Abstract
The natural habitat of Drosophila melanogaster Meigen (Diptera: Drosophilidae) is fermenting fruits, which can be rich in ethanol. For unknown reasons, temperate populations of this cosmopolitan species have higher ethanol resistance than tropical populations. To determine whether this difference is accompanied by a parallel difference in preference for ethanol, we compared two European and two tropical African populations in feeding and oviposition preference for ethanol-supplemented medium. Although females of all populations laid significantly more eggs on medium with ethanol than on control medium, preference of European females for ethanol increased as ethanol concentration increased from 2 to 6%, whereas that of African females decreased. In feeding tests, African females preferred control medium over medium with 4% ethanol, whereas European females showed no preference. Males of all populations strongly preferred control medium. The combination of preference for ethanol in oviposition, and avoidance or neutrality in feeding, gives evidence that adults choose breeding sites with ethanol for the benefit of larvae, rather than for their own benefit. The stronger oviposition preference for ethanol of temperate than tropical females suggests that this benefit may be more important in temperate populations. Two possible benefits of ethanol for which there is some experimental evidence are cryoprotection and protection against natural enemies.
Keywords: ethanol preference, geographic variation, Diptera, Drosophilidae avoidance, feeding preference, oviposition preference
Introduction
Drosophila melanogaster Meigen (Diptera: Drosophilidae) feeds and breeds on fermenting fruits, which can contain ethanol in concentrations as high as 6% (Gibson et al., 1981). Fruit-breeding Drosophila species are more resistant to ethanol than species that breed on mushrooms or other non-sweet vegetation (Merçot et al., 1994), giving evidence that ethanol in fruits is an important selective agent. Drosophila do not simply passively encounter ethanol, however – in laboratory choice tests, D. melanogaster females preferentially oviposit, and larvae preferentially feed, on medium supplemented with ethanol (Parsons, 1977, 1980; Hougouto et al., 1982; Siegal & Hartl, 1999). The reason that this preference has evolved is not well understood.
Another mysterious aspect of the Drosophila-ethanol relation is the occurrence of repeated latitudinal clines in ethanol resistance in D. melanogaster. This species originated in tropical Africa, but has spread world-wide as a human commensal, reaching the New World and Australia only in the last few hundred years (Keller, 2007). Nonetheless, in every region examined, populations from temperate latitudes have markedly higher ethanol resistance than those from the tropics or subtropics (David & Bocquet, 1975; Anderson, 1982; Cohan & Graf, 1985; David et al., 1986; Parkash et al., 1999; Montooth et al., 2006). Although the presence of parallel clines on multiple continents strongly implicates the role of selection, why selection should favor higher ethanol resistance in temperate regions, either directly or indirectly, is not clear. It seems unlikely that decaying fruits with substantial ethanol concentrations are more common in temperate regions than in the tropics; decaying palm fruits can contain ethanol concentrations approaching 10% (Dudley, 2004), and large, sweet fruits are common in tropical forests but rare in temperate forests (Levey, 2004). Another possibility that seems unlikely is that the ethanol resistance clines can be explained entirely as correlated responses to selection for resistance to physiological stresses that are more common in the temperate zone than in the tropics, such as cold (see Discussion).
A more promising possibility, suggested by the preference of larvae and ovipositing females for ethanol, is that ethanol provides a fitness benefit that is greater in temperate regions than in the tropics. This would be expected to cause selection to favor increased preference for ethanol in temperate populations compared to tropical populations, with simultaneous stronger selection for ethanol resistance. Consistent with this hypothesis, Parsons (1977, 1980) found that in Australia, larvae from temperate populations had stronger preference for ethanol than larvae from subtropical populations. There is no information, however, about whether temperate females have stronger oviposition preference for ethanol than those from tropical populations, or whether adult feeding preference for ethanol differs between temperate and tropical populations. Because larvae have limited mobility, oviposition preference is a particularly important determinant of habitat use. In this study we compared two European populations and two tropical African populations in oviposition and adult feeding preference for ethanol.
