Abstract
The nucleus reuniens (RE) of the ventral midline thalamus in strongly reciprocally connected with the hippocampus (HF) and the medial prefrontal cortex (mPFC) and has been shown to mediate the transfer of information between these structures. It has become increasingly well established that RE serves a critical role in mnemonic tasks requiring the interaction of the HF and mPFC, but not tasks relying solely on the HF. Very few studies have addressed the independent actions of RE on prefrontal executive functioning. The present report examined the effects of lesions of the ventral midline thalamus, including RE and the dorsally adjacent rhomboid nucleus (RH) in rats on attention and behavioral flexibility using the attentional set shifting task (AST). The task uses odor and tactile stimuli to test for attentional set formation, attentional set shifting, behavioral flexibility and reversal learning. By comparison with sham controls, lesioned rats were significantly impaired on reversal learning and intradimensional (ID) set shifting. Specifically, RE/RH lesioned rats were impaired on the first reversal stage of the task which required a change in response strategy to select a previously non-rewarded stimulus for reward. RE/RH lesioned rats also exhibited deficits in the ability to transfer or generalize rules of the task which consists of making the same modality-based choices (e.g., odor vs. tactile) to different sets of stimuli in the ID stage of the task. These results demonstrate that in addition to its role in tasks dependent on HF-mPFC interactions, RE/RH is also critically involved cognitive/executive functions associated with the medial prefrontal cortex. As such, the deficits in the AST task produced by RE/RH lesions suggest the ventral midline thalamus directly contributes to flexible goal directed behavior.
Keywords: nucleus reuniens, rhomboid nucleus, cognition, behavioral flexibility, reversal learning, attentional set shifting
1. Introduction
It is well recognized that the hippocampal formation (HF) and the medial prefrontal cortex (mPFC) serve a critical role in working memory in the rat. Recent evidence suggests that these structures mutually interact to process memory/working memory (WM). For instance, it has been shown that inactivation of either structure, or their disconnection, impairs spatial working memory (Floresco et al., 1997; Lee and Kesner, 2003; Yoon et al., 2008; Churchwell et al., 2010; Churchwell and Kesner, 2011; Griffin, 2015). In addition, the two structures show coherent activity during the performance of certain working memory tasks (Jones and Wilson, 2005; Colgin, 2011; Gordon, 2011; O'Neill et al., 2013). Specifically, using a continuous spatial alternation task in rats, Jones and Wilson (2005) demonstrated strong coherence and theta phase locking between the hippocampus and the infralimbic/prelimbic cortices of the ventral mPFC during successful task completion. More recently, Gordon and colleagues (O'Neill et al. 2013) reported that activity was highly synchronous between both the dorsal and ventral hippocampus and the ventral mPFC during correct choices (runs) on a spatial alternation T-maze task (O'Neill et al., 2013). Interestingly, inactivation of the ventral hippocampus with muscimol disrupted synchronous theta oscillations between the dorsal HF and the mPFC.
While the hippocampus sends dense projections to the mPFC, there are virtually no return projections from the mPFC to the HF -- suggesting that the mPFC influences the hippocampus indirectly through other routes (Swanson, 1981; Ferino et al., 1987; Jay and Witter, 1991; Carr and Sesack, 1996; Laroche et al., 2000; Vertes, 2002, 2004; Hoover and Vertes, 2007). One such route is through the nucleus reuniens (RE) of the midline thalamus. Specifically, RE receives pronounced projections from the infralimbic and prelimbic cortices of the ventral mPFC and in turn distributes heavily to the HF where it exerts predominantly excitatory actions (Dolleman-Van der Weel et al., 1997; Bertram and Zhang, 1999; Vertes, 2002; Viana Di Prisco and Vertes, 2006; Vertes et al., 2006). Furthermore, RE distributes via collaterals to the mPFC and HF, and a subset of RE cells projecting to the HF receive direct excitatory synaptic contacts from ventral mPFC neurons (Vertes et al., 2007; Hoover and Vertes, 2012; Varela et al., 2014). This suggests that RE is critical link between the mPFC and the hippocampus.
Based on these patterns of connections, several recent behavioral studies have examined the role of RE (and the dorsally adjacent rhomboid nucleus, RH) in various spatial working memory tasks (Hembrook and Mair, 2011; Hembrook et al., 2012; Loureiro et al., 2012; Cholvin et al., 2013; Hallock et al., 2013; Layfield et al., 2015). The general conclusion from these studies is that RE/RH is most directly involved in those tasks that involve the cooperative actions of the mPFC and the hippocampus (for review, Cassel et al., 2013; Vertes et al. 2015; Griffin, 2015). For instance, inactivation of RE/RH does not disrupt performance on the standard water maze task, which is dependent on the hippocampus to the essential exclusion of the mPFC (Doleman-van der Weel et al., 2009; Loureiro et al., 2012; Cholvin et al., 2013). By contrast, RE/RH lesions or inactivation have been shown to significantly impair performance on spatial tasks requiring both the HF and mPFC. In this regard, inactivation of RE/RH was shown to alter performance on a delayed non-match to sample radial arm maze (RAM) task and on a delayed non-match to position task -- both of which are sensitive to disruption of the HF or the mPFC. Performance, however, was unaltered on a variable choice paradigm in the RAM, sensitive only to hippocampal disruption (Hembrook and Mair, 2011; Hembrook et al., 2012).
Similarly, Loureiro et al. (2012) reported that inactivating RE/RH had no effect on retention on a water maze task 5 days post-acquisition compared to significant impairments 25 days post-acquisition. They attributed the difference to the respective engagement of recent (5 days) or remote (25 days) memories; that is, the recall of recent spatial memories only requires the hippocampus, whereas remote memories require both the hippocampus and the mPFC. This same group (Cholvin et al., 2013) subsequently assessed the role of RE/RH in spatial WM using a ‘double H’ water maze. Specifically, starting from a fixed position in the maze, rats learned to navigate to one of the arms of the maze and on the probe trial were released from a different start position and the choice was to continue to make the same pattern of movements to reach the platform (i.e., a right and then an immediate left turn), considered a response strategy, or alternatively to switch strategies which would lead to the previous (and correct) location of the platform (or a place response). Inactivation of RE/RH, the hippocampus or the mPFC impaired the shift to a place response, indicating that disruption of the HF, the mPFC, or the RE-mediated communication between them altered performance on this spatial WM task. Finally, Layfield et al. (2015) recently reported that suppression of RE/RH did not disrupt performance on continuous version of a T-maze alternation task, which appears to depend on the mPFC, but significantly altered performance on the delayed version of the task which reportedly enlists both the HF and the mPFC (Aggelton et al., 1986; Brito and Brito, 1990; Sanchez-Santad et al., 1997; Hock and Bunsey, 1998; Lee and Kesner, 2003; Yoon et al., 2008).
While, as discussed, RE has been linked to behaviors that depend upon interactions of the hippocampus and the mPFC, RE has also been implicated in behaviors that almost solely enlist the prefrontal cortex. In an early study, Dolleman –Van der Weel et al. (2009) found that RE lesions did not impair acquisition or retention of spatial reference memory on a water maze task, but nevertheless led to a maladaptive search strategy that was deemed a PFC and not a hippocampal-associated deficit. Consistent with this, Prasad et al. (2013) showed that RE lesions led to premature responding (on a variable intertrial interval schedule) with the 5 choice serial reaction time task (5-CSRTT), whereas other indices such as omitting responses (a measure of attention) or perseverative responses were unaffected by the lesions. Premature responding is viewed as a failure of impulse control and is also seen with lesions of the infralimbic cortex on the 5-CSRTT task (Chudasama et al., 2003).
