Abstract
Background
QUIT is the only primary care-based brief intervention that has previously shown efficacy for reducing risky drug use in the US (Gelberg et al., 2015). This pilot study replicated the QUIT protocol in one of the five original QUIT clinics primarily serving Latinos.
Design
Single-blind, two-arm, randomized controlled trial of patients enrolled from March–October 2013 with 3-month follow-up.
Setting
Primary care waiting room of a federally qualified health center (FQHC) in East Los Angeles.
Participants
Adult patients with risky drug use (4–26 on the computerized WHO ASSIST): 65 patients (32 intervention, 33 control); 51 (78%) completed follow-up; mean age 30.8 years; 59% male; 94% Latino.
Interventions and measures
Intervention patients received: 1) brief (typically 3–4 minutes) clinician advice to quit/reduce their risky drug use, 2) video doctor message reinforcing the clinician’s advice, 3) health education booklet, and 4) up to two 20–30 minute follow-up telephone drug use reduction coaching sessions. Control patients received usual care and cancer screening information. Primary outcome was reduction in number of days of drug use in past 30 days of the highest scoring drug (HSD) on the baseline ASSIST, from baseline to 3-month follow-up.
Results
Intervention patients reduced past month HSD use by 4.5 more days than controls (p<.042, 95% CI: 0.2, 8.7) by 3-month follow-up in intent-to-treat linear regression analysis. Similar significant results were found using a complete sample regression analysis: 5.2 days (p<.03, 95% CI: 0.5, 9.9). Additionally, on logistic regression analysis of test results from 47 urine samples at follow-up, intervention patients were less likely than controls to test HSD positive (p < .05; OR:0.10, 95% CI: 0.01, 0.99).
Conclusions
Findings support the efficacy of QUIT for reducing risky drug use.
Keywords: brief intervention, primary care, motivational interviewing, risky drug use, randomized controlled trial, community health centers
1. Introduction
The Mental Health Parity and Addiction Equity Act (MHPAEA) (Beronio et al., 2013) and the US Affordable Care Act (ACA) (Buck, 2011; Pating et al., 2012) ask primary care clinicians to integrate behavioral health, including drug use reduction, into routine care. Use of illicit drugs and non-medical use of prescription medications have significant impacts on public health (De Alba et al., 2004; Degenhardt and Hall, 2012; Dickey et al., 2004; Dickey et al., 2002; Grant et al., 2004; Jane-Llopis and Matytsina, 2006; Mack, 2013; McGeary and French, 2000; Mertens et al., 2003; Stein, 1999; U.S. Department of Health and Human Services (HHS) Office of the Surgeon General, 2016; Weisner et al., 2001) and healthcare costs (Barbosa et al., 2016; McAdam-Marx et al., 2010; Parthasarathy et al., 2001; Thomas et al., 2005; Zarkin et al., 2015). The National Prevention Council (National Prevention Council, 2011) and the Office of National Drug Control Policy (Hingson and Compton, 2014; Office of National Drug Control Policy, 2014) have recommended implementation of brief interventions in primary care settings to address illicit drug use and prevent the development of substance use disorders. Integrating effective BI protocols into primary care could have major public health impact for the 20 million risky drug users in the U.S. (The National Center on Addiction and Substance Use at Columbia University (CASA Columbia), 2012; U.S. Department of Health and Human Services (HHS) and Office of the Surgeon General, 2016). Some randomized controlled trials testing the efficacy of brief intervention for reducing risky drug use in primary care settings in the US have yielded negative results. (Hingson and Compton, 2014; Roy-Byrne et al., 2014; Saitz et al., 2014) However, some observational studies, (Bashir et al., 1994; Cormack et al., 1994) and clinical trials abroad have shown promise (Humeniuk et al., 2012) as have randomized trials in nonprimary care settings in the US (Bernstein et al., 2005; Blow et al., 2017; Mitchell et al., 2012).
The Quit Using Drugs Intervention Trial (QUIT) is the only primary care-based brief intervention protocol that has shown efficacy for reducing risky drug use among adults in the US (Gelberg et al., 2015; Padwa et al., 2014). The 9th US-Mexico Binational Conference on Drug Demand Reduction held in 2011 recommended a pilot comparative randomized controlled trial of the efficacy of screening for drug use and brief intervention for drug users in community health centers in the US-Mexico border regions (Office of National Drug Control Policy, 2011), and funded a randomized controlled trial, the “US-Mexico Binational QUIT Study.” Here we report on a pilot replication of QUIT in the US, in one of the original QUIT study sites at a later time period (1 year later), testing the efficacy of the brief intervention protocol in reducing risky drug use in mostly Latino patients (the now “majority minority” in Los Angeles) (U.S. Census Bureau, 2012, 2015).
2. Methods
2.1 Setting
The selected federally qualified health center (FQHC) clinic was the largest in East Los Angeles that focused on healthcare for Latino patients. Inclusion criteria for clinicians were: (1) staff providers (physicians, nurse practitioners, and physician assistants) trained in primary care; and (2) agreed to follow the intervention protocol and participate in a clinician group intervention training session averaging 15 minutes and a 1–2 minute one-on-one reminder session before conducting the first intervention.
