Abstract
Objective The endoscopic endonasal approach is being increasingly used for the resection and reconstruction of anterior skull base (ASB) lesions. Vascularized nasoseptal flaps (NSF) have become the workhorse for the reconstruction of ASB defects, resulting in a significant decrease in the incidence of cerebrospinal fluid (CSF) leaks. The objective of this study was to investigate the efficacy and safety of NSF in children.
Methods This is a retrospective analysis of the medical records of all patients under the age of 18 years who underwent endoscopic repair of ASB lesions with the use of NSF at our tertiary medical center between 1/2011 and 8/2016.
Results Twelve children underwent ASB defect repair for both benign and malignant neoplasms using the endoscopic endonasal NSF technique. Four children had previously undergone ASB surgery. The male-to-female ratio was 1:1, the average age was 12.3 years, the average hospitalization time was 8.3 days, and the maximum follow-up period was 24 months, during which craniofacial growth appeared to be unimpaired. A lumbar drain was used postoperatively in six cases. Crust formation and synechia were observed in two cases. There was one case of a major long-term complication (a CSF leak followed by meningitis).
Conclusions Endoscopic endonasal NSF was both an effective and a safe technique for ASB defect reconstruction in 12 children for both benign and malignant neoplasms. It had a high success rate and a low complication rate. No apparent negative influence on craniofacial growth was observed in our series.
Keywords: nasoseptal flap, anterior skull base, endoscopic endonasal approach, reconstruction, children, pediatric, craniofacial growth, CSF leak
Introduction
Skull base lesions are infrequent in children and are highly variable in nature. The anterior skull base (ASB) is considered to be a challenging area for surgical intervention at any age, but even more so in the pediatric population. Its close proximity to vital organs and the aesthetic consideration that need to be taken into account when approaching this area mandate careful planning and high-precision surgery. Reconstruction of an ASB defect, to prevent cerebrospinal fluid (CSF) leaks and to provide a vital barrier between the brain and the nasal cavity, is an integral part of any surgical procedure in this area. 1 2 3
The endoscopic endonasal approach (EEA) to the ASB has been increasingly used for both resection and reconstruction of lesions of the ASB. The EEA enables surgical access to midline lesions of the ASB as well as to the clival, sellar, suprasellar, and parasellar regions. The EEA has been shown to be safe and effective in several studies on adult patients. 3 4 5
The introduction of endoscopic vascularized pedicled nasoseptal flaps (NSF) by Hadad et al in 2006 succeeded in significantly lowering the rate of CSF leaks. 6 When used in a multilayer reconstruction technique, this flap reduced the rate of CSF leaks to as low as 5%. 7 8 9 Despite the fact that NSF have gradually become the workhorse reconstruction method for ASB defects, there is few data on the associated complication rate when using this technique. There is even fewer data on the use of these flaps in children.
This study was conducted to investigate the efficacy and morbidity related to the use of NSF in children undergoing ASB surgery using the EEA.
Methods
Study Design
After an institutional review board approval had been granted, we conducted a retrospective search in our department's database and retrieved the medical records of 466 patients, who had undergone skull base surgery in our medical center between 1999 and 2017. We selected the records of children under the age of 18 years, who underwent endoscopic ASB surgery and reconstruction with NSF. From these records, we collected data that included demographics, preoperative workup, operative findings, and postoperative complications and follow-up.
Presurgical and Postsurgical Care
All the children were operated on by the same team that included an otolaryngologist and a neurosurgeon. The operation was performed using a navigation system. All patients were administered with perioperative antibiotics. The postoperative care included a 24 to 48 hours hospitalization in the pediatric intensive care unit, after which the patients were transferred to the pediatric neurosurgery department for further treatment and observation. Follow-up continued at the outpatient clinic with periodic visits.