In the present report, we further examined the role of RE/RH in prefrontal executive functions using an odor/texture discrimination task, commonly referred to as the attentional set shifting task (AST) -- as adopted from Brown and colleagues (Birrell and Brown, 2000; McAlonan and Brown, 2003; Hatcher et al., 2005; O'Neill and Brown, 2007; Tait et al., 2007). The task is analogous to the intradimensional extradimensional (IED) task used to assess prefrontal functioning in primates (Sahakian and Owen, 1992; Robbins and Arnsten, 2009). The AST task was used to examine the effects of RE/RH lesions on three measures known to be affected by alterations of the prefrontal cortex: (1) attention, or forming an attentional set (Roberts et al., 1994; Crofts et al., 2001; Chase et al. 2012) associated with the orbital cortex; (2) attentional set shifting, associated with the mPFC (Birrell and Brown, 2000; Floresco et al., 2008; McGaughy et al., 2008); and (3) reversal learning or behavioral flexibility also linked to the orbital cortex (McAlonan and Brown, 2003; Clarke et al., 2005, 2007; Chase et al., 2012). In light of the fact that RE strongly targets these prefrontal cortical sites Vertes et al., 2006), we hypothesized that RE/RH lesions would impair attentional and cognitive processes as assessed with the AST task.
2. Results
2.1 Histology
Surgery was performed on 52 rats. Of these, 33 were included in the analysis of this study (RE/RH lesion = 17; sham control = 16). The schematic representations of Figure 2 depict the size of the lesion for the smallest and largest RE/RH cases. The lesions of the 17 cases that were included were restricted to RE/RH, with minimal (or no) spread to adjacent structures such as the anteromedial nucleus, submedial nucleus, or the central medial nucleus of thalamus. By comparison, the excluded cases were those with lesions that extended laterally to the ventromedial or submedial nuclei of thalamus (n = 6), dorsally to the central medial or the interanteromedial nuclei of thalamus (n = 4), or ventrally to medial regions of the hypothalamus, particularly to the paraventricular nucleus of the hypothalamus (n = 2). In addition, sham or lesion cases were excluded if the electrode produced significant damage to the overlying cortex, specifically the caudal aspect of the anterior cingulate cortex (n = 4). In these cases, significant damage was primarily observed in the superficial layers of the cortex at the entry point of the electrode into the brain. Finally, 3 rats were excluded due to the small size of the lesions. Although these lesions were restricted to RE, the cell loss was minimal and localized to a very small part of RE, leaving most of nucleus intact. Interestingly, however, the deficits observed in two of these three cases were comparable to those seen with larger RE/RH lesions (see below). Figure 3 is a photomontage of transverse sections at three rostrocaudal levels of the thalamus from a representative case showing the loss of cells restricted to the ventral midline thalamus (RE/RH).
Figure 2.
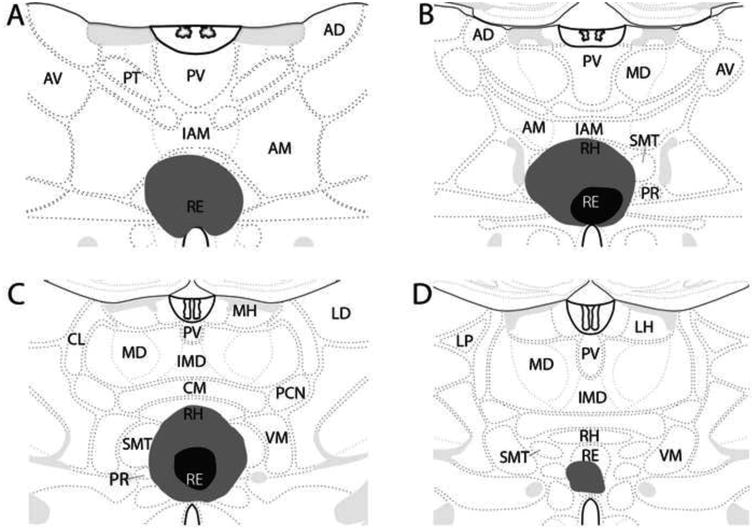
Schematic diagram showing four (A-D) rostrocaudal levels through the thalamus depicting the extent of the lesions. Black shaded areas denote the size of the smallest lesions; gray shaded areas the size of the largest lesions. Sections modified from Swanson (2004). Abbreviations: AD, anterodorsal nucleus of thalamus; AM, anteromedial nucleus of thalamus; AV, anteroventral nucleus of the thalamus; CL, central lateral nucleus of thalamus; CM, central medial nucleus of thalamus; IAM, interanteromedial nucleus of thalamus; IMD, intermediodorsal nucleus of thalamus; LD, laterodorsal nucleus of thalamus; LH, lateral habenula; LP, lateral posterior nucleus of thalamus; MD, mediodorsal nucleus of thalamus; MH, medial habenula; PCN, paracentral nucleus of thalamus; PR, perireuniens; PT, paratenial nucleus of thalamus; PV, paraventricular nucleus of thalamus; RE, reuniens nucleus of thalamus; RH, rhomboid nucleus of thalamus; SMT, submedial nucleus of thalamus; VM, ventromedial nucleus of thalamus.
Figure 3.
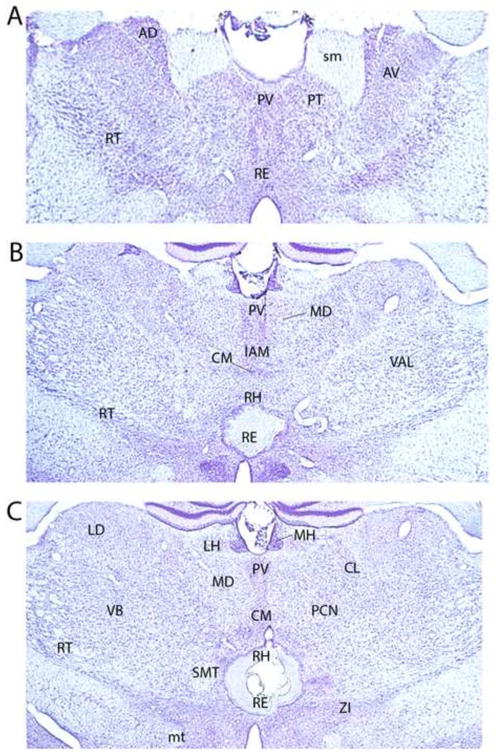
Low magnification transverse photomicrographs of Nissl stained sections at three rostrocaudal levels of the thalamus (A-C) depicting an electrolytic lesion of the ventral midline thalamus for a representative case. Abbreviations (not included with Fig. 2): mt, mammillothalamic tract; RT, reticular nucleus of thalamus; sm, stria medullaris; VB, ventrobasal nucleus of thalamus; ZI, zona incerta.
2.2. Behavioral Testing
2.2.1. Digging and Habituation
Overall, all rats successfully completed the digging and habituation phases of training which included digging establishment and two simple discriminations (SD1 and SD2) that differed in modalities. There was no difference between sham (M=15.38; SD= 5.28) and RE/RH rats (M-15.41; SD= 5.59) in the mean number of trials to establish digging behavior, F(1,31) = 0.00, p > 0.05, np2= 0.00. Similarly, there were no differences between sham (M= 10.44; SD= 6.12) and RE/RH rats (M=12.18; SD= 7.35) in mean total trials (correct trials + total errors) to reach criterion in the SD1 (odor) habituation stage F(1,31)=0.54, p > 0.05, np2= 0.02. Whereas there were also no differences in total errors (incorrect + omitted responses) during this stage, t(24.43)= -1.50, p > 0.05, RE/RH rats made more incorrect responses (M=0.53; SD= .80) than shams (M=0.06; SD=.25), t(19.27)= -2.29, p < 0.05. There were, however, no differences in errors of omissions for SD1, t (24.54)= -1.30, p > 0.05. In a similar manner, there was no difference between sham and lesioned rats in mean total trials to reach criterion, F(1,31)= 0.59, p=0.45, np2= 0.02, or errors, F(1,31)= 0.41, p > 0.05, np2= 0.01, for SD2 (digging medium) of habituation.