2.2 Enrollment in the trial
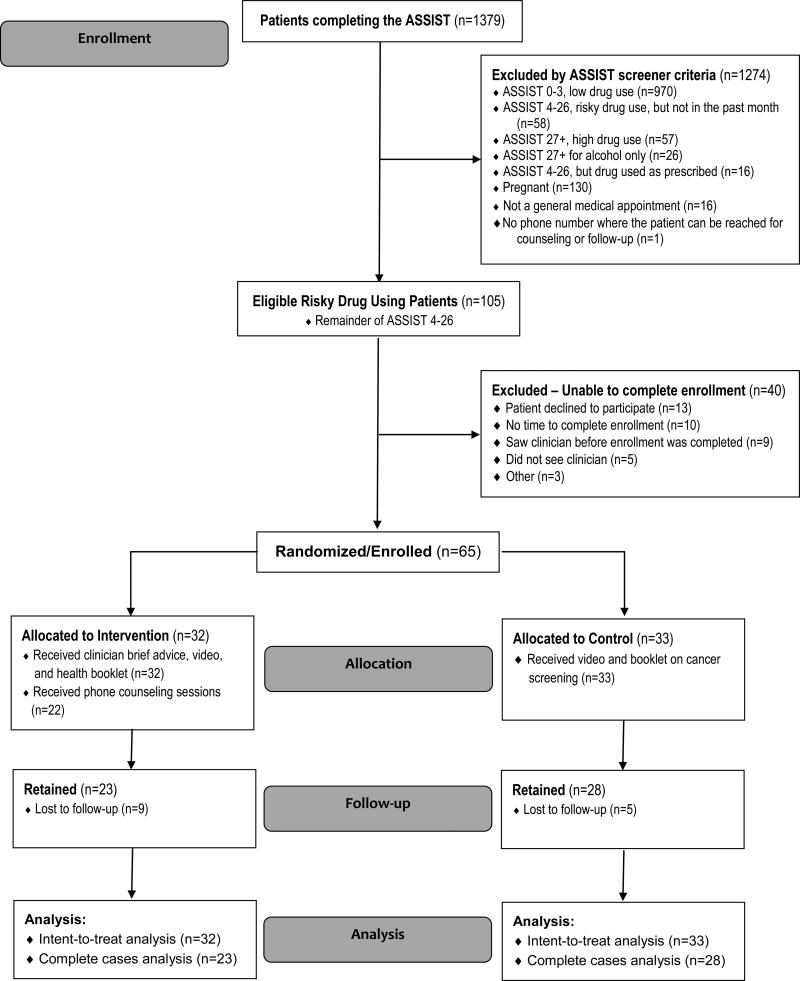
Research assistants conducted enrollment by approaching all adult patients in the waiting room before their clinician appointment in March through October, 2013. Patients self-administered all questionnaires on “talking touch-screen” tablet computers (Gelberg et al., 2015; Hahn et al., 2004; Hahn et al., 2011; Karlsson and Bendtsen, 2005; Singleton et al., 2011). The CONSORT diagram for enrollment is shown in Figure 1.
Figure 1.
CONSORT Diagram
2.3 Inclusion And Exclusion Criteria
Inclusion criteria for patients included: (1) risky drug use in the prior 3 months (ASSIST score 4–26); (2) drug used in the past 30 days; (3) 18 or older; (4) spoke English or Spanish; (5) had a primary care appointment; (6) anticipated living in the Los Angeles County area for the next 3 months (so they would be present for the 3 month outcome assessment); and (7) had an active phone number. Exclusion criteria included: (1) previously screened for this study, (2) under substance use treatment in the past 3 months, (3) scored as high users of drugs or alcohol on the ASSIST (27+), or (4) were pregnant.
Overall, 1,783 patients were approached to determine their eligibility for the ASSIST. Of those, 201 were not eligible. The most common reasons for ineligibility were that the patient was under 18 (106) or had already seen the doctor (47). In addition, 203/1582 (13%) patients eligible for the ASSIST did not complete the ASSIST because they were called in for their medical appointment before they could finish the ASSIST (156) or they declined to complete the ASSIST (47), leaving 1379 who completed the ASSIST (Figure 1).
The ASSIST screening instrument (Humeniuk et al., 2012; Humeniuk et al., 2008; McNeely et al., 2014; WHO ASSIST Working Group, 2002) was self-administered anonymously in English or Spanish, and identified risky drug use patients as “at moderate risk of health and other problems because of their drug use” (Humeniuk et al., 2012; Humeniuk et al., 2008). Patients' use of each drug category was coded as: no or low use (score 0–3); risky (moderate) use indicating clinician brief advice (score 4–26); or high use (score 27 and above). If a patient scored in the risky range for a stimulant (methamphetamine, amphetamines, cocaine), clinicians focused on that stimulant even if it was not the HSD, since our conversations with experts in addiction suggested that stimulants were the most common drugs other than marijuana used illicitly by the patient population. Time to complete the ASSIST screener averaged 4.5 minutes (SD: 5.4, median: 2 minutes); 74% of patients needed less than 6 minutes to complete it. Dropout rates in the intervention and control groups were 28% and 15%, respectively (p=.203).
Of the patients completing the ASSIST, 1,274 screened patients were not eligible for the trial -- mostly because the ASSIST found them not to be risky drug users (ASSIST 0–3). An additional 40 patients who completed the ASSIST and were eligible for the trial were excluded because they failed to complete the enrollment process. That left 65 current risky drug users who were enrolled and randomized into the trial.
2.4 Incentives And Consent
Patients were paid $30 for the initial assessment (average 42 minutes) and $50 for the follow-up assessment (average 50 minutes); those completing all study activities were eligible for a $500 lottery.
Informed consent was obtained -- orally for screening and in writing if they qualified for enrollment. The consent, screening, baseline, and follow-up surveys included questions regarding eight chronic conditions, exercise, diet, and tobacco and alcohol use history in part to mask the purpose of the study, naming it the “Living Well Study.” The research protocol was approved by UCLA’s Institutional Review Board.
2.5 Randomization
Study eligible consenting patients were assigned equally to the intervention (n=32) or control group (n=33) by a computerized (Singleton et al., 2011) adaptive urn randomization program that blocked on ASSIST scores of 4–16 versus 17–26 (Stout et al., 1994).