Materials and methods
Fly stocks and rearing conditions
We created four outbred D. melanogaster populations, two each from the temperate zone and tropics, by intercrossing isofemale lines from single collection locations in The Netherlands (n = 9 lines), Austria (n = 20), Cameroon (n = 20), and Tanzania (n = 6). Original collection locations and dates for the lines were: Leiden, The Netherlands, 52°N, 1990s (provided by D. Rand); a forested area outside of Vienna, Austria (48°N, 2004) (provided by C. Schlötterer); a village in Cameroon (8°N; 2004) (‘CD’ lines of Pool & Aquadro, 2006); and a fruit market in Dar es Salaam, Tanzania (7°S; 2001) (Dean et al., 1993). When tested with standard mortality assays, the lines showed the expected temperate-tropical difference in adult ethanol resistance (Fry, 2014; J Zhu & JD Fry, unpubl.).
Each population was maintained in 20 shell vials (ca. 20 flies per vial) on standard maize meal-molasses-Brewer’s yeast-agar medium and handled under light CO2 anesthesia. Populations were maintained on 2-week generations under continuous lighting at 25 °C and 60–70% r.h., with parental flies being removed from the vials after 5 days.
Oviposition preference
Non-virgin females were allowed to choose between ethanol medium and control medium to lay eggs. Sets of 25 2- to 4-day-old non-virgin females were kept in holding vials with regular food for 1 day, and then 50 females (from two vials) were transferred without anesthesia to a plastic cage (15 × 15 × 6 cm) for egg laying. Each cage contained 10 Petri dishes (4 cm diameter), half of which were filled with ethanol-supplemented medium (2, 4, or 6%, depending on the cage), and the other half with control medium (no ethanol, with the same amount of water added instead). To decrease evaporation, medium was allowed to cool to around 45 °C before ethanol or water was added. Sixteen hours later (overnight), the cages were frozen for about half an hour, females were discarded, and the eggs laid on ethanol or control medium were counted. Each cage was treated as a replicate, and the ‘oviposition ethanol preference index’ (OEPI) of each cage was calculated by dividing the number of eggs laid on ethanol medium by the total number of eggs laid in each cage, then subtracting 0.5. OEPI ranges from −0.5 (always lay eggs on non-ethanol medium) to +0.5 (always lay eggs on ethanol medium), and a zero OEPI shows no preference or avoidance. All the four populations were assayed simultaneously. Three replicate cages for each population and ethanol concentration (2, 4, or 6%) were assayed in each of two temporal blocks.
Feeding preference
Adult attraction or avoidance to feed on ethanol medium was measured with a two-choice feeding preference system modified from Tanimura et al. (1982). Non-virgin flies (2–4 days old, separated by sex) were starved on water at room temperature for 1 day and then single-sex groups of 50 flies were introduced into each plastic cage without CO2. (Non-virgin females were used because virgin females, other than newly emerged ones, are a rarity in the wild). As in the oviposition assay, each cage contained 10 Petri dishes, half with ethanol-supplemented medium (either 2 or 4%), and half with control medium. In half the cages, the ethanol food was colored with 0.6% blue food dye (Indigo Carmine; Fisher Scientific, Waltham, MA, USA) and the control food was colored with 0.6% red dye (Amaranth; Sigma-Aldrich, St. Louis, MO, USA), and vice versa for the other half. The flies were allowed to choose between ethanol food and control food for 4 h. Austria and Cameroon flies were assayed together in three temporal blocks, whereas Netherlands and Tanzania flies were assayed at a different time in four blocks. In each block, two replicate pairs of cages with dyes reversed were set up for each sex, population, and ethanol concentration. After feeding, flies were frozen and their abdominal coloration was checked under a stereomicroscope. Abdomens were classified as blue (B), red (R), purple blue (PB), purple red (PR), purple (P), or unstained. The ‘feeding ethanol preference index’ (FEPI) was calculated as follows (for the case where ethanol food was blue; with red ethanol food, ‘R’ and ‘B’ in the numerator should be interchanged):
FEPI ranges from −0.5 (always chose control food) to +0.5 (always chose ethanol food), with zero corresponding to no preference.
Statistical analysis
Both, oviposition and feeding preference for ethanol medium were analyzed using the MIXED procedure in SAS 9.2 (SAS Institute, Cary, NC, USA). F-tests for the fixed effects were performed using the Satterthwaite method, and random effects for which P≥0.29, as determined by the Z-tests provided by SAS, were dropped.
