Abstract
Importance
African American (AA) women have a two-fold higher incidence of breast cancers that are negative for estrogen receptor, progesterone receptor and HER2/neu (triple negative breast cancer, TNBC) compared with White/Caucasian Americans (WA). TNBC likely arises from different pathogenetic pathways compared to non-TNBC, and benign breast disease (BBD) predicts for future non-TNBC.
Objective
To determine whether AA identity remained associated with TNBC among women with a prior diagnosis of BBD.
Design
Retrospective analysis; January 1, 1994-December 31, 2005; mean follow-up 10.2 years.
Setting
Henry Ford Health System in metropolitan Detroit, Michigan; an integrated multihospital, multispecialty health care system.
Participants
2,588 AA and 3,566 WA patients age 40-70 years with biopsy-proven BBD diagnosed 1/1/1994 to 12/31/2005.
Main Outcome Measures
Subsequent breast cancer, stratified by phenotype.
Results
BBD detection and management were similar for the AA and WA patients. Subsequent breast cancers developed in approximately 4% of AA patients (mean 6.8 years following BBD diagnosis) and WA patients (mean 6.1 years). More than three-quarters of subsequent cancers in each subset were DCIS or Stage I. The 10-year probability estimate for developing TNBC was 0.56% (95% confidence interval 0.32-1.0) for AA versus 0.25% for WA (95% confidence interval 0.12-0.53). Among the 73 AA patients that developed subsequent invasive breast cancer, 24.2% were TNBC compared to 7.4% of the 111 subsequent invasive BC cases (p=0.0125) among the WA patients.
Conclusion and Relevance
AA identity persisted as a significant risk factor for TNBC in our study, the largest analysis to date of BBD and subsequent breast cancer phenotypes in a diverse patient population managed equitably. This suggests that AA identity is associated with inherent susceptibility for TNBC pathogenetic pathways.
Introduction
In comparison to White/Caucasian American (WA) women, African American (AA)women have an approximately two-fold higher incidence rate of cancers that are negative for the estrogen receptor (ER), the progesterone receptor (PR) and HER2/neu1,2,, commonly described as triple negative breast cancer (TNBC). The inherently aggressive basal breast cancer subtype defined by gene expression profiling, accounts for approximately 80% of the immunohistochemically defined TNBC phenotype.3,4 TNBC also represents a more challenging form of breast cancer because patients with these tumors are not candidates for treatment with targeted systemic agents such as endocrine therapy or anti-HER2/neu therapy. The increased frequency of TNBC among AA women therefore contributes to disparities in breast cancer outcome that are well-documented in the United States, with higher mortality rates among AA compared to WA women. TNBC is thought to arise from different pathogenetic pathways compared to non-TNBC, and it is associated with different reproductive history risk factors5-7. Histopathologic patterns of benign breast conditions (commonly referred to as benign breast disease [BBD]), such as hyperplasia, atypia, and lobular carcinoma in situ (LCIS) are associated with an increased risk for future breast cancers that are more likely to be of the ER-positive/non-TNBC phenotype.8,9 Our goal was to determine whether AA identity remained associated with TNBC among a cohort of women initially diagnosed with BBD.
Methods
Description of the Cohort
Pathology Information System, Co-Path, was used to identify women diagnosed with Benign Breast Disease (BBD) by biopsy performed between January 1, 1994 and December 31, 2005. Entry into the cohort was restricted to women between ages 40 and 70 years, with a minimum of 6 months stay with HFHS. Women with a previous history of malignancy of the breast or other organs except squamous or basal cell carcinoma of the skin were excluded. Additionally, women whose breast cancers were diagnosed within 6 months of the diagnoses of their BBD were excluded. This exclusion criterion minimized the likelihood of inadvertently evaluating cases of coexisting BBD and cancer, as some patients are found to have cancer after undergoing initial core needle biopsy revealing either benign/high-risk pathology (atypia and/or LCIS) or some discordant benign pathology followed by a subsequent diagnostic surgical biopsy revealing a synchronous breast malignancy, and other patients have a cancer biopsy following short-term observation of an initially indeterminate lesion. BBD was detected by routine screening mammography or because of physical signs and symptoms, i.e. pain and tenderness, lumps or nipple discharge.
