Abstract
Head and neck rhabdomyosarcoma (HNRMS) is a uniquely challenging site to treat given the young patient age and critical anatomy of the head and neck region. We review the characteristics, management, and future directions in the treatment of HNRMS. Most patients who present with HNRMS have unresectable disease due to functional and/or cosmetic constraints. However, surgical resection and brachytherapy serve a critical role in select patients. The treatment paradigm for the majority of patients with HNRMS consists of definitive chemotherapy and radiation therapy. As the incidence of late toxicities increases with improved survival, modern efforts must focus on ways to decrease long-term morbidity. We recommend a multimodal approach emphasizing the preservation of form and function for the treatment of HNRMS.
Keywords: pediatric rhabdomyosarcoma, head and neck neoplasms, radiotherapy, brachytherapy
Introduction
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma in children, with 350 cases annually in the United States. As multimodality therapy for RMS has improved over the past four decades, survival has also significantly improved from 25% in 1970 to >70% by the late 1990s. 1 2 3 The treatment approach for RMS is complex and depends on a variety of prognostic factors, including histology, extent of resection, and primary tumor site. Although RMS can arise from anywhere in the body, it has a predilection for certain locations including the head and neck (36%), genitourinary organs (24%), and extremities (19%). 4
Head and neck RMS (HNRMS) is divided into three anatomic subsites based on prognosis and location: parameningeal, orbital, and other head and neck ( Fig. 1 ). Parameningeal RMS comprise 16% of all of cases and include those tumors specifically arising from the nasal cavity/nasopharynx, paranasal sinuses, parapharyngeal space, pterygopalatine/infratemporal fossa, and mastoid/middle ear. Orbital RMS comprise 10% of all cases, and nonparameningeal HNRMS comprise an additional 10%, including those tumors arising from the face, oral cavity, cheek, external ear, scalp, neck, and larynx. In general, parameningeal RMS commonly presents with locally advanced disease and has a poor prognosis, with local failure the dominant form of relapse. 5 However, orbital and nonparameningeal HNRMS are associated with a more favorable prognosis. Given the young age at which these patients are treated and the many surrounding critical structures, HNRMS remains a uniquely challenging disease to treat. Here, we review the characteristics, management, and future directions in the treatment of HNRMS.
Fig. 1.
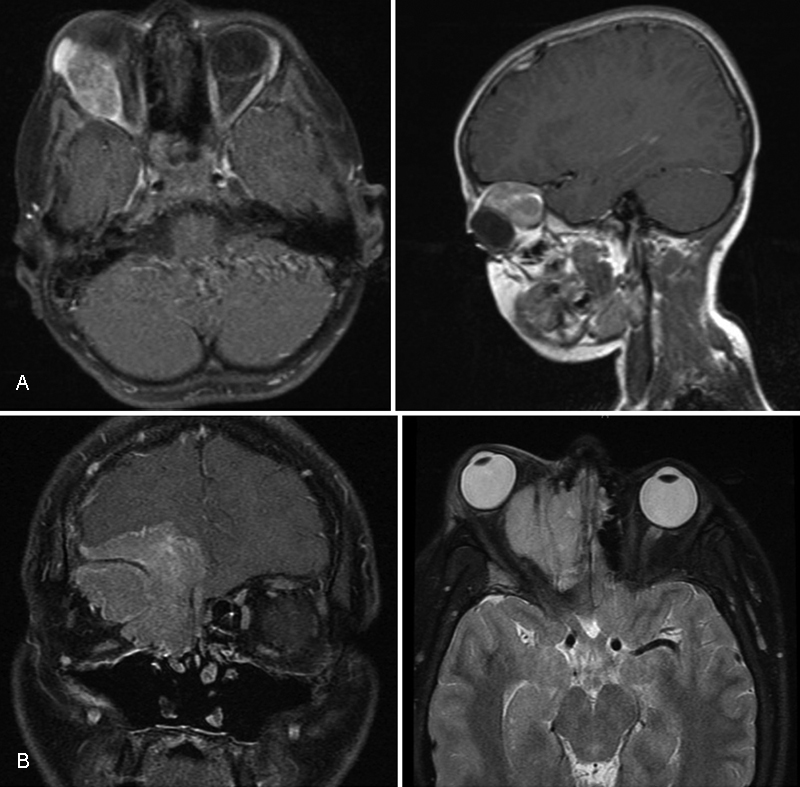
Representative images highlighting the difference between ( A ) orbital rhabdomyosarcoma and ( B ) parameningeal rhabdomyosarcoma.