In the oviposition preference experiment, population and ethanol concentration and their interaction were fixed effects; block and all possible interactions involving block were random effects. To compare temperate populations to tropical populations, an additional analysis was conducted, in which region (temperate vs. tropical) and ethanol concentration were fixed effects; block, population nested within region, and all possible interactions involving each of the two were treated as random effects. Because region*ethanol concentration interaction was significant (P<0.0001), additional analyses were conducted separately by region to estimate the effect of ethanol concentration, and separately by ethanol concentration to test for the region effect on ethanol concentration at 2, 4, or 6%.
To determine whether dye color may have affected the feeding results, we did a preliminary analysis with population, sex, ethanol concentration, and dye color in ethanol food as crossed fixed effects, and block as a random effect; all possible interactions were included, with those involving block as random effects. In separate analyses of the Austria-Cameroon and Netherlands-Tanzania pairs, which were tested at different times, dye color in ethanol food and all interactions involving it had no significant effects (P>0.05), so the dye color and all its interactions were dropped from the model. Least-square means and their standard errors from these reduced models are used for graphical purposes below.
To test for the region effect at each ethanol concentration, we used a combined analysis in which the pair effect (Austria-Cameroon or Netherlands-Tanzania), block nested within pair, and all interactions involving the two were treated as random effects. Region, sex, and their interactions were treated as fixed effects. In this model, random effects of population within region contribute to the pair*region interaction. Because the sex effect was significant (P<0.0001), this analysis was further separated by sex.
Results
Oviposition preference
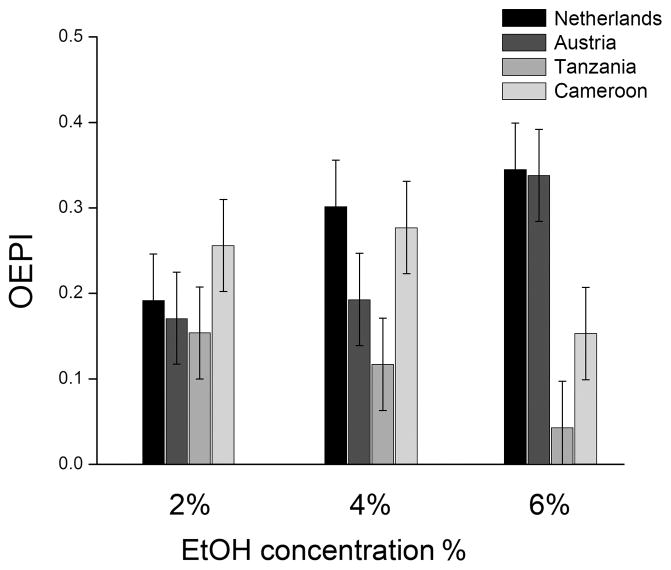
Females preferred to lay their eggs on ethanol food at all concentrations tested (Figure 1). When comparing temperate and tropical females, however, a strong region*ethanol concentration interaction was detected (P<0.0001). As ethanol concentration increased, females from the two temperate populations increased their preference for oviposition on ethanol medium (P = 0.0002 for ethanol concentration effect), whereas the females from the two tropical populations showed the opposite pattern (P = 0.02). The temperate-tropical difference increased along with ethanol concentration, with no significant region effect when ethanol concentration was 2 or 4% (P = 0.73 and 0.65, respectively), but a significant effect at 6% ethanol (P = 0.047).
Figure 1.
Mean (± SEM) preference for oviposition on ethanol medium (OEPI), with positive values showing preference, and zero showing random choice between ethanol medium and non-ethanol medium. All OEPIs are significantly higher than zero (P<0.05), except for the Tanzania population at 4 and 6% (P = 0.10 and 0.48, respectively).
