Abstract
Significance
For glaucoma patients, high intraocular pressure (IOP) is a risk factor for progressive neuropathy. Similarly, animal models used to study the disease are based on an experimental elevation of IOP. Thus, accurate IOP measurements are important in characterizing experimental models and resulting effects.
Purpose
The purpose of the present study was to investigate IOP measurements in a non-human primate model of experimental glaucoma by comparing clinical tonometry (Tono-Pen and TonoVet) to the true IOP from intracameral manometry.
Methods
A total of 17 rhesus macaque eyes from 12 animals were used for this study. Eleven eyes had no prior experimental intervention, whereas 6 eyes were at varying stages of laser induced experimental glaucoma. IOPs were adjusted by inserting a needle in the anterior chamber that was attached to a pressure transducer and syringe pump system. The anterior chamber IOP was adjusted to values between 10 and 50 mmHg and corresponding measures with Tono-Pen and TonoVet were taken.
Results
The IOPs by TonoVet and Tono-Pen were linearly related over the range of pressures tested (slope = 0.68 normal/healthy and 0.72 experimental glaucoma). For the most, TonoVet measures overestimated IOP at all anterior chamber pressure settings (mean difference of 3.17 mmHg, 95% CI 12.53 to −4.74 normal and 3.90 mmHg, 95% CI 12.90 to −6.53 experimental glaucoma). In contrast, Tono-Pen measures overestimated IOP at lower IOPs and underestimated at higher IOP (slope = −0.26 normal and −0.21 experimental glaucoma).
Conclusions
The TonoVet and TonoPen tonometers that are often used to assess IOP in both clinical and experimental settings generally reflect the status of IOP, but the results from this study suggest that the instruments need calibration with true anterior chamber pressure for accurate measures in experimental models of glaucoma.
Keywords: intraocular pressure, tonometry, non-human primate experimental glaucoma
Glaucoma is a group of optic neuropathies that can lead to irreversible blindness. Although the pathophysiology of retinal ganglion cell loss is not fully understood, intraocular pressure (IOP) is a major risk factor and clinical trials have demonstrated that lowering the IOP is an effective treatment.1–3 This mechanistic relationship is also the basis for most animal models of experimental glaucoma, which are generally created by procedures that decrease aqueous outflow and subsequently elevate IOP.
Because the ocular anatomy and visual function of monkeys are similar to those of humans, the non-human primate is an excellent model for studies of ocular pathology. Experimental glaucoma in the non-human primate is often induced by scaring of the trabecular meshwork by contiguous Argon laser burns.4–6 Subsequently, the status of experimental glaucoma (i.e. structure and function) is typically assessed as a function of cumulative IOP over post-treatment time to quantify pressure insult.7, 8 It is, therefore, important to have accurate assessments of IOP, which typically involves tonometry, often using standard clinical/veterinary instruments.
The clinical standard for IOP quantification is Goldmann tonometry, which builds on the Imbert-Fick principal. In human eyes with normal corneal thickness (500 – 570 μm), a 3.06 mm diameter applanation zone is shown to be ideal as a 0.1 gm force corresponds to 1 mmHg.9 However, IOP quantified using Goldmann tonometry is dependent on corneal biomechanics,10–12 corneal thickness,13,14 and corneal curvature.15,16 Because the non-human primate cornea is significantly steeper and thinner than that of humans, Goldmann type tonometers are not ideal. Hence, most laboratories investigating experimental glaucoma use either a Tono-Pen XL (Reichert, Inc., Depew, NY, USA) or TonoVet (icare, Vantaa, Finland) to monitor IOP.
The Tono-Pen XL is an applanation/indentation tonometer that works on the Mackay-Marg principal.17 While the sleeve of the tonometer is similar in width to that of the Goldmann applanation zone (~3.0 mm), the central plunger that is connected to the strain gauge, is significantly smaller. The TonoVet is an induction/impact tonometer in which a small probe rebounds off the cornea. The deceleration rate of the probe caused by corneal contact is recorded and used to determine IOP.18,19 In theory, IOP measures from both instruments should be repeatable, accurate and precise in both healthy and disease eyes. The purpose of this study was to investigate IOP measurements in a non-human primate model of experimental glaucoma by comparing standard tonometry to intracameral manometry. Some of the results of these studies have been presented in abstract form (McAllister F, et al. OVS 2016;93:E-abstract 160077).