Histology
RMS is a small round blue cell tumor that arises from undifferentiated skeletal tissue. Until recently, RMS was divided prognostically into two main histologic subtypes: embryonal and alveolar. Embryonal RMS, which comprises 60% of cases, typically occurs in younger patients, is most commonly located in the head and neck and genitourinary system, and has classically been associated with a more favorable prognosis than alveolar histology. Embryonal RMS can be characterized by a loss of heterozygosity on the short arm of chromosome 11(11p15.5), encoding for IGF II.
Alveolar RMS tends to occur in older children and adolescents and has been classically associated with a worse prognosis. It can be characterized by the PAX/FKHR translocation between chromosome 2;13 or less commonly 1;13. 6 Among patients with alveolar histology, fusion-negative patients (representing ∼20% of cases) tend to do better, with a similar prognosis to patients with embryonal histology. 7 8 In fact, on the Children's Oncology Group (COG) trial D9803, there was a trend toward improved event-free survival (EFS) among patients with fusion-negative alveolar RMS when compared with those with embryonal RMS (EFS 90% versus 77%). These findings suggest that fusion status may be more important prognostically than histology. As such, the current COG clinical trials now account for fusion status in addition to histology for risk stratification.
Risk Stratification
To guide management, a risk stratification system for RMS was developed based on stage, clinical group, and histology ( Table 1 ). Stage is based on the anatomic site (favorable versus unfavorable), tumor size, nodal involvement, and presence or absence of distant metastases. For HNRMS, parameningeal RMS is considered an unfavorable site, while nonparameningeal and orbital RMS are considered favorable. Clinical grouping is based on the degree of surgical resection and nodal involvement. Of note, the majority of patients with HNRMS are classified as group III given the difficulty and potential morbidity associated with obtaining a gross total resection in the head and neck. In fact, on International Rhabdomyosarcoma Study II–IV (IRS II–IV), 95% of patients with parameningeal tumors presented with group III disease. 9 In addition, ∼50% of patients with parameningeal disease present with regional disease at diagnosis. 10 11
Table 1. The intergroup rhabdomyosarcoma study.
| (a) Clinical grouping system | |||
| Group I | Localized disease, completed resection | ||
| Group II | Positive microscopic margins or resected regional disease | ||
| Group III | Incomplete resection or biopsy, with gross residual disease | ||
| Group IV | Distant metastatic disease present at onset | ||
| (b) Staging system | |||
| Stage | Site | Size | Regional and distant metastases |
| 1 | Favorable sites [orbit, non-PM head and neck, genitourinary (non-BP), biliary tract] | All | N0 or N1 |
| 2 | Unfavorable sites [BP, extremity, PM, other] | <5 cm | N0 |
| 3 | Unfavorable sites [BP, extremity, PM, other] | <5 cm >5 cm |
N1 N0 or N1 |
| 4 | Any | Any | M1 |
| (c) Risk groups | |||
| Risk group | Histology | Stage | Clinical group |
| Low risk | Embryonal | 1 | I, II, III |
| Embryonal | 2, 3 | I, II | |
| Intermediate risk | Embryonal | 2, 3 | III |
| Alveolar | 1, 2, 3 | I, II, III | |
| High risk | Embryonal | 4 | IV |
| Alveolar | 4 | IV | |
Abbreviations: BM, bladder/prostate; PM, parameningeal.
Taking into account stage, grade, and histology, low-risk RMS includes localized disease of embryonal histology at a favorable site (such as the orbit) or localized disease of embryonal histology at an unfavorable site that has been grossly resected. The intermediate-risk group includes patients with localized disease of embryonal histology at an unfavorable site with gross residual disease, as well as all patients with localized disease with alveolar histology (regardless of the site or extent of resection). The high-risk group includes patients with metastatic disease.
