Abstract
Objective
Blood products are often transfused in critically ill children, although recent studies have recognized their potential for harm. Translatability to pediatric acute respiratory distress syndrome (PARDS) is unknown given that hypoxemia has excluded PARDS patients from clinical trials. We aimed to determine whether an association exists between blood product transfusion and survival or duration of ventilation in PARDS.
Design
Retrospective analysis of prospectively enrolled cohort.
Setting
Large, academic pediatric intensive care unit.
Patients
Invasively ventilated children meeting Berlin ARDS and PALICC PARDS criteria from 2011 to 2015.
Interventions
We recorded transfusion of red blood cells (RBC), fresh frozen plasma (FFP), and platelets within the first 3 days of PARDS onset. Each product was tested for independent association with survival (Cox) and duration of mechanical ventilation (competing risk regression with extubation as primary outcome and death as competing risk). A sensitivity analysis using 1:1 propensity matching was also performed.
Measurements and Main Results
Of 357 PARDS patients, 155 (43%) received RBC, 82 (23%) received FFP, and 92 (26%) received platelets. Patients that received RBC, FFP, or platelets had higher severity of illness score, lower PaO2/FiO2, and were more often immunocompromised (all p < 0.05). Patients who received RBC, FFP or platelets had worse survival and longer duration of ventilation by univariate analysis (all p < 0.05). After multivariate adjustment for above confounders, no blood product was associated with survival. After adjustment for the same confounders, RBC were associated with decreased probability of extubation (subdistribution hazard ratio 0.65, 95% CI 0.51 to 0.83). The association between RBC and prolonged ventilation was confirmed in propensity-matched subgroup analysis.
Conclusions
RBC transfusion was independently associated with longer duration of mechanical ventilation in PARDS. Hemoglobin transfusion thresholds should be tested specifically within PARDS to establish whether a more restrictive transfusion strategy would improve outcomes.
Keywords: ARDS, PARDS, transfusions, outcome
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a heterogeneous syndrome of diverse etiologies of hypoxemic respiratory failure. In pediatrics, it is uncommon, representing only 1–4% of pediatric intensive care unit (PICU) patients (1, 2), but is associated with up to 30% of PICU deaths (3). At present, there are no specific therapies, and management in children (4) and adults focuses on supportive care with lung-protective mechanical ventilation (5) and fluid-restriction (6). Transfusion of red blood cells (RBC) is frequently prescribed in order to maximize oxygen delivery during hypoxemia. However, there is increasing recognition of the negative consequences of RBC transfusion in critical illness, with studies showing increased inflammatory mediator release (7), development or worsening of lung injury (7–9), and increased mortality (9, 10). Leukoreduction has been shown to decrease, but not eliminate, pro-inflammatory effects and transfusion-related complications (11). The 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) explicitly recommended further studies assessing the risk and benefits of transfusions in pediatric ARDS (PARDS), as well as testing of specific transfusion thresholds (12).
Trials in critically ill adults (13) and children (14) have shown that a more restrictive transfusion threshold has no difference in outcome compared with more liberal thresholds. However, PARDS patients may be underrepresented in these studies due to significant hypoxemia and hemodynamic instability, making it difficult to extrapolate results to this population. Therefore, we aimed to study the impact of blood product transfusion on outcomes of PARDS. We hypothesized that blood products would be associated with higher mortality and increased duration of mechanical ventilation.
METHODS
Patient Population
This was a retrospective analysis of a prospectively enrolled PARDS cohort. The cohort has been described in detail before (2). Briefly, patients were screened daily in the Children’s Hospital of Philadelphia (CHOP) PICU for acute lung injury (American-European Consensus Conference definition)(15) between July, 2011 and June, 2015. As the study was initiated prior to the 2012 Berlin definition (16), a minimum positive end-expiratory pressure (PEEP) was not specified; however, as our institution does not utilize PEEP < 5 cmH2O, all patients met Berlin criteria for ARDS. Similarly, as the study was initiated prior to the 2015 PALICC definition for PARDS (4), we did not stratify severity using oxygenation index (OI), but by PaO2/FiO2; however, all patients met PARDS criteria by OI. This study was approved by the CHOP Institutional Review Board and requirement for informed consent was waived.
Specific inclusion criteria were 1) acute respiratory failure requiring invasive mechanical ventilation for more than 24 hours, 2) invasive arterial access, 3) age > 1 month and < 18 years, 4) PaO2/FiO2 ≤ 300 on two consecutive arterial blood gases separated by at least 1 hour, and 5) bilateral parenchymal infiltrates on radiograph. Exclusion criteria were 1) respiratory failure from cardiac failure (determined by echocardiography), 2) exacerbation of underlying chronic respiratory disease, 3) chronic ventilator dependence, 4) intracardiac shunt, 5) mechanical ventilation for more than 7 days before PaO2/FiO2 ≤300, and 6) PARDS established outside of the CHOP PICU.
