Abstract
Here we report the case of a young girl who had vague signs and symptoms potentially attributable to hyperthyroidism and was found to have autoimmune thyroiditis and hyperthyroxinemia. The elevated serum free thyroxine levels were persistent when measured by both standard assays and equilibrium dialysis/high-pressure liquid chromatography-tandem mass spectrometry. The clinical symptoms, with discordant thyroid test results, created a diagnostic dilemma that led initially to unnecessary additional evaluations. She was ultimately found to have familial dysalbuminemic hyperthyroxinemia (FDH) and required no therapy. This case highlights the inherent difficulties in evaluating children, who typically have vague signs and symptoms of thyroid dysfunction, when, in addition, they have an unrelated acquired (autoimmune) as well as a genetic (FDH) defect. The benefit of including testing for immediate members of the family is emphasized.
Keywords: autoimmune thyroiditis, familial dysalbuminemic hyperthyroxinemia, free thyroxine assay, hyperthyroxinemia
Introduction
Familial dysalbuminemic hyperthyroxinemia (FDH) is an autosomal dominant disorder identified in 1979 by Henneman et al. (1) and Lee et al. (2). It is characterized by elevations in total serum thyroxine (T4) levels due to increased binding to an abnormal serum albumin with an approximately 15-fold increase in binding affinity for T4 and 3,3′,5′-triidothyronine (reverse T3, rT3), but not for 3,3′,5-triidothyronine (T3), compared to the common type of albumin (3). This can be caused by various mutations in the ALB ( albumin ) gene, the most common of which is a missense mutation of the ALB gene, resulting in the replacement of an arginine with a histidine (p.R218H). Two other forms of FDH result in marked increases in serum T4 concentration or binding affinity preferentially for T3 (4).
With standard serum assays, both total and free T4 levels appear to be elevated in FDH. However, assays employing equilibrium dialysis followed by radioimmunoassay (RIA), liquid chromatography, and tandem mass spectrometry should give normal free T4 results. Lack of normalization of free T4 levels using these assays can be due to causes other than FDH, although false-positive results have been previously reported (5). This poses a challenge to the clinician, who may then undertake extensive additional and unnecessary evaluations.
Subject
A 4-year-8-month-old girl was referred for evaluation of hyperactivity and palpitations. She was previously healthy, with the only past history being early-onset tooth development. She reportedly had her first tooth erupt at 3 months of age, and, recently, she shed two primary teeth. Her birth history was normal, and, developmentally, she was on target for all milestones. Her family history was notable for teenage Graves’ disease in her mother, treated by thyroidectomy.
On physical examination, all vital signs were normal for age. Her weight was 19.4 kg (79th percentile), her height was 103.5 cm (35th percentile), and her body mass index was 18 kg/m2 (94th percentile). Her growth velocity was 5 cm/year, without any note of acceleration. Her eye exam result was normal, with no proptosis or lid lag. Her thyroid gland was palpable, with no gross enlargement or tenderness. Her heart rate and peripheral pulses were normal, and the extremities were well perfused, with no tremor or hyperreflexia. Her neurologic exam result was normal, and she was Tanner stage I. The remainder of her examination result was normal.
Initial laboratory evaluations showed an elevated serum total T4 level of 22.03 μg/dL (reference range 4.5–12 μg/dL), elevated free T4 level of 3.03 ng/dL (reference range 0.58–1.64 ng/dL) (measured by direct automated immunometric assay), and a normal thyroid-stimulating hormone (TSH) concentration of 2.38 mIU/L (reference range 0.34–5.6 mIU/L). Her bone age was 5 years at a chronological age of 5 years. Repeat testing confirmed these results as well as elevated thyroperoxidase and thyroglobulin (TG) antibody titers. Thyroid-stimulating immunoglobulin and thyrotropin-binding inhibitory immunoglobulins were not detected. On subsequent follow-up, laboratory evaluations showed persistence of high serum total T4 levels with a non-suppressed TSH. A free T4 level measured using direct equilibrium dialysis coupled with tandem mass spectrometry was also elevated at 2.9 ng/dL (reference range 0.58–1.64). Specific tests to rule out antibody interference with the TSH assay produced negative results. Given these results, along with her mother’s thyroid history and the advanced dental age, an evaluation was undertaken to rule out a TSH-secreting pituitary tumor or resistance to thyroid hormone (RTH). She had normal levels of the α subunit of TSH, and no mutations in the thyroid hormone receptor β ( THRB ) gene were identified, ruling out a TSH-secreting pituitary tumor and RTH, respectively.