Data Elements
The cohort data elements were collected by manually reviewing electronic medical records (EMR). The minimum data elements included date of birth, race/ethnicity, date of biopsy and date of final follow-up. Data were collected on method of BBD biopsy and management.
Women were followed by EMRs until last encounters with the healthcare system for any reason. For women diagnosed with subsequent breast cancer, we documented information on dates of diagnosis and the associated pathology- ER, PR, HER2/neu, grade and stage of cancer. Cases of subsequent cancer included any invasive tumor or ductal carcinoma in situ (DCIS); cases of subsequent lobular carcinoma in situ were excluded.
Immunohistochemistry and Categories of Breast Cancer Phenotypes
Nuclear expression of hormone receptor (ER and PR) proteins was detected with specific monoclonal antibodies using a labeled streptavidin-biotin immunoperoxidase method. The immunohistochemical assay was performed on deparaffinized formalin-fixed tissue sections of the specimens. Monoclonal mouse antibodies to human ER (DAKO clone ID5) and to human PR (DAKO clone PgR636) were used with a DAKO automated immunostainer following the manufacturer's protocol. Immunohistochemistry for HER2/neu staining was performed using the HerceptTest (DAKO, Glostrup, Denmark), an FDA-approved clinical test that qualitatively identifies by light microscopy p185 HER2 overexpression in breast cancer cells. Molecular marker staining was interpreted in compliance with established guidelines and as per Fitzgibbons et al10-12. Tumors were scored as ER/PR-negative if they had less than 1% nuclear staining. Confirmed ER and PR positive tumors served as positive controls, and normal adjacent mammary gland ductules present in sections of tumor served as internal positive controls. Expression of HER2/neu was scored as per Fitzgibbons et al10. Grading was based on degree and intensity of membrane labeling of tumor cells, on a scale from 0-3+: grade 0 (no observable labeling or faint, incomplete, or barely detectable membrane labeling in <10% of tumor cells), 1+ (faint, incomplete, or barely detectable membrane labeling in >10% of tumor cells), 2+ (incomplete and/or weak to moderate complete membrane staining in >10% of tumor cells, or complete, intense membrane labeling in <10% of tumor cells) or 3+ (intense, complete membrane labeling in >10% of tumor cells). A specimen scored as 0 or 1+ was classified as HER2/neu negative, and specimens scored as 3+ were considered positive. Specimens with a grade of 2+ were equivocal, and fluorescent in situ hybridization was used to assess amplification of the HER2/neu gene.
Subsequent cases of DCIS were evaluated by ER and PR, subsequent invasive carcinomas by ER, PR and HER2/neu. Phenotypes were classified as “ER+ and/or PR+, HER2-”, “ER+ and/or PR+, HER2+”,“ER- andPR-, HER2+” or “ER- /PR-, HER2-” (TNBC).
Statistical Methods
We used descriptive statistics to summarize the demographic and clinicopathologic characteristics of the cohort. The variables age at BBD diagnosis and duration of stay with the health system were included in the analysis as continuous variables. Women were categorized as asymptomatic if their benign conditions were detected during routine screening mammography or clinical breast examination. Women with missing information were classified as unknown. BBD was classified as fibrocystic/proliferative/hyperplasia without atypia, fibrocystic/proliferative/hyperplasia with atypia or lobular carcinoma in situ. Differences in the distributions of the demographic and clinicopathologic variables between AA and WA women were assessed using Student's two-sided t-test, Mantel-Haenszel test of significance or Wilcoxon rank-sum test.
We applied polychotomus multivariable logistic regression to evaluate features that were significantly associated with ultimately having TNBC among those patients that developed invasive breast cancer. In these models we sought to determine if the variable “race” was associated with the risk of “ER-, PR- and HER2-” subtype of invasive breast cancer (TNBC). In developing the best fitted model, we first estimated the effect of each variable and their interaction with “race” on subtype of BC. Variables were evaluated because of their potential biological impact (histology of benign breast condition for the entire cohort; and stage as well as grade for the subsequent invasive cancer that was diagnosed on follow-up) or clinic-demographic influence (age at the time of diagnosis of benign breast condition, method of detection and treatment modality of benign breast condition). Stratification of women by BC phenotype and histologic grade yielded no observation within grade 1 and the subtype of “ER-, PR- and HER2-”. Histologic grades were dichotomized into “well/moderately differentiated’ and “poorly differentiated” categories. This step was necessary in order to satisfy the convergence criterion when developing the best fitted model. The unit for the variable age at diagnosis of benign breast condition was converted into decade before inclusion in the model. The variables with P-value <0.10 from the univariable logistic analyses were considered the candidate variables. Interactions between variables also were tested at P=0.10. The final model contained only two variables, “Race” and “Grade” at the significant P-level=0.05. A total of 157 members of the cohort who developed invasive breast cancer subsequent to their BBD contributed to odds ratios calculation for each subtypes of breast cancer. All statistical tests were two sided.