Feeding preference
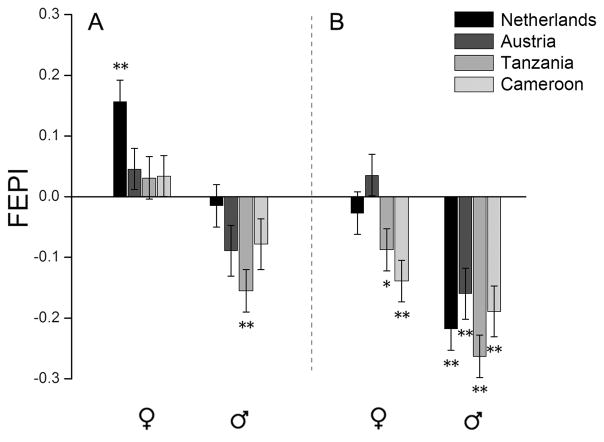
When flies were allowed to choose between control medium and medium with 2% ethanol (Figure 2A), there was a significant effect of sex (P<0.0001), but no significant region effect (P>0.3) or sex*region interaction (P>0.9). In separate analyses by sex, region effects remained non-significant (P>0.1 in males, P>0.3 in females). Males on average showed avoidance of the ethanol food [estimated from the model intercept, mean (± SE) FEPI = −0.12 ± 0.03; P<0.003], whereas females on average showed neither preference nor avoidance (FEPI = 0.033 ± 0.039; P>0.4). However, females from the Netherlands population showed preference for 2% ethanol (Figure 2A).
Figure 2.
Mean (± SEM) preference for feeding on (A) 2% or (B) 4% ethanol food (FEPI), with positive values showing preference, and negative values showing avoidance. Asterisks indicate significant difference from zero (*P<0.05, **P<0.01).
When flies were allowed to choose between control medium and medium with 4% ethanol (Figure 2B), there were significant effects of both region (P<0.03) and sex (P<0.0001), with a non-significant but suggestive sex*region interaction (P = 0.098). When analyzed separately, males showed significant avoidance of ethanol (FEPI = −0.23 ± 0.04; P<0.05), with no significant region effect (P>0.2). In contrast, females showed a significant region effect (P<0.02), with tropical females significantly avoiding ethanol (FEPI = −0.11 ± 0.03; P = 0.0025) and temperate females showing neither preference nor avoidance (FEPI = 0.00 ± 0.03; P>0.9).
Discussion
As predicted, females from more ethanol-resistant European populations showed stronger oviposition preference for ethanol than females from less resistant African populations. Females from all populations preferred to oviposit on ethanol-supplemented medium over control medium, but this preference grew stronger in European females as ethanol concentration increased, whereas growing weaker in African females. At the highest ethanol concentration (6%), European females laid nearly 85% of their eggs on the ethanol-supplemented food, vs. only about 60% for African females. Our 6% is within the range for reported ethanol concentrations of D. melanogaster breeding sites, but close to the upper limit; potential breeding sites with higher ethanol concentrations seem to be rare, at least in the temperate zone (Gibson et al., 1981; Oakeshott et al., 1982). Our results therefore suggest that temperate D. melanogaster females seek out oviposition sites with the maximum available ethanol concentration.
In contrast, in feeding choice tests, flies were more likely to avoid than prefer ethanol-supplemented medium (cf. Pohl et al., 2012). Although both European and African females preferred 4% ethanol over control medium for oviposition, European females showed no preference when given the same choice in feeding trials, whereas African females preferred to feed on control medium. Males showed stronger aversion to feeding on ethanol than females, with males from all four populations strongly avoiding 4% ethanol. One possible explanation for the sex difference in feeding preference is that female oviposition preference for ethanol predisposes them to feed on ethanol-supplemented food more than males. Females lay eggs singly, so feeding preferentially on control medium, whereas ovipositing preferentially on ethanol medium would require frequent movement between the two medium types. (Although flies were starved for 24 h before the feeding tests to increase their feeding rate and decrease their oviposition rate, the females nonetheless laid some eggs during the 4-h feeding period, albeit many fewer than in the oviposition assays).
The combination of avoidance of ethanol by feeding adults with preference by larvae and ovipositing females strongly suggests that ethanol preference evolved because of one or more benefits to larvae. Two possible benefits of choosing breeding sites rich in ethanol are cryoprotection, and escape from ethanol-sensitive natural enemies and competitors. In support of the first possibility, Waagner et al. (2013) showed that immersion in a dilute ethanol solution increased survival of springtails following cold shock, apparently by making cell membranes less rigid. If ethanol provides similar cryoprotection to larvae of D. melanogaster, a comparatively cold-sensitive species which evolved in the tropics (Gibert et al., 2001), this would be expected to favor stronger preference for ethanol in temperate than in tropical populations.