2.6 Study Groups
At baseline, intervention patients received a face-to-face brief intervention during their clinician visit. Clinicians followed a paper scripted protocol “Summary to Clinician” provided by research staff based on the patients’ HSD; the majority of clinicians reported on our post-visit “Intervention Plan” that their intervention lasted 3–4 minutes and all clinicians reported that they had counseled the patient on their HSD. The message covered drug addiction as a chronic brain disease (McLellan et al., 2000), the need to quit or reduce using drugs to prevent this disease, the physical and mental consequences of drug use, and the potential accelerated progression towards addiction caused by poly-substance use. Clinicians also told patients that they would receive telephone calls 2 and 6 weeks later from a health educator. Patients subsequently received a Drug Health Education Booklet with a Report Card for their HSD, and viewed a video doctor (2 minutes) reinforcing the clinician message (Gerbert et al., 2003; Gerbert et al., 2006; Gilbert et al., 2008). Patients were enrolled on their HSD, and it was that drug that the clinician (and health educator) focused on (even if they scored higher for alcohol); they would also briefly mention the benefits of reducing risky use of alcohol or tobacco if the patient screened positive on the ASSIST for risky use of these substances.
The 2- and 6-week telephone drug-use coaching sessions (20–30 minutes each) reinforced the clinicians’ message, and followed a patient-centered protocol, focusing on HSD use reduction. As previously described (Gelberg et al., 2015), lay health educators (HEs) were trained in motivational interviewing (Miller and Rollnick, 2002) and cognitive behavioral techniques. Weekly meetings with the PIs and project director fostered a HE “Learning Community,” where every case was discussed to maintain fidelity to the protocol. All 32 intervention patients received clinician brief advice (as reported on the clinician Intervention Plan), and 22 (69%) had at least 1 telephone session and 15 (47%) had both sessions.
Control patients completed the ASSIST but did not receive clinician brief intervention or coaching sessions; they did receive a video doctor and information booklet on cancer screening. At study exit, control patients received the intervention components of the video doctor and informational booklet.
2.7 Urine Drug Screen
Urine drug testing was conducted at baseline and follow-up to validate self-reported drug use. The Confirm BioSciences, San Diego, Integrated QuickScreen™ CLIA cup was used since it reliably tests for drugs of interest to this study (96–100% sensitivity). At baseline, 58/65 patients (89%) provided urine specimens and 47/51 (92%) did so at follow-up. Thirty-two patients tested positive for marijuana at baseline and all 32 disclosed past month marijuana use. Similarly, 2 patients tested positive for cocaine and both self-reported its use. At follow-up, 18 patients (13 control, 5 intervention) tested positive for marijuana; all of these patients reported recent marijuana use. Three control patients tested positive for cocaine and/ or amphetamines - 1 for cocaine, 1 for amphetamines and 1 for both; all 3 disclosed their use of these drugs. Thus, for all intervention and control patients with urine tests, self-reports of drug use were confirmed by the tests at both baseline and follow-up. Finally, to complement the assessment of a group difference in degree of self-reported reduction in HSD use over the study period, chi-square and logistic regression analyses were conducted to determine whether there was a group difference with respect to the objective measure of testing positive for HSD use via urine analysis at follow-up.
2.8 Measures
The outcome measure was reduction in number of days of drug use in the past 30 days (Addiction Severity Index, ASI) (McLellan et al., 2006; McLellan et al., 1992; McLellan et al., 1980) of the patients’ HSD between baseline and 3-month follow-up. The ASI is a standardized data collection tool that has excellent psychometric properties (Leonhard et al., 2000; Moos et al., 2000; Rosen et al., 2000). For this study, we employed self-reported use of substances for the past 30 days that provides similar results as the timeline follow-back method (Sobell et al., 1979; Sobell and Sobell, 1992). Patients self-administered the questionnaires and recorded their responses on the tablet computers at baseline and follow-up (research assistants were nearby in case patients needed assistance with the computer).
The Behavioral Model for Vulnerable Populations guided selection of variables used as potential covariates in analyses (Gelberg et al., 2000). Key characteristics are shown in Table 1. Perceived general health status was assessed by a five-point Likert scale item from the SF-12 (Ware et al., 1996; Ware et al., 1995); for analysis, responses were dichotomized to fair/poor health versus good, very good, or excellent health. Physical health was measured by self-reported history of 8 chronic medical conditions. Readiness to change drug use was assessed (Hile and Adkins, 1998; Rollnick et al., 1992). Baseline and follow-up questionnaires were identical.
Table 1.
Baseline characteristics of study participants: risky drug using patients in a federally qualified health center in East Los Angeles
| Characteristic | Total | Control | Intervention | Pa |
|---|---|---|---|---|
| All subjects | (n=65) | (n=33) | (n=32) | |
| PREDISPOSING | ||||
| Socio-Demographics | ||||
| Age, mean (SD) | 30.8 (12.0) | 31.6 (12.7) | 29.9 (11.4) | .586 |
| Education ≥ 12yrs, n (%) | 54 (83.1) | 29 (87.9) | 25 (78.1) | .294 |
| Male, n (%) | 38 (58.5) | 19 (57.6) | 19 (59.4) | .883 |
| Hispanic, n (%) | 61 (93.9) | 30 (90.9) | 31 (96.9) | .317 |
| U.S. Born, n (%) | 56 (87.5) | 31 (93.9) | 25 (80.7) | .142 |
| Ever married n (%) | 19 (29.7) | 9 (27.3) | 10 (32.3) | .663 |
| # children < 18 years old living with patient, mean (SD) | 1.0 (1.2) | 0.8 (1.1) | 1.1 (1.4) | .373 |
| Homeless history, n (%) | ||||
| Homeless, lifetime | 22 (34.4) | 12 (36.4) | 10 (32.3) | .730 |
| Homeless, current | 3 (4.6) | 3 (9.1) | 0 (0.0) | .239 |
| Prison or jail time, past 12 months, n (%) | 6 (9.4) | 5 (15.2) | 1(3.2) | .198 |
| Sexual abuse, childhood, n (%) | 11 (17.2) | 4 (12.0) | 7 (22.6) | .268 |