2.2.2. Odor texture attentional set shifting task
The effects of lesions of RE/RH on performance on the AST task are shown in Figures 4 and 5. Figure 4 illustrates the mean total number of trials which includes all correct trials and all errors (incorrect and omitted responses) to completion for each stage of the task for sham and RE/RH rats. Figure 5 depicts mean total errors (Fig. 5A), incorrect responses (Fig. 5B) and omitted responses (Fig. 5C) for each stage of the task for the two groups. As shown, lesions of RE/RH produced selective impairments in reversal learning as seen during the first reversal (CD RV), but performance recovers during subsequent RV stages. RE/RH rats, compared to shams, and also displayed deficits in the intradimensional shift (ID) stage.
Figure 4.
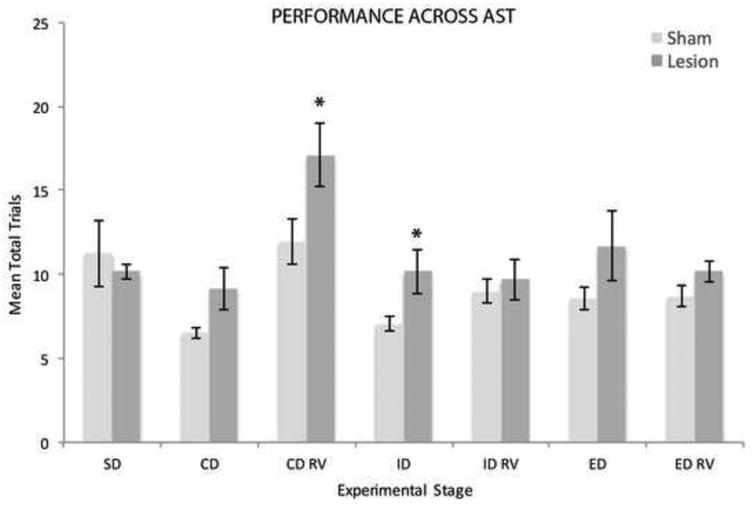
Bar graph showing the effect on performance of lesions of the nucleus reuniens (RE) and the rhomboid (RH) nucleus on mean number of total trials to complete each stage (error bars represent ± SEM) of the attentional set shifting task. As depicted, RE/RH lesioned rats required significantly more trials to complete the first compound discrimination reversal (CD RV) and the intradime nsional (ID) shift stage compared to sham controls. Abbreviations: SD, simple discrimination; CD, compound discrimination; CD RV, compound discrimination reversal; ID, intradimensional shift; ID RV, intradimensional shift reversal; ED, extradimensional shift; ED RV, extradimensional shift reversal. The * denotes p < 0.05.
Figure 5.
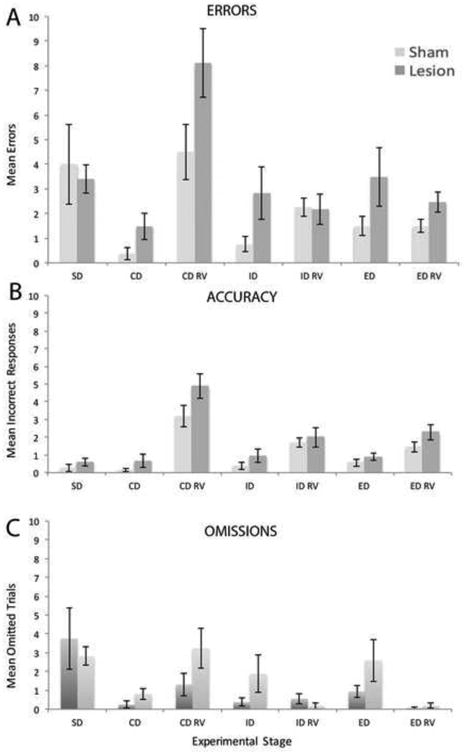
Bar graph depicting the effect of lesions of the nucleus reuniens (RE) and the rhomboid (RH) nucleus on mean number of total error trials (A), which were further analyzed by mean incorrect choices (B) and mean errors of omission (C) during each stage of the attentional set shifting task. Error bars represent ± SEM. Abbreviations: see Figure 4.
Similar to the habituation phase, there were no differences between rats in mean total number of trials, t(19.69)= 0.51, p > 0.05, and mean errors, t(18.73)= 0.34, p > 0.05, during the first stage of the AST task: simple olfactory discrimination (SD). The second stage of the AST task, or compound discrimination (CD), involved a choice between the same odor (O1) from the SD stage (still rewarded) and a new odor, and the two odors were now paired with separate mediums. This required the development of an attentional set; that is, attending to the odor exemplars and ignoring the irrelevant mediums. RE/RH rats required more trials (M=9.18; SD= 5.31) to complete the CD stage in comparison to sham control rats (M= 6.50; SD= 1.21) as assessed by mean total trials, but this only approached significance, t(17.76) = -2.02, p= 0.058. No differences in the mean number of errors, [t(23.02)= -1.86, p > 0.05], omitted responses [t(27.10)= -1.61, p > 0.05], or incorrect responses[t(17.59)=-1.33, p > 0.05] were found in the CD stages between groups (Fig. 5A-C).
The third stage of the AST task was reversal learning of the CD (CD RV), whereby the same odor/digging medium stimulus pairs were used, but the previously incorrect odor (O2) was now associated with the reward. Univariate analysis yielded a significant difference for mean number of total trials for the first reversal such that RE/RH rats needed more trials (M=17.12; SD= 7.87) to complete this stage than sham controls (M= 11.94; SD= 5.31), F(1, 31)= 4.85, p < 0.05, np2= 0.14 (Fig. 4). RE/RH rats also made considerably more errors (M=8.12; SD=5.75) than sham controls (4.50; SD= 4.49) for this stage but it only approached statistical significance, F(1,31)= 4.02, p= 0.054, np2= 0.12 (Fig. 5A). Further analysis, however, revealed no significant differences in the mean number of incorrect, F(1,31)= 3.33, p > 0.05 np2= 0.10, or omitted responses, F(1,31)= 2.37, p> 0.05, np2= 0.07, during the CD RV stage (Fig. 5B,C).
The fourth stage of the AST task was the intradimensional shift (ID), whereby two new novel odor/digging medium pairs were introduced, resulting in new odors/mediums associations. RE/RH rats (M=10.18; SD=5.49) took significantly longer to complete the ID stage in comparison to sham rats (M= 7.06; M= 1.77), as measured by mean total number of trials to reach criterion, t(19.47)= -2.20, p < 0.05 (Fig. 4). There were, however, no significant differences between RE/RH and sham rats in the mean number of errors, t(18.64)= -1.86, p > 0.05, for this stage (Fig. 5A). This is reflected by the fact that there were no differences in either omitted, t(17.34)= -1.50, p > 0.05, or incorrect responses F(1,31)= 4.68, p > 0.05, np2= 0.10 for the ID stage (Fig. 5B,C). The fifth stage was the ID reversal (ID RV), whereby the same novel odor/digging medium pairs were presented but the incorrect odor (O4) from the ID stage was now rewarded. Univariate analysis yielded no differences between the RE/RH and sham rats in mean total trials to reach criterion, F(1,31)= 0.25, p > 0.05, np2= 0.10, or total errors F(1,31)= 0.01, p > 0.05, np2= 0.00, for the ID RV (Fig. 4).
The sixth stage was the extradimensional shift (ED), whereby two novel odor/digging medium pairs were introduced (O5, O6, M5, M6). During the ED shift, the odor exemplars became irrelevant, and rats now had to make response selections based on tactile information, with a new digging medium (M5) now associated with the reward. There was no difference in mean number of total trials to reach criterion between RE/RH and sham rats for the ED stage, F(1,31)= 1.93, p > 0.05, np2= 0.06 (Fig. 4). Correspondingly, no significant differences were observed in the number of errors, t(19.33)= -1.57, p > 0.05, incorrect responses, F(1,31)= 1.21, p > 0.05, np2= 0.04, or omitted responses, t(18.42)= -1.42, p > 0.05, for the ED shift stage (Fig. 5A-C). The final stage of the AST task was the ED reversal (ED RV), whereby the previously incorrect digging medium (M6) was now paired with the reward. There was no difference in performance between RE/RH and sham controls in the number of trials to reach criterion, F(1,31)= 2.73, p > 0.05, np2= 0.08, or total errors, F(1,31)= 3.87, p > 0.05, np2= 0.11 for the ED RV stage (Fig. 4, 5A).