In support of the second possibility, Milan et al. (2012) showed that 6% ethanol in medium reduced the number of eggs that parasitoid wasps laid on D. melanogaster larvae, and increased mortality of the developing wasp larvae inside fly larvae, resulting in higher survival of the fly larvae. Moreover, parasitized larvae sought out ethanol-containing medium more than unparasitized larvae, and the sight of adult wasps caused female D. melanogaster to increase their oviposition preference for ethanol (Kacsoh et al., 2013). Due to its volatility and rapid sedating properties, ethanol also potentially deters ethanol-sensitive predators and competitors of D. melanogaster larvae. Unfortunately, because virtually all ecological studies of D. melanogaster have been conducted in the temperate zone, there is little information on whether temperate populations are more affected by natural enemies or competitors than tropical populations. In the tropics and subtropics, however, D. melanogaster is reported to be uncommon except in or near buildings (Fuyama & Watada, 1981; Yamamoto et al., 1985; J Poole, pers. comm. 2014), where exposure to natural enemies and competitors would be expected to be lower than in forests or orchards. In contrast, D. melanogaster is typically the most abundant Drosophila species in orchards in northern Europe and the northern USA, regardless of their proximity to buildings (Fleury et al., 2009). In these settings, larvae often suffer high rates of parasitoid attack (Fleury et al., 2009).
It is also possible that natural enemies and competitors of D. melanogaster in the tropics tend to be more ethanol resistant than those in the temperate zone, reducing the advantage of choosing breeding sites high in ethanol. (Highly ethanol-resistant competitors in the tropics, if a factor, would not likely include other Drosophila species, which are less ethanol resistant than even tropical D. melanogaster; Mercot et al., 1994). Observations on two common and widely-distributed figitid parasitoids of D. melanogaster, Leptopilina boulardi Barbotin et al. and Leptopilina heterotoma Thomson, give some support for this possibility. Of the two species, L. boulardi is found in warmer climates, including the tropics, where L. heterotoma appears to be absent (Fleury et al., 2009). Milan et al. (2012) showed that ethanol is considerably less effective in protecting D. melanogaster larvae from L. boulardi than L. heterotoma, a relatively ethanol-resistant species (Bouletreau & David, 1981). More generally, because fruits large and sweet enough to produce high concentrations of ethanol upon fermentation occur naturally in the tropics, but are rare outside of agricultural settings in temperate regions (Levey, 2004), natural enemies and competitors of frugivorous Drosophila in the tropics would be expected to have had longer to evolve ethanol resistance than those in temperate regions.
If the benefits of ethanol for larvae are stronger in the temperate zone than in the tropics, the following verbal argument suggests that ethanol resistance and preference for ethanol would have increased more or less simultaneously, as ancestral tropical D. melanogaster adapted to temperate regions. Any increase in ethanol’s fitness benefit for larvae would be expected to increase the ethanol concentration that yields the highest larval fitness, thus causing selection to favor preference for higher ethanol concentrations in oviposition and larval feeding. Any resulting increase in preference would then intensify selection for resistance. Moreover, even without an increase in preference, any reduction in larval mortality caused by a benefit of ethanol would increase the proportion of surviving adults in the population that had experienced selection for ethanol resistance as larvae (and whose parents had experienced such selection while ovipositing and mating), thereby increasing the average strength of selection for resistance. As resistance increased, the optimal ethanol concentration for larvae would further increase, creating selection for yet higher preference. This joint increase in preference and resistance would be accelerated if the two traits were positively correlated due to pleiotropy, but does not require such a correlation. Although there is some evidence that mutations affecting ethanol resistance can also affect preference (Devineni et al., 2011), our finding that European and African males do not differ in feeding preference for ethanol, in spite of differing strongly in resistance, suggests that the two traits may be largely independent.
Although our results suggest there is a benefit of ethanol for D. melanogaster larvae that is stronger in the temperate zone than in the tropics, other factors may have contributed to the evolution of increased resistance and preference for ethanol in temperate populations. For example, it is possible that selection for cold resistance in temperate populations increased ethanol resistance as a pleiotropic response (cf. Montooth et al., 2006). Differences in cold resistance between temperate and tropical D. melanogaster populations, however, are surprisingly slight (Gibert et al., 2001; Hoffmann et al., 2001). Moreover, the ethanol resistance of temperate D. melanogaster populations is substantially higher than that of most other Drosophila species, including those occurring only in temperate regions (Merçot et al., 1994). We also note that there is no evidence that temperate D. melanogaster populations have generally higher stress resistance than tropical populations (Hoffmann et al., 2001; 2005; Hoffmann & Weeks, 2007).