METHODS
Animal Subjects
Data were collected from a total of 17 eyes of 12 rhesus macaques. 11 eyes had no previous experimental intervention, whereas 6 eyes had laser induced experimental glaucoma. Of the normal control eyes, 6 were used to determine intra-session test-retest variability. Experimental and animal care procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Houston. The use of animals for this experiments adhered to the National Institutes of Health guidelines for the care and use of laboratory animals, and to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Animal Preparation
Animals were anesthetized with an intramuscular injection of ketamine (20-25 mg/kg) and xylazine (0.8-0.9 mg/kg) and treated with a subcutaneous injection of atropine sulfate (0.04 mg/kg). Throughout the experiment, body temperature was monitored and maintained using a thermal blanket (TC1000 temperature controller, CWE, Ardmore), while heart rate and pulse was monitored with a pulse oximeter (model 9847; Nonin, Plymouth, MN).
Laser-induced Ocular Hypertension
Application of argon laser energy to the trabecular meshwork to induce experimental glaucoma has been described previously.4–6 Briefly, for the initial laser procedure, performed through a one mirror gonioscopy lens (Ocular Kaufman; Ocular Instruments, Bellvue, WA, USA), contiguous laser burns (1.0 watt laser power, 0.5 sec duration, 50 um spot size) were applied over 270 degrees of the drainage angle. If a sustained increase in IOP was not achieved by 4 weeks after the initial laser session, additional laser treatments over 180 degrees of the drainage angle were applied until sustained elevated IOPs were achieved. For the 6 experimental glaucoma eyes used in this study, the mean IOP from the first sustained elevated pressure measured using Tono-Pen was 32.6mmHg (range 25.3-36.5 mmHg). Cannulation studies on these eyes were done on average at day 576 (range 454-693 days) from the first sustained elevated pressure.
Ocular Biometry
An ocular biometer (Lenstar LS900) was used to measure axial length, anterior chamber depth, lens thickness, anterior corneal curvature, and central corneal thickness in all eyes. The biometry data was used to assess the associated relationship with tonometry error.
Anterior Chamber IOP Control
To prevent infection, 5% ophthalmic betadine (Alcon Laboratories, Fort Worth, TX) was applied to the eyelids, instilled on the ocular surface, and subsequently washed off with sterile balanced salt solution (BSS, Alcon Laboratories, Fort Worth, TX) after a period of two minutes. The head of the animal was stabilized using mouth and occipital bars, and a sterile speculum was inserted to keep the eyelids open. The anterior chamber was then accessed using a 27G butterfly needle, inserted approximately 1 mm from the temporal limbus and extending up to 2 mm into the anterior chamber. The needle was connected with sterile microtube filled with balanced salt solution, to a pressure control system that included a capacitive pressure transmitter (Keller PR-41X, Keller America, Newport News, VA) coupled with a syringe pump (Cole-Parmer, Vernon Hills, IL) that was controlled through a MATLAB (The Mathworks, Natick, MA) program. For each of the cannulation experiments, the pressure in the anterior chamber was adjusted from 10 to 50 mmHg. After changing the pressure setting, eyes were allowed to equilibrate for at least 5 minutes before IOP was measured. Throughout the experiment, the ocular surface was kept hydrated with sterile balanced salt solution. To assess repeatability, the IOP in 6 normal control eyes was increased from 10 to 50 mmHg in 10 mmHg steps, and at each pressure measure, 3 measures from both Tono-Pen and TonoVet were acquired. Because TonoVet does not significantly indent the cornea, measures from this instrument were obtained prior to Tono-Pen with an interval of at least 2 minutes between measures. For the 6 eyes with repeated measures, all TonoVet measures were acquired prior to Tono-Pen, allowing for at least a 2 min interval between all measures. For 5 eyes of normal controls, and 6 of the experimental glaucoma eyes, anterior chamber pressure was adjusted from 10 mmHg to 50 mmHg in 5 mmHg steps. For these eyes, only one Tono-Pen and TonoVet IOP was obtained at the highest reliability for each instrument (i.e. <= 5% std dev for Tono-Pen and <= 1.0 mmHg std dev for TonoVet). Following anterior chamber cannulation and completion of data collection, the needle was removed, and topical antibiotics (polymyxin B/trimethoprim and moxifloxacin) were instilled on the eye.