For the stages showing significant results, multivariate analysis was conducted to further compare performance across stages for these tasks: the reversal and attentional (set/shift) stages. First, a repeated multivariate ANOVA with reversal stage (within subjects factor) × lesion (between subjects) was conducted. There was a significant within subjects difference across the three reversal stages, F(2, 62)= 13.71, p < 0.001, np2= 0.31, but no significant reversal stage × experimental group interaction, F(2, 62)= 2.23, p> 0.05, np2= 0.07. While RE/RH rats needed significantly more trials to complete the CD RV compared to controls, Bonferroni pairwise comparisons indicated that all rats took significantly more trials to complete the initial CD reversal stage compared to both the ID and ED reversal stages. Next a repeated measures multivariate analysis was conducted on errors across the three reversal stages. While a significant within subjects effect of stage was noted, F(2, 62)= 20.05, p < 0.001, np2= 0.39, the stage × experimental group interaction did not reach a level of significance, F(2, 62)= 3.07, p > 0.05, np2= 0.09. Multivariate analysis of variance found a significant difference for RE/RH rats in total errors across the three reversal stages, F(2, 15)= 10.48, p < 0.01, np2= 0.58. Bonferroni pairwise comparisons found that RE/RH rats made more errors during the initial CD RV stage in comparison to both the ID and ED reversals but the mean errors between ID RV and ED RV did not differ. By comparison, there was no significant difference in mean number of errors across the three reversals for control rats, F(2, 14)=3.36, p > 0.05, np2= 0.33.
Multivariate repeated measures analysis was also conducted across the attentional set and shift stages: CD, ID, and ED. There was no significant within factors effect of stage, F(2, 62)= 2.20, p > 0.05, np2= 0.07, and no significant stage × experimental group effect, F(2, 62)= 0.03, p > 0.05, np2= 0.00. This indicated that while RE/RH rats took longer to complete both the CD and ID task, performance across the CD, ID, and ED stage did not significantly differ for the RE/RH rats. There were also no differences across stages for the sham controls. Lastly, the shift cost for attentional set shifting was calculated. This was done by computing differences in mean total trials to complete the ID stage in comparison to the ED stage. Previous studies have shown that impairments in attentional set formation, as found in the CD and ID stages, are often accompanied by enhancement of performance in the ED stage (Chase et al., 2012; Wright et al., 2015). Both RE/RH (M=-1.53; SD= 8.41) and control rats (M= -1.50; SD= 2.90) displayed a minor shift cost effect, whereby more trials were needed to complete the ED shift. There were, however, no significant differences between experimental groups, t(19.96)- 0.01, p > 0.05.
2.2.3. Latencies
For each stage, two latency measures were computed: mean latency per trial and mean latency with errors. Mean latencies per trial for each stage assessed: (1) motivation to complete the task, as longer latencies per trial could indicate a lack of volition as opposed to deficits of attentional or executive functioning; and (2) impulsivity, whereby persistently shorter latencies across all trials, regardless of choice accuracy (correct or error trials), could suggest impulsive responding. Overall, there were no differences between lesioned and sham rats in mean latency per trial across stages or mean latency for incorrect choices across each stage (data is not shown). The single exception to this was found for the intradimensional reversal stage. RE/RH rats had significantly shorter latencies (M=10.19; SD= 8.19) to complete each trial for the ID RV stage than sham controls (M=21.05; SD= 13.74), t(24.17)= 2.74, p < 0.05.
3. Discussion
In the present study, we found that irreversible lesions of the ventral midline thalamus, including the nucleus reuniens (RE) and to a lesser extent the rhomboid nucleus (RH), produced impairments in attentional set and reversal learning in the rodent attentional set shifting task (AST). Specifically, lesioned rats took significantly more trials to complete the first reversal of the compound discrimination (CD RV) and showed significant impairments on the intradimensional shift (ID) stage of the task. The AST is a rodent version of the intradimensional extradimensional shift task (IED) used to assess executive functions in (nonhuman) primates and humans (Sahakian and Owen, 1992; Robbins and Arnsten, 2009). It is analogous to the classic Wisconsin Card sorting task which serves to evaluate simple stimulus discriminations, attentional set, attentional set-shifting, and cognitive flexibility (Nyhus and Barcelo, 2009).
RE lesioned rats, however, showed no deficits in discrimination learning as exemplified by the successful completion of both the olfactory and tactile simple discriminations. Although RE/RH lesioned rats displayed a small deficit in the initial olfactory discrimination during the habituation stage, as shown by a greater number of incorrect responses, their performance recovered on the subsequent olfactory SD during the AST task. RE lesioned rats, however, required more trials to complete the compound discrimination (CD) stage -- with differences approaching statistical significance. In the CD stage, rats must continue to respond to the previously learned stimulus-response pattern as the same odor used in the SD stage is rewarded in the CD stage. During this stage, attentional set is assessed as there are now two novel digging mediums, which act as distractors. The correct odor (O1) can be paired with either digging medium. If an attentional set is formed, rats will learn to ignore the irrelevant exemplars in the tactile modality and continue responding to the correct odor stimulus. The deficits of RE rats on the CD stage may be associated with attentional impairments (see also below).
3.1 Attentional set/attentional set shift
As indicated, RE/RH rats performed significantly poorer than controls on the ID set shifting stage of the task, but exhibited no deficits on the extradimensional shift. Several AST and IED studies have attributed a role for the medial prefrontal cortex in rodents (dorsolateral prefrontal cortex in primates) in attentional set shifting, measured by an increase in trials to complete the extradimensional shift (Dias et al., 1996; Birrell and Brown, 2000; Bissonette et al., 2008). It is generally the case, however, that more trials are needed to complete ED stages in comparison to other stages due to the greater complexity of shifting attention to a new modality. Atypically, some reports have described minor differences between the ID and ED stages or improved performance for the ED stage. This could reflect a failure of an attentional set formation in earlier shift stages. If a poor attentional set developed to one sensory modality, learning a shift to new modality should be easier than if a strong association was formed to the original modality (Roberts et al., 1994; Chase et al., 2012).
The interaction of striatal and prefrontal systems is thought to play a role in attentional set formation. Initial studies found that depletion of dopamine in the prefrontal cortex produced impairments in the formation of an attentional set in the IED paradigm, characterized by deficits on the CD stages (Roberts et al., 1994; Crofts et al., 2001; Boulougouris and Tsaltas, 2008; Walker et al., 2009a, b). A new modified version of the AST task described a role for both the dorsal striatum and the orbitofrontal cortex in attentional set (Chase et al., 2012; Lindgren et al., 2013). In this modified version of the AST task, attentional set is assessed by the use of multiple intradimensional shifts, followed by one extradimensional shift – and no reversal stages. If rodents formed an attentional set to one of the sensory domains, the ED stage (whereby an attentional shift occurs) should be more difficult, as indicated by an increase in number of trials to complete the ED stage compared to ID stages, known as a shift-cost. However, if rats are unable to form an attentional set to one sensory domain, performance should be impaired for subsequent ID stages, with a shift-benefit for ED stage, whereby shifting attention to another sensory domain is comparatively easier. Aggleton and colleagues (Wright et al., 2015) reported that lesions of the anterior thalamus impaired attentional set and hence performance on ID shifts in the modified AST task. However, in contrast to current results, anterior thalamic lesions also produced a shift benefit for the ED stage – or improved performance for this stage. It remains to be determined whether RE/RH lesions would produce similar effects in this version of the AST task.