Treatment
Since 1972, the International Rhabdomyosarcoma Study Group (IRSG) has led protocols that have helped to define the evolving treatment paradigms for RMS. Multimodality therapy for RMS is risk-adapted and consists of systemic therapy with either surgery and/or radiation therapy (RT). RMS is both very chemo- and radiosensitive. In contrast to adult sarcomas where surgical resection is often necessary for local control, chemotherapy with definitive RT for local control can successfully treat RMS. For HNRMS specifically, given the critical surrounding structures and efforts to preserve form and function, RT may often be preferred over attempts at surgical resection.
Chemotherapy
Multiagent chemotherapy utilized for RMS typically consists of a combination of vincristine, dactinomycin, and cyclophosphamide (VAC), ideally given on a clinical trial. Different variations and dosing of these agents are recommended for the different risk groups and continue to be explored on the COG protocols. 12
Surgical Resection
Given the anatomical constraints of the head and neck and the often infiltrative nature of HNRMS, gross total resection may be difficult to achieve without resulting in significant loss of form and function. 13 For these reasons, surgery is most commonly limited to an initial biopsy for HNRMS. If surgical resection is performed, given the technical difficulties of operating in the head and neck in young children, a multidisciplinary team consisting of otolaryngologists, neurosurgeons, maxillofacial, and plastic surgeons must be involved. Surgical access should take into account balancing adequate visualization and removal of the tumor with potential cosmetic deficits and morbidity. After surgical resection, the most common adverse events are cranial nerve paralysis (most commonly the trigeminal and facial nerves), cosmetic defects, and decreased motility of the temporomandibular joint. 14
Daya et al described the role of definitive surgery for nonorbital HNRMS. In this study, of the 48 patients with HNRMS, only 11 underwent surgical resection as definitive therapy. The cheek and parotid region were the most common locations amenable to resection. Of the 11 patients with tumors that were amenable to resection, 5 achieved complete resection (IRS group I) and were able to avoid RT. 15 Of note, patients with orbital tumors have very low rates of local failure (<5%) and excellent survival outcomes after chemoradiotherapy alone. As such, orbital exenteration is rarely if ever indicated as the primary treatment approach for orbital RMS.
Surgical resection after induction chemotherapy in RMS (also called delayed primary excision, DPE) was explored on COG D9803. In an attempt to potentially reduce the dose of RT given to patients with intermediate-risk RMS whose tumors were unresectable at diagnosis, select patients were treated with induction chemotherapy followed by DPE prior to RT. Those who achieved gross total resection at the time of DPE were then eligible for reduced dose RT with 36 to 41.4 Gy (decreased from 50.4 Gy). Local control following DPE and reduced dose RT was similar to historic results after higher doses of definitive RT. However, only tumors at select anatomic sites including the bladder dome, extremity, and trunk were considered for DPE on this study. 16 The current COG intermediate risk study, ARST1431 (NCT02567435), allows DPE for nonparameningeal head and neck sites.
While upfront resection of skull-based RMS is limited by anatomic and functional constraints, surgical salvage is critical for survival in patients who relapse locally after definitive chemoradiation. 17 As described in more detail below, a multidisciplinary approach utilized in the Netherlands involving surgical resection, brachytherapy, and reconstruction called the AMORE protocol has been successfully utilized as salvage therapy in previously irradiated patients with HNRMS. 18
Brachytherapy
Brachytherapy is a highly conformal form of localized RT that allows the delivery of high-dose radiation intraoperatively directly at the tumor site. Brachytherapy can be used for HNRMS in the upfront setting to reduce the dose of external beam RT (EBRT) required, as part of a multidisciplinary approach with surgical resection, and in the salvage setting after local recurrence. 19 The main advantage of brachytherapy is the rapid dose falloff with distance that permits the delivery of a high dose of radiation to the target with relative sparing of nearby normal tissues. For example, a separation of just 8mm results in a 50% reduction in dose when using iridium 192 high-dose brachytherapy. 20 See Fig. 2 for an example of a brachytherapy radiation treatment plan versus an external beam treatment plan for RMS of the external auditory canal. 21
Fig. 2.
