Abstract
Juvenile angiofibroma (JA) is a benign, highly vascular tumor which is diagnosed on the basis of clinical and imaging features. It has a characteristic pattern of spread commonly involving the pterygopalatine fossa and pterygoid base. The mainstay of treatment is surgery, while radiotherapy is rarely used for the treatment of recurrent lesion. Endoscopic endonasal surgery is currently the treatment of choice for small to intermediate size JAs, and is feasible even for advanced lesions; however, this should only be practiced in well-experienced centers.
Keywords: juvenile angiofibroma, endoscopic surgery, surgical treatment of juvenile angiofibroma
Introduction
Juvenile angiofibroma (JA) is a rare benign vascular lesion of the skull base that affects young adolescent males. The management of JA is challenged by the abundant vascular blood supply of the lesion, along with the complex anatomy of the skull base and the young age of the affected population. While minimal surgical approaches are accompanied by high rates of residual or recurrent lesions, expanded external approaches may result in facial growth disturbance in children in addition to cosmetic and functional complications. Despite its rarity, the management of JA has dramatically evolved during the last decades, mainly due to the advent of endoscopic approaches as well as advancement in preoperative embolization techniques. In this article, we present an overview of the main features of JA and current strategies in its management.
Clinical Presentation
JA typically affects the male population, most commonly between 9 and 19 years of age. 1 The most frequent symptoms are nasal obstruction and epistaxis. Nasal obstruction may be bilateral despite the unilaterality of the lesion, due to nasopharyngeal extension as well as deviation of the nasal septum by the expansile lesion. Epistaxis is usually brisk and intermittent. Purulent nasal discharge and facial pain can be due to sinus drainage pathway obstruction, and conductive hearing loss indicates obstruction of the eustachian tube. Nasal endoscopy commonly shows a hypervascularized lobulated mass with a smooth surface typically bulging behind the tail of the middle turbinate, obstructing the choana or completely filling the nasal fossa ( Fig. 1 ). Advanced lesions can present with proptosis and facial swelling due to orbital and infratemporal fossa (ITF) extension, respectively. Ophthalmoplegia due to cranial nerve compromise is rare, but may complicate orbital apex and cavernous sinus invasion.
Fig. 1.
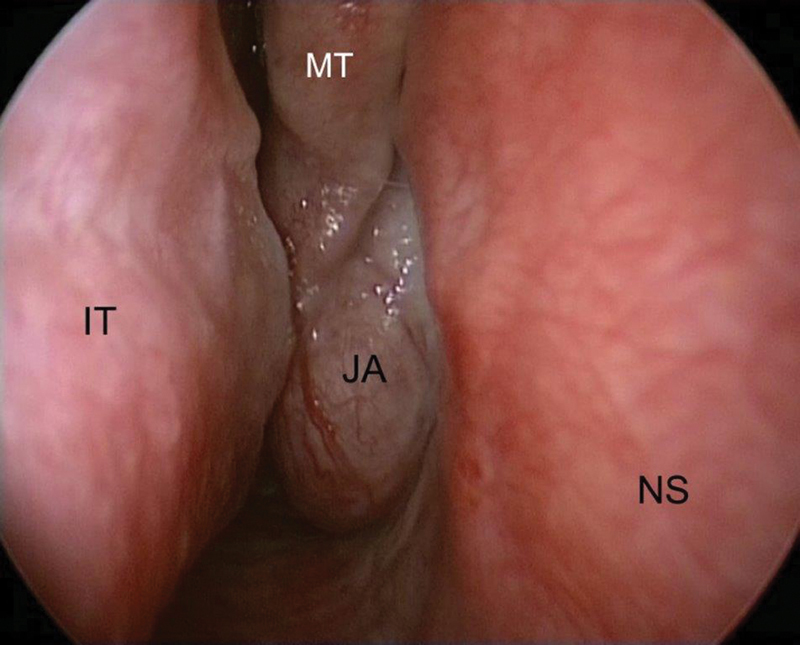
Endoscopic view of the right nasal fossa. Juvenile angiofibroma (JA) obstructing the nasal cavity and protruding between the middle turbinate (MT) and the nasal septum (NS). Abbreviation: IT, inferior turbinate.
Diagnosis
Diagnosis is made by typical clinical and imaging findings, while tissue biopsy is unnecessary and may lead to brisk hemorrhage. At imaging, JA appears as a highly vascularized and expansile lesion centered on the pterygopalatine fossa (PPF) in both contrast-enhanced computerized tomography (CT) and gadolinium-enhanced magnetic resonance imaging (MRI); ( Fig. 2A ). CT and MRI are complementary in the diagnosis of JA, as CT emphasizes skull base bony involvement while MRI is superior in the demonstration of intracranial, orbital, and cavernous sinus invasion. On both T1 and T2 weighted, unenhanced MRI, the lesion shows flow-void spotty signals, due to enlarged blood vessels.
Fig. 2.
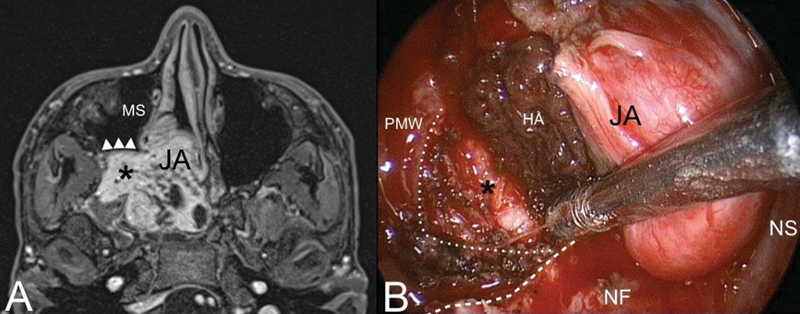
( A ) Axial contrast-enhanced T1-weighted magnetic resonance (MR). Juvenile angiofibroma (JA) with typical extension into the pterygopalatine and infratemporal fossa (black asterisk). The posterior wall of the maxillary sinus (MS) is pushed forward by the lesion (white arrowheads). ( B ) Identification of the dissection plane cutting the posterior periosteum of the posterior maxillary wall (PMW) (white dotted line). Inferior turbinate and medial maxillary wall have been removed to adequately expose the posterior maxillary wall. White dashed line: inferior limit of the medial maxillary wall. Abbreviations: HA, hemostatic agent; NF, nasal floor; NS, nasal septum.