The present impairment in the ID stage of the task may not, however, be the result of altered set formation, as there was no predicted shift benefit from the ID to the ED stages (see above). In fact, a small, but insignificant, shift-cost (poorer performance) was seen for the ED stage. In this stage, two novel odor and digging medium pairs were introduced, but rats still used the odors to guide response selection. The intradimensional shift occurred within the same sensory modality so that the reward contingencies learned during CD stage could be applied to ID response selection. In the primate analogue of the AST task, selective deficits at the ID stage involve difficulties with rule abstraction, or the ability to apply a previously learned rule to novel stimuli (O'Reilly et al., 2002; Robbins, 2007; Leeson et al., 2009). Rule abstraction reflects a form of cognitive flexibility wherein learned rules guiding choices with one set of stimuli are applied to other sets of stimuli. Primate studies have described a mediolateral distinction across the PFC whereby damage to the orbitofrontal cortex impairs ID shifts and dorsolateral PFC (analogous to the ventral mPFC in rats) lesions alter extradimensional shifts (Dias et al., 1997). A deficit in “rule abstraction” appears to characterize the present dysfunction with RE/RH rats in that they showed marked impairment from the CD to the ID stages of the task. In effect, then, RE/RH lesions impair the ability to learn successful rules/strategies that would transfer (or generalize) across a very comparable set of tasks/stimuli.
3.2. Reversal learning
RE/RH lesioned rats were also impaired in the first reversal CD RV stage. Lesioned rats took significantly more trials to complete this reversal than did shams, as measured by mean number of total trials, indicating a deficit in behavioral flexibility. Reversal learning is defined as adapting to changes in stimulus-reward contingencies whereby an organism needs to stop responding to a previously rewarded stimulus and initiate responding to a previously unrewarded (incorrect) stimulus. RE/RH rats also made more total errors than sham controls during the first reversal – which could involve incorrect choices or omitted responses. To address whether the impairments in reversal learning resulted from errors of commission (incorrect responses) or omission (failure to make a response), the two types of errors were analyzed separately. Incorrect responses would denote compulsive behavior (perseveration), while the omitted responses would signify lapses of attention or volition. There were, however, no statistically significant differences in numbers of incorrect choices or omitted responses in RE rats compared to sham controls. This alternatively suggests RE rats, while not exhibiting perseverative behavior, were impulsive – or failed to inhibit responses to the previously positive but now inappropriate choices.
Successful completion of any stage requires 6 consecutive correct responses, so that a significant increase in the number of trials across a stage represents the sum of incorrect and corrects trials. As Boulougouris and Tsltas (2009) state: “behavioral inflexibility may take the form of impulsivity -- hastily responding with no regard for consequences.” RE/RH rats appeared to develop a similar impulsive behavioral pattern for the first reversal such that they were unable to change their response selection from the previously incorrect choice to the now correct stimulus. In this regard, Prasad et al. (2013) reported that rats with RE lesions exhibited impulsive behavior on the 5-choice serial reaction time task (5-CSRTT). Specifically, rats made more premature responses (prior to stimulus onset), particularly when the intertrial interval was varied and unpredictable. Alternatively, factors contributing to deficits in reversal learning may be analogous to those reportedly responsible for reversal deficits following MD lesions in monkeys; that is, an inability to adequately utilize information gained from making positive choices (leading to rewards) to guide future behavior (Chakraborty et al., 2016). In this regard, during the first reversal, rats made a number of correct choices but were not able to sustain that behavior, reverting to incorrect choices – thus leading to a significant increase in the total number of trials during CD-RV.
It has been reported that lesions of the orbitofrontal cortex or serotonergic depletion of the orbitofrontal cortex produce profound impairments of reversal learning across all stages of the AST and IED task (McAlonan and Brown, 2003; Clark et al., 2004, 2005, 2007; Dalley et al., 2004; Roberts and Parkinson, 2006; Rolls, 2006). Unlike previous studies showing that damage to the orbitofrontal cortex produced persistent impairments in reversal learning, RE rats were only deficient on the first reversal, but not on subsequent ones (ID and ED reversal stages) – thus indicating improved performance with repeated exposures to the reversal paradigm. There was no difference in performance on the ID and ED reversals in the lesion group demonstrating recovery after the initial deficit. This improvement in performance over the course of reversal learning has been previously been demonstrated with cortical lesions. For instance, Lapiz-Bluhm et al. (2009) reported that chronic unpredictable stress, as well as serotonin depletion with systemic injections of para-chlorophenylalanine, produced profound deficits in reversal learning on the CD RV stage of the AST task that were not seen on the subsequent ID and ED reversals. In a similar manner, Klanker et al. (2013) showed that high frequency disruptive stimulation of the lateral orbitofrontal cortex impaired performance on the initial but not on subsequent reversals of a spatial operant conditioning (lever press) task. The demonstration, then, that RE rats show initial deficits in reversal learning followed by restoration suggests that RE may pivotally contribute to behavioral flexibility in a novel situation, but is essentially not required once the task, or rule guiding the task, is well established. Whereas RE involvement in reversal learning could, in part, stem from pronounced projections to the mPFC, it more likely results from equally dense RE projections to the orbital cortex (Vertes et al., 2006) – the main cortical site for reversal learning.
Interestingly, a recent report by Prasad et al (2016) examining the effects of excitotoxic RE lesions on visual discrimination and reversal tasks, described findings at odds with the present and several previous reports (for review, Cassel et al, 2013; Griffin, 2015; Vertes, 2015). Specifically, RE lesioned rats showed enhanced (or improved) performance in discriminating simple visual stimuli displayed on a touch screen. However, no differences (enhancement or deficits) were seen the reversal stage of this task. By contrast, we found no improvements or deficits in performance in RE rats for the SD stage of the AST task, but profound impairments on reversal learning, restricted to the first reversal. While the conflicting findings are difficult to reconcile, there may be modality specific differences as the discriminative stimuli in the current task were olfactory and tactile. Differences may also stem from different locations or the extent of RE lesions in these reports. For example, recent studies have shown that RE projections to its main targets in the orbital and medial PFC and the hippocampus largely originate from separate regions of RE (Hoover and Vertes, 2012; Varela et al., 2014) suggesting that differing locations of RE lesions could have separate effects on specific behaviors.
3.3. Thalamic roles in cognition
While cognitive flexibility is classically associated with the prefrontal cortex, it involves a large network of forebrain structures comprised of both thalamo-prefrontal and striatal-prefrontal connections (Chudasama and Robbins, 2006). It is well established that thalamo-prefrontal systems contribute to cognitive flexibility. To date, however, research on the role of the thalamus in cognitive flexibility/executive functions has centered on the mediodorsal (MD) nucleus of the thalamus, including an MD involvement in behavioral flexibility and reversal learning (Mitchell, 2015; Chakraborty et al., 2016). For instance, Chudasama et al. (2001) reported that MD lesions in rats impaired reversal learning to visual stimuli in an operant chamber. Parnaudeau et al. (2015) recently described reversal deficits with a similar paradigm in mice using designer receptors exclusively activated by designer drugs (DREADDs) to temporarily inactivate MD. In like manner, Floresco and colleagues (Block et al., 2007) examined the role of MD in behavioral flexibility using a response-shifting paradigm in a cross maze with rats. Rats first learned to guide their response using an egocentric spatial strategy (i.e., to turn right down one arm) but once established, they were required to shift response strategy by using visual cues to obtain a reward. Inactivation of MD produced perseverative responses on the task, and also impaired the ability to develop a novel strategy to guide future response selection (Block et al., 2007). Reversal learning deficits have also been described with MD lesions/inactivation in olfactory discrimination tasks (Slotnick and Risser, 1990; McBride and Slotnick, 1997). Finally, Alcaraz et al. (2016) recently reported that rats with MD lesions were significantly impaired in the ability to correctly choose spatial locations associated with higher food reward value following satiety-induced devaluation. The deficit was described as a failure of “MD rats in adapting their choice according to goal value”.
