Abstract
A 4 year male presented with rapid onset cranial nerve palsy and ataxia. Brain MRI revealed a pontine mass lesion with discordant conventional and advanced MR imaging. A stereotactic core biopsy revealed glioblastoma with immunostaining suggestive of histone H3K27M and TP53 mutation, consistent with diffuse intrinsic pontine glioma. Three month post-radiotherapy MRI revealed extensive new leptomeningeal metastatic disease involving both the supra- and infratentorial brain as well as the imaged portion of the spine. Tissue procured at the time of needle biopsy following consent to our institutional tissue banking protocol has undergone striking in vivo expansion as an orthotopic xenograft.
Keywords: pontine glioma, leptomeningeal metastasis, hypofractionated radiotherapy, H3K27M, xenograft, stereotactic biopsy
Brief Report
Case Report
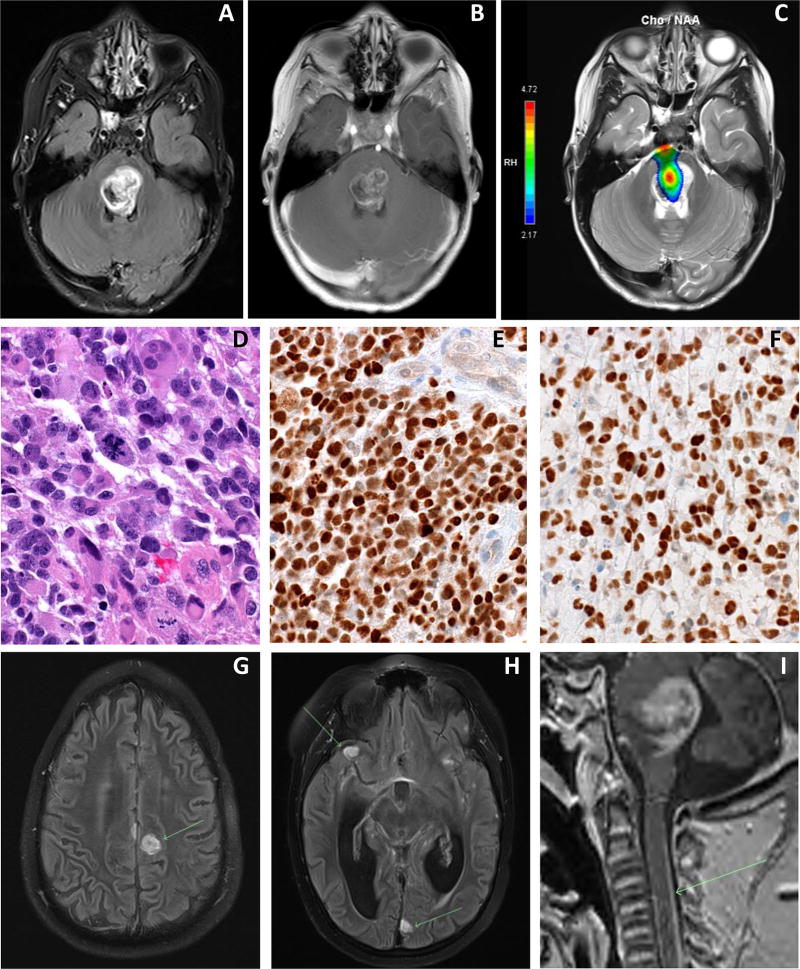
A 4 year old male presented with one week history of left eye esotropia and several days of ataxia and left lower extremity weakness. Brain MRI revealed a relatively well circumscribed, enhancing brainstem mass lesion centered in the dorsal pons compatible with a low grade process, yet with perfusion and spectroscopy suggestive of a high grade lesion (Fig. 1a, b and c). There were no signs of leptomeningeal metastasis within the imaged brain. Given discrepant MR imaging features, he underwent a stereotactic biopsy without complications which revealed glioblastoma (WHO grade IV) with immunostaining suggestive of histone H3K27M and TP53 mutation, consistent with diffuse intrinsic pontine glioma (Fig. 1d, e and f). He was treated with hypofractionated intensity modulated radiation therapy (IMRT) to 45 Gy over 15 daily fractions of 3 Gy1 with a 1 cm clinical target volume. He tolerated radiotherapy well, yet with minimal improvement in symptoms and remained steroid-dependent. Brain MRI three months following radiotherapy revealed extensive new leptomeningeal metastatic disease involving both the supra- and infratentorial brain as well as the imaged portion of the spine (Fig. 1g, h and i). The expansile pontine mass did not appear significantly changed in size. Of note, tissue procured at the time of needle biopsy following consent to our institutional tissue banking protocol has undergone striking in vivo expansion as an orthotopic xenograft. At the time of progression the family opted for home hospice and against CSF diversion. The patient was kept on high dose glucocorticoids, yet experienced progressive symptoms related to hydrocephalus and died two weeks following this MRI. Autopsy was declined.