Perhaps the most important step in the diagnosis of JA is understanding of its distinct pattern of spread, which should guide the radiologist and clinician in differentiating this tumor from other vascular and invasive lesions involving the pediatric skull base, such as schwannoma, meningioma, hemangioma, hemangiopericytoma, and sarcoma. JA originates from the superior margin of the sphenopalatine foramen where the sphenoid process of the palatine bone meets the pterygoid base and the horizontal ala of vomer. The lesion spreads in a submucosal and subperiosteal fashion, and progresses not only through minor resistance regions, but also through fissures and sutures and even directly to cancellous bone by means of pressure erosion. Bony involvement can have one of three patterns: (1) bone remodeling by means of expansion, thinning, and displacement (anterior displacement of the posterior maxillary wall, enlarged vidian canal, and inferior orbital fissure [IOF]) ( Fig. 2A ); (2) cancellous bone invasion (pterygoid base) ( Fig. 3 ); and (3) bone resorption and destruction (greater wing of sphenoid bone destruction by large tumor invading the middle cranial fossa) ( Fig. 4 ). From the nasal cavity and nasopharynx, JA has two characteristic pathways of spread: the more common anterolateral route and the posterolateral route. Anterolaterally, JA spreads anterior to the pterygoid plates to the PPF, which is involved in more than 70% of cases 2 3 4 ( Fig. 2 ). From the PPF, anterior progression of JA will displace the posterior wall of maxillary sinus anteriorly. Laterally, the tumor spreads through the pterygomaxillary fissure to the ITF. Pushing posteriorly, the tumor erodes the pterygoid plates and extends into the pterygoid fossa. Sphenoid sinus involvement usually follows erosion and expansion of the vidian canal ( Figs. 3 and 4A, B ). Superiorly JA spreads through the IOF to involve the orbit and through the superior orbital fissure (SOF) and foramen rotundum until it reaches the cavernous sinus ( Fig. 4 ). A posterolateal route of extension is less common, and consists of tumor spread from the nasopharnx to the pharyngeal recess, and laterally to the pterygoid fossa posterior to the medial pterygoid plate or through erosion of the medial pterygoid plate ( Fig. 5 ). From the pterygoid fossa, JA may progress to the parapharyngeal space laterally, while superior extension will involve the foramen lacerum and carotid canal. Intracranial extension occurs in 10 to 20% of cases, while intradural extension is very rare. 5 Intracranial extension may be classified as medial or lateral to the cavernous sinus and internal carotid artery (ICA), depending on the route of extension: 3 direct erosion of the greater sphenoid wing to the middle cranial fossa or progression through the IOF and SOF to the cavernous sinus. Less commonly, intracranial extension follows direct erosion of the sphenoid sinus walls or, through the ethmoid fovea and cribriform plate, reaching the anterior cranial fossa.
Fig. 3.
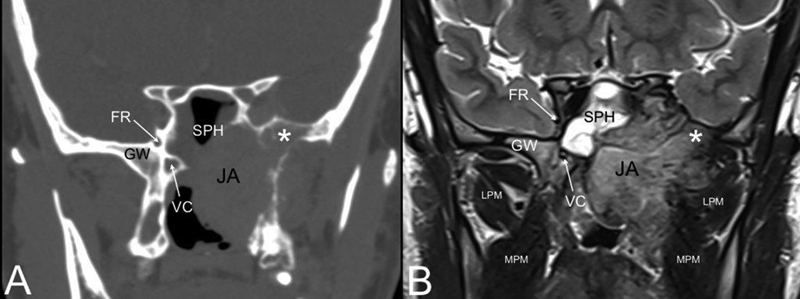
Coronal computed tomography (CT) ( A ) and T2-weighted magnetic resonance (MR) ( B ). Juvenile angiofibroma (JA) eroding the left greater wing (GW) of the sphenoid (white asterisk). Abbreviations: FR, foramen rotundum; LPM, lateral pterygoid muscle; MPM, medial pterygoid muscle; SPH, sphenoid sinus; VC, vidian canal.
Fig. 4.
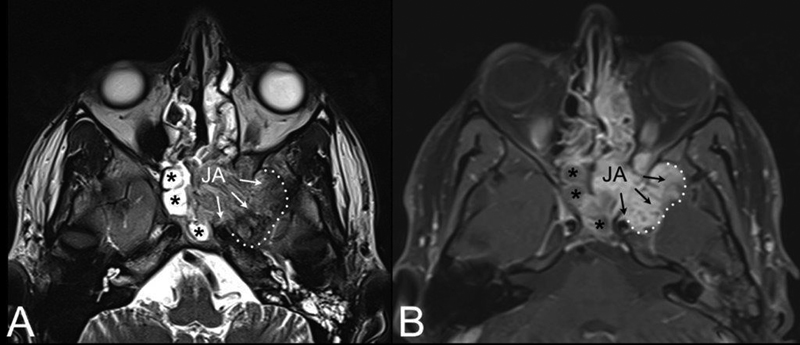
AxialT2-weighted ( A ) and contrast-enhanced T1-weighted magnetic resonance (MR) ( B ). Juvenile angiofibroma (JA) with intracranial extension (black arrows) into the middle cranial fossa through the pterygoid erosion and inferior orbital fissure. Extension to the anterior portion of the left cavernous sinus is also visible. Black asterisks: sphenoidal inflammatory tissue.
Fig. 5.
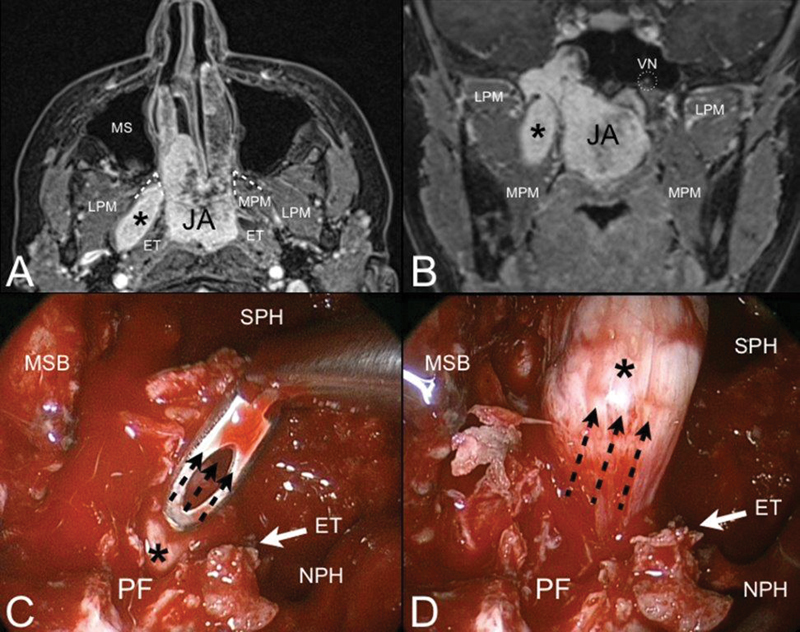
Axial ( A ) and coronal ( B ) contrast-enhanced T1-weighted magnetic resonance (MR). Juvenile angiofibroma (JA) with finger-like retropterygoid extension (black asterisk). Through the bone of the pterygoid root, JA extents to the pterygoid fossa (PF) and follows the direction of the medial pterygoid muscle (MPM) staying behind the auditory tube (ET). Endoscopic view of the right nasal fossa during the endoscopic procedure ( C, D ). The transpterygoid approach allows to expose the pterygoid fossa and pull up (black dashed arrows) the retropterygoid extension of the lesion. Abbreviations: LPM, lateral pterygoid muscle; MS, maxillary sinus; MSB, middle skull base; NPH, nasopharynx; SPH, sphenoid sinus; VN, vidian nerve.