Kaplan-Meier methods were applied to generate 5- and 10-year estimates of breast cancer incidence for the AA and WA patients separately. These probabilities were calculated for all cancer phenotypes, as well as for TNBC versus non-TNBC. Statistical significance was tested by generating logrank p values.
Analyses were performed using SAS v. 9.3 (SAS Institute, Cary, NC).
Results
Table 1 demonstrates the clinicopathologic features of the study cohort, stratified by racial/ethnic identity as AA (2,588 patients) versus WA (3,566 patients). The average age at BBD diagnosis was similar for both subsets, at approximately 52 years. Mean follow-up of ten years after BBD detection was also similar. There were no statistically significant differences in method of BBD detection; 78.4% of the AA and 78.9% of the WA cases were biopsied for evaluation of a screening mammography abnormality. Approximately 12% of both subsets underwent biopsy of a palpable mass. Histopathologic patterns of the benign breast conditions were also similar for the two groups- more than 90% of both subsets had hyperplasia without atypia; there was a slightly higher (but statistically significant) frequency of atypical hyperplasia among the WAs (8.0% versus 5.8%; p=0.0012); and only 0.1% of each group had lobular carcinoma in situ. The small volume of cases with atypia and lobular carcinoma in situ precluded subset analysis for these high-risk/benign histopathologies.
Table 1. Clinicopathologic characteristics of the benign breast disease (BBD) cohort.
| Variable | African-Americans N=2,588 (%) | White-Americans N=3,566 (%) | P-Value 95% CI |
|---|---|---|---|
|
| |||
| Age at diagnosis of BBD | 51.7 (± 8.3) | 52.1 (±8.4) | 0.0692 |
| Mean (± SD) | |||
|
| |||
| Stay with the system post BBD diagnosis | |||
| Mean Years (± SD) | 10.3 (± 4.5) | 10.2 (± 4.8) | 0.318 |
|
| |||
| Method of BBD Detection | |||
| Asymptomatic/Screening Mammogram | 2,019 (78.4) | 2,800 (78.9) | 0.468 |
| Symptomatic | 555 (21.6) | 748 (21.1) | |
| Clinical Breast Examination | 96 | 94 | |
| Pain and Tenderness | 53 | 57 | |
| Lump | 309 | 436 | |
| Clear Discharge | 49 | 126 | |
| Bloody Discharge | 28 | 18 | |
| Other Symptoms | 20 | 17 | |
| Unknown/Missing | 14 | 18 | |
|
| |||
| Extent of BBD Management | |||
| Excision/Lumpectomy | 376 (14.7) | 583 (16.5) | 0.653 |
| Biopsy (Needle/Core) | 2,126 (83.3) | 2,799 (79.4) | |
| Incidental to Surgery1 | 50 (2.0) | 144 (4.0) | |
| Unknown | 36 | 40 | |
|
| |||
| Histology of Benign Lesions | |||
| Fibrocystic changes/Hyperplasia without Atypia | 2,438 (94.2) | 3,283 (92.0) | 0.0012 |
| Hyperplasia with Atypia | 150 (5.8) | 283 (8.0) | |
| Ductal | 122 | 207 | |
| Lobular | 19 | 52 | |
| Ductal and Lobular | 6 | 21 | |
| Lobular Carcinoma in Situ | 3 | 3 | |
Incidental to Surgery: diagnosis of benign breast disease incidental to cosmetic surgery
Thirty AA (1.6%) and 30 WA (0.8%) patients were diagnosed with subsequent DCIS (p=0.262) at a mean age of 58.4 and 61.8 years respectively (p=0.16). These DCIS cases were diagnosed at a mean follow-up interval of 6.5 and 6.1 years, respectively (p=0.64). Hormone receptor expression for these DCIS cases was similar for AA and WA patients; ER was positive in 86.4% and 88.9% of AAand WA cases respectively (p=0.81) and progesterone receptor expression was positive in 77.3% and 77.8% respectively (p=0.97)
Table 2 demonstrates the clinicopathologic profiles for the patients that developed subsequent invasive breast cancer, detected in 73 (2.8%) of the AA and 111 (3.1%) of the WA patients; p=0.58. These cancers were diagnosed at a similar 6.9 and 6.2 years following the prior benign breast biopsy for the AA and WA patients and at similar mean ages (61.6 and 61.9 years, respectively). Approximately half of both subsets of women with subsequent invasive breast cancer were diagnosed with Stage I disease. A numerically higher (but not statistically significant) frequency of high grade tumors was found in the AA (39.1%) compared to WA (27.8%) cases.