Reconstruction with a NSF: Surgical Technique
A NSF is usually elevated at the beginning of a surgery when a CSF leak is expected. The nasal cavity is decongested with 1% lidocaine and adrenaline (10:1) solution and infiltrated with 2% lidocaine and adrenaline (1:100000) solution. The septal mucosa is incised using a #10 blade scalpel and a monopolar cautery. The NSF is elevated in an anterior–posterior direction using a Freer elevator ( Fig. 1 ). Once elevated, it is tucked into the nasopharynx or the maxillary sinus for the duration of surgery ( Fig. 2 ). To prepare the region of the ASB defect for receiving the flap, drilling of bony septations in the vicinity of the defect and removal of the circumferential mucosa are performed. Reconstruction of ASB defects is usually done using a multilayer closure technique, which includes an underlay placement of abdominal fat or, occasionally, fascia lata. The NSF is then placed as an overlay layer to cover the defect ( Fig. 3 ). Care is taken, when placing the NSF, to avoid torsion of the pedicle and to ensure that the appropriate orientation of the NSF mucosal surface is kept.
Fig. 1.
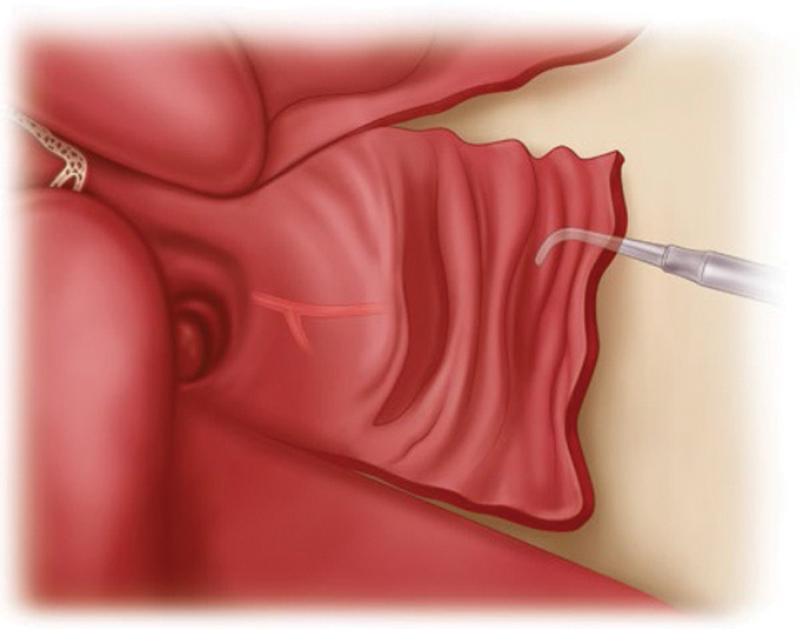
The nasoseptal flap is meticulously elevated with a Freer retractor. (Reprinted with permission from Atlas of Surgical Approaches to Paranasal Sinuses and the Skull Base; Authors: Fliss, Dan M., Gil, Ziv; Springer 2016.)
Fig. 2.
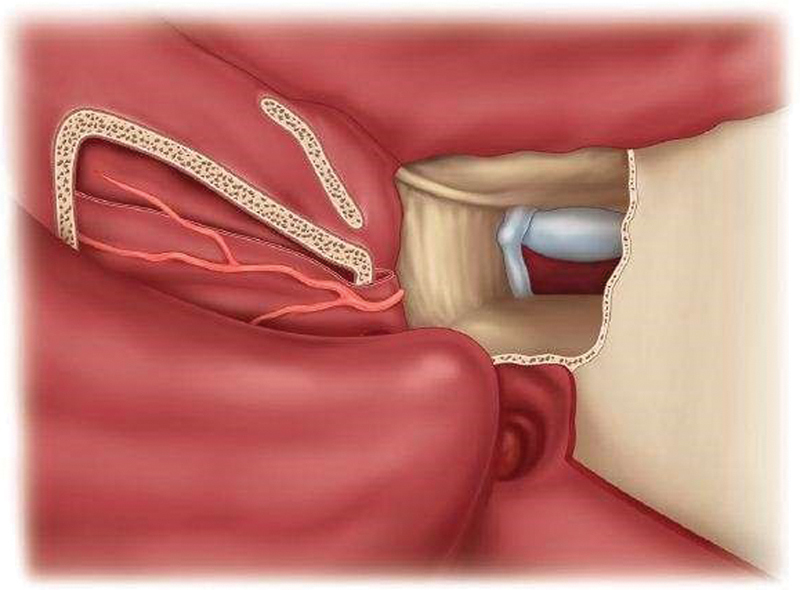
The flap is elevated laterally up to the level of the sphenopalatine foramen. It is stored in the choana or in the maxillary antrum. (Reprinted with permission from Atlas of Surgical Approaches to Paranasal Sinuses and the Skull Base; Authors: Fliss, Dan M., Gil, Ziv; Springer 2016.)