IOP Control System Calibration
Calibration of the pressure control system was assessed prior to the start of experiments and after all data collection. The system was calibrated by setting up the pressure transducer, syringe pump, tubing and needle as would be done during a cannulation experiment. The tubing connected to the needle was taped to a vertical meter ruler that was aligned with the pressure transducer. The pressure setting was adjusted from 10 to 60 mmHg, and the rise of fluid in the tubing was measured in centimeters of water, and subsequently converted to mmHg (1.0 cmH2O = 0.736 mmHg). Overall, there was excellent agreement between the rise of the water column and the pressure setting (Fig 1A), with 95% confidence limits of about 0.3 mmHg for the mamometer-transducer relationship (Fig. 1B).
Figure 1.
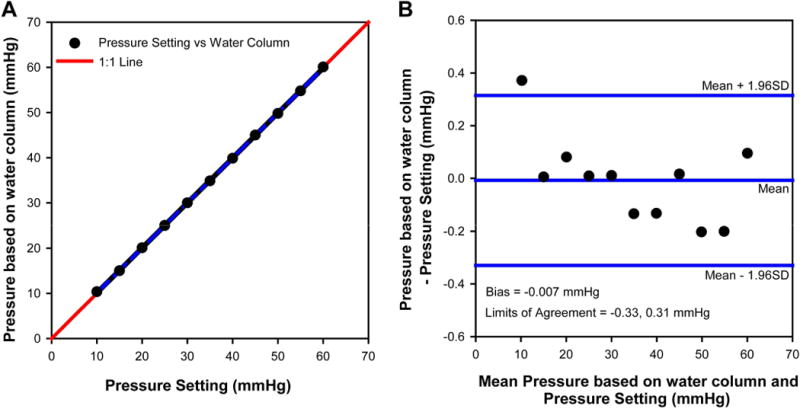
(A) Correspondence between pressure setting and rise of water column converted to mmHg and comparison to the 1:1 line. (B) Bland-Altman plot for the agreement between pressure setting and water column rise, illustrating the bias and 95% limits of agreement.
Statistical Analysis
All of the tonometry measurements were acquire by one investigator (NBP). Measurement error and repeatability was assessed for 6 healthy eyes. Linear regression was used to determine the relationship IOP measures from the two tonometers. To determine accuracy of the Tono-Pen and TonoVet, the difference between IOP and pressure transducer were plot against the pressure setting/pressure transducer reading for each animal. To evaluate if there were differences in calibration with disease, data were separated for normal control and experimental glaucoma eyes.
RESULTS
Data were collected from 11 normal control and 6 experimental glaucoma eyes. Of the 11 normal controls, repeatability was assessed in 6 eyes. All animals maintained good systemic health and no ocular complications from cannulation and IOP quantification were noted during the experiment or at follow up evaluation of each animal. Table 1 illustrates the baseline biometry data for normal and experimental non-human primate glaucoma eyes. The only significant difference between the two groups was the longer axial lengths in experimental glaucoma eyes (p=0.02).
Table 1.
Optical biometry measures for normal (n=11) and experimental glaucoma (n=6) eyes. Statistically significant differences (p<0.05) between the two groups are noted by an*
| Normal control | Experimental Glaucoma | |
|---|---|---|
| Axial Length (mm) | 18.9±0.67 | 19.9±0.75 * |
| Corneal Curvature (D) | 53.8±2.31 | 52.8±2.24 |
| Central Corneal Thickness (μm) | 461.7±22.85 | 490.5±34.34 |
| Anterior Chamber Depth (mm) | 3.33±0.28 | 3.07±0.23 |
| Lens Thickness (mm) | 3.39±0.07 | 3.42±0.20 |
The measurement errors of both tonometers were assessed using the estimated within-subject standard deviation (Sw) from repeated measures in only 6 normal eyes.20 Table 2 illustrates the repeatability (2.77×Sw) from these 6 eyes, for pressure settings from 10 to 50 mmHg. In general, there is an increase in test-retest error with increasing IOP for both tonometers.