Table 2. Clinicopathologic characteristics of patients subsequently diagnosed with invasive breast cancer.
| Variable | African-Americans N= 73(%) | White-Americans N= 111(%) | P-Value |
|---|---|---|---|
|
| |||
| Age at Diagnosis of Cancer | |||
| Mean (± SD) | 61.6(± 9.4) | 61.9 (± 9.2) | 0.734 |
|
| |||
| Length of time between diagnosis of BBD and cancer (years) | |||
| Mean (± SD) | 6.9 (± 4.4) | 6.2 (± 3.8) | 0.226 |
|
| |||
| Estrogen Receptor | 0.036 | ||
| Positive | 49 (70.0) | 90 (83.3) | |
| Negative | 21 (30.0) | 18 (16.7) | |
| Missing | 3 | 3 | |
|
| |||
| Progesterone Receptor | 0.009 | ||
| Positive | 43 (65.6) | 85 (79.2) | |
| Negative | 27 (34.4) | 22 (20.8) | |
| Missing | 3 | 4 | |
|
| |||
| HER2 | 0.472 | ||
| Positive | 9 (13.6) | 17 (17.9) | |
| Negative | 57 (86.6) | 78 (82.1) | |
| Missing1 | 7 | 16 | |
|
| |||
| AJCC Stage | 0.769 | ||
| IA | 21 (20.8) | 30 (21.6) | |
| IB | 24 (23.8) | 39 (28.0) | |
| IIA | 9 (8.9) | 11 (7.9) | |
| IIB | 11(10.9) | 20 (14.3) | |
| IIIA | 3 (3.0) | 4 (2.9) | |
| III C | 1 (1.0) | 0 | |
| IV | 2 (2.0) | 5 (3.6) | |
| Missing | 2 | 2 | |
|
| |||
| Histologic Grade | 0.079 | ||
| 1 | 13 (18.8) | 30 (27.8) | |
| 2 | 29 (42.0) | 48 (44.4) | |
| 3 | 27 (39.1) | 30 (27.8) | |
| Missing | 4 | 3 | |
HER2/neu testing is not routinely performed for cases of ductal carcinoma in situ; it became a standardized component of invasive breast cancer biomarker assays at the Henry Ford Health System in 2001.
As shown in Table 3, statistically significant differences were observed between the phenotype frequencies in the two subsets of women diagnosed with subsequent invasive breast cancer. Among the 184 subsequent invasive cancers that developed, TNBC was detected in three times as many of the AA compared to WA patients (24.2% vs. 7.4%; p=0.0125).
Table 3.
Distribution of phenotypes among 73 African-American and 111 White-American members of the cohort that developed subsequent invasive breast cancer.
| Sub-type | African-Americans N (%) | White-Americans N (%) | P-Value |
|---|---|---|---|
| ER+ and/or PR+, HER2- | 41 (62.1) | 70 (74.5) | 0.0125 |
| ER+ and/or PR+, HER2+ | 6 (9.1) | 11 (11.7) | |
| ER-, PR-, HER2+ | 3 (4.5) | 6 (6.4) | |
| ER-, PR- and HER2- | 16 (24.2) | 7 (7.4) |
ER=estrogen receptor; PR=progesterone receptor
Table 4 demonstrates the results of the polychotomous logistic regression analysis, demonstrating that AA identity and high-grade pathology were the two statistically significant features associated with having TNBC. AA identity remained significantly associated with TNBC even after adjusting for tumor grade. Among those who developed invasive breast cancer, the odds of TNBC versus the reference breast cancer phenotype was 4.34 times (95% CI=1.28-14.68; p=0.018) higher in AA compared to WA patients.