Figure 1.
Diagnostic MRI of axial T2 FLAIR (A), T1 post-contrast (B), and spectroscopy (C) revealing a hyperintense, enhancing mass centered in the tegmentum and dorsal portion of the upper pons with a focus of significantly elevated choline/N-acetylaspartate (NAA) ratio within the lesion. Histologic characterization of needle biopsy specimen revealing frequent mitosis on hematoxilin and eosin staining (40×) (D), and immunohistochemical analysis of H3K27M mutation in DIPG tumor cells (40×) (E) and p53 immunoreactivity (40×) (F), the pattern of which is indicative of TP53 mutation. Three-month post-radiotherapy MRI of axial post-contrast T2 FLAIR images (G and H) and sagittal 3D T1 MPRAGE (I) demonstrating numerous rounded nodules of abnormal hyperintensity throughout the supra and infratentorial compartments, as well as a thin layer of abnormal enhancement coating sulci and cisternal spaces within the brain and dorsal aspect of the imaged spinal cord as depicted by arrows.
Discussion
Distant leptomeningeal spread, while relatively rare in DIPG, has been documented in upwards of 20% of cases, and has raised the concern for complete neuraxis staging at diagnosis.2 A spine MRI was not obtained at diagnosis for our patient, as our imaging approach with suspected DIPG has been a dedicated brain MRI with spine imaging for concerning symptoms or findings on brain imaging. Interestingly, recent detailed histo-pathologic and genetic analysis of brain autopsy specimens has revealed extensive intracranial dissemination in DIPG.3 With the increasing application of molecular diagnostics, it will be important to explore what, if any, molecular alterations may predict an elevated risk of leptomeningeal spread. However, given limited tissue resources, coupled with the relative rarity with which distant metastatic disease is observed in DIPG, this will likely be a difficult question to answer. Of note, molecular analysis of a matched primary progressive DIPG tumor and concomitant metastatic deposits from a single patient failed to reveal significant differences in mutational spectra, suggesting at least in this limited sample primary tumor directed therapy may be relevant for sites of metastasis.4
Hypofractionated radiotherapy, or the delivery of larger doses over smaller number of treatments, has recently emerged as a potential treatment approach in DIPG. Several retrospective reports from Europe have suggested this regimen may provide similar survival outcomes with a side effect profile in line with that seen with conventionally fractionated RT.1,5,6 A recent randomized phase III trial from Egypt evaluated 80 patients with DIPG treated to 39 Gy in 13 fractions or 54 Gy in 30 fractions. While technically exceeding the pre-specified non-inferiority margin,7 this study ultimately showed no significant difference in outcome between the two regimens.8 While most of these studies did not address the impact of hypofractionation on the incidence and kinetics of symptom palliation, all patients experienced rapid symptom improvement in the report by Janssens et al.5 There is additional data from adult patients with metastatic cancers that suggest that hypofractionated regimens may provide more rapid and durable symptom relief.9,10 In our case, the patient’s family opted for shorter course RT after a discussion of the above data and review of outcomes with both regimens. The decision was also influenced by the referral-based nature of our practice, given that patients often travel a significant distance for care. Counter balancing the potential benefit of early symptom improvement is the concern that more aggressive hypofractionation may lead to a higher rate of symptomatic radiation necrosis and lower median overall survival.11