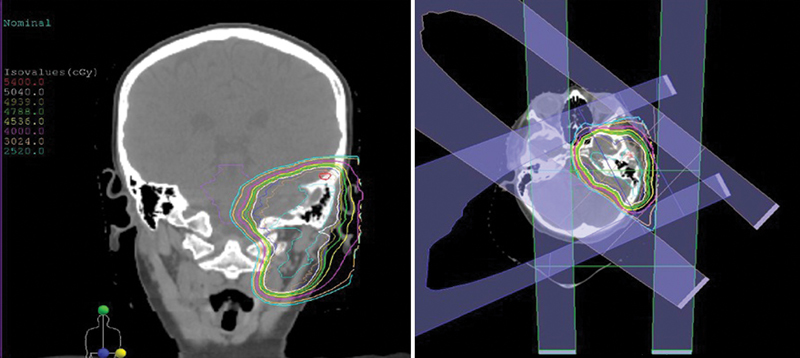
Example of a conformal treatment plan for a patient with head and neck rhabdomyosarcoma treated with proton therapy.
Brachytherapy has been used as part of a multidisciplinary treatment approach for the treatment of nonorbital HNRMS in the Netherlands since 1990. This approach, named the AMORE technique, involves consecutive Ablative Surgery, MOld technique with afterloading brachytherapy and immediate surgical REconstruction after induction chemotherapy. Specifically, patients undergo macroscopic radical tumor resection, followed by custom mold creation and placement based on the surgical defect, followed by brachytherapy with the mold in place, and finally surgical reconstruction. 22 A team consisting of head and neck surgeons, plastic surgeons, radiologists, radiation oncologists, physicists, pediatric oncologists, and pathologists is needed for successful execution of this approach. In addition, AMORE is only utilized for select cases where a nonmutilating resection is feasible. 23 Blank et al described the outcomes of 42 patients with nonorbital HNRMS (29 parameningeal, 13 nonparameningeal) treated on this protocol. Of the 42 patients, 31 were treated in the upfront setting as their primary treatment, and 9 of these 31 (29%) relapsed locally. 22 The other 11 patients were treated in the salvage setting. The 5-year overall survival was 70% for the primary treatment group (65% for patients with parameningeal disease and 90% for those with nonparameningeal disease).
External Beam Radiation Therapy
Given the difficulties of obtaining a gross total resection, EBRT plays a critical role in the management of the majority of patients with HNRMS. The dose and timing of RT depend on the histology, site of the primary tumor, nodal involvement, and extent of resection. All patients with clinical group II–IV RMS receive EBRT. In addition, all patients with alveolar RMS require EBRT, regardless of the extent of resection. This was exemplified on IRS I and II, where the 10-year overall survival of patients with alveolar or undifferentiated RMS who received adjuvant RT after complete resection was 82% compared with 52% in those who did not receive adjuvant RT. 24 The only group that RT is not recommended for includes those patients with embryonal histology who undergo complete resection (group I).
For patients with embryonal histology and microscopic residual disease (group II), a dose of 36 Gy is currently recommended. The same dose is recommended for patients with alveolar histology who undergo complete resection. 25 A dose of 50.4 Gy is utilized for the treatment of all gross disease. Dose escalation for gross disease beyond 50.4 Gy to 59.4 Gy with hyperfractionation was tested in a randomized fashion on IRS-IV and did not result in improved local control for any tumor sites. 26 For orbital tumors specifically, dose reduction with 45 Gy was tested on D9602, resulting in a local failure rate of 16% (compared with 5% seen on IRS IV), and a 5-year failure-free survival and overall survival of 86% and 96%, respectively. 27 A dose of 45 Gy was shown to result in unacceptable rates of local control for orbital RMS again on ARST0331 in those without a complete response to chemotherapy. Currently, it is recommended that all orbital tumors be treated with similar doses as other primary sites with 36 Gy to the prechemotherapy volume and a boost to 50.4Gy to any residual disease after week 12. Regarding timing of RT, it is typically given at week 13 of chemotherapy for intermediate-risk RMS and at week 20 for high-risk RMS.