Table 4.
Adjusted odds ratio for demographic characteristics and histologic grade. A total of 157 members of the cohort who developed invasive breast cancer subsequent to their BBD contributed to risk estimation for each subtypes of breast cancer using polychotomous multivariate logistic regression technique.
| Adjusted for histologic grade | ||||||||
|---|---|---|---|---|---|---|---|---|
| African Americans N (%) | White Americans N (%) | African Americans N (%) | White Americans N (%) | African Americans N (%) | White Americans N (%) | |||
| TNBC | 16 (25.0) | 7 (7.5) | HR-, HER2+ | 3 (1.9) | 6 (6.4) | HR+, HER2+ | 6 (9.4) | 11 (11.8) |
| HR+, HER2- | 39 (60.9) | 69 (74.2) | HR+, HER2- | 39 (60.9) | 69 (74.2) | HR+, HER2- | 39 (60.9) | 69 (74.2) |
| OR=4.34 (95% CI=1.28-14.69) P=0.018 | OR= 0.93 (95% CI=0.20-4.44) P=0.930 | OR=0.94 (95% CI=0.32-2.92) P=0.957 | ||||||
| Adjusted for race/ethnicity | ||||||||
| Grade 3 N (%) | Grades1&2 N (%) | Grade 3 N (%) | Grades1&2 N (%) | Grade 3 N (%) | Grades1&2 N (%) | |||
| TNBC | 21 (91.3) | 2 (8.7) | HR-, HER2+ | 7 (77.8) | 2 (22.2) | HR+, HER2- | 7 (41.2) | 10 (58.8) |
| HR+, HER2- | 14 (13.0) | 94 (87.0) | HR+, HER2- | 14 (13.0) | 94 (87.0) | HR+, HER2- | 14 (13.0) | 94 (87.0) |
| OR=72.12 (95% CI=14.65-355.02) P<0.0001 | OR=22.95 (95% CI=4.32-121.88) P=0.0002 | OR=4.60 (95% CI=1.50-14.06) P=0.007 | ||||||
HR=hormone receptor (estrogen receptor and/or progesterone receptor); OR=odds ratio
Results from the Kaplan-Meier risk estimates up to twelve years are shown in Figures 1a (probability of any invasive breast cancer following a diagnosis of BBD), 1b (probability of TNBC) and 1c (probability of non-TNBC). There were no significant differences in the risk of subsequent invasive breast cancer for AAs compared to WAs when all phenotypes were grouped together (logrank p = .45), with estimated 10 year incidence of 2.5% [95% CI: 1.9%, 3.2%] and 3.2% [95% CI: 2.6%, 4.0%], respectively. Most of the subsequent invasive BC tumors were non-TNBC for both AAs and WAs, however the risk of subsequent TNBC was significantly higher for AAs compared to WAs (logrank p=0.0042), and the risk of subsequent non-TNBC was higher for WAs compared to AAs (logrank p=0.048). The ten-year estimates for incidence of TNBC were 0.56 (95% confidence interval 0.32-1.0%) and 0.25% (95% confidence interval 0.12-0.53%) for AAs and WAs, respectively. The ten-year estimates for incidence of non-TNBC were 1.76% (95% confidence interval 1.27-2.43%) and 2.85% (95% confidence interval 2.30-3.55%).
Figure 1.