| Highest Scoring Drug (HSD)b History and Beliefs | ||||
| Duration of HSD use(mean years, SD) | 12.9 (12.9) | 15.4 (13.7) | 10.4 (11.7) | .102 |
| Perception has problem with HSD, n (%) | .609 | |||
| Do not have drug problem | 37 (56.9) | 17 (51.5) | 20 (62.5) | |
| Probably have drug problem | 19 (29.2) | 10 (30.3) | 9 (28.1) | |
| Definitely have drug problem | 9 (13.9) | 6 (18.2) | 3 (9.4) | |
| Interest in reducing/ stopping HSD, n (%) | .911 | |||
| Very | 17 (26.2) | 8 (24.2) | 9 (28.1) | |
| Somewhat | 28 (43.1) | 15 (45.4) | 13 (40.6) | |
| Not at all | 20 (30.8) | 10 (30.3) | 10 (31.3) | |
| ENABLING | ||||
| Income ≤ $500/month, n (%) | 45 (75.0) | 25 (78.1) | 20 (71.4) | .550 |
| Insurance, past 3 months, n (%) | 36 (56.3) | 21 (63.6) | 15 (48.4) | .219 |
| NEED | ||||
| Fair or poor general health, n (%) | 24 (37.5) | 13 (39.4) | 11 (35.5) | .747 |
| # Chronic medical conditions, mean (SD)c | 0.7 (1.1) | 0.8 (1.1) | 0.6 (1.0) | .228 |
| Baseline # Tobacco Use Days past month, mean (SD) | 6.8 (11.0) | 6.4 (10.2) | 7.2 (11.8) | .773 |
| Baseline Any binge drinking day, past month, n (%)d | 40 (61.5) | 19 (57.6) | 21 (65.6) | .505 |
| Baseline HSD ASSIST Score, mean (SD)e | 14.4 (6.2) | 14.5 (6.5) | 14.4 (6.0) | .942 |
| Drug Type of HSD, n (%) | .665 | |||
| Cannabis | 44 (67.7) | 24 (72.7) | 20 (62.5) | |
| Cocaine/Crack | 6 (9.2) | 3 (9.1) | 3 (9.4) | |
| Amphetamines | 5 (7.7) | 3 (9.1) | 2 (6.3) | |
| Sedatives | 2 (3.1) | 0 (0.0) | 2 (6.3) | |
| Opiates | 8 (12.3) | 3 (9.1) | 5 (15.6) | |
| Other (inhalants, hallucinogens) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| HSD Use at Baseline, # days past 30 days, mean (SD) | 11.9 (10.8) | 12.4 (10.6) | 11.4 (11.2) | .699 |
| Polydrug use (risky use of multiple drugs, past 30 days) | 21 (32.3) | 10 (30.3) | 11 (34.4) | .756 |
Based on chi-square, two-sample t, or two-sample Wilcoxon test
HSD = Highest scoring drug in risky range (4–26) on WHO ASSIST
Number of 8 chronic medical conditions in lifetime: asthma, hepatitis, epilepsy, cancer, tuberculosis, HTN, diabetes, or HIV/AIDS
Binge drinking day is defined as 5+ drinks for men <65 yo, 4+ for men >= 65yo and all women
Baseline ASSIST Score for Highest Scoring Drug on the ASSIST conducted at screening
2.9 Statistical Analysis
Reduction in past month HSD use between baseline and follow-up was approximately normally distributed and was assessed with linear regression analysis. Baseline variables in Table 1 associated with reduction in HSD use at the 0.05 level were candidate covariates. A parsimonious final model was obtained by manually removing covariates one at a time in descending order of p values until only those associated with reduction in HSD use at the 0.10 level remained and multi-collinearity was not a problem (Committee for Medicinal Products for Human Use (CHMP), 2015). A priori power testing for efficacy was not conducted for this pilot study.
Since 14 of the total sample of 65 patients were lost to follow up, intention-to-treat analysis was performed using multiple imputation (SAS 9.3 Procs MI and MIANALYZE) to impute their missing outcome values rather than carrying forward the last observation (LOCF) to accommodate the very real possibility of change over time (Hall et al., 2001; White et al., 2012; White et al., 2011). Baseline variables in Table 1 related to loss-to-follow-up were included in the imputation model (Siddique et al., 2008), along with analytic variables. Twenty sets of imputed values were produced.
Two separate regression analyses were compared to check the sensitivity of our estimates of the effects of QUIT on drug use reduction. One was the intent-to-treat analysis including all 65 cases (Table 2b). The other used the 51 complete cases with both baseline and follow-up data (Table 2c).
Table 2.
Effect of the QUIT intervention on reduction in risky drug use among primary care patients of a federally qualified health center
| a. Profile of past 30-day highest scoring druga (HSD) use at baseline and 3-month follow-up, for patients completing the study (n=51) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Days Used HSD, Past 30 Days |
Reductionb In HSD Use Over Time |
Adjusted Group Difference In HSD Reduction, Controlling for Baseline HSD Usee |
|||||||
| Baseline | Follow-up | ||||||||
| Program | Mean | pc | Mean | pc | Mean | Pd | Mean | 95% CI | P |
| Group Difference | .620 | .003 | 5.28 | −0.06, 10.63 | .053 | ||||
| Control Group (n=28) | 12.64 | 12.93 | −0.29 | .557 | |||||
| QUIT Intervention Group (n=23) | 11.04 | 6.61 | 4.43 | .001 | |||||
| b. Intent-to-treat linear regression for group difference in reductiona in number of days used HSDb in past 30 days (n=65) | ||||
|---|---|---|---|---|
| Decrease in Days of HSD Use, Past 30 Days | ||||
| Measure | Coeff | s.e. | P | 95% CI |
| QUIT Intervention Group (ref: Control group) | 4.46 | 2.2 | .042 | 0.17, 8.74 |
| Baseline HSD Use | 0.22 | 0.1 | .038 | 0.01, 0.42 |
| High School Graduate | 8.51 | 3.0 | .005 | 2.63, 14.40 |
| # Children <18 years old at home | 2.91 | 0.9 | .002 | 1.10, 4.73 |
| Sexually assaulted < 18 years old | −5.32 | 2.8 | .057 | −10.80, 0.16 |
| c. Completed sample linear regression for group difference in reductiona in number of days used HSDb in past 30 days, at 3 month follow-up (N=51) | |||
|---|---|---|---|
| Reduction in use from Baseline to 3 months | |||
| Measure | Coeff | s.e. | P |
| Intervention Group (ref: Control group) | 5.22 | 2.3 | .030 |
| Baseline HSD Use | 0.28 | 0.1 | .010 |
| High School Graduate | 11.33 | 3.3 | .001 |
| # Children <18 years old at home | 3.60 | 1.1 | .002 |
| Sexually assaulted < 18 years old | −6.23 | 2.8 | .029 |
HSD = Highest scoring drug in risky range (4–26) on baseline WHO ASSIST
Baseline minus follow-up HSD use
Wilcoxon two-sample test for group difference
Paired t test for change
Program difference of HSD use differences over time adjusted for baseline HSD use
Baseline minus 3 month follow-up HSD use
HSD = Highest scoring drug in risky range (4–26) on baseline WHO ASSIST
Baseline minus 3 month follow-up HSD use
HSD = Highest scoring drug in risky range (4–26) on baseline WHO ASSIST
Additionally, to investigate whether patients might have compensated for reducing their HSD use by increasing their use of alcohol and tobacco, we assessed changes in use of these substances among patients who reduced their HSD use by 1 day or more.