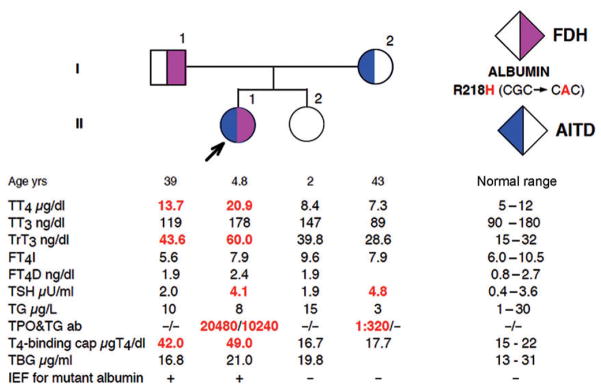
Without a clear diagnosis, further testing including genetic analysis was undertaken in the patient and members of her immediate family. The study was approved by the Institutional Review Board of the University of Chicago, where the analyses were performed. After obtaining informed consents, we investigated the family members in terms of their thyroid function tests ( Figure 1 ). The normal free T4 index, calculated from the total T4 and the resin T4 uptake ratio, combined with increased T4-binding capacity not due to excess T4-binding globulin (TBG), suggested the presence of an abnormal serum T4-binding substance. Furthermore, the normal total serum T3 with high rT3 concentrations, together with similar abnormalities found in the father, was compatible with a dominantly inherited defect, likely FDH. The presence of an abnormal T4-binding albumin was confirmed by isoelectric focusing analysis. Sequencing of the ALB gene revealed the most common mutation associated with FDH, c.653G > A producing p.R218H, in both the patient (proband) and her father. In light of this finding, a free T4 level was again measured, this time using equilibrium dialysis coupled with RIA. This assay produced a normal free T4 measurement of 2.4 ng/dL (reference range 0.8–2.7), consistent with the genetic diagnosis of FDH. The symptoms of hyperactivity and palpitations were not replicated during any of the examinations and ultimately resolved spontaneously during the course of the evaluation. At follow-up 2 years later, the girl was asymptomatic, growing and developing appropriately, and euthyroid without requiring anti-thyroid therapy.
Figure 1.
Pedigree showing the results of thyroid function tests and presence of ALB gene mutation. Square symbols indicate males and circles indicate females. The proband is indicated with an arrow. Results are aligned with each symbol. Abnormal values are in bold numbers. Ages represent those at the time of blood sampling. FT4D indicates free T4 measured by equilibrium dialysis in the presence of radiolabeled T4 and calculated using the total T4 determination. For all other abbreviations, see the text.
Discussion
Euthyroid hyperthyroxinemia refers to a broad category of conditions that share the unusual combination of high serum thyroxine with detectable thyrotropin levels and lack of clinical signs of hyperthyroidism ( Table 1 ). Typically, the hyperthyroxinemia is discovered while evaluating vague signs and symptoms possibly associated with thyroid dysfunction.
Table 1.
Differential diagnosis of hyperthyroxinemia with normal or increased TSH.
| Diagnosis | Total T4 | Free T4 | TSH | Comments |
|---|---|---|---|---|
| Resistance to thyroid hormone | ↑ | ↑ | Normal or ↑ | Defect in THRB gene |
| TSH-secreting pituitary tumor | ↑ | ↑ | ↑ | α subunit of TSH (SU) ↑ ( α SU/TSH > 1) |
| Familial dysalbuminemic hyperthyroxinemia | ↑ | ↑ or normal | Normal | Mutant albumin gene |
| Medications (amiodarone) | ↑ | ↑ | Normal | Inhibits T4 → T3 |
| Deiodinase defect | ↑ | ↑ | Normal or ↑ | SBP2 gene mutation |
| Gain of function mutation of transthyretin (TTR) | ↑ | ↑ or normal | Normal | Normal serum TTR levels |
| TBG excess | ↑ | Normal | Normal | High TBG concentration |
| Anti-T4 antibody (assay interference) | ↑ or ↓ | Normal, ↑ or ↓ | Normal | Falsely high or low total T4 |
Most clinical laboratories measure free T4 by automated immunometric assays. The analytical performance of these assays has been questioned, and there have been significant biases reported due to either endogenous factors (abnormal binding proteins, dialyzable protein binding competitors, heterophile antibodies, and autoantibodies) or in vitro factors (free fatty acid, assay antibodies, analogs, and intrinsic dilution). An ideal method to measure free T4 should employ a step to separate the free fraction from the protein-bound fraction before quantitative analysis. The separation step should not disturb the endogenous equilibrium. Understanding the mechanisms, advantages, and weaknesses of the various free T4 assays is therefore critical for an accurate evaluation of patients with thyroid disorders. The various free T4 assays are briefly reviewed here (6).