The cognitive functions associated with MD are thought to involve strong MD projections to the medial and orbital prefrontal cortices (Leonard, 1969; Krettek and Price, 1977; Reep et al., 1996; Ongur and Price, 2000; Hoover and Vertes, 2007). The demonstration that RE lesions produced similar deficits in rule generalization and reversal learning as shown for MD suggests that RE/RH is also part of an interconnected circuitry involving MD and the orbitomedial prefrontal cortex, responsible for executive functioning.
In accord with present findings, a number of reports, using various paradigms, have shown that RE lesions/inactivation impair behavioral flexibility. For instance, Dolleman-van der Weel et al. (2009) reported that while RE lesions had no effect on the acquisition or retention of spatial reference memory using a standard water maze task, they nonetheless resulted in an ineffective search strategy in the probe test which was described as inflexible behavior to changed contingencies. Consistent with this, Cassel and colleagues (Cholvin et al., 2013) recently showed that inactivation of RE impaired the shifting of strategies on a ‘double H’ water maze task. Essentially, RE rats were impaired in the ability to switch from an incorrect response strategy (repeating the same sequence of movements to reach a target) to a correct place response that involved returning to the same quadrant of the maze in which the escape platform had initially been located. Finally, Prasad et al. (2016) reported that RE lesioned rats showed deficits in working memory in a modified non-match to sample task RAM task, which were considered perseverative in nature. The present findings further demonstrate a direct role for RE in behavioral flexibility as evidenced by deficits in reversal learning and attentional set shifting. As such, RE appears critically involved in prefrontal networks regulating both mnemonic and executive functions.
4. Experimental procedures
4.1 Subjects
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 275-300 grams upon arrival were used in the experiment. Rats were housed in pairs in a climate-controlled colony on a 12 h light/dark cycle (lights on at 7 AM). Rats were given food and water ad libitum upon arrival and throughout the surgical and postsurgical recovery period and then placed on a food restricted diet where they were maintained at 85-90% of their free feeding weight. These experiments were approved by the Florida Atlantic University Institutional Animal Care and Use Committee and conform to all federal regulations and National Institutes of Health guidelines for the care and use of laboratory animals.
4.2. Surgical Procedure
Electrolytic lesions of the ventral midline thalamus, targeting the reuniens and rhomboid nuclei, were made using stereotaxic procedures. Rats were anesthetized with isoflurane (4-5% induction, 1-3% maintenance), placed in the stereotaxic apparatus, and an incision was made to expose the skull. A burr hole was drilled over the midline thalamus and a tungsten electrode was lowered to RE/RH. Positive direct current was applied through a Grass stimulator (Model 88) coupled with a high-voltage stimulator at 1 mA for 30-35 seconds. Shams underwent the same procedure without applying the current. Following surgery, rats were allowed 7 days of postsurgical recovery and were then placed on a food-restricted diet before beginning behavioral procedures.
4.3. Apparatus
Figure 1 is a schematic diagram of the AST apparatus. It consisted of an opaque rectangular Plexiglas enclosure: 40.5 cm wide × 62 cm length × 37 cm height. A stationary central opaque divider at one end of the arena ran parallel to the long axis of the apparatus (22 cm) and divided one third of the apparatus into two sections (choice areas). Each of these sections housed a ramekin (7 cm in diameter) during the training/test trials. Two movable opaque dividers (22 cm width × 37 cm height) slid perpendicular to the parallel divider to close off the choice areas between trials. The ramekins contained discriminative odors and digging mediums and were fixed to the floor with Velcro to prevent their displacement.
Figure 1.
Schematic representation of the apparatus used in the attentional set shifting task. The Plexiglas apparatus has a stationary central opaque divider at one end of the arena that runs parallel to one third of the length of the apparatus dividing this section into two choice areas. Each of these sections housed a ramekin during the training/test trials. Two movable opaque dividers, indicated by a dashed line, slid perpendicular to the parallel divider to close off the choice areas between trials. See Experimental procedures for dimensions on the apparatus.
4.4. Behavioral testing
4.4.1. Habituation
Following the postsurgical recovery period (7 days), rats were placed on a food-restricted diet for 7 days prior to behavioral testing and maintained at 85-90% of their free feeding weight. Testing was conducted 12-20 days postsurgery. During this time, rats were transferred from their housing area to the test room daily for acclimation to the transport and the testing environment. On the day prior to habituation and training, rats were placed in the empty testing apparatus for 5 mins to freely explore it and to acclimate to the ramekins and food rewards (fruit loops).
One day prior to testing, rats were exposed to the training stages to establish digging behavior and to associate cues (odors, mediums) with appropriate responses. To establish digging behavior, the ramekins were filled with an unscented digging medium (recycled cellulose bedding) and placed in each of the choice areas of the testing apparatus. Following this, rats were placed in the start location of the apparatus and trials began when the dividers slid open to allow access to the ramekins. Rats were given 5 min to retrieve food rewards from both ramekins. On the first trial, considered exploratory, fruit loops were placed on top of each bowl allowing for easy retrieval, and rats remained in the apparatus for the entire 5 min period to familiarize themselves with the arena and the ramekins -- regardless of how quickly they obtained the food rewards. For subsequent trials, the food reward was buried in the bottom of each ramekin, and trials ended when both rewards were retrieved or when 5 min had elapsed. Training continued until rats successfully retrieved the food reward from both ramekins for 12 non-consecutive trials. If no or only one food reward was retrieved during the 5 min trial, the trial was considered unsuccessful (aborted). If a rat aborted on three consecutive trials, the rat was placed in its home cage for 5 minutes before resuming trials to reach criteria.
Establishment of digging was followed by two simple discriminations. In the simple discrimination (SD) tasks, rats learn to discriminate between two exemplars in a single domain to retrieve a food reward, while a second domain remained constant between the two choices. The first simple discrimination (SD1) was an odor discrimination: ramekins were scented with clove (correct choice paired with food reward) or with nutmeg (no reward) and both ramekins were filled with the same digging medium, recycled cellulose bedding. The second simple discrimination (SD2) was a tactile discrimination wherein the two exemplars were unscented digging mediums: one ramekin was filled with plastic beads (correct choice) and the other with felt pebbles (incorrect choice). For the SD habituation stages, the first four trials were exploratory. For these trials, lasting 2 min each, the rats were allowed to explore the ramekins and retrieve the food reward from either ramekin. If digging first occurred in the incorrect bowl, the trial was marked as an error. The rat, however, was not removed from the testing apparatus, but allowed to retrieve food from the other bowl (thereby making the correct association) -- or until 2 min elapsed. If correct choices were made, the trial was immediately terminated and the next trial began. After the exploratory phase, trials were terminated once rats began to dig in one of the ramekins, whether rats performed a correct or incorrect choice. If a rat did not dig in either of the choices after two minutes, the trial was terminated and considered an aborted trial (omitted response). If rats aborted on three consecutive trials, they were placed in their home cage for 5 min before testing resumed. Six consecutive correct choices were required to successfully complete each SD stage of the task. Errors during the SD trials were of two types: digging in the incorrect ramekin (incorrect choice) or failure to select either ramekin (error of omission). The odors and the unscented mediums used for the olfactory (SD1) and tactile (SD2) discriminations in the habitation stage were not used again in the AST task.
4.4.2. AST task
Twenty four hours following habituation, rats were tested in the AST across seven stages: (1) simple discrimination (SD); (2) compound discrimination (CD); (3) reversal learning (RL) of the compound discrimination (CD RV); (4) intradimensional shift (ID), (5) RL of the intradimensional shift (ID RV); (6) extradimensional shift (ED); and (7) RL of the extradimensional shift (ED RV). Ramekins were scented using essential oils for all stages of the experiment. Table 1 lists the odor and digging medium exemplars used for all stages of the experiment.
Table 1.