3. Results
Baseline characteristics (Table 1) show that 94% were Latino; on average had used their HSD for 12.9 years; had a mean HSD ASSIST score at baseline of 14.4 (range 4–26); and their most common HSD was cannabis (68%), followed by stimulants (17%). Intervention and control groups did not differ on baseline characteristics.
For the 51 patients with follow-up data, the mean number of days of HSD use in the past 30 days was balanced at baseline (Intervention: 11.0 days, Control: 12.6 days) (Table 2a). Past 30-day HSD use at follow-up was significantly lower for intervention patients (Intervention: 6.6, Control: 12.9 days). While the control group reported no change in HSD use over time (−0.29 days), the intervention group reported a significant unadjusted mean reduction of 4.4 days from baseline to follow-up (40% reduction, p<.001). Among the 47 participants who provided urine samples, those in the intervention group were less likely than controls to test positive for their HSD (25% vs. 56%; P < 0.05). A logistic regression analysis for testing HSD positive that controlled self-reported baseline HSD use confirmed that intervention group participants were less likely than those in the control group to test HSD positive at follow-up (p < 0.05; adjusted OR: 0.10, 95% CI: 0.01, 0.99) (not shown).
In the intent-to-treat linear regression model with multiple imputation of missing values (Table 2b), intervention patients reduced their HSD use an average of 4.5 (95% CI: 0.2, 8.7; p=.042) more days in the past month than did controls, controlling for baseline HSD use, high school graduation, number of children under 18 living with them, and having been sexually assaulted before they were 18 years old. The complete sample regression with the same covariates for the 51 patients with follow-up data produced similar results (Table 2c), with intervention patients reducing their HSD use an average of 5.2 more days than controls (p<.03; 95%CI: 0.5,9.9,).
Finally, among the 32 patients in the complete sample who reduced their HSD use by a day or more, 28 patients who reported risky alcohol use reduced that use by an average of 0.3 days (median=0) and 17 patients who disclosed smoking reduced their tobacco use by an average of 2.5 days (median=0). Neither change was significant (p>0.05, Wilcoxon signed rank test).
4. Discussion
In this study of mostly Latino primary care patients of an FQHC, the QUIT brief intervention group reported a 40% decline in mean HSD use, corresponding to an adjusted 4.5-day reduction in reported past month HSD use by 3-month follow-up compared to controls (5.2 day reduction in the complete case analysis); there was no compensatory increase in use of alcohol or tobacco. This degree of drug use reduction is meaningful clinically according to norms for reductions in marijuana use in clinical trials (Babor TF and The Marijuana Treatment Project Research Group., 2004; Coffey et al., 2002). The trial has clinical significance as its findings could apply to 12% of our study clinic patients that screen positive for risky drug use (ASSIST 4–26) (see Figure 1), and represents significant potential public health impact for the 20 million risky drug users in the US if replicated in other clinic populations (The National Center on Addiction and Substance Use at Columbia University (CASA Columbia), 2012; U.S. Department of Health and Human Services (HHS) Office of the Surgeon General, 2016). The findings are important given the limited number of randomized trials of screening and brief intervention for risky drug use in primary care, and notable in that the findings affirm the positive findings of the QUIT trial.
Some distinctive characteristics of the QUIT intervention that may contribute to its greater success than other brief intervention protocols designed to address risky drug use in primary care (Humeniuk et al., 2012; Roy-Byrne et al., 2014; Saitz et al., 2014) include: (1) use of primary care clinicians to deliver brief advice messages about drug use; (2) regular weekly “learning community” meetings among health coaches and the study team; (3) incorporation of quality of life issues patients spontaneously raised as barriers to drug use reduction into telephone coaching sessions; (4) embedding of drug use consent and patient assessment questions within a larger behavioral health paradigm to conceal the study’s drug focus and minimize potential contamination of the control group; and (5) patient self-administered assessment of drug use on tablet computers.
The original QUIT study, showed a significant reduction in HSD in 30-day risky drug use (2.2 day reduction in the ITT analysis using LOCF (last observation carried forward), 3.5 day reduction in the completer analysis) in intervention compared to control patients (Gelberg et al., 2015). Of particular importance for considering QUIT implementation -- risky drug use reduction was observed in each of the original study’s 5 FQHC organizations controlling for baseline HSD use, although we lacked the power to test for clinic specific significance: Clinic#1 4.5 day reduction, Clinic#2 11.8 days, Clinic#3 3.2 days, Clinic#4 1.5 days, Clinic#5 5.2 days. Also the original study’s FQHCs had varying characteristics (Gelberg et al., 2015), including location (in different areas of Los Angeles County), clinic size (serving 8,799 to 20,877 patients per year) (California Office of Statewide Health Planning and Development (OSHPD), 2012), and study patient characteristics: age (mean 32.2 to 49.2); male (42–75%); White/Asian (9–62%), African-American (3–66%), Latino (14–88%); and currently homeless (1.4–50%). The positive outcomes in all of these different clinics bolstered by positive outcomes from this pilot replication suggest that QUIT may prove effective and implementable in a variety of settings and across a variety of patient demographics.
