Abstract
Personalized genetic testing for vulnerability to mental disorders is expected to become increasingly common. It is therefore important to understand whether learning about one’s genetic risk for a mental disorder has negative clinical implications, and if so, how these might be counteracted. Among participants with depressive symptoms, we administered a sham biochemical test purportedly revealing participants’ level of genetic risk for major depression. Participants told that they carried a genetic predisposition to depression expressed significantly lower confidence in their ability to cope with depressive symptoms than participants told they did not carry this predisposition. A short intervention providing education about the non-deterministic nature of genes’ effects on depression fully mitigated this negative effect, however. Given the clinical importance of patient expectancies in depression, the notion that pessimism about one’s ability to overcome symptoms could be exacerbated by genetic information—which will likely become ever more widely available— represents cause for concern. Education and counseling about the malleability of genetic effects may be an important tool for counteracting clinically deleterious beliefs that can be evoked by genetic test results. Genetic counselors may be able to help patients avoid becoming demoralized by learning they have a genetic predisposition to depression by providing education about the non-deterministic role of biology in depression, and a brief audiovisual intervention appears to be an effective approach to delivering such education.
Keywords: Depression, Genetics, Health Beliefs, Prognostic Pessimism, Biological Explanations
Introduction
Personalized health-related genetic information is increasingly accessible to the general public, as demonstrated by a growing market for direct-to-consumer genetic testing. This development has been both hailed as a promising trend with the potential to benefit public health and criticized as an alarming development that risks a variety of harmful consequences (Hogarth, Javitt, & Melzer, 2008; McBride, Koehly, Sanderson, & Kaphingst, 2010).
On one hand, personalized genetic information could allow for accurate prediction of individualized risk levels and customization of intervention approaches. As a result, it has been argued that such information could motivate positive changes in health behaviors (but see Hollands et al., 2016) and inform better interventions and treatment plans (Drmanac, 2011; McBride et al., 2010)
On the other hand, some have expressed concern that direct-to-consumer genetic testing results could be misunderstood or be misleading to members of the public, resulting in problematic healthcare decisions and harmful health beliefs (Hogarth et al., 2008). In particular, it has been argued that genetic causal attributions can lead to “prognostic pessimism” — the belief that symptoms are relatively permanent (Kvaale, Haslam, & Gottdiener, 2013; Phelan, Yang, & Cruz-Rojas, 2006).
The reason that receiving personalized genetic test results may cause prognostic pessimism is explained by the theory of “genetic essentialism.” Genetic essentialism refers to laypeople’s widely held, false belief that DNA represents the fundamental, immutable essence of a person (Dar-Nimrod & Heine, 2011; Haslam, 2011). As such, a genetic essentialist perspective on health would be to view DNA as an unchangeable underlying cause that gives rise to symptoms deterministically and permanently. For example, the more individuals attributed their own depressive symptoms to neurochemical and genetic factors, the more pessimistic they were about their own prognoses (Lebowitz, Ahn, & Nolen-Hoeksema, 2013).
Nonetheless, speculation about the potential harms of receiving personalized genetic test results has outpaced the available evidence (Caulfield, Chandrasekharan, Joly, & Cook-Deegan, 2013). The current study examines the effects of receiving genetic feedback about depression susceptibility and, more centrally, how to mitigate potential harms to individuals who might adopt negative assumptions based on learning that they have a genetic predisposition to depression. Here, we first discuss critical gaps in the literature and how the current research addresses them.
First, little evidence exists demonstrating that receiving personalized information about genetic susceptibility to health problems actually causes prognostic pessimism [but see below (Dar-Nimrod, Zuckerman, & Duberstein, 2013) for a rare exception)]. Indeed, a recent review concluded that studies had failed to demonstrate that receiving personalized genetic risk information decreases people’s perceived control over their own risk (Collins, Wright, & Marteau, 2011), but there are methodological complications with the studies reviewed.
For example, some studies merely had participants pretend that they had tested positive for elevated genetic susceptibility to a certain disease (Frosch, Mello, & Lerman, 2005; Sanderson, Persky, & Michie, 2010). Although manipulation checks indicated that participants could do so, it is difficult to argue that the psychological impact of such imaginary scenarios would measure up to those of reacting to genetic testing perceived as real (Persky, Kaphingst, Condit, & McBride, 2007).