Data Collection
RBC, fresh frozen plasma (FFP), and platelet transfusions were retrospectively abstracted during the first 3 days after PARDS diagnosis. Products were ordered at the discretion of the physicians caring for the patients with no pre-determined protocol. RBC and platelets were leukoreduced pre-storage, and irradiated prior to transfusion. All platelets were obtained by apheresis.
Definitions
The designation “immunocompromised” was given for patients with a potentially immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency, as we have previously done (17). Vasopressor score (18) was: dopamine (μg/kg/min) x 1 + dobutamine (μg/kg/min) x 1 + epinephrine (μg/kg/min) x 100 + norepinephrine (μg/kg/min) x 100 + phenylephrine (μg/kg/min) x 100 + vasopressin (U/kg/min) x 10,000 + milrinone (μg/kg/min) x 10. Non-pulmonary organ failures at time of PARDS diagnosis were identified using accepted pediatric definitions (19). Severity of illness score used was the Pediatric Risk of Mortality (PRISM) III at 12 hours.
Outcomes
The co-primary outcomes were 28-day all-cause mortality and duration of mechanical ventilation. All mention of “mechanical ventilation” in this study implies invasive ventilation, and non-invasive support was not counted toward total ventilator days. “Day 1” was initiation of invasive ventilation. Liberation from invasive ventilation for > 24 hours defined successful extubation. Ventilator-free days (VFD) at 28 days are also reported, and are equal to 28 minus the number of ventilator days, with non-survivors and ≥ 28 days of ventilation assigned VFD = 0.
Statistics
Analyses were performed in Stata 14. Data are expressed using either percentages or median values [IQR, inter-quartile ranges]. Differences between distributions of categorical variables were analyzed by Fisher’s exact test. Continuous variables were compared using Wilcoxon rank sum. A non-parametric test of trend was used to assess whether increasing volume of transfusion were associated with either higher mortality, duration of ventilation in survivors, or probability of extubation.
Multivariate Cox regression was used to test for an association between transfusions and 28-day mortality, after adjustment for PRISM III, immunocompromised status, and PaO2/FiO2 24 hours after PARDS onset. We have shown PRISM III and immunocompromised status to be associated with mortality and duration of ventilation (20), and PaO2/FiO2 at 24 hours accurately stratifies PARDS severity (2). Additional potential confounders with univariate association with mortality (p < 0.10, Table 1) were also considered. Non-pulmonary organ failures, vasopressor score, and fluid balance were all co-linear with PRISM III, and were not included given the low mortality rate and concerns for over-fitting. The proportional hazard assumption was tested by examination of scaled Schoenfeld residuals.
Table 1.
Characteristics of the PARDS Cohort
| Variable | All patients (n = 357) | Survivors (n = 310) | Non-survivors (n = 47) | p value |
|---|---|---|---|---|
| Age (years) | 4.1 [1.3, 12.1] | 3.9 [1.3, 11.4] | 6.3 [1.4, 15.1] | 0.132 |
| Female/male (%/%) | 154/203 (43/57) | 132/178 (43/57) | 22/25 (47/53) | 0.637 |
| PRISM III at 12 hours | 10 [5, 17] | 9 [5, 15] | 20 [11, 31] | < 0.001 |
| Immunocompromised (%) | 68 (19) | 43 (14) | 25 (53) | < 0.001 |
| Cause of PARDS (%) | ||||
| Infectious pneumonia | 203 (57) | 180 (58) | 23 (49) | 0.572 |
| Aspiration pneumonia | 34 (10) | 30 (10) | 4 (8.5) | |
| Non-pulmonary sepsis | 73 (20) | 62 (20) | 11 (23) | |
| Trauma | 26 (7) | 21 (7) | 5 (11) | |
| Other | 21 (6) | 17 (5) | 4 (8.5) | |
| Non-pulmonary organ dysfunctions at PARDS onset | 2 [1, 3] | 1 [1, 2] | 3 [2, 4] | < 0.001 |
| Vasopressor score | 9 [3, 18] | 8 [3, 16] | 17 [5, 30] | 0.004 |
| Fluid balance at 72 hours (mL/kg) | 90 [31, 175] | 88 [30, 170] | 121 [46, 265] | 0.017 |
| PARDS onset | ||||
| PaO2/FiO2 | 160 [113, 217] | 161 [118, 218] | 136 [81, 205] | 0.099 |
| OI | 10 [7, 16] | 10 [7, 15.5] | 11.8 [6.6, 25] | 0.107 |
| PIP (cmH2O) | 30 [25, 35] | 30 [25, 35] | 30.5 [28, 36.8] | 0.174 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 12] | 10 [8, 12] | 0.926 |
| ΔP (cmH2O) | 21 [17, 25] | 20 [17, 24] | 21 [18, 26] | 0.176 |
| VT (mL/kg) | 7.5 [6.7, 8.5] | 7.5 [6.7, 8.5] | 7.6 [6.5, 8.3] | 0.771 |
| 24 hours after PARDS onset | ||||
| PaO2/FiO2 | 226 [165, 280] | 231 [172, 284] | 174 [106, 236] | < 0.001 |
| OI | 6.8 [5, 10.9] | 6.5 [4.8, 10.5] | 9.2 [6.2, 19.6] | < 0.001 |
| PIP (cmH2O) | 27 [24, 32] | 27 [23, 31] | 30 [26, 35] | 0.005 |
| PEEP (cmH2O) | 10 [8, 10] | 10 [8, 10] | 10 [8, 12] | 0.051 |
| ΔP (cmH2O) | 18 [14, 22] | 17 [14, 22] | 21 [15, 23] | 0.020 |
| VT (mL/kg) | 7.3 [6.5, 8.1] | 7.3 [6.5, 8.1] | 7.1 [6.2, 8] | 0.617 |
| Transfusions at 72 hours (%) | ||||
| Any | 190 (53) | 153 (49) | 37 (79) | < 0.001 |