Depicts the successive stages and combinations of stimuli used for the habitation stage and the attentional set-shifting task (AST). The stimulus associated with rewards for each stage is shown in bold.
| Stage | Exemplars | Stimulus Combinations | ||
|---|---|---|---|---|
| Habituation | ||||
| Digging Establishment | Cellulose Bedding | |||
| Simple Discrimination (SD) 1: Odor | Clove | Nutmeg | ||
| SD 2: Tactile | Beads | Felt | ||
| AST | Odor | Medium | + | - |
| SD | O1 - Ginger | Cellulose Bedding | O1 | O2 |
| O2 - Mint | Cellulose Bedding | |||
| Compound Discrimination (CD) | O1 - Ginger | M1 - Aspen | O1/M1 | O2/M2 |
| O2 - Mint | M2 - Paper | O1/M2 | O2/M1 | |
| CD Reversal | O1 - Ginger | M1 - Aspen | O2/M1 | O1/M2 |
| O2 - Mint | M2 - Paper | O2/M2 | O1/M1 | |
| Intradimensional shift (ID) | O3 -Cinnamon | M3 - Bugles | O3/M3 | O4/M4 |
| O4 - Fennel | M4 - Rubber | O3/M4 | O4/M3 | |
| ID Reversal | O3 -Cinnamon | M3 - Bugles | O4/M3 | O3/M4 |
| O4 - Fennel | M4 - Rubber | O4/M4 | O3/M3 | |
| Extradimensional shift (ED) | O5 -Citronella | M5 - Beads | M5/O5 | M6/O6 |
| O6 - Lemon | M6 - Gravel | M5/O6 | M6/O5 | |
| ED Reversal | O5 -Citronella | M5 - Beads | M6/O5 | M5/O6 |
| O6 - Lemon | M6 - Gravel | M6/O6 | M5/O5 | |
In the simple discrimination (SD), choice selection differed along one dimension: odor (O1 rewarded). Recycled cellulose bedding, the same digging medium used for digging establishment was used for O1. For the compound discrimination (CD), the same odor (O1) was rewarded, but for each trial odors were paired with one of two possible mediums such that on any trial, odor 1 could be paired with medium 1 and odor 2 could be paired with medium 2 (O1/M1 and O2/M2) or alternatively odor 1 could be paired with medium 2 and odor 2 with medium one (O1/M2 and O2/M1). For compound discrimination, then, rats must learn to form an attentional set, and respond to the correct odor, irrespective of the digging medium it was paired with. The intradimensional shift (ID) introduced two novel odor/digging medium pairs for which rats need to use the previously learned “rule” and attend to the correct odor while ignoring irrelevant digging mediums that differed between the two odors. The extradimensional shift (ED), a test of attentional set shifting, introduced another pair of novel odor/digging medium pairs, however rats must now learn to ignore (irrelevant) odors and attend to a correct digging medium for reward. For the ED, rats must shift their attention to the previously ignored sensory domain (e.g., medium) in order to guide their response selection. Reversal learning (RL), an assessment of behavioral flexibility, for all stages entails choosing the previously incorrect choice (odor for SD, CD, and ID and digging medium for ED) which is now the correct choice. For RL stages, rats must extinguish responding to a previously rewarded stimulus and shift their response to the now correct but previously incorrect choice (odor or medium).
Similar to the habituation SD stages (see above), the first four trials were exploratory trials and rats were allowed to explore both bowls. If the rat dug in the incorrect bowl during these exploratory trials, an error was recorded but the rat was allowed to continue to explore and retrieve the reward in the correct bowl. Following the four trials, once a rat made a choice (correct or incorrect) trials were terminated. If rats did not make a choice and started to dig after 2 min had elapsed, the trial was considered an abort (error of omission). Rats had to complete six consecutive correct trials to finish each stage. Rats were given 40 trials to complete each stage. If rats failed to complete any stage of the AST task, they remained on a food-restricted diet and were generally retested 7 days after the previous testing. The combination of exemplar pairs as well as the position of correct exemplar choices were presented in a pseudo-randomized order for all rats. In addition, intertrial intervals remained constant and ramekins were always removed between trials to eliminate auditory cues.
4.5. Histology
Following testing, rats were deeply anesthetized with 250 mg/kg of Euthasol, perfused intracardially with 150 mL heparinized saline, followed by 300-400 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were removed and postfixed for another 24-48 hours in 4% paraformaldehyde, then placed in a 30% sucrose solution for another 48 hours. Following sucrose cryoprotection, 50 μm coronal sections were cut on a freezing sliding microtome, and every 6th section throughout the thalamus and surrounding areas was collected. Sections were mounted on chrome-alum gelatin slides, stained with cresyl violet, and coverslipped with Permount. The Nissl stained sections were used to identify the nuclear boundaries of thalamic nuclei and the extent of each lesion.
4.6 Data Analysis
Univariate analysis of variance (ANOVA) was conducted on the following measures during digging establishment: mean total number of trials to establish digging behavior (correct trials + unsuccessful and aborted trials). In addition, univariate analysis was conducted on the following measures during the two habituation simple discrimination stages: incorrect choices (whereby the rat dug in the ramekin containing the incorrect odor or medium), errors of omission (whereby the rat failed to make a response selection by not digging in either ramekin during the 120 sec trial), and mean errors (the total number of trials in which the rat did not select the correct ramekin -- or incorrect choices + errors of omission). Significant differences between sham and lesioned rats in the habituation stage indicated deficits in acquisition of the SD. ANOVAs were further conducted on mean total number of trials to complete each stage, mean errors, mean incorrect trials, and mean errors of omission for each stage, with a between subject factor of experimental group. The dependent variables assessed frontal-executive functioning, specifically attentional set formation, attentional set shifting, and behavioral flexibility. Impaired performance in any AST stage could be the result of several executive dysfunctions. For example, if lesioned rats showed significant impairments on reversal stages, by requiring a greater number of total trials to meet criterion, this could involve preservative behavior, and consequently increased numbers of incorrect choices, or alternatively it could result from impaired volition and hence more errors of omission.
Multivariate repeated measures analysis was also conducted to assess differences in performance across stages. For the reversal stages, the total number of trials, incorrect trials, and errors of commission served as the within subjects factor (for CD RV, ID RV, and ED RV) with a between subjects factor of experimental group. This served to determine if reversal learning would stabilize or improve across the three RL stages. The repeated measures multivariate analysis was also done for the shift stages (CD to ID to ED stages) to determine if there were differences in attentional set, or specifically differences when the shift required intra-sensory judgements versus shifting attention to a new sensory domain. Univariate analysis of variance was also conducted for the mean latency of trials in each stage. Latencies were collected by a stopwatch and measured in seconds. While this was a self-paced task, significant differences in latencies across trials for a treatment group, regardless of correct or incorrect responses, may reflect motivational differences, whereas a reduction in latencies across trials could signify impulsive responding.
For all univariate and multivariate measures, homogeneity of variance was done to examine differences in variances between groups. In univariate analysis, homogeneity of variance was analyzed using Levene's test. In the repeated measures/multivariance analysis, Mauchly's test of sphericity was used to test whether or not or covariance could be assumed. Pairwise comparisons using the Bonferroni test examined differences across the within subjects factors. If homogeneity of variance could not be met in univariate analysis, an unequal variances t-test was conducted. In multivariate repeated measures analysis, if sphericity could not be assumed, the Greenhouse Geisser correction was used. Estimates of effect size were calculated using partial eta squared (np2). All measures calculated used an alpha level of 0.05 to reach statistical significance. Statistics tests were computed using SPSS 22.0.