Staging
Multiple staging systems have been proposed for JA ( Table 1 ). These systems mainly depend on defining the extent of tumor, skull base involvement, ITF extension, and intracranial invasion. Since Sessions et al 6 first proposed a staging system for JA in 1981, many other authors suggested a staging system that can better assist in tumor classification and treatment. The most commonly used staging systems are those proposed by Andrews et al 7 and Radkowski et al. 8 A staging system proposed by Onerci et al 9 attempted to better define tumors amenable to endoscopic resection versus tumors that are better managed by an external or combined approach. In 2010, Snyderman et al 10 proposed the University of Pittsburgh Medical Center (UPMC) staging system, emphasizing the role of residual tumor vascularization by the ICA following embolization and the route of intracranial extension (medial or lateral to ICA and cavernous sinus) as the most important factors for determining the feasibility of endoscopic resection and the risk of residual or recurrent tumor. Tumors in stages I and II receive their entire blood supply from branches of the external carotid artery (ECA) and are readily excised (following embolization) using endoscopic techniques with little morbidity. Stage III tumors are intermediate risk and are characterized by skull base erosion or lateral extension, but without residual vascularity. Stage IV and V tumors are challenging to treat because of significant blood supply from the ICA. Routes of intracranial extension may be from the sphenoid sinus medial to the cavernous sinus or lateral to the ICA through the orbital fissures to the middle cranial fossa. 11
Table 1. Staging systems most commonly adopted in the literature.
| Andrews et al 7 | |
|---|---|
| I | Limited to the nasopharynx and nasal cavity. Bone destruction negligible or limited to the sphenopalatine foramen |
| II | Invading the pterygopalatine fossa or the maxillary, ethmoid, or sphenoid sinus with bone destruction |
| III | • Invading the infratemporal fossa or orbital region without intracranial involvement |
| • Invading the infratemporal fossa or orbit with intracranial extradural (parasellar) involvement | |
| IV | • Intracranial intradural without infiltration of the cavernous sinus, pituitary fossa, or optic chiasm |
| • Intracranial intradural with infiltration of the cavernous sinus, pituitary fossa, or optic chiasm | |
| Radkowski et al 8 | |
| I | • Limited to posterior nares and/or nasopharyngeal vault |
| • Involving the posterior nares and/or nasopharyngeal vault with involvement of at least one paranasal sinus | |
| II | • Minimal lateral extension into the pterygopalatine fossa |
| • Full occupation of pterygopalatine fossa with or without superior erosion orbital bones | |
| • Extension into the infratemporal fossa or extension posterior to the pterygoid plates | |
| III | • Erosion of skull base (middle cranial fossa/base of pterygoids)—minimal intracranial extension |
| • Extensive intracranial extension with or without extension into the cavernous sinus | |
| Onerci et al 9 | |
| I | Nose, nasopharyngeal vault, ethmoidal-sphenoidal sinuses, or minimal extension to pterygopalatine fossa |
| II | Maxillary sinus, full occupation of pterygopalatine fossa, extension to the anterior cranial fossa, and limited extension to the infratemporal fossa |
| III | Deep extension into the cancellous bone at the base of the pterygoid or the body and the greater wing of sphenoid, significant lateral extension to the infratemporal fossa, or to the pterygoid plates posteriorly or orbital region, cavernous sinus obliteration |
| IV | Intracranial extension between the pituitary gland and internal carotid artery, tumor localization lateral to internal carotid artery, middle fossa extension, and extensive intracranial extension |
| Snyderman et al 10 | |
| I | No significant extension beyond the site of origin and remaining medial to the midpoint of the pterygopalatine fossa |
| II | Extension to the paranasal sinuses and lateral to the midpoint of the pterygopalatine fossa |
| III | Locally advanced with skull base erosion or extension to additional extracranial spaces, including orbit and infratemporal fossa, no residual vascularity following embolization |
| IV | Skull base erosion, orbit, infratemporal fossa Residual vascularity |
| V | Intracranial extension, residual vascularity M: Medial extension L: Lateral extension |
Preoperative Embolization
Preoperative embolization is used for all cases of JA except the very small lesions. Advancements in preoperative embolization has resulted in significant reduction in intraoperative bleeding, and despite some reports of safe resection without embolization 12 13 it is considered to be the standard of care at most centers. 14 15 16 The most commonly used approach for embolization is transarterial embolization (TAE). This technique most frequently uses particle material (polyvinyl alcohol [PVA], microspheres, etc.) that is introduced by a superselective catheterization of the feeding vessel or vessels. Glue, coils, or more recently ethylene vinyl alcohol copolymer (Onyx) are also used as embolic agents. Early stage juvenile nasopharyngeal angiofibroma (JNA) has a distinct blood supply from the ipsilateral ECA, most commonly the internal maxillary artery, ascending pharyngeal, sphenopalatine artery, and descending palatine artery, which can easily be embolized. Advanced lesions receive multiple blood supplies from the ipsilateral ECA as well as the contralateral one and from the ICA, most commonly through the vidian artery, ophthalmic artery, and inferolateral and meningiohypophyseal trunks. Supply from the ICA is present in ∼30% of cases when the tumor extends to the sphenoid sinus, parapharyngeal space, orbit, or the intracranial cavity. 14 17 Embolization of ICA branches can be done by temporary distal occlusion of the ICA, but carries significant risk for stroke and other neurologic complications caused by dislodgement of particles, and thus is highly discouraged. The effectiveness of TAE depends on the ability to occlude all tumor feeders, which is limited in cases of multiple, small, and tortuous vessels, previous operation with ligation of main branches of ECA, and in cases of significant blood supply from the ICA. Additionally, the presence of dangerous extracranial-to-intracranial anastomoses and particle reflux into the intracranial circulation may increase the risk of neurologic complications. Direct intratumoral embolization (DIE) has been introduced to overcome some of these limitations. Although there are many reports on its efficiency, 18 19 20 21 22 the method has not been widely adopted. DIE relies on direct intraparenchymal injection of an embolic agent such as Onyx by percutaneous or endonasal injection. The injection is done under fluoroscopic control to prevent reflux of injected material to the main arterial system and ICA.
Surgical Treatment
The goal of surgical treatment is complete tumor removal with minimal morbidity. When complete surgical resection is not achieved, consideration for further intervention is based on symptoms and the risk for critical neurovascular structure involvement by the tumor. Large skull base residual lesions are associated with increased risk for recurrent severe epistaxis and damage to critical neurovascular structures and warrant further intervention. Surgery for such lesions can be hazardous and patients may be referred to radiotherapy. As radiotherapy has the potential to be associated with significant complications in a growing child, it is reserved as last choice for unresectable lesions with a high risk of recurrence.