Limitations of the study include: generalizability of the sample to other Latino populations, potential for social desirability bias to influence the primary outcome of self-reported drug use reduction which we tried to minimize by patients’ self-administration of survey items on a tablet computer, loss to follow-up, and small sample size which limits subgroup analysis.
5. Conclusion
The ACA and the MHPAEA expanded behavioral health coverage to 62 million people, who might benefit from brief intervention programs for risky drug use in primary care settings such as FQHCs (Buck, 2011; Pating et al., 2012). An effectiveness/implementation study of QUIT in FQHCs is needed to confirm its general applicability to fulfill this need.
Highlights.
QUIT (Quit Using Drugs Intervention Trial) brief intervention reduced past month drug use by 4.5 days.
QUIT protocol was efficacious in a variety of primary care settings.
Screening for substance use could be implemented in federally qualified health centers (FQHCs).
Acknowledgments
Role of funding Source
This research, the “US-Mexico Binational Quit Using Drugs Intervention Trial” (UCLA-Mexico Binational QUIT Study), was primarily funded by grants from NIDA (3P30DA027828-02S1; P30DA027828-02S2) [supplements to the NIDA Center for Prevention Implementation Methodology (Ce-PIM) for Drug Abuse and HIV Sexual Risk Behavior (P30-DA027828 NIDA/OBSSR, PI CH Brown)] and the US State Department’s Bureau of International Narcotics and Law Enforcement (INL) (SMX53012-GR186).
We gratefully acknowledge the following, without whose support we could not have conducted this study: Nora Volkow MD, Wilson Compton MD, MPE, Harold Perl PhD, and Jacqueline Lloyd PhD, of the National Institute on Drug Abuse of the US National Institutes of Health; Terry Zobeck PhD, Assistant Deputy Director, of the U.S. Office of National Drug Control Policy, Executive Office of the President; the U.S. State Department’s Bureau of International Narcotics and Law Enforcement (INL); and Dr. C. Hendricks Brown and Juan Villamar from the Center for Prevention Implementation Methodology (Ce-PIM). We are indebted to Dr. Abdolmonem Afifi, Dean Emeritus, UCLA School of Public Health, Professor of Biostatistics & Biomathematics for his careful review and comments on the analyses used in this article. We greatly appreciate the following. Our hard working clinic partners: the clinicians and staff of our study clinic including Dr. Michael Hochman, Dr. Sandra Pisano, Michael Eaton; and the patients, who were willing to consider drug use behavior change. Our colleagues and collaborators: Miriam Arroyo Belmonte, MSc, National Institute of Psychiatry Ramón de la Fuente Muñiz (Mexico), Robert Ali, MD, University of Adelaide; Corey Arnold, PhD, UCLA Medical Imaging Informatics; Alex Bui, PhD, UCLA Medical Imaging Informatics; Adeline Nyamathi, ANP PhD FAAN, UCLA School of Nursing; Keith Heinzerling, MD, MPH, UCLA Department of Family Medicine; Mario González Zavala, MD, National Council Against Addictions (Mexico). Our health educators: Nell Baldwin and Melvin Rico. Our research assistants: Rahul Abraham, Belinda Aguirre, Claire Alvarenga, Melissa Avila, Nataly Barragan, Daniel Benhuri, Ben Benhuri, Magaly Chavez, Pauline Do, Perla Elenes, Chelsea Emery, Marianna Garcia, Lea Heller, Marissa Hernandez, Blake Johnson, Jinsol (Gene) Lee, Aida Martinez, Frania Mendoza Lua, Hannah Mendoza, Crystal Munoz, Keila Perez, Francesca Rozo, John Scholtz, Jose Serrano, Cyrus Sinai, Ashley Torkan, Darlene Vera, Anmy Vu, Julia Yacenda-Murphy.
Footnotes
Trial Registration
Brief Title: Binational Quit Using Drugs Intervention Trial (BiN-QUIT)
Other study ID: BINAT 3P30DA027
Contributors
Lillian Gelberg: Drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, study concept and design. Ronald M. Andersen: Drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtained funding, study concept and design. Melvin W. Rico: Acquisition of data, study supervision, critical revision of the manuscript for important intellectual content. Guillermina Natera Rey: Acquisition of data, study supervision, critical revision of the manuscript for important intellectual content. Mani Vahidi: Acquisition of data, study supervision, critical revision of the manuscript. Steve Shoptaw: Critical revision of the manuscript for important intellectual content. Barbara D. Leake: Statistical analysis, critical revision of the manuscript for important intellectual content. Martin Serota: Critical revision of the manuscript for important intellectual content. Sebastian E. Baumeister: Statistical analysis, critical revision of the manuscript for important intellectual content. Kyle Singleton: Critical revision of the manuscript for important intellectual content.