Table 2.
Average and repeatability of IOP measures for Tono-Pen and TonoVet tonometers at increasing pressure levels (n=6 eyes).
| Pressure Setting (mmHg) |
TONO-PEN | TONOVET | ||
|---|---|---|---|---|
| Average IOP (mmHg) |
Repeatability (2.77 × Sw, mmHg) |
Average IOP (mmHg) |
Repeatability (2.77 × Sw, mmHg) |
|
| 10 | 10.8 | 1.67 | 12.8 | 1.13 |
| 20 | 18.2 | 2.83 | 22.1 | 1.56 |
| 30 | 25.8 | 3.48 | 33.3 | 2.66 |
| 40 | 33.6 | 2.51 | 44.3 | 3.75 |
| 50 | 40.7 | 4.82 | 54.4 | 2.69 |
When compared to the IOP’s as set by the transducer system, the IOPs measured by the TonoVet were on average 3-4 mmHg higher across all pressure settings (Figs 2A&B), but the slopes of the linear regressions were near unity (difference from slope of 1, p=0.45 normal, and p=0.86 glaucoma) with high correlation coefficients. Consequently, the analyses of agreement between the transducer pressure and the TonoVet measurement illustrated small biases, but relatively large limits of agreement (Figs. 2C&D). In addition, there was no difference in the mean bias and 95% confidence interval for healthy normal (mean difference = 3.17 mmHg, 95% confidence interval 12.53 – −4.74 mmHg) or eyes with experimental glaucoma - (mean difference = 3.90 mmHg, 95% confidence interval 12.90 – −6.53 mmHg). It is important to note that the standard deviation of errors was greater at higher pressure settings (2.3 vs. 4.1 mmHg in normal, p=0.3 and 1.7 vs 4.1 mmHg, p=0.13 in experimental glaucoma eyes at 10 and 50 mmHg, respectively), but did not reach statistical significance. These findings are consistent with the increase in measurement error with increase in IOP (Table 2).
Figure 2.
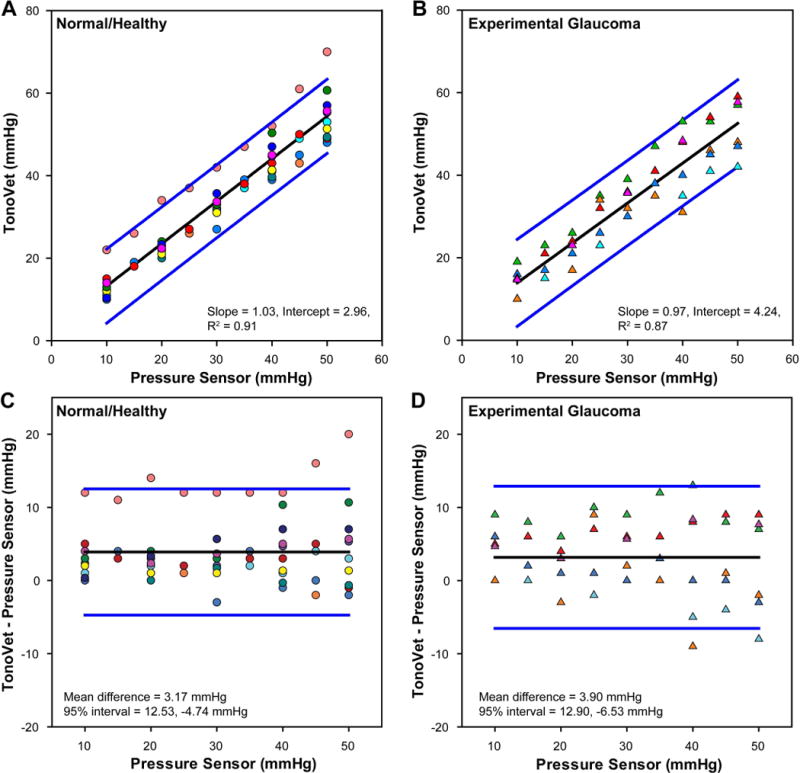
(A & B) The relationship between TonoVet IOP and intracameral pressure in normal healthy and experimental glaucoma eyes. (C & D) Difference in tonometer IOP measures from that of the pressure transducer. Healthy eyes are represented by circles and experimental glaucoma with triangles. Each animal is represented by a unique color.