While controversial,12,13 minimally invasive biopsy of DIPG offers an opportunity to establish a pathologic diagnosis. Furthermore, this will permit the development of relevant experimental model systems which may help unlock the molecular underpinnings of, and elucidate effective therapies for, this dreadful disease. Our current institutional practice for patients with pontine-centered mass lesions thought not to be resectable is to proceed with diagnostic biopsy if the following two criteria are met: 1) there is diagnostic uncertainty based on symptomatology and/or imaging, and 2) stereotactic biopsy is felt to be safe based on multidisciplinary case review with neurosurgical evaluation. In our case, biopsy was diagnostically informative, however, further genetic profiling to potentially assist therapy recommendations was not pursued. Of note, consent was obtained at the time of this post-radiotherapy MRI for our institutional tumor and germline next generation sequencing (NGS) protocol, Genome for Kids (NCT# 02530658), and results from the analysis are pending. Precedent has been set for the application of molecular genetic analysis to help inform therapeutic options for DIPG, including the recently completed phase II trial from the Dana Farber Cancer Center (NCT# 01182350), and the ongoing Pediatric Neuro-Oncology Consortium’s (PNOC) PNOC 003 study (NCT# 02274987) among others (NCT# 02233049). However, given the lack of published therapeutic clinical trials incorporating systemic agents thought to be specific to driver mutations in DIPG, successful or otherwise, we do not currently base upfront systemic therapy on results of tumor genetic analysis.
The development of human-derived cell line and xenograft mouse models of DIPG represent important advances in the field of molecular pathogenesis of pediatric high grade glioma. Initial success came in the form of autopsy-based material cultured in neural stem cell conditions which yielded a human DIPG-specific neurosphere cell line and a subsequent orthotopic xenograft mouse model following stereotactic transplantation of dissociated neurospheres.14 Several groups have gone on to derive treatment-naïve human DIPG cell lines and xenograft mouse models through procurement of biopsy tissue obtained at diagnosis.15,16 These cell line and xenograft models have been used for both evaluation of novel treatment strategies17,18 and investigation of the underlying biology of DIPG.19 Based on findings from several of these studies, a number of clinical trials have been developed including the ongoing Children’s Oncology Group ADVL 1217 phase I trial evaluating radiation and the Wee-1 inhibitor MK-1775 (NCT# 01922076) and the Pediatric Brain Tumor Consortium 047 phase I/II trial testing the histone deacetylase inhibitor panobinostat in the recurrent setting (NCT# 02717455). Following stereotactic biopsy of our patient’s tumor, cells were directly dissociated and stereotactically implanted into the mouse cerebrum. Tumor developed within 7 weeks as an initial passage, several weeks to months earlier than most PDXs we have derived from autopsy samples. H3F3A K27M mutation has been confirmed through targeted DNA sequencing. Ongoing efforts include further molecular characterization including whole genome/exome sequencing, RNAseq, and DNA methylation, as well as cell line derivation. While autopsy was declined in our case, analysis of primary/recurrent tumor samples may provide for evaluation of clonal divergence following therapeutic interventions.20
Acknowledgments
The authors would like to thank Xiaoyan Zhu for her efforts with PDX generation.