Labeled analog method
This is a one-step method that is designed to give a signal that is proportional to the free T4 concentration in the presence of binding proteins. Serum is incubated with an immobilized T4-specific antibody and a chemically modified labeled T4 analog that binds to the T4-specific antibody but is unable to bind to serum proteins. After equilibrium is reached, the immobilized antibody containing the bound T4 is washed and the occupancy of antibody binding sites is measured as for a typical competitive immunoassay. The greater the amount of free T4 available, the less room there is for the labeled analog and the lower the signal ( Figure 2 ). This method tends to perform poorly at extremes of thyroid hormone-binding capacity, at very low or high TBG, or with significant changes in T4-binding protein affinity.
Figure 2.
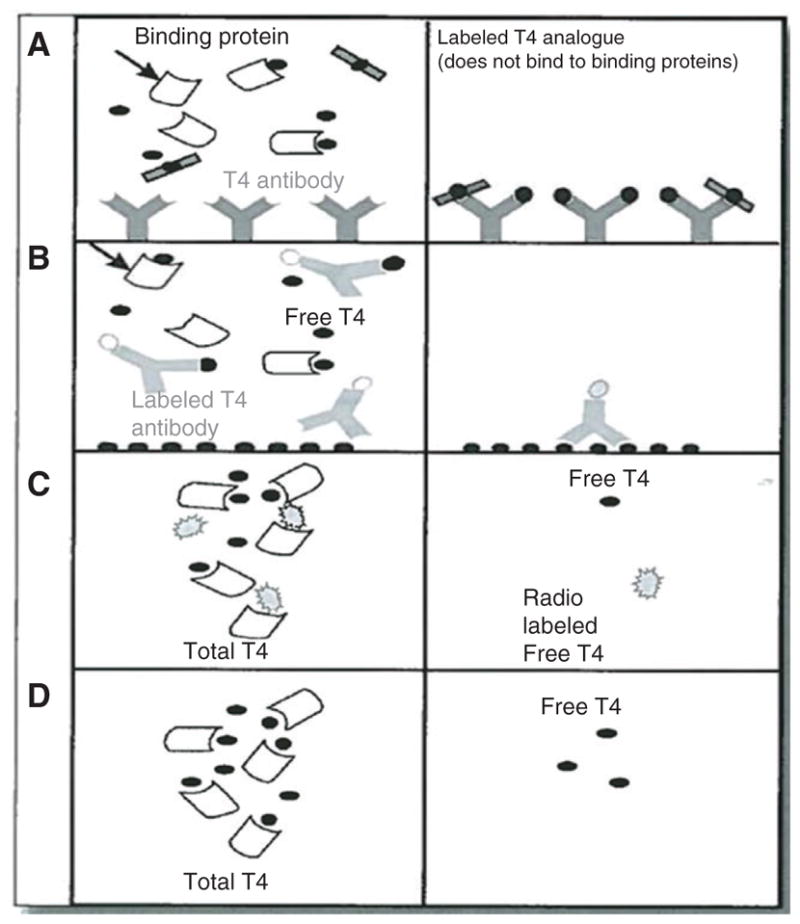
(A) In the one-step analog method, labeled T4 is altered to prevent it from interacting with binding proteins. After equilibrium is achieved, the serum is removed and the occupancy of the antibody binding site is measured. The more the free T4, the less room there is for the labeled analog and the lower the signal. (B) In the labeled antibody method, free T4 competes with solid-phase T4 for a limited number of labeled antibody binding sites. The resulting solid-phase bound label is inversely proportional to the original free T4 concentration. (C) Indirect (tracer) equilibrium dialysis involves addition of radiolabeled T4 with free T4 calculated as the fraction of the total radiolabeled T4 that has crossed the dialysis membrane. (D) Direct equilibrium dialysis requires quantitative measurement of the free T4 present in the compartment from which the protein-bound T4 is excluded by radioimmunoassay or mass spectrometry. [Reproduced with permission from the editor of Ref. (6).]
Labeled antibody method
This method uses a T4-specific antibody rather than a T4 analog, which is labeled ( Figure 2 ). This has successfully addressed some of the problems associated with labeled analog methods. Although less affected by extremes of thyroid hormone binding capacity, the labeled antibody method may still show some low bias when binding capacity is extremely low (as in TBG deficiency).
Equilibrium dialysis
This method includes the indirect (tracer) and the direct methods. The indirect method involves dialysis of a serum mixture containing radiolabeled T4 and is less often used. The indirect (tracer) equilibrium dialysis involves dialysis of a serum mixture containing radiolabeled T4 ( Figure 2C). The free T4 is calculated as the fraction of the total radiolabeled T4 that has crossed the dialysis membrane. An accurate assessment is highly dependent on the use of a very pure, nondegradable tracer.