| RBC only | 71 (20) | 67 (22) | 4 (9) | 0.048 |
| FFP only | 11 (3) | 9 (3) | 2 (4) | 0.644 |
| Platelets only | 12 (3) | 12 (4) | 0 | 0.379 |
| RBC + FFP | 16 (4) | 13 (4) | 3 (6) | 0.453 |
| RBC + platelets | 25 (7) | 13 (4) | 12 (26) | < 0.001 |
| FFP + platelets | 12 (3) | 10 (3) | 2 (4) | 0.663 |
| RBC + FFP + platelets | 43 (12) | 29 (9) | 14 (30) | < 0.001 |
FFP: fresh frozen plasma; OI: oxygenation index; PARDS: pediatric acute respiratory distress syndrome; PEEP: positive end-expiratory pressure; PICU: pediatric intensive care unit; PIP: peak inspiratory pressure; ΔP: driving pressure (PIP minus PEEP); PRISM: Pediatric Risk of Mortality; RBC: red blood cells; VT: tidal volume; VFD: ventilator-free days
Because VFD is a composite endpoint incorporating both death and length of ventilation, it is a problematic endpoint from which to identify variables associated predominantly with ventilator duration. Thus, we did not analyze VFD. Additionally, using Kaplan-Meier or Cox regression analysis of time to extubation would censor a patient who dies before the end of the time period assuming non-informative censoring, which is likely a false assumption.
We therefore used competing risk regression to look at the association between blood product transfusion and duration of mechanical ventilation, using extubation as the primary outcome and death as the competing risk. Observations were censored at 28 days. As the two outcomes are interrelated (increased mortality decreases duration of ventilation), competing risk analysis using the Fine and Gray method (21) generates a cumulative incidence function and allows calculation of a subdistribution hazard ratio (SHR) to estimate the risk of extubation while accounting for the competing risk of death. This allows for an estimation of the hazard (the SHR) of transfusion on probability of extubation while explicitly accounting for the fact the mortality contributes to the decreased probability of extubation for patients who die. This is contrasted with traditional Cox regression, in which patients who die are censored, which is assumed (incorrectly in our case) to be non-informative. As with mortality analysis, the competing risk regression models were adjusted for PRISM III, immunocompromised status, and PaO2/FiO2 at 24 hours after PARDS onset. The proportional hazard assumption was assessed by testing for interaction with a time-dependent covariate.
Additionally, as we detected an association with RBC transfusion and outcome in our primary analyses, we performed propensity score matching to specifically examine the relationship of RBC transfusions and outcomes. We preferred propensity score matching over propensity score stratification due to the improved covariate balance with matching, even at the expense of reduced sample size and precision (22). We created a propensity score to estimate likelihood of RBC transfusion, using the variables age, PRISM III, immunocompromised status, non-pulmonary organ failures, vasopressor score, PaO2/FiO2 at PARDS onset and at 24 hours. Variables were chosen for univariate association with either RBC transfusion (Supplementary Table 1) or mortality (Table 1). Fluid balance at 72 hours was not included because it occurred entirely after the exposure (RBC transfusion in the first 72 hours). The vasopressor score (highest in first 24 hours) and PaO2/FiO2 at 24 hours were included because they were strongly associated with both RBC exposure and outcomes, and greatly improved the propensity score matching, despite being partly concurrent with RBC exposure. The C statistic for the propensity score was 0.78, suggesting good discrimination for probability of receiving RBC. The propensity scores were used to perform a 1:1 match using a greedy nearest-neighbor caliper (width 0.05) matching (without replacement) algorithm (23), with only successfully matched pairs used for analysis. Greedy nearest-neighbor cycles through subjects one at a time, matching an RBC transfused subject with the best available non-transfused, even if that non-transfused subject is a better match for a later RBC transfused subject. “Without replacement” implies that an RBC transfused subject is matched only to a single non-transfused subject. Balance of covariates was assessed after matching using both standardized mean differences (SMD) and p values.
RESULTS
Description of the Cohort
Between 2011 and 2015, 357 patients with PARDS were enrolled. Demographics of the cohort are summarized in Table 1. Infectious pneumonia, non-pulmonary sepsis, and aspiration pneumonia were the most common triggers for PARDS. Overall mortality was 13%, and 19% of patients were immunocompromised. As expected, non-survivors had a higher PRISM III score, were more likely to be immunocompromised, had more organ failures, and had worse PaO2/FiO2 at 24 hours.
