Adipose tissues are distributed in multiple depots in the body of animals including human beings and rodents1. In the past decades, the biology of adipose tissues has been intensely studied. Currently, it is well accepted that the existence of adipose tissues is essential to maintain normal physiological activities and health. However, excessive accumulation of adipose tissues in their original depots and ectopic accumulation of lipids especially in the liver and skeletal muscles lead to obesity and insulin resistance, which will further cause lethal cardiovascular complications2. Mechanistically, it has been established that macrophage infiltration and oxidative stress in adipose tissues cause malfunction of the adipose tissue resulting in the secretion of numerous inflammatory factors and adipocyte-derived hormones which negatively regulate functions of the target organs such as heart and blood vessels, and eventually lead to cardiac and vascular diseases3. Notably, adipose tissue may be originally differentiated from cells in the vasculature or share the same precursors with vasculature cells4. Also, adipogenesis always is companied by angiogenesis of blood vessels5. Thus, the relationship between adipose tissue and blood vessels is drawing much attention on aspects relating to both physiology and shared origin.
One of the primary functions of adipose tissue is to store extra triglycerides in the form of lipid droplets. However, the sizes and distributions of lipid droplets are considerably different in different adipose tissue depots. Most adipose tissues in the human body comprise adipocytes containing a single large lipid droplet with fewer mitochondria, which is called white adipose tissue (WAT) and is distributed in visceral and subcutaneous regions. It is well accepted that visceral WAT is positively associated with development of cardiovascular diseases (CVDs) and related complications, while subcutaneous WAT may be inversely associated with CVDs1. Indeed, recent studies have shown that subcutaneous WAT harbors a few particular adipocytes containing multiple smaller size lipid droplets and more mitochondria. Immunohistological, gene and protein profile studies demonstrated that these cells are classical brown adipocytes. Therefore, subcutaneous WAT actually is a mixture of white and brown adipose tissue (BAT), which was recognized as beige adipose tissue (BeAT)6. Importantly, many compounds such as rosiglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) ligand, growth factors such as fibroblast growth factor 21 (FGF21) and hormones such as irisin or the stimulation with cold or β3-adrenergic receptor agonists significantly increased the numbers of characteristically brown adipocytes in WAT, which is called the “browning” process7. Studies focusing on browning WAT are extremely relevant and critical because these particular adipocytes express uncoupling protein-1 (UCP-1), which promotes heat production over ATP production in the mitochondria8. This process leads to more energy expenditure, which underlies the apparent opposite aspects of WAT function in alternative depots or physiological contexts. Therefore, it is hypothesized that accumulation of BeAT may be positively associated with CV protection1. Unfortunately, the investigation of BAT has been largely ignored because of the long-held believe that BAT was not present in adult human beings. Interestingly, the adult humans do have BAT in very specific body regions9, even though the amount of such BAT is small (~32–85g) when compared to the body weight of adult human beings. Its capability for energy expenditure in the whole body and herein its effects in preventing obesity and CVDs remain largely unknown10. We believe that BAT is not only a “heater”, but also an endocrine organ8 like WAT which secrets numerous factors and interacts with neighboring organs.