Fig. 3.
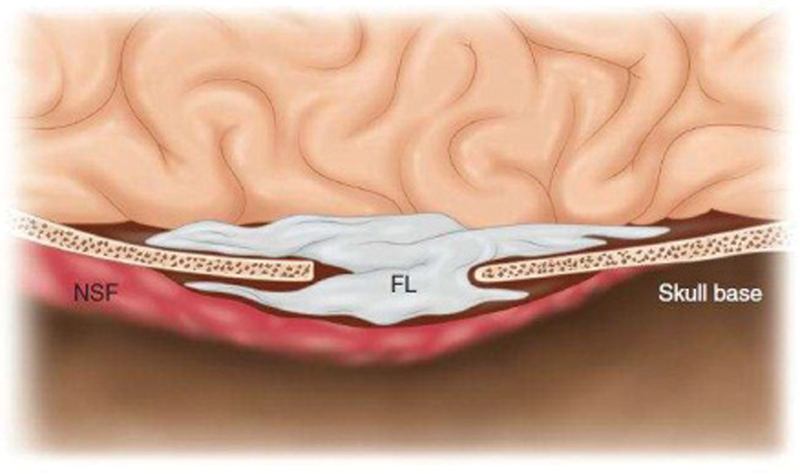
Cerebrospinal fluid leak is sealed with a fat flap harvested from the abdomen (Reprinted with permission from Atlas of Surgical Approaches to Paranasal Sinuses and the Skull Base; Authors: Fliss, Dan M., Gil, Ziv; springer 2016.)
Results
Between 1/2011 and 8/2016, 12 patients under the age of 18 years underwent EEA for the repair of ASB lesions, using the endoscopic NSF technique. The average age of the patients was 10.3 years (range 1–17) and the male-to-female ratio was 1.4:1. In six patients (50%), the pathologies were of benign nature (pituitary adenomas, dermoid cysts, and encephaloceles). The other (50%) had a malignant or locally aggressive disease. Six children (50%) had undergone prior ASB surgery and were reoperated by our team, for either the treatment of CSF leaks associated with a prior operation or for the treatment of a persistent underlying pathology. Patient demographics and characteristics are summarized in Table 1 .
Table 1. Patients characteristics.