All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Babor TF The Marijuana Treatment Project Research Group. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J. Consult. Clin. Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. [DOI] [PubMed] [Google Scholar]
- Barbosa C, Cowell A, Landwehr J, Dowd W, Bray J. Cost of screening, brief intervention, and referral to treatment in health care settings. J. Subst. Abuse Treat. 2016;60:54–61. doi: 10.1016/j.jsat.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Bashir K, King M, Ashworth M. Controlled evaluation of brief intervention by general practitioners to reduce chronic use of benzodiazepines. Br. J. Gen. Pract. 1994;44:408–412. [PMC free article] [PubMed] [Google Scholar]
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Beronio K, Glied S, Po R, Skopec L. Affordable Care Act will expand mental health and substance use disorder benefits and parity protections for 62 million Americans, U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation 2013 [Google Scholar]
- Blow FC, Walton MA, Bohnert ASB, Ignacio RV, Chermack S, Cunningham RM, Booth BM, Ilgen M, Barry KL. A randomized controlled trial of brief interventions to reduce drug use among adults in a low-income urban emergency department: the HealthiER You study. Addiction. 2017 doi: 10.1111/add.13773. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff. (Millwood) 2011;30:1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- California Office of Statewide Health Planning and Development (OSHPD) Healthcare Information Division: Annual Utilization Report of Primary Care Clinics. State of California: 2012. [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: An Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Committee for Medicinal Products for Human Use (CHMP) European Medicines Agency. London, United Kingdom: 2015. Guideline on adjustment for baseline covariates in clinical trials. [Google Scholar]
- Cormack M, Sweeney KG, Hughes-Jones H, Foot GA. Evaluation of an easy, cost-effective strategy for cutting benzodiazepine use in general practice. Br. J. Gen. Pract. 1994;44:5–8. [PMC free article] [PubMed] [Google Scholar]
- De Alba I, Samet JH, Saitz R. Burden of medical illness in drug- and alcohol-dependent persons without primary care. Am. J. Addict. 2004;13:33–45. doi: 10.1080/10550490490265307. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Dickey B, Dembling B, Azeni H, Normand SL. Externally caused deaths for adults with substance use and mental disorders. J. Behav. Health Serv. Res. 2004;31:75–85. doi: 10.1007/BF02287340. [DOI] [PubMed] [Google Scholar]
- Dickey B, Normand S-LT, Weiss RD, Drake RE, Azeni H. Medical morbidity, mental illness, and substance use disorders. Psychiatr. Serv. 2002;53:861–867. doi: 10.1176/appi.ps.53.7.861. [DOI] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Afifi AA, Leake BD, Arangua L, Vahidi M, Singleton K, Yacenda-Murphy J, Shoptaw S, Fleming MF, Baumeister SE. Project QUIT (Quit Using Drugs Intervention Trial): A randomized controlled trial of a primary care-based multi-component brief intervention to reduce risky drug use. Addiction. 2015;110:1777–1790. doi: 10.1111/add.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelberg L, Andersen RM, Leake BD. The Behavioral Model for Vulnerable Populations: Application to medical care use and outcomes for homeless people. Health Serv. Res. 2000;34:1273–1302. [PMC free article] [PubMed] [Google Scholar]
- Gerbert B, Berg-Smith S, Mancuso M, Caspers N, McPhee S, Null D, Wofsy J. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promot. Pract. 2003;4:249–261. doi: 10.1177/1524839903004003009. [DOI] [PubMed] [Google Scholar]
- Gerbert B, Danley DW, Herzig K, Clanon K, Ciccarone D, Gilbert P, Allerton M. Reframing "prevention with positives": Incorporating counseling techniques that improve the health of HIV-positive patients. AIDS Patient Care STDS. 2006;20:19–29. doi: 10.1089/apc.2006.20.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, McPhee SJ, Calderon SH, Bogetz A, Gerbert B. Interactive "Video Doctor" counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Hahn EA, Cella D, Dobrez D, Shiomoto G, Marcus E, Taylor SG, Vohra M, Chang C, Wright BD, Linacre JM, Weiss BD, Valensuela V, Chaing H, Webster K. The talking touchscreen: A new approach to outcomes assessment in low literacy. Psychooncol. 2004;13:86–95. doi: 10.1002/pon.719. [DOI] [PubMed] [Google Scholar]
- Hahn EA, Choi SW, Griffith JW, Yost KJ, Baker DW. Health literacy assessment using talking touchscreen technology (Health LiTT): A new item response theory-based measure of health literacy. J. Health. Commun. 2011;16(Suppl 3):150–162. doi: 10.1080/10810730.2011.605434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D, Tsoh JY, Niaura R. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine Tob. Res. 2001;3:193–202. doi: 10.1080/14622200110050411. [DOI] [PubMed] [Google Scholar]
- Hile MG, Adkins RE. The impact of substance abusers' readiness to change on psychological and behavioral functioning. Addict. Behav. 1998;23:365–370. doi: 10.1016/s0306-4603(98)00016-1. [DOI] [PubMed] [Google Scholar]
- Hingson R, Compton WM. Screening and brief intervention and referral to treatment for drug use in primary care: Back to the drawing board. JAMA. 2014;312:488–489. doi: 10.1001/jama.2014.7863. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, McRee B, Newcombe D, Pal H, Poznyak V, Simon S, Vendetti J. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Jane- Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: A review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug Alcohol Rev. 2006;25:515–536. doi: 10.1080/09595230600944461. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Bendtsen P. Acceptability of a computerized alcohol screening and advice routine in an emergency department setting--A patient perspective. Addict. Behav. 2005;30:767–776. doi: 10.1016/j.addbeh.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Leonhard C, Mulvey K, Gastfriend DR, Shwartz M. The Addiction Severity Index: A field study of internal consistency and validity. J. Subst. Abuse Treat. 2000;18:129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Mack KA. Drug-Induced Deaths—United States, 1999–2010. CDC Health Disparities and Inequalities Report—United States, 2013. 2013;62:161. [PubMed] [Google Scholar]
- McAdam-Marx C, Roland CL, Cleveland J, Oderda GM. Costs of opioid abuse and misuse determined from a Medicaid database. J. Pain Pall. Care Pharmacother. 2010;24:5–18. doi: 10.3109/15360280903544877. [DOI] [PubMed] [Google Scholar]