4.7. A note on the lesioning technique
As indicated, electrolytic lesions were made of the ventral midline thalamus (RE/RH). This lesioning technique was selected for several reasons, the foremost of which is that allowed for very accurate control of the size and the shape of the lesions. This is not always possible with excitotoxic-induced lesions. Specifically, in virtually all instances, the electrolytic lesions were very symmetrical (or round) in shape, and when centered in RE/RH, very symmetrically affected tissue on each side of RE/RH – in effect the lesions were not skewed. In addition, with the present electrolytic lesions, the extent of damage and associated cell loss could be unequivocally determined as it was virtually confined to the region of tissue loss and the very immediately surrounding area. With chemically-induced lesions, it is sometimes difficult to determine the degree to which seemingly altered cells at the vicinity of the lesion remain functional. While it is recognized that electrolytic lesions not only destroy cells but also coursing, this is much less of a problem with nucleus reuniens as the nucleus ends at the rostral thalamus where the ventricles converge. In effect, fibers coursing rostrally to the rostral pole of RE do not continue forward beyond that point. In fact, as RE stretches over a considerable rostrocaudal extent of the thalamus, it is difficult to lesion a large region of the nucleus in the rostral-caudal plane (whatever the lesioning technique). As such, electrolytic lesions may be preferable to other methods in that by damaging cells at the lesion site as well as fibers passing rostrally through it, electrolytic lesions would affect a far greater (longitudinal) region of the nucleus than would lesions confined to a single RE site. It is also important to note that an earlier report using excitotoxic lesions of RE/RH with the AST task yielded very similar findings to those described in the present study (Linley et al., 2008).
Highlights.
Lesions of the ventral midline thalamus (VMT) in the rat impaired reversal learning.
Rats also exhibited were also impaired in establishing an attentional set.
These deficits resulted in an inefficient response strategy to novel stimuli.
Results suggest VMT directly contributes to flexible goal directed behavior.
Acknowledgments
The authors thank the following individuals for their participation in the collection of the behavioral data and analysis: Brittney Clark, Randy Ellis, Patricia Pinedo and Tatiana Viena. This research was supported by NIMH grant MH099590 to RPV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 1986;19:133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Alcaraz F, Naneix F, Desfosses E, Marchand AR, Wolff M, Coutureau E. Dissociable effects of anterior and mediodorsal thalamic lesions on spatial goal-directed behavior. Brain Struct Funct. 2016;221(1):79–89. doi: 10.1007/s00429-014-0893-7. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang DX. Thalamic excitation of hippocampal CA1 neurons: a comparison with the effects of CA3 stimulation. Neuroscience. 1999;92:15–26. doi: 10.1016/s0306-4522(98)00712-x. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–46. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP. The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol. 2013;111:34–52. doi: 10.1016/j.pneurobio.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Kolling N, Walton ME, Mitchell AS. Critical role for the mediodorsal thalamus in permitting rapid reward-guided updating in stochastic reward environments. Elife. 2016:e13588. doi: 10.7554/eLife.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase EA, Tait DS, Brown VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci. 2012;36:2368–2375. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De SND, Raingard H, Cassel JC. The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci. 2013;33:8772–8783. doi: 10.1523/JNEUROSCI.0771-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011;225:389–95. doi: 10.1016/j.bbr.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from “on-line” processing. J Neurosci. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van Der Weel MJ, Morris RG, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct. 2009;213:329–342. doi: 10.1007/s00429-008-0200-6. [DOI] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL. Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci. 2015;9:1–8. doi: 10.3389/fnsys.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013;127:860–866. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher PD, Brown VJ, Tait DS, Bate S, Overend P, Hagan JJ, Jones DN. 5-HT6 receptor antagonists improve performance in an attentional set shifting task in rats. Psychopharmacology. 2005;181:253–259. doi: 10.1007/s00213-005-2261-z. [DOI] [PubMed] [Google Scholar]
- Hembrook JR, Mair RG. Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus. 2011;21:815–826. doi: 10.1002/hipo.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, Mair RG. Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus. 2012;22:853–860. doi: 10.1002/hipo.20945. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct. 2012;217:191–209. doi: 10.1007/s00429-011-0345-6. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:12. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Post G, Joosten R, Feenstra M, Denys D. Deep brain stimulation in the lateral orbitofrontal cortex impairs spatial reversal learning. Behav Brain Res. 2013;245:7–12. doi: 10.1016/j.bbr.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Pina AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–446. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Layfield DM, Patel M, Hallock H, Griffin AL. Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem. 2015;125:163–167. doi: 10.1016/j.nlm.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci. 2003;117:1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnesm TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication types and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain Res. 1969;12:321–343. doi: 10.1016/0006-8993(69)90003-1. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Wickens R, Tait DS, Brown VJ, Dunnett SB. Lesions of the dorsomedial striatum impair formation of attentional set in rats. Neuropharmacology. 2013;71:148–53. doi: 10.1016/j.neuropharm.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Linley SB, Steigerwald ML, Hoover WB, Hughes KM, Vertes RP. Effects of excitotoxic lesions of the midline thalamus on attention and working memory in the rat. Soc Neurosci. 2008 Abstract 388.13. [Google Scholar]
- Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A. The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci. 2012;32:9947–9959. doi: 10.1523/JNEUROSCI.0410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McBride SA, Slotnick B. The olfactory thalamocortical system and odor reversal learning examined using an asymmetrical lesion paradigm in rats. Behav Neurosci. 1997;111:1273–1284. doi: 10.1037//0735-7044.111.6.1273. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev. 2015;54:76–88. doi: 10.1016/j.neubiorev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Nyhus E, 1, Barceló F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- O'Neill PK, Gordon JA, Sigurdsson T. Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci. 2013;33:14211–1424. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Brown VJ. The effect of striatal dopamine depletion and the adenosine A2A antagonist KW-6002 on reversal learning in rats. Neurobiol Learn Mem. 2007;88:75–81. doi: 10.1016/j.nlm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biol Psychiatry. 2015;77:445–453. doi: 10.1016/j.biopsych.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad JA, MacGregor EM, Chudasama Y. Lesions of the thalamic reuniens cause impulsive but not compulsive responses. Brain Struct Funct. 2013;218:85–96. doi: 10.1007/s00429-012-0378-5. [DOI] [PubMed] [Google Scholar]
- Prasad JA, Abela AR, Chudasama Y. Midline thalamic reuniens lesions improve executive behaviors. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.071. in press. doi:10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 1996;111:215–232. doi: 10.1007/BF00227299. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Parkinson J. A componential analysis of the functions of primate orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006. pp. 237–263. [Google Scholar]
- Roberts AC, De Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The neurophysiology of the orbitofrontal cortex. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006. pp. 95–124. [Google Scholar]
- Sánchez-Santed F, 1, de Bruin JP, Heinsbroek RP, Verwer RW. Spatial delayed alternation of rats in a T-maze: effects of neurotoxic lesions of the medial prefrontal cortex and of T-maze rotations. Behav Brain Res. 1997;84:73–79. doi: 10.1016/s0166-4328(97)83327-x. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Risser JM. Odor memory and odor learning in rats with lesions of the lateral olfactory tract and mediodorsal thalamic nucleus. Brain Res. 1990;529:23–29. doi: 10.1016/0006-8993(90)90807-n. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, Wilson MA. Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct. 2014;219:911–929. doi: 10.1007/s00429-013-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol. 2002;442:163–187. doi: 10.1002/cne.10083. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, do Valle AC, Sherman A, Rodriguez JJ. Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol. 2006;499:768–796. doi: 10.1002/cne.21135. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti K, Leranth C. Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull. 2007;71:601–609. doi: 10.1016/j.brainresbull.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Di Prisco G, Vertes RP. Excitatory actions of the ventral midline thalamus (rhomboid/reuniens) on the medial prefrontal cortex in the rat. Synapse. 2006;60:45–55. doi: 10.1002/syn.20271. [DOI] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex. 2009a;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SC, Robbins TW, Roberts AC. Response disengagement on a spatial self-ordered sequencing task: effects of regionally selective excitotoxic lesions and serotonin depletion within the prefrontal cortex. J Neurosci. 2009b;29:6033–6604. doi: 10.1523/JNEUROSCI.0312-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Vann SD, Aggleton JP, Nelson AJD. A critical role for the anterior thalamus in directing attention to task-relevant stimuli. J Neurosci. 2015;35:5480–5488. doi: 10.1523/JNEUROSCI.4945-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem. 2008;15:97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]