| Pathology | Prior surgery | Age (y) | Gender | Patient |
|---|---|---|---|---|
| Dermoid cyst | No | 2 | F | 1 |
| Encephalocele | Yes | 9 | M | 2 |
| Osteosarcoma | Yes | 1 | M | 3 |
| Pituitary adenoma | Yes | 13 | F | 4 |
| Encephalocele | Yes | 13 | F | 5 |
| Craniopharyngioma | No | 17 | M | 6 |
| Pituitary adenoma | No | 17 | F | 7 |
| Chordoma | No | 17 | M | 8 |
| Encephalocele | No | 1 | M | 9 |
| Craniopharyngioma | Yes | 13 | M | 10 |
| Craniopharyngioma | No | 17 | F | 11 |
| Craniopharyngioma | Yes | 4 | M | 12 |
In seven patients (58.3%), we placed a continuous CSF lumbar continuous drainage (CD) for 3 to 5 days postsurgery. This procedure was performed when some difficulty was expected with the ASB repair (e.g., a high-flow CSF leak or a large dural defect). Its purpose is to lower the CSF pressure and to allow a better adhesion of the NSF. There are different surgical approaches to the ASB. Factors that may influence a surgeon's decision to choose one approach for an ASB defect repair over the other are the location of the defect, patient's anatomy, and personal experience and preference. In our series, the transsphenoid approach was used in seven patients (58.3%), the transcribriform in four (41.6%), and transclival in one (8.3%). The repair in seven cases (58.3%) was done by means of a multilayer technique using both an overlay NSF and an underlay of additional material, such as abdominal fat or fascia lata. Intraoperative details are listed in Table 2 .
Table 2. Intraoperative data.
| CD placement | Reconstruction technique | Approach | Patient |
|---|---|---|---|
| Yes | NSF + fat | Transcribriform | 1 |
| No | NSF | Transsphenoid | 2 |
| Yes | NSF + fascia lata | Transcribriform | 3 |
| No | NSF | Transsphenoid | 4 |
| Yes | NSF + fascia lata | Transcribriform | 5 |
| Yes | NSF + fat | Transsphenoid | 6 |
| Yes | NSF | Transsphenoid | 7 |
| Yes | NSF + fat | Transclivus | 8 |
| No | NSF+ bone | Transcribriform | 9 |
| No | NSF | Transsphenoid | 10 |
| Yes | NSF + fat | Transsphenoid | 11 |
| No | NSF | Transsphenoid | 12 |
Abbreviations: CD, cerebrospinal fluid lumbar continuous drainage; NSF, nasoseptal flap.
The average total postsurgical hospitalization time was 7.83 days. The long-term postoperative complications were divided into minor (crust formation, synechiae) and major (meningitis, need for revision). Only one patient (8.3%) developed a long-term complication: a 13-year-old girl who had a large cribriform encephalocele and a craniofacial malformation. She developed meningitis, 2 years after the first operation, due to recurrence of the encephalocele and a persistent CSF leak. Her condition mandated a revision surgery. The postoperative data are brought in Table 3 .
Table 3. Postoperative data.
| Long-term Complications |
Long-term CSF leak | Hospitalization (days) |
Patient |
|---|---|---|---|
| No | No | 4 | 1 |
| Minor (crusting) | No | 6 | 2 |
| No | No | 15 | 3 |
| No | No | 5 | 4 |
| Meningitis, revision | Yes | 11 | 5 |
| No | No | 10 | 6 |
| No | No | 9 | 7 |
| Minor (synechiae) | No | 7 | 8 |
| No | No | 6 | 9 |
| No | No | 7 | 10 |
| No | No | 7 | 11 |
| No | No | 7 | 12 |
Abbreviations: CSF, cerebrospinal fluid.
Discussion
ASB surgery for adults has become safe and widespread because of improved techniques of endoscopic endonasal skull base surgery and the ability to reliably reconstruct the skull base defect. 5 10 Skull base surgery in children is especially demanding since ASB lesions are rare at young age, carry great diversity in their pathology, and raise technical issues related to operating in and around small cavities. Vascularized pedicled NSF were shown to be an effective reconstructive tool in adult ASB surgery. 6 8 11 12 In this report, we describe and discuss our experience using NSF in children undergoing endoscopic resection of ASB lesions.