Over the last two decades, radiation techniques have drastically evolved from three-dimensional conformal radiation therapy (3D-CRT) to intensity-modulated radiation therapy (IMRT) and proton therapy in an attempt to decrease dose to the surrounding normal tissues. IMRT improved target dose coverage compared with 3D-CRT on protocol D9803 and has been found to result in outstanding local control despite using a reduced margin. 28 There is promise that proton therapy may be able to further decrease dose to normal structures and consequently late effects from RT. Protons are a highly conformal form of RT that exhibit a rapid fall off in dose after depositing their energy at the target. Dosimetric analyses have shown that proton therapy can provide greater sparing of ipsilateral and contralateral structures in the head and neck. 29 See Fig. 3 for a proton plan for a patient with HNRMS.
Fig. 3.
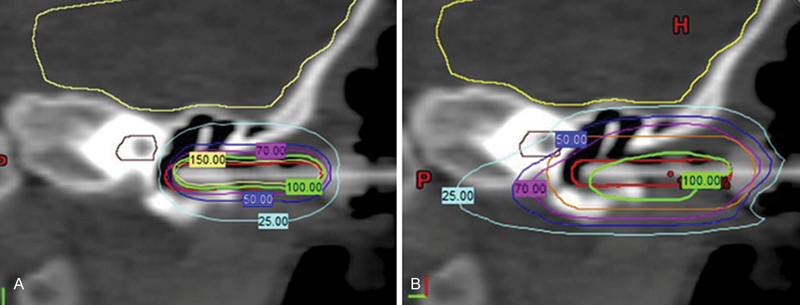
Treatment planning images for a patient with rhabdomyosarcoma of the external auditory canal utilizing ( A ) high-dose rate brachytherapy versus ( B ) intensity-modulated radiation therapy.
Of note, in Europe, attempts are made to avoid RT by giving more second or third line chemotherapy before proceeding with definitive RT or surgery. However, despite being able to avoid RT in some patients, it is important to note that the local control and survival outcomes for patients treated this way are lower than what is seen in the United States where RT is routinely used. 30 31 For example, in a pooled analysis including 1105 patients with parameningeal disease treated on North American and European cooperative group trials from 1984 to 2004, overall survival in those who received RT was 68.5% compared with 40.8% in those who did not. 32 In addition, for orbital RMS specifically, the SIOP MMT 89 study used upfront chemotherapy followed by second-line chemotherapy for poor responders, with the goal of avoiding the effects of RT and/or surgery. However, on this trial, EFS was 53%, much lower than the 89% failure-free survival seen on IRS IV. 33
Outcomes
Specific local control and survival outcomes for HNRMS depend on the anatomic subsite. On IRS IV and D9803, local failure for patients with parameningeal RMS was 16% and 19%, respectively. The 5-year failure-free and overall survival for patients with parameningeal disease treated on IRS II–IV were 69% and 73%, respectively. As described above, prognosis for orbital RMS is excellent, with a local failure rate of 5% on IRS IV and a 3-year failure-free and overall survival of 89% and 100%, respectively. 26 For nonparameningeal HNRMS treated on IRS III and IV, local failure was 19% (9% on IRS IV and 20% on IRS III). The failure-free and overall survival were 76% and 83%, respectively. 34 35
Late Effects
As with all pediatric tumors, it is critical to minimize risks of long-term toxic effects without compromising survival outcomes. Late effects are especially important to consider for HNRMS, where children are often <10 years old and the tumors are surrounded by several critical organs such as the brain, inner ears, optical apparatus, salivary glands, pituitary gland, growing facial bones, and developing teeth. Long-term toxicities include growth abnormalities, cosmetic defects such as facial hypoplasia, endocrine deficiencies, impaired vision, decreased auditory acuity, cataract formation, retinopathy, dental complications, and second cancers. 36 37
IMRT, which allows for the sparing of more normal tissue structures than 3D-CRT, is now commonly used for the treatment of HNRMS. Lockney et al described late toxicities after IMRT for 30 patients with HNRMS treated at Memorial Sloan Kettering. 38 With a median follow-up of 7.7 years, the most common late toxicity observed was facial disfigurement, seen in 77% of patients. Children treated at younger ages (median 6.0 years) and those with infratemporal fossa tumors were more likely to develop severe facial deformity. Other common late effects seen after IMRT in this cohort included growth hormone deficiency (37%), cataracts (34%), and dental problems (33%). There were no secondary solid neoplasms. Regarding the AMORE technique, a recent analysis compared late effects after treatment of HNRMS with AMORE versus EBRT. After a median follow-up of 10.5 years, those who were treated on the AMORE protocol had less late grade 3 and 4 adverse events than those who received EBRT (53% versus 77%). 39 However, the RT techniques utilized in many of the patients in the EBRT group are now considered historical by current standards.