Challenges in surgical treatment of JA include control of intraoperative bleeding, addressing the different routes of skull base spread of the tumor, and preventing morbidity. Despite preoperative embolization, intraoperative hemorrhage is the hallmark of surgical treatment of large JAs, and a surgical approach should provide adequate visualization in a bloody surgical field as well as early control of vascular supply. JA develops in a deep narrow space, namely, the PPF, and spreads through the fissures and foramens of skull base as well as through direct bone invasion, thus, complex, and combined skull base approaches are often warranted. Because children have a small intravascular blood volume compared with adults, they have a reduced tolerance to operative blood loss. Additionally, in young patients, the potential for osteotomies to affect facial growth should also be considered when planning the surgical approach.
Depending on the tumor extension, different skull base approaches are used. External approaches were the mainstay of surgical treatment for decades, and these included the transpalatal approach, Le Fort I osteotomies, lateral rhinotomy, midfacial degloving, facial translocation, anterior craniofacial, and lateral infratemporal/subtemporal approaches. The transpalatal approach has been largely abandoned as it affords poor exposure for large tumors, resulting in a greater chance of recurrence. However, Mishra et al 23 recently presented their experience on the resection of 63 JAs using modified transpalatal approaches. A transpalatal-circumaxillary approach, where the incision is extended to the retromolar area to expose the pterygoid plates and PPF, was appropriate for tumors involving the PPF and medial ITF, while a transpalatal–circumaxillary–sublabial approach, where the incision is extended to include the sublabial region, was appropriate for tumors involving the ITF, cheek, and even the temporal fossa. Straight and angled endoscopes were used to visualize the sphenopalatine region and to ensure complete tumor resection. Midfacial degloving gained popularity over lateral rhinotomy due to the avoidance of facial incisions and better cosmetic results. Additionally, it provides access to the bilateral nasopharynx and can be extended to control orbital and intracranial invasion. 2 5 The lateral infratemporal approaches, as proposed by Fisch, 24 are appropriate for tumors with extensive lateral infratemporal, cheek, and temporal fossa invasion. Craniofacial approaches are designed to control extensive intracranial invasion.
External approaches are associated with high rate of morbidity. These approaches usually involve extensive osteotomies which are associated with increased blood loss, increase operative time, and which may interfere with the normal facial growth of the adolescent patient. Facial scars are another concern of utmost importance. Cerebrospinal fluid leak, facial and infraorbital nerve damage, lacrimal dysfunction, facial deformities, trismus, and dental malocclusion have been reported with transfacial approaches. 7 25 26
The Endonasal Endoscopic Approach: An Overview
During the last decades, an endoscopic approach for the resection of JA has gained increasing popularity due to its obvious advantages over external transfacial approaches, which include the avoidance of facial incisions, osteotomies, and bone plating, which do not expose young patients to the risk of craniofacial alterations. In addition, the magnified field of view and angled view “behind the corner” may be associated with more complete inspection of the resection cavity and shorter hospitalization time. Early reports on the exclusive use of endoscopic approach were restricted to early stage JAs, which were confined to the nasal cavity, nasopharynx, ethmoid, and sphenoid sinuses with limited PPF extension (Radkowski et al 8 stages Ia–IIb, Andrews et al (modified Fisch) 7 stages I–II). 25 27 28 29 30 31 32 33 34 35 36 37 38 Relapse rates were similar to those achieved with external approaches or even more favorable (0–15% 8 38 39 ). Later on, some experienced centers successfully expanded the indications to more advanced lesions involving the ITF and parasellar area. 15 16 40 41 42 43 In 2010, Nicolai et al 14 presented a series of 46 JAs that were treated exclusively by an endoscopic approach after vascular embolization. In this series, 17 of 46 (37%) had Andrews et al 7 stage IIIa or IIIb disease. Feeding vessels from the ICA were reported in 14 (30%) patients. Mean intraoperative blood loss was 580 mL. In four (8.7%) cases, suspicious residual disease was detected by MRI. All four residual lesions involved the root of pterygoid. In one patient, a residual lesion was successfully resected, while the other three lesions remained stable during follow-up by MRI.
Cloutier et al 44 compared their surgical outcomes based on a 10-year experience on 72 patients, who were divided into two groups based on year of resection: group 1 (2000–2005) and group 2 (2005–2010). The rate of endoscopic approach was significantly higher in group 2 than group 1 (82.9% vs. 45%). Approximately half of cases had advanced disease (stage IIIA or higher according to Radkowski et al 8 ), with no significant difference between the two groups. The rate of recurrence was 8.3% and did not differ between groups. While massive and large tumors were treated through an external approach during the earlier period, in the second period all tumors were addressed endoscopically, except in case of middle cranial fossa invasion or optic nerve or ICA encasement.
Huang et al 45 recently reported a single institutional case series that compared outcomes between open and endoscopic approaches. The study included 162 patients, 96 treated by a transpalatal or transmaxillary approach, and 66 treated using a transnasal endoscopic approach, with or without labiogingival incision. Approximately half of patients who were treated by an open approach and 60% of patients who were treated endoscopically had advanced lesions (Radkowski et al 8 stage IIc, IIIa, and IIIb). Compared with the open surgery group, the endoscopic surgery group had a lower median blood loss and a lower number of complications. The overall recurrence rate was 31.4%, with no significant difference between groups.
Recently, a retrospective study on the experience of five academic tertiary or quaternary care otolaryngology departments reviewed a series of 72 males with advanced stage JAs (Radkowski et al 8 stage IIIA or IIIB) treated via endonasal/endoscopic approach. 46 Fifty-two (71.9%) and 18 (28.1%) patients had a Radkowski et al IIIA and Radkowski et al IIIB tumor, respectively. Patients with massive intracranial invasion were excluded. Seventy-one patients underwent preoperative angiography and embolization, and 14 (19.1%) showed feeders from the ICA. Mean hospital stay was 6.1 days and the mean intraoperative blood loss of 1279.7 mL. Postoperative complications were reported in five patients including trigeminal nerve (V2) anesthesia, trigeminal neuralgia, transient abducens nerve palsy, and palatal insufficiency. The mean follow-up was 37.9 months (54 patients included), with 33.3% residual disease (18 of 54 patients). Two residual cases received radiotherapy and one underwent surgical resection, while the majority was followed by serial MRIs. All 18 cases remain asymptomatic and periodic MRI showed no growth of the residual tumors after a mean follow-up of 35.6 months.
The Endoscopic Endonasal Approach: Technical Notes
The key principle in the endoscopic approach to JA is to expose the lesion as extensively as possible without traumatizing its surface to minimize bleeding. The resection of large volume lesions can rarely be achieved in a single bloc and it is preferable to disassemble the JA by first removing the nasal-nasopharyngeal portion, and subsequently addressing the deepest peripheral projections.