In contrast, when IOPs from the Tono-Pen were compared to the pressures set by the transducer system, there were systematic errors as a function of pressure. For lower pressure settings, the Tono-Pen overestimated IOPs while underestimating IOPs at higher pressures (Figs 3A&B). The IOP relationship was linear (R2 > 0.9) and although the slopes from linear regression were slightly different (−0.26 for normal eyes and −0.21 in eyes with experimental glaucoma), the difference was not significant (p=0.45). These relationships are also illustrated by the analyses of agreement between the transducer pressure and the Tono-Pen measurements Figs. 3C&D) which also demonstrated relatively large limits of agreement. Similar to measures from the TonoVet, the standard deviation of error of Tono-Pen measures increased at higher pressure settings (2.6 vs. 2.8 mmHg in normal, p=0.86 and 1.8 vs 4.6 mmHg, p=0.04 in experimental glaucoma eyes at 10 and 50 mmHg respectively).
Figure 3.
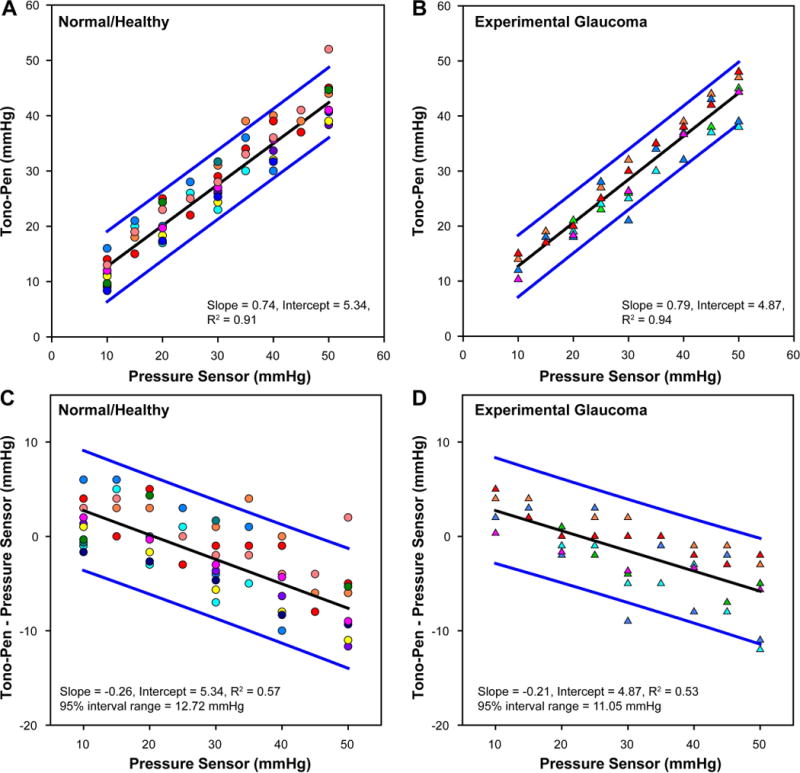
(A & B) The relationship between Tono-Pen IOP and intracameral pressure in normal healthy and experimental glaucoma eyes. (C & D) Difference in tonometer IOP measures from that of the pressure transducer. Healthy eyes are represented by circles and experimental glaucoma with triangles. Each animal is represented by a unique color.
IOP measures from the Tono-Pen and TonoVet were linearly related for both normal and experimental glaucoma eyes (Fig 4). Although there was good correspondence between Tono-Pen and TonoVet IOP measures, for both normal eyes (slope = 0.68, R2 = 0.90) and eyes with glaucoma (slope = 0.72, R2 = 0.88), the slope of the best fit linear functions for IOP measures from both instruments were significantly different from unity (p<0.01). Although the function was slightly steeper for eyes with glaucoma, the slopes were not statistically different t (p = 0.46). However, the limits of agreement between the two tonometers were as relatively large and, while the width of the 95% confidence interval band was greater for experimental glaucoma (16.3 mmHg vs. 13.8 mmHg), the difference was within measurement error.