Most dialysis assays use the direct method, which eliminates the need for adding a radiolabeled tracer to the sample before dialysis. The direct equilibrium dialysis method is considered the gold standard ( Figure 2 ). The free T4 that crosses the dialysis membrane can be measured by RIA or tandem mass spectrometry (6).
In the case presented here, the initial total and free T4 assays were performed using the labeled antibody method. This is common as the method of choice for initial testing in most centers due to wide availability and low cost. To confirm or better delineate an abnormal result, direct equilibrium dialysis is the next test of choice, as it was in our patient. While this method has a better ability to accurately report true free T4 values in the face of confounding variables, false positives may still occur. Hoshikawa et al. (5) have reported false-positive results of elevated free T4 assays using equilibrium dialysis/RIA, and they suggest that this assay is not an ultimate standard for diagnosing FDH, especially in patients with the R218P mutation of the ALB gene. They further recommend ultrafiltration as an added measure to increase the accuracy of testing prior to RIA.
Conclusions
Our case is unique, since, to the best of our knowledge, this is the first report of a case of FDH where the use of equilibrium dialysis coupled with tandem mass spectrometry resulted in a false-positive result of an elevated free T4.
Given the signs and symptoms reported by the family, combined with the inherent difficulty of accurately assessing a 4-year-old child’s complaints, therapies and evaluations were undertaken that otherwise may have been avoided had inclusion of the family in the testing been undertaken earlier. This case highlights the inherent difficulties in evaluating children with vague signs and symptoms of thyroid dysfunction. In cases where clinical symptomatology is incongruous with the results of thyroid function tests, and FDH is of high clinical suspicion, the clinician is best served by a combination of familial and genetic testing and by a close scrutiny of the results obtained even with gold standard assays.
Acknowledgments
This work was supported in part by grant DK15070 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Contributor Information
Abha Choudhary, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock, AR, USA.
Chutintorn Sriphrapradang, Department of Medicine, The University of Chicago, Chicago, IL, USA.
Samuel Refetoff, Department of Medicine, The University of Chicago, Chicago, IL, USA; and Department of Pediatrics and Genetics, The University of Chicago, Chicago, IL, USA.
Zoltan Antal, Zoltan Antal, MD, Department of Pediatrics, Division of Endocrinology, Weill Cornell Medical College, New York Presbyterian Hospital, 525 East 68th Street, Box 103, New York, NY 10065, USA.
References
- 1.Henneman G, Docter R, Krenning EP, Bos G, Otten M, et al. Raised total thyroxine and free thyroxine index but normal free thyroxine. A serum abnormality due to inherited increased affinity of iodothyronines for serum binding protein. Lancet. 1979;i:639–42. doi: 10.1016/s0140-6736(79)91080-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee WN, Golden MP, Van Herle AJ, Lippe BM, Kaplan SA. Inherited abnormal thyroid hormone-binding protein causing selective increase of total serum thyroxine. J Clin Endocrinol Metab. 1979;49:292–9. doi: 10.1210/jcem-49-2-292. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz M, Rajatanavin R, Young RA, Taylor C, Brown R, et al. Familial dysalbuminemic hyperthyroxinemia: a syndrome that can be confused with thyrotoxicosis. N Engl J Med. 1982;306:635–9. doi: 10.1056/NEJM198203183061103. [DOI] [PubMed] [Google Scholar]
- 4.Pannain S, Feldman M, Eiholzer U, Weiss RE, Scherberg NH, et al. Familial dysalbuminemic hyperthyroxinemia in a Swiss family caused by a mutant albumin (R218P) shows an apparent discrepancy between serum concentration and affinity for thyroxine. J Clin Endocrinol Metab. 2000;85:2786–92. doi: 10.1210/jcem.85.8.6746. [DOI] [PubMed] [Google Scholar]
- 5.Hoshikawa S, Mori K, Kaise N, Nakagawa Y, Ito S, et al. Artifactually elevated serum-free thyroxine levels measured by equilibrium dialysis in a pregnant woman with familial dysalbuminemic hyperthyroxinemia. Thyroid. 2004;14:155–60. doi: 10.1089/105072504322880409. [DOI] [PubMed] [Google Scholar]
- 6.Nakamoto J. Laboratory diagnosis of multiple pituitary hormone deficiencies: issues with testing of the growth and thyroid axes. Pediatr Endocrinol Rev. 2009;6:291–7. [PubMed] [Google Scholar]