Bleeding control is mainly achieved by preoperative embolization which is done 24 to 48 hours before surgery. Early detection and control of the internal maxillary artery and other feeding vessels reduce intraoperative bleeding. Even in cases of feeding vessels from the ICA, which are not amenable to embolization, these tributaries can be coagulated. A subperiosteal and submucosal plane of dissection should be followed to minimize bleeding, as well as the use of a monopolar electrocautery or diode laser to minimize bleeding during tumor disassembly. Hemostatic materials should be used to control venous bleeding from the cavernous sinus, pterygoid plexus, and basilar plexus ( Fig. 2 ). Hemostasis is also achieved by continuous irrigation with warm water (40–45°C). For resection of large JAs, when extensive bleeding is encountered, staging of the operation with delayed resection of the skull base component should be considered to allow patients to recover, equilibrate blood volume, and correct hemorrhage-induced coagulopathies before addressing the residual part of the lesion.
Exposure of the surgical field is made by first completing an ethmoidectomy, sphenoidotomy, and a large middle antrostomy extended posteriorly to expose the crucial area of the sphenopalatine foramen should be performed. By modulating the opening of the meatal wall of the maxillary sinus, lateral exposure of its posterior wall can be effectively increased ( Fig. 2 ). Medial maxillectomy can be anteriorly enlarged by sectioning the lacrimal duct at the inferior limit of the lacrimal sac or also removing the medial part of the anterior maxillary wall with type D endoscopic medial maxillectomy. 47 Moreover, the resection of the posterior third of nasal septum allows exposure of the nasopharyngeal portion of the lesion and enables easier use of the “4-hand 2-nostril” technique. In this way, the assistant helps the surgeon to maintain a proper cleavage plain by gentle traction on the lesion itself.
ITF dissection is achieved by incising the periosteum after removal of the posterior maxillary wall. It is noteworthy that the maxillary periosteum can be easily confused with the surface of the JA, leading the surgeon along the wrong surgical plane of dissection ( Fig. 2 ). Careful dissection and continuous gentle traction also allow to progressively pull out far lateral projections of JA that extend to the infratemporal/temporal fossa. To minimize morbidity, special attention must be paid to identify the maxillary nerve. The dissection plane into the PPF is posterior with respect to the palatine nerves; therefore, only in lesions with a small lateral extension can the nerves be preserved. Moreover, it is advisable to identify and clip the maxillary artery early, not only to reduce intraoperative bleeding but also to prevent its accidental damage during the procedure.
Extension to the nasopharynx commonly occurs following the submucosal plane. By eroding the pterygoid process of the sphenoid bone, JA can also invade the pterygoid fossa and the upper parapharyngeal space ( Fig. 5 ). In this case, the inferior turbinate and the medial wall of the maxillary sinus should be resected to the floor of the nasal cavity to optimize exposure.
Drilling of the basisphenoid and other bony areas involved by the lesion is recommended to remove microscopic nests of the lesion that may not be visible and prevent their regrowth. JA has the tendency to invade the vidian canal and basisphenoid, and extensive drilling of these areas is important to avoid recurrence 48 ( Fig. 6 ). Even if the vidian nerve is sacrificed to ensure a radical resection at the site of origin of the JA, patients rarely complain of dry eye.
Fig. 6.
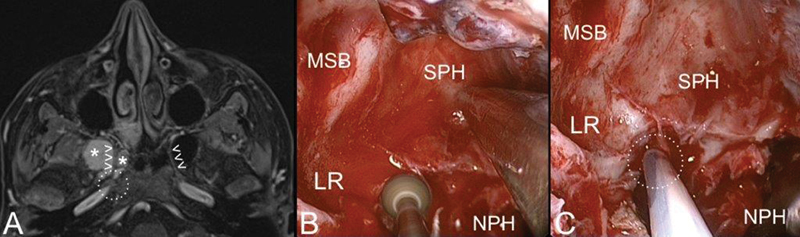
Axial contrast-enhanced T1-weighted magnetic resonance (MR) ( A ) and endoscopic view ( B, C ) of juvenile angiofibroma (white asterisks) extending along the right vidian canal (white arrowheads). After removal of the vidian portion of the lesion, the endoscopic procedure is completed by extensive drilling of the pterygoid root ( B ) till the foramen lacerum ( C ; dashed white circle). Abbreviations: LR, lateral recess; MSB, middle skull base; NPH, nasopharynx; SPH, sphenoid sinus.
As mentioned, cavernous sinus involvement can follow one of two pathways: (1) through the IOF, orbit, and then SOF; (2) following the maxillary nerve. Although at primary treatment, JA does not commonly show tight adhesions with adjacent neurovascular structures, whenever a portion of JA is in close contact with the cavernous sinus or ICA, it should be addressed as a last step after achieving wide exposure of the critical area and possibly resecting the nasal-nasopharyngeal part of the lesion. The use of intraoperative navigation and Doppler is mandatory to precisely identify the artery and avoid its injury ( Fig. 6 ). The dissection of JA from ICA should be cautiously performed with the help of small cottonoids to avoid any direct traumatic pressure on the vessel. Until the intervention is completed, the presence of an interventional radiologist in the hospital who can manage uncontrolled ICA bleeding is another precautionary measure that should be considered. One should keep in mind that residual lesions in critical areas such as the cavernous sinus may be followed rather than aggressively approached, as they can remain asymptomatic or even spontaneously regress. 25 41 49
Intracranial extension is present in 10 to 20% of advanced lesion; however, dural invasion is very rare 5 ( Fig. 4 ). This fact translates into the need to combine gentle movements of tumor traction and dissection from critical structures (i.e., ICA, cavernous sinus, dura) during skull base dissection and obviate the need for craniectomy and intradural dissection and complex skull base reconstruction techniques.
Identification of the posterior and inferior margin of the lesion within the nasopharynx at the end of the surgical procedure may be difficult due to several factors, including poor visualization in a bloody surgical field. The use of two rubber catheters passed transnasally to retract the palate anteriorly can be used to isolate the posteroinferior nasopharyngeal attachment of the JA and to effectively remove the mass from the mouth.
Light packing is placed in the nasal cavities for 24 to 48 hours and a third-generation cephalosporin is administered starting the day before surgery until nasal packing is removed. Cleaning of the surgical cavity is performed under endoscopic control to remove clots and fibrin, and the patient is instructed to perform daily irrigations of the sinonasal cavity with saline solution to moisten secretions and minimize crust formation.
Imaging surveillance after surgery is always required because persistences or recurrences are typically localized submucosally, and inaccessible to endoscopic evaluation. Moreover, postoperative MRI within 72 hours after surgery has shown to be effective in differentiating vascularized nodules of persistence in the early postoperative period. Imaging should be performed every 6 to 8 months for at least 3 years after surgery. Persistent JAs, either intentionally unresected due to unacceptable surgical hazard or detected by routine follow-up scans, require close surveillance with contrast-enhanced MRI to assess its possible growth before establishing that treatment is actually required.