- McGeary KA, French MT. Illicit drug use and emergency room utilization. Health Serv. Res. 2000;35:153–169. [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Cacciola JC, Alterman AI, Rikoon SH, Carise D. The Addiction Severity Index at 25: Origins, contributions and transitions. Am. J. Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J. Nerv. Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McNeely J, Strauss SM, Wright S, Rotrosen J, Khan R, Lee JD, Gourevitch MN. Test-retest reliability of a self-administered Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in primary care patients. J. Subst. Abuse Treat. 2014;47:93–101. doi: 10.1016/j.jsat.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: Comparison with matched controls. Arch. Intern. Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people for change. Guilford Press; New York, NY: 2002. [Google Scholar]
- Mitchell SG, Gryczynski J, Gonzales A, Moseley A, Peterson T, O'Grady KE, Schwartz RP. Screening, brief intervention, and referral to treatment (SBIRT) for substance use in a school-based program: Services and outcomes. Am. J. Addict. 2012;21(Suppl. 1):S5–13. doi: 10.1111/j.1521-0391.2012.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Finney JW, Federman EB, Suchinsky R. Specialty mental health care improves patients' outcomes: Findings from a nationwide program to monitor the quality of care for patients with substance use disorders. J. Stud. Alcohol. 2000;61:704–713. doi: 10.15288/jsa.2000.61.704. [DOI] [PubMed] [Google Scholar]
- National Prevention Council. National Prevention Strategy. Department of Health and Human Services, Office of the Surgeon General; Washington, D.C: 2011. [Google Scholar]
- Office of National Drug Control Policy. 9th US–Mexico Binational Conference on Drug Demand Reduction; Mexico D.F., Mexico. 2011. [Google Scholar]
- Office of National Drug Control Policy. National Drug Control Strategy. Executive Office of the President, White House; Washington, D.C: 2014. [Google Scholar]
- Padwa H, Ni YM, Barth-Rogers Y, Arangua L, Andersen R, Gelberg L. Barriers to drug use behavior change among primary care patients in urban United States community health centers. Subst. Use Misuse. 2014;49:743–751. doi: 10.3109/10826084.2013.866962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S, Weisner C, Hu T-W, Moore C. Association of outpatient alcohol and drug treatment with health care utilization and cost: Revisiting the offset hypothesis. J. Stud. Alcohol. 2001;62:89. doi: 10.15288/jsa.2001.62.89. [DOI] [PubMed] [Google Scholar]
- Pating DR, Miller MM, Goplerud E, Martin J, Ziedonis DM. New systems of care for substance use disorders: Treatment, finance, and technology under health care reform. Psychiatr. Clin. North Am. 2012;35:327–356. doi: 10.1016/j.psc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Heather N, Gold R, Hall W. Development of a short 'readiness to change' questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br. J. Addict. 1992;87:743–754. doi: 10.1111/j.1360-0443.1992.tb02720.x. [DOI] [PubMed] [Google Scholar]
- Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self-administered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95:419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Bumgardner K, Krupski A, Dunn C, Ries R, Donovan D, West II, Maynard C, Atkins DC, Graves MC, Joesch JM, Zarkin GA. Brief intervention for problem drug use in safety-net primary care settings: A randomized clinical trial. JAMA. 2014;312:492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, Meli SM, Chaisson CE, Samet JH. Screening and brief intervention for drug use in primary care: The ASPIRE randomized clinical trial. JAMA. 2014;312:502–513. doi: 10.1001/jama.2014.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique J, Brown CH, Hedeker D, Duan N, Gibbons RD, Miranda J, Lavori PW. Missing data in longitudinal trials - part B, analytic issues. Psychiatr. Ann. 2008;38:793–801. doi: 10.3928/00485713-20081201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KW, Lan M, Arnold C, Vahidi M, Arangua L, Gelberg L, Bui AAT. Wireless data collection of self-administered surveys using tablet computers. AMIA Annu. Symp. Proc. 2011;2011:1261–1269. [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav. Res. Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Timeline Follow-Back: A Technique For Assessing Self-Reported Ethanol Consumption. Allen J, Litten RZ, editors. Human Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stein MD. Medical consequences of substance abuse. Psychiatr. Clinics North Am. 1999;22:351–370. doi: 10.1016/s0193-953x(05)70081-2. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J. Stud. Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- The National Center on Addiction and Substance Use at Columbia University (CASA Columbia) Addiction Medicine: Closing the Gap between Science and Practice. New York, NY: 2012. [Google Scholar]
- Thomas MR, Waxmonsky JA, Gabow PA, Flanders-McGinnis G, Socherman R, Rost K. Prevalence of psychiatric disorders and costs of care among adult enrollees in a Medicaid HMO. Psychiatr. Serv. 2005;56:1394–1401. doi: 10.1176/appi.ps.56.11.1394. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. U.S. Census Bureau Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now. [Retrieved June 2, 2016];2012 from http://www.census.gov/newsroom/releases/archives/population/cb12-243.html.
- U.S. Census Bureau. State and County QuickFacts. Data derived from Population Estimates, Census of Population and Housing. [Retrieved June 2, 2016];2015 May 28; 2015 from http://quickfacts.census.gov/qfd/states/06/06037.html.
- U.S Department of Health and Human Services (HHS) and Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, D.C: 2016. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (HHS) Office of the Surgeon General. Facing Addiction in America: The Surgeon General's Report on Alcohol, Drugs, and Health. HHS. Washington, D.C: 2016. [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. SF-12: How to score the SF-12 physical and mental health summary scales. Health Institute, New England Medical Center; 1995. [Google Scholar]
- Weisner C, Mertens J, Parthasarathy S, Moore C, Lu Y. Integrating primary medical care with addiction treatment: A randomized controlled trial. JAMA. 2001;286:1715–1723. doi: 10.1001/jama.286.14.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Carpenter J, Horton NJ. Including all individuals is not enough: Lessons for intention-to-treat analysis. Clin. Trials. 2012;9:396–407. doi: 10.1177/1740774512450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Horton NJ, Carpenter J, statistics, r.i.m., social. Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342 doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ASSIST Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): Development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Zarkin G, Bray J, Hinde J, Saitz R. Costs of screening and brief intervention for illicit drug use in primary care settings. J. Stud. Alcohol Drugs. 2015;76:222–228. doi: 10.15288/jsad.2015.76.222. [DOI] [PMC free article] [PubMed] [Google Scholar]