Regarding the relationship between adipose tissue and CVDs, the perivascular adipose tissue (PVAT), the adipose tissue specifically surrounding blood vessels, was extensively studied in recent years4, 11–14. All the adipose tissues surrounding the blood vessel tree in the cardiovascular system should be classified as PVAT (Figure 1). Particularly, mesenteric PVAT was traditionally recognized as visceral WAT, which is believed to be positively associated with CVDs3. It is well known that obesity is one of the risk factors for hypertension. Interestingly, in lean individuals PVAT has anticontractile properties, and consistently, PVAT from obese individuals loses its anticontractile properties. So far, multiple substances secreted by PVAT contribute to the PVAT anticontractile role1. H2O2 is one of the anticontractile factors in both aortic and mesenteric PVAT. Previous studies documented that aortic PVAT has a BAT phenotype, while mesenteric PVAT has a WAT phenotype in rodents4. Friederich-Persson et al15 in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology investigated the adipose phenotype in different depots in mice by analyzing mRNA levels of markers for WAT, BAT and BeAT. The results indicated that mesenteric PVAT displayed expression of both WAT and BeAT specific markers, hence establishing that mesenteric PVAT is not pure WAT, but a BeAT-like adipose tissue. As discussed above, BeAT shares partial characteristics of classic BAT. Friederich-Persson et al demonstrated that, similarly to mesenteric and aortic PVAT, interscapular BAT releases H2O2 and exerts an anticontractile effect as well, which does not directly influence endothelial-dependent and –independent vascular relaxation. Since Nox4 is predominately expressed in BAT and produces H2O2, BAT in the context of Nox4 deficiency fails to exert the anticontractile effect. Even though H2O2 in BAT is partially the result of dismutation of O2·−, BAT from animals deficient for the O2·− producing enzymes Nox1 and 2 still exerts anticontractile effects on blood vessels. The mechanisms underlying the anticontractile properties of PVAT are largely unknown. Vascular smooth muscle cells (VSMCs) are the targets for factors released from PVAT to regulate vascular tone4. Friederich-Persson et al15 documented that only Cyclic GMP-dependent kinase G (PKG)-1 in VSMCs is a contractility-relevant target of H2O2, even though H2O2 also activates protein kinase A (PKA). Yet, PKA in VSMCs does not appear to be involved in the anticontractile effect of H2O2 released from BAT. Indeed, incubation of vascular tissue with BAT results in reduced phosphorylation of vascular MYPT1 and MLC20, both downstream targets of active PKG-1 in VSMCs and induces smooth muscle contraction. Unlike pure BAT, BeAT-like mesenteric PVAT exerts anticontractile effects through different mechanisms, which are yet unclear. Significantly, further browning of resident BeAT-like mesenteric PVAT increased anticontractility through a mechanism similar to interscapular pure BAT, suggesting perhaps a continuum of changing adaptive (and, eventually, maladaptive) responses depending on the degree of browning of the PVAT.
Figure 1.
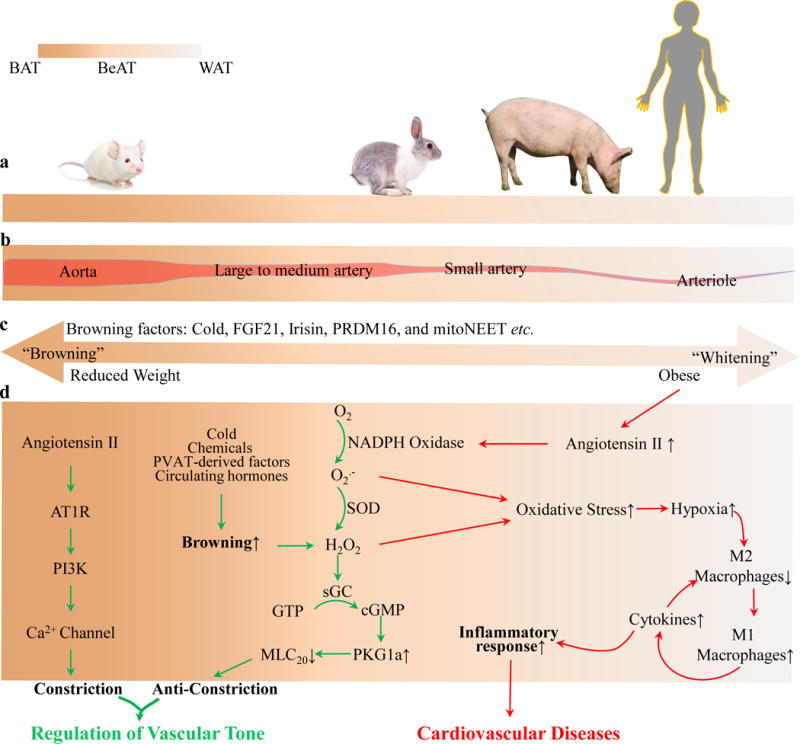
The PVAT phenotype and vascular homeostasis. (a). PVAT shows stronger BAT-like phenotype in smaller animals such as rodents, whereas it shows relatively increasing WAT-like phenotype in larger animals such as rabbits, pigs or humans. (b). PVAT is BeAT in nature, and surrounds most blood vessels with varying phenotypes in different vascular beds. (c). PVAT could be turned into BAT- or WAT-like phenotype in response to bioactive factors, temperature, nutrition status and obesity. (d) In healthy conditions, BAT-like PVAT effectively regulates homeostatic vascular tone by secreting adipocyte-derived hormones. On the other end of the continuum, WAT-like PVAT shows exacerbated oxidative stress and inflammatory phenotype and promotes CVDs. The continuum afforded by the different degrees of BeAT-like phenotypes during “browning” of WAT and, conversely, the transition to a WAT-like phenotype from BAT during disease progression, can operate as an adaptive response that can be brought about by either mild oxidative stress, initial adaptive inflammatory response, pharmacological treatment or weight loss interventions. This phenomenon could also underlie the obesity paradox associated with the relative protection against CVDs observed in overweight conditions.