Endoscopic versus External Approaches
External and endoscopic approaches seem to have comparable outcomes regarding tumor recurrence and residual tissue, and selecting an approach depends mainly on the surgeon's experience. Boghani et al 50 recently published a systematic review focusing on outcomes of endoscopic, endoscopic-assisted, and open surgical approaches. A total of 85 studies comprising 1,047 surgical cases were identified. When analyzing studies reporting on aggregate of all patients, they found that a purely endoscopic approach had significantly fewer recurrences or residual tissue compared with both endoscopic-assisted and open approaches. However, there was no difference in recurrence rate when they analyzed the individual patient data controlled for Radkowski/Sessions grading. Regarding blood loss, there was a significant difference between the endoscopic group (average of 544 mL) and the open group (average of 1579.5 mL). In the endoscopic cases, patients with preoperative embolization had significantly less blood loss (average of 406.7 mL) than nonembolized patients (average 828.3 mL).
Despite the advancements in endoscopic approaches, endoscopic treatment of advanced JA with extensive skull base and ITF involvement should only be practiced in highly specialized centers, with surgeons experienced in endoscopic approaches as well as adequate and dedicated equipment and support of interventional radiology, neurosurgery, and intensive care unit. External approaches should be considered in JAs with large invasion of the skull base, extensive vascular feeders from the ICA, or critical encasement of ICA. The possibility of a combined endoscopic and external approach should also be considered. Advanced JAs, especially those with residual vascularity from ICA after embolization, can show massive intraoperative bleeding with a considerable increase in surgical risk and need for intraoperative transfusion. In such a situation, it is wise to consider a multistage treatment, a possibility that should be preoperatively discussed with the patient. Another strategy for very advanced JAs with critical intracranial extension and unacceptable surgical hazard is to endoscopically resect the extracranial portion intentionally leaving residual disease and subsequently evaluate for “wait and see” monitoring or surgical treatment based on the rate of growth as demonstrated radiologically. The management of residual tumors involving critical areas or neurovascular structures (i.e., ICA, optic nerve, cavernous sinus, dura, cerebral arteries) should always be carefully discussed considering the need for external approaches due to the impact that adhesions could have on the possibility to perform a safe dissection.
The Role of Radiotherapy
External beam radiation has been shown to be effective as primary treatment as well as postsurgical treatment for residual lesions, and most series report 80 to 85% local control rates (complete resolution or asymptomatic residual disease), although tumor involution may take up to 3 years 51 52 53 54 55 56 (57–62). The radiation dose used was variable, ranging between 3,000 and 5,500 cGy (more commonly 3,000–3,600 cGy). Despite the efficacy of radiotherapy, a high rate of side effects as well as concern about radiation-induced malignancy has limited its role. To minimize the adverse effects of radiotherapy, more recently conformal radiotherapy has been applied. Chakraborty et al 57 reported on eight patients who were deemed inoperable owing to extensive intracranial/intraorbital extension or proximity to optic nerve. They were treated by intensity-modulated radiotherapy (IMRT) with a median dose of 39.6 (30–46) Gy. Local control at 2 years was 87.5%. Although one patient died1 month after therapy due to massive epistaxis, no long-term complications were found other than chronic rhinitis. Stereotactic radiosurgery using gamma knife was first introduced by Dare et al 58 who successfully treated two patients for residual tumor after surgery. Álvarez et al 59 reported on 10 cases of advanced JNA (Andrews et al 7 stage IV) who were treated by a multimodal approach including surgery and radiosurgery for residual tumor. They did not encounter remarkable adverse effects.
Radiotherapy may have a role for residual or recurrence lesions involving the critical neurovascular structures of the skull base which are not suitable for surgical excision. In our experience, radiotherapy has a very limited role in the management of JA. As mentioned previously, a recent retrospective multicenter study on the management of 74 advanced JAs that were treated endoscopically, only 2 of 18 cases with residual tumor received radiotherapy. 46 It is important to differentiate true recurrent symptomatic lesions from asymptomatic, nongrowing residual tissue. Small tumor remnants or tissue enhancement detected on MRI in asymptomatic patients may undergo involution after time. 25 49 60 Therefore, further intervention, whether surgical or by radiation, should be carefully indicated for symptomatic and growing lesions only.
References
- 1.Lund V J, Stammberger H, Nicolai P et al. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl. 2010;22:1–143. [PubMed] [Google Scholar]
- 2.Antonelli A R, Cappiello J, Di Lorenzo D, Donajo C A, Nicolai P, Orlandini A. Diagnosis, staging, and treatment of juvenile nasopharyngeal angiofibroma (JNA) Laryngoscope. 1987;97(11):1319–1325. doi: 10.1288/00005537-198711000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Szymańska A, Szymański M, Czekajska-Chehab E, Szczerbo-Trojanowska M. Two types of lateral extension in juvenile nasopharyngeal angiofibroma: diagnostic and therapeutic management. Eur Arch Otorhinolaryngol. 2015;272(01):159–166. doi: 10.1007/s00405-014-2965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanation A M, Mitchell C A, Rose A S.Endoscopic skull base techniques for juvenile nasopharyngeal angiofibroma Otolaryngol Clin North Am 20124503711–730., ix ix . [DOI] [PubMed] [Google Scholar]
- 5.Danesi G, Panciera D T, Harvey R J, Agostinis C. Juvenile nasopharyngeal angiofibroma: evaluation and surgical management of advanced disease. Otolaryngol Head Neck Surg. 2008;138(05):581–586. doi: 10.1016/j.otohns.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Sessions R B, Bryan R N, Naclerio R M, Alford B R. Radiographic staging of juvenile angiofibroma. Head Neck Surg. 1981;3(04):279–283. doi: 10.1002/hed.2890030404. [DOI] [PubMed] [Google Scholar]