Figure 4.
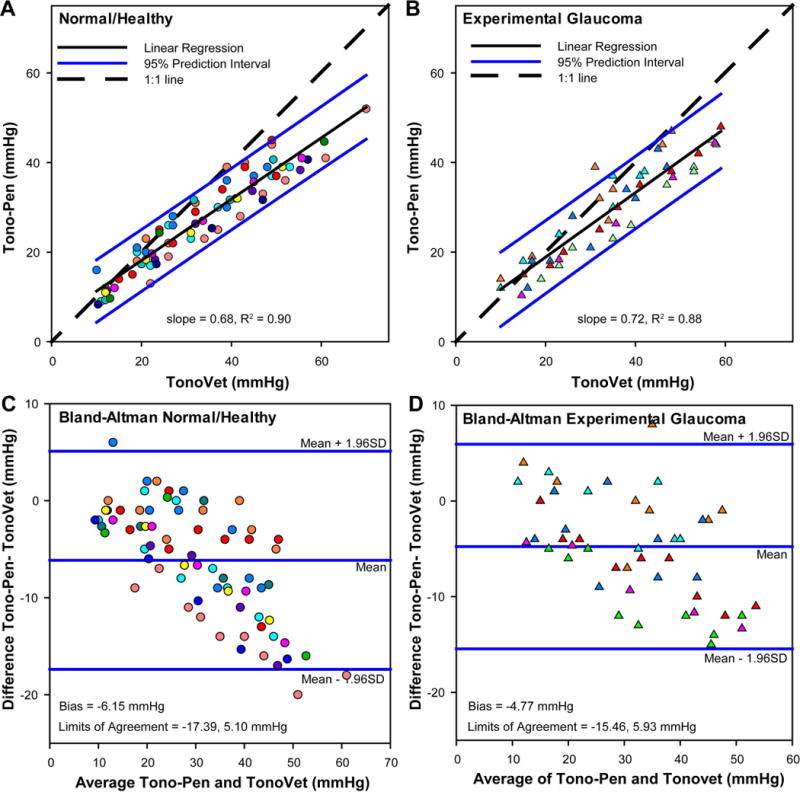
Scatter plots illustrating the comparison of Tono-Pen and TonoVet IOP measures in normal (A) and experimental glaucoma (B) eyes for all anterior chamber pressure settings. Bland-Altman plot for the agreement between the two tonometers in normal (C) and experimental glaucoma (D) eyes, illustrating the bias and 95% limits of agreement. Each animal is represented by a unique color.
Multiple regression analysis was used to test if the IOP error was related to the IOP setting and ocular biometry measures. The analysis showed that the difference of measured versus anterior chamber IOP was significantly (p<0.05) related to the IOP setting and central corneal thickness for Tono-Pen (F=25.63, p<0.01) and central corneal thickness for TonoVet (F=10.93, p<0.01). However, independently, the relationship between central corneal thickness and the mean difference for TonoVet measures was not statistically significant (p=0.08). However, the two animals with the largest difference seen on Fig 2A (pink circle) & 2B (green square), also had the thickest corneas. As Tono-Pen error was related to anterior chamber IOP, the slopes for error measures for individual animals was compared to central corneal thickness, and no significant relationship was found (p=0.99).
DISCUSSION
The main finding from this study was that in rhesus macaques, although IOP measures from Tono-Pen and TonoVet instruments typically were different, they were highly correlated. However, neither instrument had good accuracy when compared to the anterior chamber pressure settings. The precision of both tools for repeated measures on a single subject was better for lower IOPs and decreased with higher IOPs, but more remarkable are the larger inter-subject limits of agreement for both tonometers. Overall, the findings from this study support animal specific instrument calibration for experimental glaucoma studies.
For average anterior chamber IOP levels in healthy eyes (<20mmHg), the error in Tono-Pen measures was smaller. However, IOP was overestimated for pressures less than ~15mmHg, and underestimated for higher pressure settings. This finding is similar to that reported in the cynomologus monkey where the pressure crossing was between 17-25 mmHg.21 The data in figure 3 also illustrate the variability in true versus measured IOP. For example, the error in IOP measured at an intracameral pressure of 30 mmHg can range from 2 to −6mmHg. While these differences might seem small as a ratio or percentage of the true pressure, they exponentially add when computing the pressure-time insult to the nerve.