Demographics of transfusion groups are shown in Supplementary Table 1. Of the 357 patients, 155 received RBC (43%), 82 received FFP (20%), and 92 received platelets (22%) within 72 hours of PARDS onset. Patients getting RBC, FFP, and platelets were all more likely to be immunocompromised (p < 0.001), have a higher PRISM (p < 0.001) and vasopressor scores (p < 0.001), more non-pulmonary organ dysfunction (p < 0.001), lower PaO2/FiO2 at 24 hours after PARDS diagnosis (p < 0.05), and worse outcomes (Supplementary Table 2). Of patients receiving RBC, the median pre-transfusion hemoglobin was 7.8 g/dL [7.1, 8.6] and the median post-transfusion hemoglobin was 11.0 g/dL [10, 12.1].
Primary Analysis of the Association between Transfusions and Outcomes
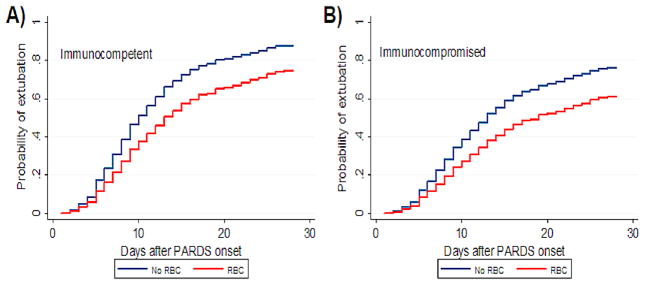
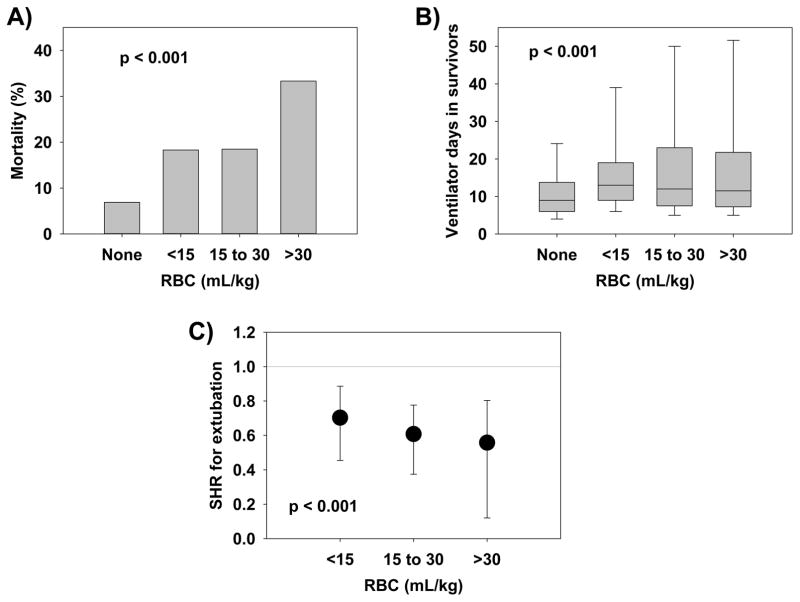
After controlling for confounders, neither RBC (HR 2.01, 95% CI 0.96 to 4.22, p = 0.063), FFP (HR 0.98, 95% CI 0.44 to 2.21, p = 0.963) nor platelet transfusion (HR 1.10, 95% CI 0.47 to 2.60, p = 0.822) demonstrated independent association with mortality (Table 2). In competing risk regression (Table 3), transfusion of RBC was associated with a decreased likelihood of being successfully extubated (SHR 0.65, 95% CI 0.51 to 0.83, p = 0.001; Figure 1). Neither FFP nor platelets showed independent association with likelihood of extubation. A dose-response curve was generated for RBC transfusion in 15 mL/kg increments (Figure 2), which showed stepwise increase in mortality (p < 0.001), increased ventilator days among survivors (p < 0.001) and decrease in SHR for successful extubation (p < 0.001) with greater incremental volume of transfusion. Transfused patients had significantly lower hemoglobin values than non-transfused patients (p < 0.001) but there were no differences between the different levels of transfusion either before transfusion (p > 0.05) or after transfusion (p > 0.05) (Supplementary Table 3).
Table 2.