These findings raise extremely interesting issues with regard to the study itself, its insights into understanding the physiology and pathophysiology of BeAT-like or BeAT-potential of PVAT in humans and its implications for targeted therapy of CVDs. Because PVAT tightly surrounds most blood vessels, PVAT-derived factors, including H2O2, will locally affect immediate neighboring cells in the vessel walls, leading to either physiological benefit or pathophysiological harm for the vessel walls. Currently, it is unclear whether PVAT is associated with CVDs, especially hypertension, atherosclerosis and aneurysms. Phenotypic changes in PVAT ultimately may affect the development of CVDs. Apart from the specific roles of endothelial cells and VSMCs in CVDs, there is no doubt that further understanding of CVDs in humans will require extensive research to understand the various phenotypes of resident PVAT, as underscored in this article. Browning of human PVAT is not just a means to improve a “heater” function, but, as a paracrine organ, it can be a powerful therapeutic target for CVDs.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL122664 (to L.C), HL068878 (to Y.E.C.) and P50-HL117929 (to M.G.B.).
References
- 1.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease: A review of current research and animal models. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malin SK, Kashyap SR. Type 2 diabetes treatment in the patient with obesity. Endocrinology and metabolism clinics of North America. 2016;45:553–564. doi: 10.1016/j.ecl.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes & metabolism. 2014;40:16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue Y, Lim S, Brakenhielm E, Cao Y. Adipose angiogenesis: Quantitative methods to study microvessel growth, regression and remodeling in vivo. Nature protocols. 2010;5:912–920. doi: 10.1038/nprot.2010.46. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidossis L, Kajimura S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation. 2015;125:478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nature reviews. Endocrinology. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 9.Brychta RJ, Chen KY. Cold-induced thermogenesis in humans. European journal of clinical nutrition. 2016 doi: 10.1038/ejcn.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15o pet measurement of blood flow and oxygen consumption in cold-activated human brown fat. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013;54:523–531. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1631–1636. doi: 10.1161/ATVBAHA.114.303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayala-Lopez N, Martini M, Jackson WF, Darios E, Burnett R, Seitz B, Fink GD, Watts SW. Perivascular adipose tissue contains functional catecholamines. Pharmacology research & perspectives. 2014;2:e00041. doi: 10.1002/prp2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Jarallah A, Oriowo MA. Loss of anticontractile effect of perivascular adipose tissue on pregnant rats: A potential role of tumor necrosis factor-alpha. Journal of cardiovascular pharmacology. 2016;67:145–151. doi: 10.1097/FJC.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 15.Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM. Brown adipose tissue regulates small artery function through NADPH Oxidase 4–derived hydrogen peroxide and redox-sensitive protein kinase G-1α. Arteriosclerosis, thrombosis, and vascular biology. doi: 10.1161/ATVBAHA.116.308659. Originally published December 22, 2016. [DOI] [PubMed] [Google Scholar]