In contrast to the Tono-Pen, IOP error from rebound tonometry was not related to the anterior chamber IOP setting. However, there was greater variability in the measured error between subjects. Specifically, while the TonoVet accurately measured IOP for several animals, there was one subject for which the error exceeded 10 mmHg for all pressure settings. In addition, the data show that the variability in error increases with pressure (Figure 2). The results in this study are in contrast to a previous publication where excellent correspondence was reported, but was only tested in two animals.22 In a different species of non-human primate (Macaca fascicularis, n=3), the TonoVet was shown to have good correspondence with manometry, but underestimated IOP by 1.72 ±5.5 mmHg.
Central corneal thickness has been shown to be a factor influencing IOP measures using both the Tono-Pen and rebound tonometer in clinical populations.23, 24 In the present study, although central corneal thickness was identified on multiple regression analysis, it was not significantly related to IOP error on its own. However, it is noteworthy that the two animals with higher rebound tonometry error also were the animals with the thickest corneas. Overall, the lack of relationship might be a reflection on the number of subjects included in the study, and the strength of the relationship itself in the non-human primate.
The similarity in results from healthy and experimental glaucoma eyes would suggest that repeat calibration of tonometers on the same eye might not be necessary. It is possible that multiple repeat calibrations on the same eye might result in short term IOP damage to the optic nerve head that might confound longitudinal studies. However, because there are connective tissue biomechanical changes of the cornea, it is important that longitudinal calibration studies are done.
There are several limitations to this study. While central corneal thickness was measured prior to inserting the needle, change in corneal thickness with IOP was not investigated. Inserting a needle at the limbus has the potential of changing corneal biomechanics and, subsequently, the IOPs quantified by the instruments. Ideally pressure would be controlled through cannulation of the vitreous chamber, however this method carries added risk of complications that can confound findings in experimental glaucoma. In addition, all of the tonometry measurements were made by one user, and intra-user variability could not be assessed. Only one time point for all eyes was quantified, including the eyes with experimental glaucoma, and intersession variability was not investigated.
Based on the results of this study, tonometers used to monitor IOP in non-human primate experimental glaucoma should be calibrated to the individual eye. Although these clinical tools provide important methods to assess IOP, IOP is known to fluctuate during the day, and it is important that tools are developed to continuously and accurately monitor IOP in experimental models.25 The findings from this study can also be extended to clinical practice. While IOP measures from clinical tonometers relate well to intracameral pressure, there are significant inter-individual differences that prevent from using a single, simple formula to convert measured IOP to real IOP. However, monitoring change in IOP over time with or without treatment should follow similar trends in eyes with high or low error using Tono-Pen or rebound tonometry.
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: Baseline Factors That Predict the Onset of Primary Open-Angle Glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DR. Collaborative Normal Tension Glaucoma Study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. Factors for Progression and Glaucoma Treatment: The Early Manifest Glaucoma Trial. Curr Opin Ophthalmol. 2004;15:102–6. doi: 10.1097/00055735-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Hohman RM. Laser Energy Levels for Trabecular Meshwork Damage in the Primate Eye. Invest Ophthalmol Vis Sci. 1983;24:1305–7. [PubMed] [Google Scholar]