In conclusion, we have shown that D. melanogaster females from two temperate populations have stronger oviposition preference for ethanol than those from two tropical populations, paralleling the well-documented temperate-tropical difference in ethanol resistance in this species. Our results are consistent with the hypothesis that choosing breeding sites high in ethanol may benefit temperate populations more than tropical populations. More work will be needed to confirm this hypothesis and establish the nature of the benefit.
Acknowledgments
We thank D Begun, D Rand, J Pool, and C Schlötterer for providing flies. J Jaenike, J Werren, and two anonymous reviewers made helpful comments on earlier versions of the manuscript. Supported by NIH grant R01AA016178 to JDF.
References
- Anderson D. Alcohol dehydrogenase activity and ethanol tolerance along the Adh cline in Australia. In: Lakovaara S, editor. Advances in Genetics, Development, and Evolution of Drosophila. Plenum; New York, NY, USA: 1982. pp. 31–62. [Google Scholar]
- Bouletreau M, David JR. Sexually dimorphic response to host habitat toxicity in Drosophila parasitic wasps. Evolution. 1981;35:395–399. doi: 10.1111/j.1558-5646.1981.tb04898.x. [DOI] [PubMed] [Google Scholar]
- Cohan FM, Graf J-D. Latitudinal cline in Drosophila melanogaster for knockdown resistance to ethanol fumes and for rates of response to selection for further resistance. Evolution. 1985;39:278–293. doi: 10.1111/j.1558-5646.1985.tb05666.x. [DOI] [PubMed] [Google Scholar]
- David JR, Bocquet C. Similarities and differences in latitudinal adaptation of two Drosophila sibling species. Nature. 1975;257:588–590. doi: 10.1038/257588a0. [DOI] [PubMed] [Google Scholar]
- David JR, Merçot H, Capy P, McEvey SF, van Herrewege J. Alcohol tolerance and Adh gene frequencies in European and African populations of Drosophila melanogaster. Genetics Selection Evolution. 1986;18:405–416. doi: 10.1186/1297-9686-18-4-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean MD, Ballard KJ, Glass A, William J, Ballard O. Influence of two Wolbachia strains on population structure of East African Drosophila simulans. Genetics. 2003;165:1959–1969. doi: 10.1093/genetics/165.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, McClure KD, Guarnieri DJ, Corl AB, Wolf FW, et al. The genetic relationships between ethanol preference, acute ethanol sensitivity and ethanol tolerance in Drosophila melanogaster. Fly. 2011;5:191–199. doi: 10.4161/fly.5.3.16987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integrative Comparative Biology. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- Fleury F, Gibert P, Ris N, Allemand R. Ecology and life history of frugivorous Drosophila parasitoids. Advances in Parasitology. 2009;70:3–44. doi: 10.1016/S0065-308X(09)70001-6. [DOI] [PubMed] [Google Scholar]
- Fry JD. Mechanisms of naturally-evolved ethanol resistance in Drosophila melanogaster. Journal of Experimental Biology. 2014;217:3996–4003. doi: 10.1242/jeb.110510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuyama Y, Watada M. The microdistribution of Drosophila melanogaster and Drosophila simulans: a survey in the Bonin Islands. Zoological Magazine. 1981;90:62–68. [Google Scholar]
- Gibert P, Moreteau B, Pétavy G, Karan D, David JR. Chill-coma tolerance, a major climatic adaptation among Drosophila species. Evolution. 2001;55:1063–1068. doi: 10.1554/0014-3820(2001)055[1063:cctamc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gibson JB, May TW, Wilks AV. Genetic variation at the alcohol dehydrogenase locus in Drosophila melanogaster in relation to environmental variation: Ethanol levels in breeding sites and allozyme frequencies. Oecologia. 1981;51:191–198. doi: 10.1007/BF00540600. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Weeks AR. Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica. 2007;129:133–147. doi: 10.1007/s10709-006-9010-z. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Hallas R, Sinclair C, Mitrovski P. Levels of variation in stress resistance in Drosophila among strains, local populations, and geographic regions: patterns for desiccation, starvation, cold resistance, and associated traits. Evolution. 2001;55:1621–1630. doi: 10.1111/j.0014-3820.2001.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Shirriffs J, Scott M. Relative importance of plastic vs genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Functional Ecology. 2005;19:222–227. [Google Scholar]