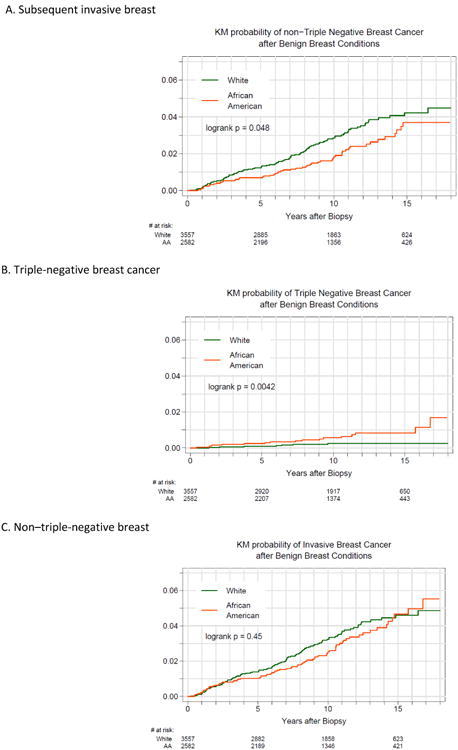
Probability of subsequent invasive breast cancer after benign breast disease (log rank, P = .45). B, Probability of triple-negative breast cancer after benign breast disease (log rank, P = .004). C, Probability of non–triple-negative breast cancer after benign breast disease (log rank, P = .048)
Discussion
Gene expression profiling has demonstrated that breast cancer is a heterogeneous disease, comprised of various subtypes associated with different prognostic and therapeutic features. The basal subtype has the most aggressive nature. Approximately 80% of basal breast cancers are negative for the three biomarkers utilized to define systemic therapy needs, and TNBC has therefore become a surrogate for identifying basal breast tumors in clinical practice3-5. TNBC differs from non-TNBC- it is a marker of hereditary breast cancer susceptibility; it responds briskly to neoadjuvant chemotherapy; it is more common among younger women; and is detected at a disproportionately higher rate among AA women.
Epidemiologic studies have demonstrated that many of the risk factors historically correlated with breast cancer risk are actually predictive for developing non-TNBC tumors. Multiple pregnancies, for example, reduce the likelihood of developing ER-positive breast cancer, but several studies reveal that multiparity increases the risk of TNBC.5,13-15
Fibrocystic breast pathology resulting in multiple biopsies is a well-established breast cancer risk factor, and is a key element of the Gail model for individualized breast cancer risk assessment.16 Benign breast hyperplasia/BBD without atypia is associated with a nearly two-fold increased breast cancer risk.17-20 The presence of histopathologic indices of abnormal proliferation (such as atypia and lobular carcinoma in situ) within an otherwise benign breast biopsy confers higher risks for future breast cancer: four-to-five-fold relative risk for atypia and ten-fold for LCIS.18-24 Several studies have furthermore confirmed that biopsy-proven BBD is a risk factor for future breast cancer in both AA and WA women.25-28
Until recently, most studies correlating BBD and subsequent breast cancer risk have looked at all phenotypes grouped together. Insights regarding the diversity of breast cancer subtypes have prompted further scrutiny of BBD patterns and their associations with particular breast cancer subtypes. One model of breast cancer pathogenesis suggests that fibrocystic proliferative changes can serve as precursors for the relatively more indolent patterns of breast cancer, including ER-positive disease.6 Indeed, the Mayo Clinic Benign Breast Disease Cohort demonstrated that 84% of the 1273 cancers that were detected among more than thirteen thousand women initially diagnosed with benign breast pathology on prior biopsy were estrogen receptor positive.8 Similarly, the Cancer and Steroid Hormone Study found that benign breast disease was only associated with an increased risk for luminal A breast cancer subtypes, but not hormone receptor-negative or TNBC disease.9 Ongoing research seeks to identify precursors that are specifically associated with TNBC pathogenesis, such as microglandular adenosis.7
The etiology of the association between TNBC and AA identity is poorly-understood, but environmental, reproductive and genetic factors have been proposed.29-33 The contribution of germline genetic factors is supported by studies demonstrating increased frequency of TNBC among western, sub-Saharan women, a population likely to have shared ancestry with AA women as a consequence of the colonial-era trans-Atlantic slave trade.29,34-38
This study sought to determine whether AA identity or fibrocystic histopathology would predominate in estimating the likelihood of subsequent breast cancer phenotype. While the majority of cancers that developed in our cohort of AA and WA women with BBD were ER-positive, it was notable that AA identity persisted as a statistically significant risk factor for TNBC in multivariate analysis and in estimates of risk over time. This finding suggests to us that African ancestry is indeed associated with some inherent susceptibility for pathways leading to the development of triple negative disease in mammary tumors.