The primary challenge in pediatric endoscopic skull base surgery is operating in small cavities and less pneumatized sinuses compared with the adult anatomy. This may cause impaired visualization and limited maneuverability on the part of the surgeon. Much progress has, nevertheless, been made in the field of pediatric skull base surgery. This progress is largely thanks to the experience gained from adult endoscopic skull base surgery, improved skills and techniques, better endoscopic equipment, and newer endoscopic hemostatic products. Moreover, guided navigation systems have facilitated endoscopic procedures for resection and reconstruction of skull base lesions in children. 6 13 14
We found that resection of the inferior part of the middle turbinate helped expose the pedicled the NSF and made it easier to elevate and rotate this flap in the children's small nasal cavities. Our success rate with the NSF in pediatric endoscopic skull base surgery was high and we did not encounter early postoperative CSF leaks or meningitis. The development of a late (2 years postsurgery) CSF leak in one patient might be related to increased intracranial pressure that caused recurrence of the encephalocele in a patient with a known developmental craniofacial malformation.
Chivukula et al summarized their results of endoscopic resection of skull base lesions in 133 pediatric patients. 15 They used NSF for reconstruction in 55 patients. These authors demonstrated that using NSF, to reconstruct a resected tumor from the ASB, reduced postoperative CSF leaks from 12.5% to 8.9%.
It has been well established that the rate of cranial growth exceeds the rate of facial growth early in life. An earlier report by Shah et al raised concern that NSF might not adequately cover large defects in young children. 16 Our experience with 12 patients showed that the use of NSF in pediatric ASB surgery is feasible and efficient at any age. We used a combination of NSF and fat or fascia lata to achieve adequate coverage in larger defects.
None of our patients developed postoperative CSF leak or meningitis related to inadequate coverage. Our single patient, who developed meningitis 2 years after the first surgery, had a recurrent encephalocele.
CSF leaks and meningitis are the more frequently reported complications related to the use of NSF reconstruction, but there are others that have been rarely mentioned in the literature. 17 Soudry et al 18 reported an overall complication rate of 30% in association with the NSF procedure in adults, most of them (60%) related to the donor site. These authors examined NSF reconstruction in adults and found local complications in 27 patients (35%) that were mainly crust formation (14%), synechia (7%), and anosmia (7%). Only two of our patients (16.6%) experienced a minor complication: one had synechia and the other suffered from prolonged crusting.
Postoperative endoscopic follow-up, nasal irrigation, and debridement are important for avoiding complications, but may be problematic to carry out in young children. The child's parents should be explained of the necessity of continuing these treatments after the child has been released from hospital. When cooperation was limited, we occasionally performed the first postoperative follow-up and debridement treatment under light sedation.
Another major concern in pediatric skull base surgery is the potential for the development of a craniofacial asymmetry. We believe that minimally invasive endoscopic surgery with NSF reconstruction is a better alternative for preserving symmetry in comparison to open surgery with free or local flap reconstruction. There is always the concern of impairing nasal growth when using NSF. However, this may be avoided by leaving the contralateral septal mucosa intact. Reports on long-term effects of endoscopic sinonasal surgery on children's facial growth did not show impaired craniofacial development as measured by cephalometric parameters. 19 Despite a relatively short follow-up time, there was no apparent negative influence of endoscopic surgery with NSF on craniofacial growth in our series.
Conclusion
In conclusion, endoscopic reconstruction of ASB defects using NSF is both an effective and a safe technique for the reconstruction of ASB defects for both benign and malignant neoplasms in children. It has a high success rate and a low complication rate. No apparent negative influence on craniofacial growth was observed in our series.