- 5.Gaasterland D, Kupfer C. Experimental Glaucoma in the Rhesus Monkey. Invest Ophthalmol. 1974;13:455–7. [PubMed] [Google Scholar]
- 6.Rasmussen CA, Kaufman PL. Primate Glaucoma Models. J Glaucoma. 2005;14:311–4. doi: 10.1097/01.ijg.0000169409.01635.bc. [DOI] [PubMed] [Google Scholar]
- 7.Harwerth RS, Smith EL, 3rd, DeSantis L. Experimental Glaucoma: Perimetric Field Defects and Intraocular Pressure. J Glaucoma. 1997;6:390–401. [PubMed] [Google Scholar]
- 8.Yang H, Thompson H, Roberts MD, et al. Deformation of the Early Glaucomatous Monkey Optic Nerve Head Connective Tissue after Acute IOP Elevation in 3-D Histomorphometric Reconstructions. Invest Ophthalmol Vis Sci. 2011;52:345–63. doi: 10.1167/iovs.09-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitacre MM, Stein R. Sources of Error with Use of Goldmann-Type Tonometers. Surv Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Roberts CJ. Influence of Corneal Biomechanical Properties on Intraocular Pressure Measurement: Quantitative Analysis. J Cataract Refract Surg. 2005;31:146–55. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton KE, Pye DC. Young’s Modulus in Normal Corneas and the Effect on Applanation Tonometry. Optom Vis Sci. 2008;85:445–50. doi: 10.1097/OPX.0b013e3181783a70. [DOI] [PubMed] [Google Scholar]
- 12.Elsheikh A, Alhasso D, Gunvant P, Garway-Heath D. Multiparameter Correction Equation for Goldmann Applanation Tonometry. Optom Vis Sci. 2011;88:102–12. doi: 10.1097/OPX.0b013e3181fc3453. [DOI] [PubMed] [Google Scholar]
- 13.Ehlers N, Bramsen T, Sperling S. Applanation Tonometry and Central Corneal Thickness. Acta Ophthalmol (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 14.Whitacre MM, Stein RA, Hassanein K. The Effect of Corneal Thickness on Applanation Tonometry. Am J Ophthalmol. 1993;115:592–6. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 15.Shimmyo M, Ross AJ, Moy A, Mostafavi R. Intraocular Pressure, Goldmann Applanation Tension, Corneal Thickness, and Corneal Curvature in Caucasians, Asians, Hispanics, and African Americans. Am J Ophthalmol. 2003;136:603–13. doi: 10.1016/s0002-9394(03)00424-0. [DOI] [PubMed] [Google Scholar]
- 16.Mark HH. Corneal Curvature in Applanation Tonometry. Am J Ophthalmol. 1973;76:223–4. doi: 10.1016/0002-9394(73)90164-5. [DOI] [PubMed] [Google Scholar]
- 17.Hessemer V, Rossler R, Jacobi KW. Tono-Pen, a New Tonometer. Int Ophthalmol. 1989;13:51–6. doi: 10.1007/BF02028638. [DOI] [PubMed] [Google Scholar]
- 18.Kontiola A. A New Electromechanical Method for Measuring Intraocular Pressure. Doc Ophthalmol. 1996;93:265–76. doi: 10.1007/BF02569066. [DOI] [PubMed] [Google Scholar]
- 19.Kontiola AI, Goldblum D, Mittag T, Danias J. The Induction/Impact Tonometer: A New Instrument to Measure Intraocular Pressure in the Rat. Exp Eye Res. 2001;73:781–5. doi: 10.1006/exer.2001.1088. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Measurement Error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JA, Kiland JA, Croft MA, Kaufman PL. Intraocular Pressure Measurement in Cynomolgus Monkeys. Tono-Pen Versus Manometry. Invest Ophthalmol Vis Sci. 1996;37:1197–9. [PubMed] [Google Scholar]
- 22.Yu W, Cao G, Qiu J, et al. Evaluation of Monkey Intraocular Pressure by Rebound Tonometer. Mol Vis. 2009;15:2196–201. [PMC free article] [PubMed] [Google Scholar]
- 23.Rao A, Kumar M, Prakash B, Varshney G. Relationship of Central Corneal Thickness and Intraocular Pressure by Icare Rebound Tonometer. J Glaucoma. 2014;23:380–4. doi: 10.1097/IJG.0b013e318279b819. [DOI] [PubMed] [Google Scholar]
- 24.Bhan A, Browning AC, Shah S, et al. Effect of Corneal Thickness on Intraocular Pressure Measurements with the Pneumotonometer, Goldmann Applanation Tonometer, and Tono-Pen. Investigative Ophthalmology & Visual Science. 2002;43:1389–92. [PubMed] [Google Scholar]
- 25.Downs JC. Iop Telemetry in the Nonhuman Primate. Exp Eye Res. 2015;141:91–8. doi: 10.1016/j.exer.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