The Henry Ford Health System is well-suited for the study of breast cancer disparities related racial-ethnic identity as well as socioeconomic status. Comprised of multiple facilities and hospitals providing care to the diverse metropolitan Detroit and southeast Michigan communities, this integrated health care system features a robust employee-based medical insurance plan (the Health Alliance Plan) while also assuming care for a large indigent inner-city population. Our study revealed similar management and follow-up intervals for the Henry Ford AA and WA patients with BBD; furthermore, similarly high proportions of the subsequent breast cancers were detected at very early stages (Stage 0/DCIS or Stage I disease). These patterns suggest equitable quality of care delivered to this diverse patient population.
To our knowledge, this is the largest reported series specifically addressing benign breast disease in AA compared to WA patients in the context of defining racial-ethnic identity versus breast tumor precursors as risk factors for TNBC. Ongoing evaluation of our cohort will incorporate other breast cancer risk factors into our analyses, such as obesity and family history. Future studies by other investigators should seek to confirm the patterns that we identified in this diverse Midwest metropolitan population.
Acknowledgments
Partially supported by a grant from the National Institute of Health/National Cancer Institute, Early Detection Research Network (CA113916-08)
References
- 1.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011 featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015 Jun;107(6) doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer. 2011 Jun 15;117(12):2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol Dec. 2010;7(12):683–692. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 4.Alluri P, Newman LA. Basal-like and triple-negative breast cancers: searching for positives among many negatives. Surg Oncol Clin N Am. 2014 Jul;23(3):567–577. doi: 10.1016/j.soc.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman LA, Reis-Filho JS, Morrow M, Carey LA, King TA. The 2014 Society of Surgical Oncology Susan G. Komen for the Cure Symposium: triple-negative breast cancer. Ann Surg Oncol. 2015 Mar;22(3):874–882. doi: 10.1245/s10434-014-4279-0. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchio C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010 Aug;57(2):171–192. doi: 10.1111/j.1365-2559.2010.03568.x. [DOI] [PubMed] [Google Scholar]
- 7.Geyer FC, Lacroix-Triki M, Colombo PE, et al. Molecular evidence in support of the neoplastic and precursor nature of microglandular adenosis. Histopathology. 2012 May;60(6B):E115–130. doi: 10.1111/j.1365-2559.2012.04207.x. [DOI] [PubMed] [Google Scholar]
- 8.Visscher DW, Frost MH, Hartmann LC, et al. Clinicopathologic features of breast cancers that develop in women with previous benign breast disease. Cancer. 2016 Feb 1;122(3):378–385. doi: 10.1002/cncr.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011 Nov;130(2):587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Sang D, Xu B, et al. Value of Breast Cancer Molecular Subtypes and Ki67 Expression for the Prediction of Efficacy and Prognosis of Neoadjuvant Chemotherapy in a Chinese Population. Medicine. 2016 May;95(18):e3518. doi: 10.1097/MD.0000000000003518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010 Jul;6(4):195–197. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013 Nov 1;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 13.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008 May;109(1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinde SS, Forman MR, Kuerer HM, et al. Higher parity and shorter breastfeeding duration: association with triple-negative phenotype of breast cancer. Cancer. 2010 Nov 1;116(21):4933–4943. doi: 10.1002/cncr.25443. [DOI] [PubMed] [Google Scholar]
- 15.Phipps AI, Chlebowski RT, Prentice R, et al. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011 Mar 16;103(6):470–477. doi: 10.1093/jnci/djr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989 Dec 20;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 17.Castells X, Domingo L, Corominas JM, et al. Breast cancer risk after diagnosis by screening mammography of nonproliferative or proliferative benign breast disease: a study from a population-based screening program. Breast Cancer Res Treatan. 2015 Jan;149(1):237–244. doi: 10.1007/s10549-014-3208-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985 Jan 17;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 19.London SJ, Connolly JL, Schnitt SJ, Colditz GA. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992 Feb 19;267(7):941–944. [PubMed] [Google Scholar]