- Hougouto N, Lietaert MC, Libion-Mannaert M, Feytmans E, Elens A. Oviposition-site preference and ADH activity in Drosophila melanogaster. Genetica. 1982;58:121–128. [Google Scholar]
- Kacsoh BZ, Lynch ZR, Mortmer NT, Schlenke TA. Fruit flies mediate offspring after seeing parasites. Science. 2013;339:947–950. doi: 10.1126/science.1229625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. Drosophila melanogaster’s history as a human commensal. Current Biology. 2007;17:R77–R81. doi: 10.1016/j.cub.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Levey DJ. The evolutionary ecology of ethanol production and alcoholism. Integrative Comparative Biology. 2004;44:284–289. doi: 10.1093/icb/44.4.284. [DOI] [PubMed] [Google Scholar]
- Merçot H, Defaye D, Capy P, Pla E, David JR. Alcohol tolerance, ADH activity, and ecological niche of Drosophila species. Evolution. 1994;48:746–757. doi: 10.1111/j.1558-5646.1994.tb01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan NF, Kacsoh BZ, Schlenke TA. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Current Biology. 2012;22:488–493. doi: 10.1016/j.cub.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montooth KL, Siebenthall KT, Clark AG. Membrane lipid physiology and toxin catabolism underlie ethanol and acetic acid tolerance in Drosophila melanogaster. Journal of Experimental Biology. 2006;209:3837–3850. doi: 10.1242/jeb.02448. [DOI] [PubMed] [Google Scholar]
- Oakeshott JG, May TW, Gibson JB, Willcocks DA. Resource partitioning in five domestic Drosophila species and its relationship to ethanol metabolism. Australian Journal of Zoology. 1982;30:547–556. [Google Scholar]
- Parkash R, Karan D, Munjal AK. Geographical variation in AdhF and alcoholic resource utilization in Indian populations of Drosophila melanogaster. Biological Journal of the Linnean Society. 1999;66:205–214. [Google Scholar]
- Parsons PA. Larval reaction to alcohol as an indicator of resource utilization differences between Drosophila melanogaster and D. simulans. Oecologia. 1977;30:141–146. doi: 10.1007/BF00345417. [DOI] [PubMed] [Google Scholar]
- Parsons PA. Larval responses to environmental ethanol in Drosophila melanogaster: Variation within and among populations. Behavior Genetics. 1980;10:183–190. doi: 10.1007/BF01066268. [DOI] [PubMed] [Google Scholar]
- Pohl JB, Baldwin BA, Dinh BL, Rahman P, Smerek D, et al. Ethanol preference in Drosophila melanogaster is driven by its caloric value. Alcoholism: Clinical and Experimental Research. 2012;36:1903–1912. doi: 10.1111/j.1530-0277.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool JE, Aquadro CF. History and structure of sub-Saharan populations of Drosophila melanogaster. Genetics. 2006;174:915–929. doi: 10.1534/genetics.106.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal ML, Hartl DL. Oviposition-site preference in Drosophila following interspecific gene transfer of the Alcohol dehydrogenase locus. Behavior Genetics. 1999;29:199–204. doi: 10.1023/a:1021648103496. [DOI] [PubMed] [Google Scholar]
- Tanimura T, Isono K, Takamura T, Shimada I. Genetic dimorphism in taste sensitivity to trehalose in Drosophila melanogaster. Journal of Comparative Physiology A. 1982;147:265–269. [Google Scholar]
- Waagner D, Bouvrais H, Ipsen JH, Holmstrup M. Linking membrane physical properties and low temperature tolerance in arthropods. Cryobiology. 2013;67:383–385. doi: 10.1016/j.cryobiol.2013.09.164. [DOI] [PubMed] [Google Scholar]
- Yamamoto AH, Fuyama Y, Watada M. Habitat selection of two sibling species, Drosophila melanogaster and D. simulans: a further survey in the Bonin Islands. Zoological Science. 1985;2:265–270. [Google Scholar]