Cox regression for 28-day survival (hazard ratio > 1 means associated with worse survival)
| Model | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Any transfusion | |||
| PRISM III at 12 hours | 1.08 | 1.05 to 1.11 | < 0.001 |
| Immunocompromised (yes) | 2.83 | 1.45 to 5.56 | 0.002 |
| PaO2/FiO2 at 24 h (per increase in 20) | 0.92 | 0.84 to 1.00 | 0.045 |
| Any transfusion (yes) | 1.33 | 0.55 to 3.21 | 0.524 |
| Any RBC | |||
| PRISM III at 12 hours | 1.08 | 1.05 to 1.10 | < 0.001 |
| Immunocompromised (yes) | 2.83 | 1.49 to 5.40 | 0.002 |
| PaO2/FiO2 at 24 h (per increase in 20) | 0.93 | 0.86 to 1.02 | 0.110 |
| Any RBC (yes) | 2.01 | 0.96 to 4.22 | 0.063 |
| Any FFP | |||
| PRISM III at 12 hours | 1.08 | 1.05 to 1.11 | < 0.001 |
| Immunocompromised (yes) | 3.06 | 1.55 to 6.04 | 0.001 |
| PaO2/FiO2 at 24 h (per increase in 20) | 0.91 | 0.84 to 0.99 | 0.038 |
| Any FFP (yes) | 0.98 | 0.44 to 2.21 | 0.963 |
| Any platelets | |||
| PRISM III at 12 hours | 1.08 | 1.05 to 1.11 | < 0.001 |
| Immunocompromised (yes) | 2.92 | 1.39 to 6.13 | 0.030 |
| PaO2/FiO2 at 24 h (per increase in 20) | 0.91 | 0.84 to 1.00 | 0.039 |
| Any platelets (yes) | 1.10 | 0.47 to 2.60 | 0.822 |
FFP: fresh frozen plasma; PRISM: Pediatric Risk of Mortality; RBC: red blood cells
Table 3.
Competing risk regression for successful extubation (subdistribution hazard ratio > 1 means associated with earlier extubation) by 28 days after pediatric acute respiratory distress syndrome onset
| Model | Subdistribution hazard ratio | 95% CI | p value |
|---|---|---|---|
| Any transfusion | |||
| PRISM III at 12 hours | 0.98 | 0.96 to 0.99 | 0.001 |
| Immunocompromised (yes) | 0.66 | 0.46 to 0.94 | 0.020 |
| PaO2/FiO2 at 24 h (per increase in 20) | 1.11 | 1.09 to 1.14 | < 0.001 |
| Any transfusion (yes) | 0.79 | 0.63 to 1.01 | 0.056 |
| Any RBC | |||
| PRISM III at 12 hours | 0.98 | 0.96 to 0.99 | 0.001 |
| Immunocompromised (yes) | 0.69 | 0.48 to 0.98 | 0.037 |
| PaO2/FiO2 at 24 h (per increase in 20) | 1.11 | 1.08 to 1.14 | < 0.001 |
| Any RBC (yes) | 0.65 | 0.51 to 0.83 | 0.001 |
| Any FFP | |||
| PRISM III at 12 hours | 0.97 | 0.96 to 0.99 | < 0.001 |
| Immunocompromised (yes) | 0.62 | 0.43 to 0.89 | 0.009 |
| PaO2/FiO2 at 24 h (per increase in 20) | 1.11 | 1.09 to 1.15 | < 0.001 |
| Any FFP (yes) | 0.99 | 0.72 to 1.37 | 0.970 |
| Any platelets | |||
| PRISM III at 12 hours | 0.97 | 0.96 to 0.99 | 0.001 |
| Immunocompromised (yes) | 0.65 | 0.45 to 0.94 | 0.024 |
| PaO2/FiO2 at 24 h (per increase in 20) | 1.12 | 1.08 to 1.15 | < 0.001 |
| Any platelets (yes) | 0.90 | 0.64 to 1.26 | 0.526 |
FFP: fresh frozen plasma; PRISM: Pediatric Risk of Mortality; RBC: red blood cells
Figure 1.
Probability of successful extubation by 28 days after pediatric acute respiratory distress syndrome (PARDS) onset, given the competing risk of death (subdistribution hazard ratio 0.65, 95% CI 0.51 to 0.83). Patients that received red blood cells (RBC; red) and those that did not receive RBC (blue). Curves are adjusted for Pediatric Risk of Mortality (PRISM) III and PaO2/FiO2 at 24 hours after PARDS onset, and shown separately for (A) immunocompetent and (B) immunocompromised patients.
Figure 2.
Dose response curves showing the associations between increasing amounts of red blood cells (RBC) and (A) mortality, (B) ventilator days among survivors, and (C) subdistribution hazard ratio (SHR) from competing risk regression reflecting probability of extubation. RBC doses are expressed as milliliters per kilogram (mL/kg) of actual body weight.
Propensity Score Analysis
Additionally, we developed a propensity score for RBC transfusion and performed 1:1 matching. One hundred ninety-eight subjects (n = 99 per group) were matched. The matched cohort demonstrated good balance of covariates, with reduction in SMD after matching (Supplementary Tables 4 and 5). Unmatched patients (Supplementary Table 6) were older (6.2 years 3.5 years, p = 0.021), had lower vasopressor scores (8 versus 10, p = 0.036), fewer organ failures (1 versus 2, p = 0.012), shorter ventilator duration (9 versus 11 days, p = 0.030), and were over-represented at the lowest (least likely to receive RBC) and highest (most likely to receive RBC) propensity score distribution (Supplementary Figure 1). The 99 matched patient pairs demonstrated no difference in mortality (12% RBC versus 8% no RBC, p = 0.480; Supplementary Table 4 and Supplementary Figure 2). However, the RBC transfused cohort had longer duration of ventilation in survivors (12 versus 10 days, p =0.023), fewer VFD (15 versus 18 VFD, p = 0.025), and a decreased likelihood of extubation given the competing risk of death (SHR 0.73, 95% CI 0.54 to 0.99, p = 0.044; Supplementary Figure 2).