- 7.Andrews J C, Fisch U, Valavanis A, Aeppli U, Makek M S. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope. 1989;99(04):429–437. doi: 10.1288/00005537-198904000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Radkowski D, McGill T, Healy G B, Ohlms L, Jones D T. Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg. 1996;122(02):122–129. doi: 10.1001/archotol.1996.01890140012004. [DOI] [PubMed] [Google Scholar]
- 9.Onerci M, Oğretmenoğlu O, Yücel T. Juvenile nasopharyngeal angiofibroma: a revised staging system. Rhinology. 2006;44(01):39–45. [PubMed] [Google Scholar]
- 10.Snyderman C H, Pant H, Carrau R L, Gardner P. A new endoscopic staging system for angiofibromas. Arch Otolaryngol Head Neck Surg. 2010;136(06):588–594. doi: 10.1001/archoto.2010.83. [DOI] [PubMed] [Google Scholar]
- 11.Snyderman C H, Pant H. Endoscopic management of vascular sinonasal tumors, including angiofibroma. Otolaryngol Clin North Am. 2016;49(03):791–807. doi: 10.1016/j.otc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 12.El Sharkawy A A. Endonasal endoscopic management of juvenile nasopharyngeal angiofibroma without angiographic embolization. Eur Arch Otorhinolaryngol. 2013;270(07):2051–2055. doi: 10.1007/s00405-012-2315-x. [DOI] [PubMed] [Google Scholar]
- 13.El-Banhawy O A, Ragab A, El-Sharnoby M M. Surgical resection of type III juvenile angiofibroma without preoperative embolization. Int J Pediatr Otorhinolaryngol. 2006;70(10):1715–1723. doi: 10.1016/j.ijporl.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Nicolai P, Villaret A B, Farina D et al. Endoscopic surgery for juvenile angiofibroma: a critical review of indications after 46 cases. Am J Rhinol Allergy. 2010;24(02):e67–e72. doi: 10.2500/ajra.2010.24.3443. [DOI] [PubMed] [Google Scholar]
- 15.Hackman T, Snyderman C H, Carrau R, Vescan A, Kassam A. Juvenile nasopharyngeal angiofibroma: the expanded endonasal approach. Am J Rhinol Allergy. 2009;23(01):95–99. doi: 10.2500/ajra.2009.23.3271. [DOI] [PubMed] [Google Scholar]
- 16.Roger G, Tran Ba Huy P, Froehlich P et al. Exclusively endoscopic removal of juvenile nasopharyngeal angiofibroma: trends and limits. Arch Otolaryngol Head Neck Surg. 2002;128(08):928–935. doi: 10.1001/archotol.128.8.928. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Franco J A, Lee A, Campos-Navarro L A, Tenorio-Sánchez J, Zenteno M, Osorio-Alvarado A R. Bilateral non-superselective embolization with particles under transient occlusion of the internal carotid artery in the management of juvenile nasopharyngeal angiofibroma: technical note. Vasc Endovascular Surg. 2012;46(07):559–564. doi: 10.1177/1538574412456436. [DOI] [PubMed] [Google Scholar]
- 18.Lv M M, Fan X D, Su L X, Chen D. Preoperative direct puncture embolization of advanced juvenile nasopharyngeal angiofibroma in combination with transarterial embolization: an analysis of 22 consecutive patients. Cardiovasc Intervent Radiol. 2013;36(01):111–117. doi: 10.1007/s00270-012-0404-2. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann M, Ulrich S, Reineke U, Hamberger U, Dietrich U, Sudhoff H. Intratumoral Onyx embolisation in the management of juvenile nasopharyngeal angiofibroma [in German] HNO. 2010;58(08):853–857. doi: 10.1007/s00106-010-2146-2. [DOI] [PubMed] [Google Scholar]
- 20.Hira A, Chao K. Direct endoscopic intratumoral injection of Onyx for the preoperative embolization of a recurrent juvenile nasal angiofibroma. Interv Neuroradiol. 2011;17(04):477–481. doi: 10.1177/159101991101700413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman B, Bublik M, Ruiz J, Younis R. Endoscopic embolization with onyx prior to resection of JNA: a new approach. Int J Pediatr Otorhinolaryngol. 2011;75(01):53–56. doi: 10.1016/j.ijporl.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Aziz-Sultan M A, Moftakhar R, Wolfe S Q, Elhammady M S, Herman B, Farhat H. Endoscopically assisted intratumoral embolization of juvenile nasopharyngeal angiofibroma using Onyx. J Neurosurg Pediatr. 2011;7(06):600–603. doi: 10.3171/2011.2.PEDS09442. [DOI] [PubMed] [Google Scholar]
- 23.Mishra A, Mishra S C, Verma V et al. In defence of transpalatal, transpalatal-circumaxillary (transpterygopalatine) and transpalatal-circumaxillary-sublabial approaches to lateral extensions of juvenile nasopharyngeal angiofibroma. J Laryngol Otol. 2016;130(05):462–473. doi: 10.1017/S0022215116000773. [DOI] [PubMed] [Google Scholar]
- 24.Fisch U. The infratemporal fossa approach for nasopharyngeal tumors. Laryngoscope. 1983;93(01):36–44. doi: 10.1288/00005537-198301000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Herman P, Lot G, Chapot R, Salvan D, Huy P T. Long-term follow-up of juvenile nasopharyngeal angiofibromas: analysis of recurrences. Laryngoscope. 1999;109(01):140–147. doi: 10.1097/00005537-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Pryor S G, Moore E J, Kasperbauer J L. Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma. Laryngoscope. 2005;115(07):1201–1207. doi: 10.1097/01.MLG.0000162655.96247.66. [DOI] [PubMed] [Google Scholar]
- 27.Kamel R H. Transnasal endoscopic surgery in juvenile nasopharyngeal angiofibroma. J Laryngol Otol. 1996;110(10):962–968. doi: 10.1017/s0022215100135467. [DOI] [PubMed] [Google Scholar]
- 28.Fagan J J, Snyderman C H, Carrau R L, Janecka I P. Nasopharyngeal angiofibromas: selecting a surgical approach. Head Neck. 1997;19(05):391–399. doi: 10.1002/(sici)1097-0347(199708)19:5<391::aid-hed5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Zicot A F, Daele J. Endoscopic surgery for nasal and sinusal vascular tumours: about two cases of nasopharyngeal angiofibromas and one case of turbinate angioma. Acta Otorhinolaryngol Belg. 1996;50(03):177–182. [PubMed] [Google Scholar]
- 30.Tseng H Z, Chao W Y. Transnasal endoscopic approach for juvenile nasopharyngeal angiofibroma. Am J Otolaryngol. 1997;18(02):151–154. doi: 10.1016/s0196-0709(97)90107-1. [DOI] [PubMed] [Google Scholar]
- 31.Mitskavich M T, Carrau R L, Snyderman C H, Weissman J L, Fagan J J. Intranasal endoscopic excision of a juvenile angiofibroma. Auris Nasus Larynx. 1998;25(01):39–44. doi: 10.1016/s0385-8146(97)10006-2. [DOI] [PubMed] [Google Scholar]
- 32.Bernal-Sprekelsen M, Vázquez A A, Pueyo J, Carbonell Casasús J. Endoscopic resection of juvenile nasopharyngeal fibromas [in German] HNO. 1998;46(02):172–174. doi: 10.1007/s001060050217. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, Kawasaki M, Higuchi Y, Seki S, Takahashi S. Transnasal endoscopic resection of juvenile nasopharyngeal angiofibroma with KTP laser. Eur Arch Otorhinolaryngol. 1999;256(04):212–214. doi: 10.1007/s004050050143. [DOI] [PubMed] [Google Scholar]