Protons provide further hope in sparing normal tissue and subsequently decreasing the incidence late effects. A phase II study from MGH using protons showed that after a median follow-up 47 months, among 57 patients with RMS, late toxicity of any grade was seen in 15/57 patients (35%). 40 For patients with HNRMS specifically, endocrine abnormalities were seen in 9%, facial hypoplasia in 9%, dry eye in 9%, cataracts in 3%, and there were no secondary cancers. While initial results seem promising for proton therapy, longer follow-up is necessary to continue to follow for adverse events that may take >4 years to develop, including endocrine deficiencies, facial asymmetry, and second cancers. Additionally, for tumors directly abutting critical normal structures in the head and neck, it is important to keep in mind that even with proton therapy dose to normal structures cannot be avoided.
Future Directions and Conclusions
Ongoing trials continue to explore the optimal dose of radiation needed to balance long-term disease control with late morbidity. For example, based on results from D9803 showing worse local control for tumors ≥5cm in size, patients with tumors measuring 5cm or more are boosted to 59.4 Gy on ARST 1431. Additionally, those patients with a complete response after week 12 of chemotherapy and those with tumors amenable to DPE receive a reduced dose of 36 Gy on this trial. For fusion-negative alveolar RMS (where the prognosis is favorable and more similar to embryonal RMS), it remains to be tested whether RT can be entirely omitted after a complete resection as is already done for clinical group I embryonal RMS. Lastly, retrospective data have suggested that response on positron emission tomography (PET) after induction chemotherapy has been correlated with outcomes in patients with RMS. 41 Ongoing trials including ARST 0531, ARST 1431, and ARST 08P1 are further evaluating the predictive value of PET. Modification of therapy based on PET response as is done in other pediatric tumors like Hodgkin lymphoma is an area of future exploration. This could potentially allow for lower RT doses or even the omission of RT in good responders.
In summary, HNRMS is a distinctly challenging site to treat given the young patient age and critical anatomy of the head and neck region. Most patients present with unresectable disease due to anatomical, functional, and/or cosmetic constraints. However, surgical resection and brachytherapy play an important role in select patients, and this role will continue to evolve as surgical techniques improve. For now, most patients with HNRMS are treated with definitive chemotherapy and RT. Modern RT techniques continue to advance as efforts are focused on ways to spare long-term morbidity. We recommend a multimodal individualized approach emphasizing the preservation of form for the successful treatment of HNRMS.
Footnotes
Conflict of Interest None.
References
- 1.Crist W M, Anderson J R, Meza J L et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 2.Pappo A S, Shapiro D N, Crist W M, Maurer H M. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13(08):2123–2139. doi: 10.1200/JCO.1995.13.8.2123. [DOI] [PubMed] [Google Scholar]
- 3.Maurer H M, Gehan E A, Beltangady M et al. The intergroup rhabdomyosarcoma study-II. Cancer. 1993;71(05):1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Ries L A, Smith M A, Gurney J G . Bethesda, MD: National Cancer Institute, SEER program, National Institutes of Health; 1999. Cancer incidence and survival among children and adolescents: United States SEER program. Publication no. 99-4649. [Google Scholar]
- 5.Raney R B, Jr, Tefft M, Newton W A et al. Improved prognosis with intensive treatment of children with cranial soft tissue sarcomas arising in nonorbital parameningeal sites. A report from the intergroup rhabdomyosarcoma study. Cancer. 1987;59(01):147–155. doi: 10.1002/1097-0142(19870101)59:1<147::aid-cncr2820590129>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Turc-Carel C, Lizard-Nacol S, Justrabo E, Favrot M, Philip T, Tabone E.Consistent chromosomal translocation in alveolar rhabdomyosarcoma Cancer Genet Cytogenet 198619(3-4):361–362. [DOI] [PubMed] [Google Scholar]