Sensitivity Analyses
Because of the possibility of ongoing bleeding confounding the relationship between RBC transfusion and outcomes, we excluded 14 patients in whom RBC transfusions were recorded for hemorrhage or post-operative bleeding. Results were consistent with the primary analysis: no association was seen with RBC transfusion and survival (HR 1.78, 95% CI 0.82 to 3.84, p = 0.148), whereas an association with reduced probability of extubation was confirmed (SHR 0.67, 95% CI 0.52 to 0.86, p = 0.002).
Finally, as patients with refractory PARDS requiring extracorporeal membrane oxygenation (ECMO) frequently require RBC transfusions at ECMO initiation, we excluded the 15 patients placed on ECMO and repeated the analyses. Results remained consistent, with a marginal association with increased mortality (HR 2.08, 95% CI 1.00 to 4.34, p = 0.050) and a consistent association with reduced probability of extubation (SHR 0.69, 95% CI 0.54 to 0.87, p = 0.002) with RBC transfusions.
DISCUSSION
In this PARDS cohort, there was an increase in duration of ventilation in patients transfused RBC compared with those patients who were not transfused after controlling for known risk markers for poor outcomes. There was no association of any blood product with mortality. Results were confirmed in propensity-matched subgroups and sensitivity analyses. Further, increasing amounts of RBC were associated with incrementally worse outcomes. This supports the notion that transfusion in an attempt to increase oxygen delivery early in the course of PARDS may have adverse effects on the overall disease course.
Previous literature has revealed conflicting results with regards to outcomes associated with transfusions of blood products in PARDS. One single center study showed a small worsening of oxygenation index after RBC transfusion, but as with our cohort, a longer duration of mechanical ventilation (24). An older single center study of transfusion in PARDS, on the other hand, showed increased mortality only in association with FFP, with only a trend toward mortality and increased duration of mechanical ventilation with RBC. However, multivariate adjustment was not done for severity of illness in that study (25).
There are several possible explanations for the results presented here. Previous studies in PARDS have shown that increasing fluid positivity early in PARDS patients is associated with increased mortality and fewer ventilator free days (26). In our cohort, those transfused blood products had a more positive fluid balance at 72 hours, but fluid balance was not independently associated with either mortality (HR 1.00, 95% CI 0.99 to 1.00, p = 0.907) or likelihood of successful extubation (SHR 1.00, 95% CI 0.99 to 1.00, p = 0.638), after adjusting for PRISM, immunocompromised status, and PaO2/FiO2.
Another possible explanation would be that RBC transfusion imparts additional injury to the lung. Transfusion related acute lung injury (TRALI) is a well described phenomenon in which respiratory distress develops within 6 hours post transfusion of blood products (27). Mechanistically, it is thought to occur via either a single hit involving donor antibodies that activate recipient neutrophils or via a double hit etiology in systemically inflamed patients. The first hit involves upregulation of vascular adhesion molecules on the pulmonary endothelium in the setting of systemic inflammation, leading to a setup for stimulus-induced pulmonary dysfunction via activation of sequestered neutrophils (27). When RBC are transfused, antibodies from the small amount of plasma transfused with red blood cells or biologic mediators such as microlipids produced as a result of the storage process induce these primed polymorphonuclear cells to release proinflammatory mediators such as IL-6, IL-8, phospholipase A2, and reactive oxygen species, leading to capillary leak syndrome, and lung injury (28). This could add additional hits to injured lungs, worsening existing PARDS early in the course of disease and thereby prolonging the duration of the disease and mechanical ventilation with it. If TRALI is the primary etiology for the demonstrated prolonged ventilation, it is notable that FFP and platelets did not demonstrate associations with outcomes, as FFP has a higher incidence of TRALI than RBC in prior studies (29). However, associations between FFP and TRALI are largely historical, and modern blood banking practices, such as limited use of female donors, have greatly reduced the incidence of TRALI (30). Additionally, it is possible we were underpowered to detect an association between FFP or platelets and outcomes, as substantially fewer patients received these products, relative to RBC.
It seems possible, therefore, that transfused RBC may negatively influence PARDS through multiple mechanisms. Recently, it has been recognized that RBC play a significant role in the control of vasoactivity through release or sequestration of nitric oxide via compounds called S-nitrosothiols (SNOs) during oxygen loading and unloading (31). It has further been demonstrated that in a porcine model of hypoxemia, there is a deficiency of SNO-hemoglobin, which impairs vasodilator ability, manifested in the lung as worsening ventilation/perfusion (V/Q) mismatch and increased pulmonary vascular resistance due to unopposed vasoconstriction (32). Storage of RBC has a similar effect in depleting SNO-Hgb, with up to 80% lost by 7 days (33). It is possible that transfusion of low SNO-Hgb cells may exacerbate deficiencies in V/Q matching and PVR, leading to worsening oxygenation impairment and the need to provide further ventilator support to overcome this deficit, leading to the potential for further ventilator induced lung injury. In addition to preclinical data and data from adults with ARDS, there is accumulating evidence in critically ill pediatric patients that transfusion may negatively impact respiratory function. Two recent prospective studies showed in a cohort of critically ill PICU patients an association between transfusion of RBC and increased new or worsening respiratory dysfunction as measured by PaO2/FiO2, and an increased risk of new organ dysfunction, compared with those who were not transfused (34, 35).