- 34.Schick B, el Rahman el Tahan A, Brors D, Kahle G, Draf W. Experiences with endonasal surgery in angiofibroma. Rhinology. 1999;37(02):80–85. [PubMed] [Google Scholar]
- 35.Newlands S D, Weymuller E A., Jr Endoscopic treatment of juvenile nasopharyngeal angiofibroma. Am J Rhinol. 1999;13(03):213–219. doi: 10.2500/105065899781389812. [DOI] [PubMed] [Google Scholar]
- 36.Jorissen M, Eloy P, Rombaux P, Bachert C, Daele J. Endoscopic sinus surgery for juvenile nasopharyngeal angiofibroma. Acta Otorhinolaryngol Belg. 2000;54(02):201–219. [PubMed] [Google Scholar]
- 37.Khalifa M A. Endonasal endoscopic surgery for nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg. 2001;124(03):336–337. doi: 10.1067/mhn.2001.113510. [DOI] [PubMed] [Google Scholar]
- 38.Carrau R L, Snyderman C H, Kassam A B, Jungreis C A. Endoscopic and endoscopic-assisted surgery for juvenile angiofibroma. Laryngoscope. 2001;111(03):483–487. doi: 10.1097/00005537-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Ungkanont K, Byers R M, Weber R S, Callender D L, Wolf P F, Goepfert H. Juvenile nasopharyngeal angiofibroma: an update of therapeutic management. Head Neck. 1996;18(01):60–66. doi: 10.1002/(SICI)1097-0347(199601/02)18:1<60::AID-HED8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Nicolai P, Berlucchi M, Tomenzoli D et al. Endoscopic surgery for juvenile angiofibroma: when and how. Laryngoscope. 2003;113(05):775–782. doi: 10.1097/00005537-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Onerci T M, Yücel O T, Oğretmenoğlu O. Endoscopic surgery in treatment of juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol. 2003;67(11):1219–1225. doi: 10.1016/j.ijporl.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Wormald P J, Van Hasselt A. Endoscopic removal of juvenile angiofibromas. Otolaryngol Head Neck Surg. 2003;129(06):684–691. doi: 10.1016/s0194-5998(03)01580-8. [DOI] [PubMed] [Google Scholar]
- 43.Pasquini E, Sciarretta V, Frank G et al. Endoscopic treatment of benign tumors of the nose and paranasal sinuses. Otolaryngol Head Neck Surg. 2004;131(03):180–186. doi: 10.1016/j.otohns.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Cloutier T, Pons Y, Blancal J P et al. Juvenile nasopharyngeal angiofibroma: does the external approach still make sense? Otolaryngol Head Neck Surg. 2012;147(05):958–963. doi: 10.1177/0194599812454394. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Liu Z, Wang J, Sun X, Yang L, Wang D. Surgical management of juvenile nasopharyngeal angiofibroma: analysis of 162 cases from 1995 to 2012. Laryngoscope. 2014;124(08):1942–1946. doi: 10.1002/lary.24522. [DOI] [PubMed] [Google Scholar]
- 46.Langdon C, Herman P, Verillaud B et al. Expanded endoscopic endonasal surgery for advanced stage juvenile angiofibromas: a retrospective multi-center study. Rhinology. 2016;54(03):239–246. doi: 10.4193/Rhino15.104. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber A, Ferrari M, Rampinelli V et al. Modular endoscopic medial maxillectomies: quantitative analysis of surgical exposure in a preclinical setting. World Neurosurg. 2017;100:44–55. doi: 10.1016/j.wneu.2016.12.094. [DOI] [PubMed] [Google Scholar]
- 48.Howard D J, Lloyd G, Lund V. Recurrence and its avoidance in juvenile angiofibroma. Laryngoscope. 2001;111(09):1509–1511. doi: 10.1097/00005537-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Jones G C, DeSanto L W, Bremer J W, Neel H B., III Juvenile angiofibromas. Behavior and treatment of extensive and residual tumors. Arch Otolaryngol Head Neck Surg. 1986;112(11):1191–1193. doi: 10.1001/archotol.1986.03780110067009. [DOI] [PubMed] [Google Scholar]
- 50.Boghani Z, Husain Q, Kanumuri V V et al. Juvenile nasopharyngeal angiofibroma: a systematic review and comparison of endoscopic, endoscopic-assisted, and open resection in 1047 cases. Laryngoscope. 2013;123(04):859–869. doi: 10.1002/lary.23843. [DOI] [PubMed] [Google Scholar]
- 51.Cummings B J, Blend R, Keane Tet al. Primary radiation therapy for juvenile nasopharyngeal angiofibroma Laryngoscope 198494(12 Pt 1):1599–1605. [PubMed] [Google Scholar]
- 52.Lee J T, Chen P, Safa A, Juillard G, Calcaterra T C.The role of radiation in the treatment of advanced juvenile angiofibroma Laryngoscope 2002112(7 Pt 1):1213–1220. [DOI] [PubMed] [Google Scholar]
- 53.Fields J N, Halverson K J, Devineni V R, Simpson J R, Perez C A. Juvenile nasopharyngeal angiofibroma: efficacy of radiation therapy. Radiology. 1990;176(01):263–265. doi: 10.1148/radiology.176.1.2162070. [DOI] [PubMed] [Google Scholar]
- 54.Reddy K A, Mendenhall W M, Amdur R J, Stringer S P, Cassisi N J. Long-term results of radiation therapy for juvenile nasopharyngeal angiofibroma. Am J Otolaryngol. 2001;22(03):172–175. doi: 10.1053/ajot.2001.23458. [DOI] [PubMed] [Google Scholar]
- 55.McAfee W J, Morris C G, Amdur R J, Werning J W, Mendenhall W M. Definitive radiotherapy for juvenile nasopharyngeal angiofibroma. Am J Clin Oncol. 2006;29(02):168–170. doi: 10.1097/01.coc.0000203759.94019.76. [DOI] [PubMed] [Google Scholar]
- 56.Amdur R J, Yeung A R, Fitzgerald B M, Mancuso A A, Werning J W, Mendenhall W M. Radiotherapy for juvenile nasopharyngeal angiofibroma. Pract Radiat Oncol. 2011;1(04):271–278. doi: 10.1016/j.prro.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Chakraborty S, Ghoshal S, Patil V M, Oinam A S, Sharma S C. Conformal radiotherapy in the treatment of advanced juvenile nasopharyngeal angiofibroma with intracranial extension: an institutional experience. Int J Radiat Oncol Biol Phys. 2011;80(05):1398–1404. doi: 10.1016/j.ijrobp.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 58.Dare A O, Gibbons K J, Proulx G M, Fenstermaker R A.Resection followed by radiosurgery for advanced juvenile nasopharyngeal angiofibroma: report of two cases Neurosurgery 200352051207–1211., discussion 1211 [PubMed] [Google Scholar]
- 59.Álvarez F L, Suárez V, Suárez C, Llorente J L. Multimodality approach for advanced-stage juvenile nasopharyngeal angiofibromas. Head Neck. 2013;35(02):209–213. doi: 10.1002/hed.22947. [DOI] [PubMed] [Google Scholar]
- 60.Glad H, Vainer B, Buchwald C et al. Juvenile nasopharyngeal angiofibromas in Denmark 1981-2003: diagnosis, incidence, and treatment. Acta Otolaryngol. 2007;127(03):292–299. doi: 10.1080/00016480600818138. [DOI] [PubMed] [Google Scholar]