- 7.Barr F G, Smith L M, Lynch J C et al. Examination of gene fusion status in archival samples of alveolar rhabdomyosarcoma entered on the intergroup rhabdomyosarcoma study-III trial: a report from the Children's Oncology Group. J Mol Diagn. 2006;8(02):202–208. doi: 10.2353/jmoldx.2006.050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skapek S X, Anderson J, Barr F G et al. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: a children's oncology group report. Pediatr Blood Cancer. 2013;60(09):1411–1417. doi: 10.1002/pbc.24532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalski J M, Meza J, Breneman J C et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in intergroup rhabdomyosarcoma study group trials II through IV. Int J Radiat Oncol Biol Phys. 2004;59(04):1027–1038. doi: 10.1016/j.ijrobp.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 10.Turner J H, Richmon J D. Head and neck rhabdomyosarcoma: a critical analysis of population-based incidence and survival data. Otolaryngol Head Neck Surg. 2011;145(06):967–973. doi: 10.1177/0194599811417063. [DOI] [PubMed] [Google Scholar]
- 11.Yang J C, Wexler L H, Meyers P A, Wolden S L. Parameningeal rhabdomyosarcoma: outcomes and opportunities. Int J Radiat Oncol Biol Phys. 2013;85(01):e61–e66. doi: 10.1016/j.ijrobp.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Raney R B, Maurer H M, Anderson J R et al. The intergroup rhabdomyosarcoma study group (IRSG): major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5(01):9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meazza C, Ferrari A, Casanova M et al. Rhabdomyosarcoma of the head and neck region: experience at the pediatric unit of the Istituto Nazionale Tumori, Milan. J Otolaryngol. 2006;35(01):53–59. doi: 10.2310/7070.2005.4091. [DOI] [PubMed] [Google Scholar]
- 14.Radzikowska J, Kukwa W, Kukwa A, Czarnecka A, Krzeski A. Rhabdomyosarcoma of the head and neck in children. Contemp Oncol (Pozn) 2015;19(02):98–107. doi: 10.5114/wo.2015.49158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daya H, Chan H S, Sirkin W, Forte V. Pediatric rhabdomyosarcoma of the head and neck: is there a place for surgical management? Arch Otolaryngol Head Neck Surg. 2000;126(04):468–472. doi: 10.1001/archotol.126.4.468. [DOI] [PubMed] [Google Scholar]
- 16.Rodeberg D A, Wharam M D, Lyden E R et al. Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: a report from the Children's Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer. 2015;137(01):204–211. doi: 10.1002/ijc.29351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulino A C, Bauman N, Simon J H, Nguyen T X, Ritchie J M, Tannous R. Local control of parameningeal rhabdomyosarcoma: outcomes in non-complete responders to chemoradiation. Med Pediatr Oncol. 2003;41(02):118–122. doi: 10.1002/mpo.10312. [DOI] [PubMed] [Google Scholar]
- 18.Buwalda J, Blank L E, Schouwenburg P F et al. The AMORE protocol as salvage treatment for non-orbital head and neck rhabdomyosarcoma in children. Eur J Surg Oncol. 2004;30(08):884–892. doi: 10.1016/j.ejso.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Folkert M R, Tong W Y, LaQuaglia M P et al. 20-year experience with intraoperative high-dose-rate brachytherapy for pediatric sarcoma: outcomes, toxicity, and practice recommendations. Int J Radiat Oncol Biol Phys. 2014;90(02):362–368. doi: 10.1016/j.ijrobp.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 20.DeLaney T F, Chen G T, Mauceri T C et al. Intraoperative dural irradiation by customized 192iridium and 90yttrium brachytherapy plaques. Int J Radiat Oncol Biol Phys. 2003;57(01):239–245. doi: 10.1016/s0360-3016(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 21.King M T, Voros L, Cohen G N et al. High-dose-rate brachytherapy of rhabdomyosarcoma limited to the external auditory canal. Brachytherapy. 2017;16(01):181–185. doi: 10.1016/j.brachy.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank L E, Koedooder K, Pieters B R et al. The AMORE protocol for advanced-stage and recurrent nonorbital rhabdomyosarcoma in the head-and-neck region of children: a radiation oncology view. Int J Radiat Oncol Biol Phys. 2009;74(05):1555–1562. doi: 10.1016/j.ijrobp.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 23.Buwalda J, Freling N J, Blank L E et al. AMORE protocol in pediatric head and neck rhabdomyosarcoma: descriptive analysis of failure patterns. Head Neck. 2005;27(05):390–396. doi: 10.1002/hed.20164. [DOI] [PubMed] [Google Scholar]