These data question the safety of transfusing RBC in the PARDS population outside of the indication of severe anemia. The Transfusion Strategy for Patients in PICU (TRIPICU) study (14) showed no difference in development of subsequent multi-system organ dysfunction when comparing a liberal threshold and a restrictive transfusion threshold among pediatric patients with critical illness, though this study was not restricted to PARDS. The present data, in combination with these recent studies of blood transfusions early on in critical illness, suggests that it would be important to assess whether RBC transfusions actually improve tissue oxygenation in PARDS, and whether a more restrictive transfusion threshold would be appropriate even in the setting of the significant hypoxemia seen in PARDS.
Because of the retrospective nature of the study, indications for transfusions were not confidently recorded. It is possible that the association between RBC and prolonged ventilation reflects a reluctance to extubate patients with significant bleeding. However, we believe this not to sufficiently explain our findings. First, the exposure we tested was limited to the first 3 days after PARDS onset, whereas subjects were ventilated for a median 10 [IQR 6, 16] days. Second, only a minority of patients (14 of 155, 9%) exposed to RBC were transfused for bleeding, and results of our sensitivity analysis excluding these subjects were consistent with the primary analysis. A future prospective cohort study specifically querying the indication for RBC transfusion in PARDS could more appropriately address this limitation.
The present study represents the largest dataset to date examining an association between transfusion and respiratory outcomes in PARDS patients using modern blood banking practices and detailed data collection. However, several important limitations must be noted. As this study was conducted at a single center, conclusions drawn from the data may not be generalizable, though it did provide for more homogeneity in management. Patient demographics, illness severity, and transfusion practices were also similar to other published data sets. Mortality in our cohort was low (13%), although consistent with that reported by recent PARDS cohorts (36, 37). This may have rendered us underpowered to confidently claim that RBC transfusion had no association with mortality. Analysis of the exposure (transfusions) was restricted to the first 3 days after PARDS onset, and we did not assess the impact of later transfusions. While this is consistent with prior analyses in critically ill children (9), this limits the generalizability of our conclusions regarding the association between RBC and prolonged ventilation. We also analyzed individual blood products recognizing that these patients received combinations of blood products, which complicates the ability to draw conclusions about the effect of transfusing individual blood products. This does, however, represent real world conditions as critically ill patients often receive multiple, sometimes simultaneous transfusions of blood products. Transfusion decisions were also left up to the judgment of the caring providers, leading to heterogeneous thresholds and indications. While this also represents real world practice, confounding by indication is a possibility. Transfused patients may represent the more severely ill, and while statistical methods cannot address unmeasured confounding, we attempted to minimize bias by performing a both multivariate analysis and propensity matching, as well as sensitivity analyses. While the conclusion regarding duration of ventilation was confirmed, the effect size was attenuated in the propensity-matched cohort. This should be interpreted with caution, as only 55% of patients could be matched, with only 20 deaths, thereby reducing both power and precision at the expense of improved covariate balance between RBC-transfused and non-transfused subjects. We also did not have detailed data regarding the age of blood for our patient population, though studies have thus far not shown a difference in outcomes with less fresh blood (38–40).
CONCLUSIONS
In PARDS patients, RBC transfusion is associated with prolonged duration of mechanical ventilation. Future studies should focus on whether tissue oxygenation improves after transfusion in PARDS and whether a lower transfusion threshold in PARDS patients would impact outcomes.
Supplementary Material
Distribution of propensity scores in successfully matched (solid) and not matched (dash) subjects. Subjects which were not matched were over-represented at the lowest (least likely to receive RBC) and highest (most likely to receive RBC) extremes of propensity scores.
(A) Probability of survival and (B) probability of extubation by 28 days after pediatric acute respiratory distress (PARDS) onset, given the competing risk of mortality, for the propensity-matched subgroups (n = 99 per group), showing patients that received (red) and did not receive (blue) red blood cell (RBC) transfusions. Receipt of RBC was associated with reduced probability of extubation (subdistribution hazard ratio [SHR] 0.73, 95% CI 0.54 to 0.99).
Acknowledgments
Financial Support: NIH K12-HL109009 (NY); NIH K23-136688 (NY)
Footnotes
Plan for Reprints: No
Institution: Children’s Hospital of Philadelphia
Copyright form disclosure: Dr. Thomas’ institution received funding from GeneFluidics, and he received funding from Therabron and CareFusion. Dr. Friedman received funding from Cord Blood of America and Kline Theroux law firm. Dr. Yehya’s institution received funding from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute, and he received support for article research from the NIH. Dr. Zubrow has disclosed that he does not have any potential conflicts of interest.