References
- 1.Gil Z, Abergel A, Leider-Trejo L et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17(01):25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fliss D M, Abergel A, Cavel O, Margalit N, Gil Z. Combined subcranial approaches for excision of complex anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2007;133(09):888–896. doi: 10.1001/archotol.133.9.888. [DOI] [PubMed] [Google Scholar]
- 3.Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello D M. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117(04):699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]
- 4.Cappabianca P, Cavallo L M, de Divitiis E.Endoscopic endonasal transsphenoidal surgery Neurosurgery 20045504933–940., discussion 940–941 [DOI] [PubMed] [Google Scholar]
- 5.Kassam A B, Prevedello D M, Carrau R L et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg. 2011;114(06):1544–1568. doi: 10.3171/2010.10.JNS09406. [DOI] [PubMed] [Google Scholar]
- 6.Hadad G, Bassagasteguy L, Carrau R L et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 7.Kassam A, Carrau R L, Snyderman C H, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19(01):E8. [PubMed] [Google Scholar]
- 8.Patel M R, Taylor R J, Hackman T G et al. Beyond the nasoseptal flap: outcomes and pearls with secondary flaps in endoscopic endonasal skull base reconstruction. Laryngoscope. 2014;124(04):846–852. doi: 10.1002/lary.24319. [DOI] [PubMed] [Google Scholar]
- 9.Hu F, Gu Y, Zhang X et al. Combined use of a gasket seal closure and a vascularized pedicle nasoseptal flap multilayered reconstruction technique for high-flow cerebrospinal fluid leaks after endonasal endoscopic skull base surgery. World Neurosurg. 2015;83(02):181–187. doi: 10.1016/j.wneu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Kono Y, Prevedello D M, Snyderman C H et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol. 2011;32(01):77–83. doi: 10.1086/657635. [DOI] [PubMed] [Google Scholar]
- 11.Eloy J A, Choudhry O J, Friedel M E, Kuperan A B, Liu J K. Endoscopic nasoseptal flap repair of skull base defects: is addition of a dural sealant necessary? Otolaryngol Head Neck Surg. 2012;147(01):161–166. doi: 10.1177/0194599812437530. [DOI] [PubMed] [Google Scholar]
- 12.Harvey R J, Smith J E, Wise S K, Patel S J, Frankel B M, Schlosser R J. Intracranial complications before and after endoscopic skull base reconstruction. Am J Rhinol. 2008;22(05):516–521. doi: 10.2500/ajr.2008.22.3223. [DOI] [PubMed] [Google Scholar]
- 13.Kassam A, Thomas A J, Snyderman Cet al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients J Neurosurg 2007106(2, Suppl):75–86. [DOI] [PubMed] [Google Scholar]
- 14.Tatreau J R, Patel M R, Shah R N et al. Anatomical considerations for endoscopic endonasal skull base surgery in pediatric patients. Laryngoscope. 2010;120(09):1730–1737. doi: 10.1002/lary.20964. [DOI] [PubMed] [Google Scholar]
- 15.Chivukula S, Koutourousiou M, Snyderman C H, Fernandez-Miranda J C, Gardner P A, Tyler-Kabara E C. Endoscopic endonasal skull base surgery in the pediatric population. J Neurosurg Pediatr. 2013;11(03):227–241. doi: 10.3171/2012.10.PEDS12160. [DOI] [PubMed] [Google Scholar]
- 16.Shah R N, Surowitz J B, Patel M R et al. Endoscopic pedicled nasoseptal flap reconstruction for pediatric skull base defects. Laryngoscope. 2009;119(06):1067–1075. doi: 10.1002/lary.20216. [DOI] [PubMed] [Google Scholar]
- 17.Lai L T, Trooboff S, Morgan M K, Harvey R J. The risk of meningitis following expanded endoscopic endonasal skull base surgery: a systematic review. J Neurol Surg B Skull Base. 2014;75(01):18–26. doi: 10.1055/s-0033-1353365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soudry E, Psaltis A J, Lee K H, Vaezafshar R, Nayak J V, Hwang P H. Complications associated with the pedicled nasoseptal flap for skull base reconstruction. Laryngoscope. 2015;125(01):80–85. doi: 10.1002/lary.24863. [DOI] [PubMed] [Google Scholar]
- 19.Van Peteghem A, Clement P AR. Influence of extensive functional endoscopic sinus surgery (FESS) on facial growth in children with cystic fibrosis. Comparison of 10 cephalometric parameters of the midface for three study groups. Int J Pediatr Otorhinolaryngol. 2006;70(08):1407–1413. doi: 10.1016/j.ijporl.2006.02.009. [DOI] [PubMed] [Google Scholar]