- 24.Wolden S L, Anderson J R, Crist W M et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: a report from the intergroup rhabdomyosarcoma studies I to III. J Clin Oncol. 1999;17(11):3468–3475. doi: 10.1200/JCO.1999.17.11.3468. [DOI] [PubMed] [Google Scholar]
- 25.Breneman J, Meza J, Donaldson S S et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: a report from the Children's Oncology Group D9602 study. Int J Radiat Oncol Biol Phys. 2012;83(02):720–726. doi: 10.1016/j.ijrobp.2011.06.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donaldson S S, Meza J, Breneman J C et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51(03):718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 27.Raney R B, Walterhouse D O, Meza J L et al. Results of the intergroup rhabdomyosarcoma study group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29(10):1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolden S L, Wexler L H, Kraus D H, Laquaglia M P, Lis E, Meyers P A. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61(05):1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Kozak K R, Adams J, Krejcarek S J, Tarbell N J, Yock T I. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74(01):179–186. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson S S, Anderson J R. Rhabdomyosarcoma: many similarities, a few philosophical differences. J Clin Oncol. 2005;23(12):2586–2587. doi: 10.1200/JCO.2005.11.909. [DOI] [PubMed] [Google Scholar]
- 31.Benk V, Rodary C, Donaldson S S et al. Parameningeal rhabdomyosarcoma: results of an international workshop. Int J Radiat Oncol Biol Phys. 1996;36(03):533–540. doi: 10.1016/s0360-3016(96)00362-8. [DOI] [PubMed] [Google Scholar]
- 32.Merks J H, De Salvo G L, Bergeron C et al. Parameningeal rhabdomyosarcoma in pediatric age: results of a pooled analysis from North American and European cooperative groups. Ann Oncol. 2014;25(01):231–236. doi: 10.1093/annonc/mdt426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens M C, Rey A, Bouvet N et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology--SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23(12):2618–2628. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 34.Pappo A S, Meza J L, Donaldson S S et al. Treatment of localized nonorbital, nonparameningeal head and neck rhabdomyosarcoma: lessons learned from intergroup rhabdomyosarcoma studies III and IV. J Clin Oncol. 2003;21(04):638–645. doi: 10.1200/JCO.2003.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Kraus D H, Saenz N C, Gollamudi S et al. Pediatric rhabdomyosarcoma of the head and neck. Am J Surg. 1997;174(05):556–560. doi: 10.1016/s0002-9610(97)00171-2. [DOI] [PubMed] [Google Scholar]
- 36.Raney R B, Asmar L, Vassilopoulou-Sellin R et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: a descriptive report from the intergroup rhabdomyosarcoma studies (IRS)-II and - III. Med Pediatr Oncol. 1999;33(04):362–371. doi: 10.1002/(sici)1096-911x(199910)33:4<362::aid-mpo4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Paulino A C, Simon J H, Zhen W, Wen B C. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2000;48(05):1489–1495. doi: 10.1016/s0360-3016(00)00799-9. [DOI] [PubMed] [Google Scholar]
- 38.Lockney N A, Friedman D N, Wexler L H, Sklar C A, Casey D L, Wolden S L. Late toxicities of intensity-modulated radiation therapy for head and neck rhabdomyosarcoma. Pediatr Blood Cancer. 2016;63(09):1608–1614. doi: 10.1002/pbc.26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoot R A, Slater O, Ronckers C M et al. Adverse events of local treatment in long-term head and neck rhabdomyosarcoma survivors after external beam radiotherapy or AMORE treatment. Eur J Cancer. 2015;51(11):1424–1434. doi: 10.1016/j.ejca.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Ladra M M, Szymonifka J D, Mahajan A et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32(33):3762–3770. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casey D L, Wexler L H, Fox J J et al. Predicting outcome in patients with rhabdomyosarcoma: role of [(18)f]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys. 2014;90(05):1136–1142. doi: 10.1016/j.ijrobp.2014.08.005. [DOI] [PubMed] [Google Scholar]