References
- 1.Santschi M, Jouvet P, Leclerc F, et al. Acute lung injury in children: Therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11:681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 2.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43:937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 3.Schouten LR, Veltkamp F, Bos AP, et al. Incidence and mortality of acute respiratory distress syndrome in children: A systematic review and meta-analysis. Crit Care Med. 2016;44:819–829. doi: 10.1097/CCM.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 4.P. A. L. I. C. C. Group. Pediatric acute respiratory distress syndrome: Consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.N. Acute Respiratory Distress Syndrome. Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.L. National Heart, N. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 7.Fransen E, Maessen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–1239. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 8.Zilberberg MD, Carter C, Lefebvre P, et al. Red blood cell transfusions and the risk of acute respiratory distress syndrome among the critically ill: A cohort study. Crit Care. 2007;11:R63. doi: 10.1186/cc5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bateman ST, Lacroix J, Boven K, et al. Anemia, blood loss, and blood transfusions in north american children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 10.Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: Potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 11.Fergusson D, Hebert PC, Lee SK, et al. Clinical outcomes following institution of universal leukoreduction of blood transfusions for premature infants. JAMA. 2003;289:1950–1956. doi: 10.1001/jama.289.15.1950. [DOI] [PubMed] [Google Scholar]
- 12.Valentine SL, Nadkarni VM, Curley MA, et al. Nonpulmonary treatments for pediatric acute respiratory distress syndrome: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16:S73–85. doi: 10.1097/PCC.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 13.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, canadian critical care trials group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, et al. The american-european consensus conference on ards. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med. 2014;15:e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11:234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 20.Yehya N, Servaes S, Thomas NJ, et al. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med. 2015;41:1658–1666. doi: 10.1007/s00134-015-3953-4. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Elze MC, Gregson J, Baber U, et al. Comparison of propensity score methods and covariate adjustment: Evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69:345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasekaran S, Sanfilippo D, Shoemaker A, et al. Respiratory impairment after early red cell transfusion in pediatric patients with ali/ards. Crit Care Res Pract. 2012;2012:646473. doi: 10.1155/2012/646473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Church GD, Matthay MA, Liu K, et al. Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009;10:297–302. doi: 10.1097/PCC.0b013e3181988952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flori HR, Church G, Liu KD, et al. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 2011;2011:854142. doi: 10.1155/2011/854142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: Epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 28.Vlaar AP, Hofstra JJ, Determann RM, et al. Transfusion-related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: A prospective nested case-control study. Crit Care Med. 2012;40:2813–2820. doi: 10.1097/CCM.0b013e31825b8e20. [DOI] [PubMed] [Google Scholar]
- 29.Silliman CC. Transfusion-related acute lung injury. Transfus Med Rev. 1999;13:177–186. doi: 10.1016/s0887-7963(99)80031-5. [DOI] [PubMed] [Google Scholar]
- 30.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: Incidence and risk factors. Blood. 2012;119:1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doctor A, Platt R, Sheram ML, et al. Hemoglobin conformation couples erythrocyte s-nitrosothiol content to o2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of s-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander JT, El-Ali AM, Newman JL, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53:2619–2628. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleiber N, Lefebvre E, Gauvin F, et al. Respiratory dysfunction associated with rbc transfusion in critically ill children: A prospective cohort study. Pediatr Crit Care Med. 2015;16:325–334. doi: 10.1097/PCC.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 35.Demaret P, Tucci M, Karam O, et al. Clinical outcomes associated with rbc transfusions in critically ill children: A 1-year prospective study. Pediatr Crit Care Med. 2015;16:505–514. doi: 10.1097/PCC.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 36.Spicer AC, Calfee CS, Zinter MS, et al. A simple and robust bedside model for mortality risk in pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2016;17:907–916. doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parvathaneni K, Belani S, Leung D, et al. Evaluating the performance of the pediatric acute lung injury consensus conference definition of acute respiratory distress syndrome. Pediatr Crit Care Med. 2017;18:17–25. doi: 10.1097/PCC.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 38.Lacroix J, Hebert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–1418. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 39.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. Long-term blood storage on mortality after transfusion. N Engl J Med. 2016;375:1937–1945. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 40.Fergusson DA, Hebert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: The aripi randomized trial. JAMA. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of propensity scores in successfully matched (solid) and not matched (dash) subjects. Subjects which were not matched were over-represented at the lowest (least likely to receive RBC) and highest (most likely to receive RBC) extremes of propensity scores.
(A) Probability of survival and (B) probability of extubation by 28 days after pediatric acute respiratory distress (PARDS) onset, given the competing risk of mortality, for the propensity-matched subgroups (n = 99 per group), showing patients that received (red) and did not receive (blue) red blood cell (RBC) transfusions. Receipt of RBC was associated with reduced probability of extubation (subdistribution hazard ratio [SHR] 0.73, 95% CI 0.54 to 0.99).