase by curcumin-aminoacid conjugates. Biochem Biophys Res Commun. 2012;424:691–696. doi: 10.1016/j.bbrc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 110.Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, Maiti P, Frautschy SA, Zeitlin S, Levine MS, Chesselet MF. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol Neurodegener. 2012;7:12. doi: 10.1186/1750-1326-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sundaram JR, Poore CP, Sulaimee NHB, Pareek T, Cheong WF, Wenk MR, Pant HC, Frautschy SA, Low C-M, Kesavapathy Curcumin ameliorates neuroinflmmation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmraks of Alzheimer’s disease. 2017 doi: 10.3233/JAD-170093. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cashman JR, Gagliardi S, Lanier M, Ghirmai S, Abel KJ, Fiala M. Curcumins promote monocytic gene expression related to beta-amyloid and superoxide dismutase clearance. Neurodegener Dis. 2012;10:274–276. doi: 10.1159/000333123. [DOI] [PubMed] [Google Scholar]
- 113.Yang MW, Wang TH, Yan PP, Chu LW, Yu J, Gao ZD, Li YZ, Guo BL. Curcumin improves bone microarchitecture and enhances mineral density in APP/PS1 transgenic mice. Phytomedicine. 2011;18:205–213. doi: 10.1016/j.phymed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 114.Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. 2010;285:28472–28480. doi: 10.1074/jbc.M110.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ahmed T, Enam SA, Gilani AH. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience. 2010;169:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav. 2009;91:554–559. doi: 10.1016/j.pbb.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 117.Pan R, Qiu S, Lu DX, Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin Med J (Engl) 2008;121:832–839. [PubMed] [Google Scholar]
- 118.Brondino N, Re S, Boldrini A, Cuccomarino A, Lanati N, Barale F, Politi P. Curcumin as a therapeutic agent in dementia: a mini systematic review of human studies. ScientificWorldJournal. 2014;2014:174282. doi: 10.1155/2014/174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rainey-Smith SR, Brown BM, Sohrabi HR, Shah T, Goozee KG, Gupta VB, Martins RN. Curcumin and cognition: a randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br J Nutr. 2016;115:2106–2113. doi: 10.1017/S0007114516001203. [DOI] [PubMed] [Google Scholar]
- 120.Veldman ER, Jia Z, Halldin C, Svedberg MM. Amyloid binding properties of curcumin analogues in Alzheimer’s disease postmortem brain tissue. Neurosci Lett. 2016;630:183–188. doi: 10.1016/j.neulet.2016.07.045. [DOI] [PubMed] [Google Scholar]