Abbreviations
- DIPG
Diffuse infiltrating pontine glioma
- IMRT
Intensity modulated radiation therapy
- CSF
Cerebrospinal spinal fluid
- MRI
Magnetic resonance imaging
- WHO
World Health Organization
- NGS
Next generation sequencing
- RNAseq
Ribonucleic acid sequencing
- PDX
Patient-derived xenograft
- DNA
Deoxyribonucleic acid
- NAA
N-acetylaspartate
Footnotes
Conflict of Interest Statement: Christopher Tinkle: No conflict of interest; Brent Orr: No conflict of interest; John Lucas: No conflict of interest; Paul Klimo: No conflict of interest; Zoltan Patay: No conflict of interest; Suzanne Baker: No conflict of interest; Alberto Broniscer: No conflict of interest, Ibrahim Qaddoumi: No conflict of interest.
References
- 1.Negretti L, Bouchireb K, Levy-Piedbois C, et al. Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution's experience. J Neurooncol. 2011;104(3):773–777. doi: 10.1007/s11060-011-0542-4. [DOI] [PubMed] [Google Scholar]
- 2.Gururangan S, McLaughlin CA, Brashears J, et al. Incidence and patterns of neuraxis metastases in children with diffuse pontine glioma. J Neurooncol. 2006;77(2):207–212. doi: 10.1007/s11060-005-9029-5. [DOI] [PubMed] [Google Scholar]
- 3.Nikbakht H, Panditharatna E, Mikael LG, et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nature communications. 2016;7:11185. doi: 10.1038/ncomms11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nazarian J, Mason GE, Ho CY, et al. Histological and molecular analysis of a progressive diffuse intrinsic pontine glioma and synchronous metastatic lesions: a case report. Oncotarget. 2016 doi: 10.18632/oncotarget.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssens GO, Gidding CE, Van Lindert EJ, et al. The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. International journal of radiation oncology, biology, physics. 2009;73(3):722–726. doi: 10.1016/j.ijrobp.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. International journal of radiation oncology, biology, physics. 2013;85(2):315–320. doi: 10.1016/j.ijrobp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Roos DE, Smith JG. Randomized trial on radiotherapy for paediatric diffuse intrinsic pontine glioma (DIPG) Radiother Oncol. 2014;113(3):425. doi: 10.1016/j.radonc.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 8.Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol. 2014;111(1):35–40. doi: 10.1016/j.radonc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta oncologica. 2012;51(5):584–588. doi: 10.3109/0284186X.2011.652741. [DOI] [PubMed] [Google Scholar]
- 10.Amini A, Altoos B, Bourlon MT, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: Is RCC truly radioresistant? Pract Radiat Oncol. 2015;5(6):e589–596. doi: 10.1016/j.prro.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankinson TC, Patibandla MR, Green A, et al. Hypofractionated Radiotherapy for Children With Diffuse Intrinsic Pontine Gliomas. Pediatric blood & cancer. 2016;63(4):716–718. doi: 10.1002/pbc.25836. [DOI] [PubMed] [Google Scholar]
- 12.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. Journal of neurosurgery. 2007;107(1 Suppl):1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 13.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children's Cancer Group. Neurosurgery. 1993;33(6):1026–1029. doi: 10.1227/00006123-199312000-00010. discussion 1029–1030. [DOI] [PubMed] [Google Scholar]
- 14.Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108(11):4453–4458. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashizume R, Smirnov I, Liu S, et al. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J Neurooncol. 2012;110(3):305–313. doi: 10.1007/s11060-012-0973-6. [DOI] [PubMed] [Google Scholar]
- 16.Truffaux N, Philippe C, Paulsson J, et al. Preclinical evaluation of dasatinib alone and in combination with cabozantinib for the treatment of diffuse intrinsic pontine glioma. Neuro Oncol. 2015;17(7):953–964. doi: 10.1093/neuonc/nou330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller S, Hashizume R, Yang X, et al. Targeting Wee1 for the treatment of pediatric high-grade gliomas. Neuro Oncol. 2014;16(3):352–360. doi: 10.1093/neuonc/not220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. doi: 10.1038/nm.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KM, Fang D, Gan H, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27(9):985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill RM, Kuijper S, Lindsey JC, et al. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27(1):72–84. doi: 10.1016/j.ccell.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]