- 20.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005 Jul 21;353(3):229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann LC, Degnim AC, Dupont WD. Atypical hyperplasia of the breast. N Engl J Med. 2015 Mar 26;372(13):1271–1272. doi: 10.1056/NEJMc1501046. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast--risk assessment and management options. N Engl J Med. 2015 Jan 1;372(1):78–89. doi: 10.1056/NEJMsr1407164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEvoy MP, Coopey SB, Mazzola E, et al. Breast Cancer Risk and Follow-up Recommendations for Young Women Diagnosed with Atypical Hyperplasia and Lobular Carcinoma In Situ (LCIS) Ann Surg Oncol. 2015 Aug 5; doi: 10.1245/s10434-015-4747-1. [DOI] [PubMed] [Google Scholar]
- 24.King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol. 2015 Nov 20;33(33):3945–3952. doi: 10.1200/JCO.2015.61.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worsham MJ, Raju U, Lu M, et al. Risk factors for breast cancer from benign breast disease in a diverse population. Breast Cancer Res Treat. 2009 Nov;118(1):1–7. doi: 10.1007/s10549-008-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Qiu S, Raju U, Wolman SR, Worsham MJ. Benign breast disease heterogeneity: association with histopathology, age, and ethnicity. Breast Cancer Res Treat. 2008 Sep;111(2):289–296. doi: 10.1007/s10549-007-9775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007 Mar-Apr;13(2):115–121. doi: 10.1111/j.1524-4741.2007.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cote ML, Ruterbusch JJ, Alosh B, et al. Benign breast disease and the risk of subsequent breast cancer in African American women. Cancer Prev Res (Phila) 2012 Dec;5(12):1375–1380. doi: 10.1158/1940-6207.CAPR-12-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman LA. Disparities in Breast Cancer and African Ancestry: A Global Perspective. Breast J. 2015 Jan 9; doi: 10.1111/tbj.12369. [DOI] [PubMed] [Google Scholar]
- 30.Newman LA. Breast cancer disparities: high-risk breast cancer and African ancestry. Surg Oncol Clin N Am. 2014 Jul;23(3):579–592. doi: 10.1016/j.soc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer Causes Control. 2013 Dec 17; doi: 10.1007/s10552-013-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haddad SA, Ruiz-Narvaez EA, Haiman CA, et al. An exome-wide analysis of low frequency and rare variants in relation to risk of breast cancer in African American Women: the AMBER Consortium. Carcinogenesis. 2016 Jun 7; doi: 10.1093/carcin/bgw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham JE, Walters CA, Hill EG, Ford ME, Barker-Elamin T, Bennett CL. Mind the gap: racial differences in breast cancer incidence and biologic phenotype, but not stage, among low-income women participating in a government-funded screening program. Breast Cancer Res Treat. 2013 Jan;137(2):589–598. doi: 10.1007/s10549-012-2305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Der EM, Gyasi RK, Tettey Y, et al. Triple-Negative Breast Cancer in Ghanaian Women: The Korle Bu Teaching Hospital Experience. Breast J. 2015 Nov-Dec;21(6):627–633. doi: 10.1111/tbj.12527. [DOI] [PubMed] [Google Scholar]
- 35.Jiagge E, Bensenhaver JM, Oppong JK, Awuah B, Newman LA. Global surgical oncology disease burden: addressing disparities via global surgery initiatives: the University of Michigan International Breast Cancer Registry. Ann Surg Oncol. 2015 Mar;22(3):734–740. doi: 10.1245/s10434-014-4345-7. [DOI] [PubMed] [Google Scholar]
- 36.Ly M, Antoine M, Dembele AK, et al. High incidence of triple-negative tumors in sub-saharan Africa: a prospective study of breast cancer characteristics and risk factors in Malian women seen in a Bamako university hospital. Oncology. 2012;83(5):257–263. doi: 10.1159/000341541. [DOI] [PubMed] [Google Scholar]
- 37.Nwafor CC, Keshinro SO. Pattern of hormone receptors and human epidermal growth factor receptor 2 status in sub-Saharan breast cancer cases: Private practice experience. Niger J Clin Pract. 2015 Jul-Aug;18(4):553–558. doi: 10.4103/1119-3077.156905. [DOI] [PubMed] [Google Scholar]
- 38.Jiagge E, Jibril AS, Chitale D, et al. Comparative Analysis of Breast Cancer Phenotypes in African American, White American, and West Versus East African patients: Correlation Between African Ancestry and Triple-Negative Breast Cancer. Ann Surg Oncol. 2016 Jul 28; doi: 10.1245/s10434-016-5420-z. [DOI] [PubMed] [Google Scholar]