Other studies that failed to find evidence of genetic essentialism used results from actual genetic testing, but they tested participants who already knew that they had high familial risk. For instance, one study recruited adults who had already been clinically diagnosed with familial hypercholesterolemia and manipulated only whether they additionally learned personalized information about their genetic risk of hypercholesterolemia (Marteau et al., 2004). The additional feedback about their genetic susceptibility may not have affected levels of fatalism merely because participants already held fatalistic beliefs about that disorder due to their knowledge of its hereditary etiology.
Given the small number of these studies and their methodological limitations, further investigation of the effect of personalized genetic information on prognostic pessimism is needed. Indeed, the aforementioned review that synthesized these findings noted that the existing evidence was sparse and that additional research is needed (Collins et al., 2011).
To address this need, the present research utilized factitious genetic testing to randomly determine which participants were told that their test results indicated increased genetic susceptibility. This random assignment enhances our ability to draw causal conclusions from the results, as it allows us to conclude that any effects of receiving “genetic feedback” are due to the genetic information itself (as opposed to actual genetic differences between participants).
Using similar factitious genetic testing methods, a recent study demonstrated negative consequences of receiving genetic feedback (Dar-Nimrod et al., 2013). Participants in the study took a purported genetic test for alcoholism susceptibility. Individuals who supposedly tested positive for the risk-conferring allele expressed more willingness to enroll in a workshop on responsible drinking. Consistent with genetic essentialism, however, they also perceived themselves to have less control over their drinking behaviors, compared to individuals who supposedly tested negative.
The current study extends these methods to examine how genetic feedback about depression susceptibility affects individuals with depressive symptoms. It has been noted that “certain individuals or groups may be more sensitive and, thus, more likely to react poorly to genetic ‘bad news’” (Caulfield et al., 2013, p. 23). The present study is motivated, in part, by the possibility that depressed individuals may be such a group. That is, general pessimism is a feature of depression, and depressed individuals show a tendency to become preoccupied by negative information (Gotlib & Joormann, 2010). In particular, heightened processing of self-relevant negative information has been posited to be a signature of cognition in depression (Beck & Clark, 1988). Genetic test results indicating elevated susceptibility to a health problem may be an example of the type of self-relevant negative information that depressed individuals are prone to processing more deeply, and liable to have more difficulty disengaging from, than non-depressed individuals. As such, the clinical implications of the questions addressed by the present research are especially pronounced in this population, motivating us to focus on these individuals.
We attempt to demonstrate, for the first time, the potential impact of receiving personalized genetic feedback about depression susceptibility, using tightly controlled experimental methods. The present study focused on studying such effects among individuals who screened positive for significant levels of depressive symptomatology. After carrying out the factitious genetic test, some participants were randomly told that they were genetically susceptible to depression, while others were told that they were not. We hypothesized that, due to genetic essentialism, participants told they had a genetic predisposition to depression would feel less control over their mood compared to those who learned that they are not genetically susceptible to depression.
Another major contribution of the current study is that we examine an intervention strategy to mitigate negative effects of learning that one is genetically predisposed to depression, which is likely to be particularly relevant in a genetic counseling context. If learning about one’s genetic susceptibility to depression does have the potential to bring about harms such as prognostic pessimism, particularly among people with symptoms of depression, these consequences would be clinically problematic because patients’ outcome expectancies are a key determinant of actual prognosis (Meyer et al., 2002; B. R. Rutherford, Wager, & Roose, 2010). For example, in one study, 90% of depressed patients who expected a treatment to be “very effective” responded positively to that treatment, compared to only 33.3% of patients who expected the treatment to be less effective (Krell, Leuchter, Morgan, Cook, & Abrams, 2004). Clearly, while concerns about the negative consequences of genetic testing for susceptibility to psychiatric disorders have not been definitively substantiated, they are potentially serious. Given the possibility that receiving “bad news” from a genetic test could have at least some negative consequences, particularly among people with pre-existing depression— and the likelihood that personalized psychiatric genomics will become increasingly common—it is notable that little research has addressed how such potential negative effects can be reduced.
One strategy for reducing psychological harm stemming from psychiatric genetic test results, to the extent that potential for such harm exists, is to counteract essentialist biases (Lebowitz et al., 2013). Specifically, by teaching laypeople about the non-deterministic nature of gene effects (e.g., because of gene-by-environment interactions and epigenetics) and related neurobiology (i.e., because of neuroplasticity), we sought to dispel fatalism about the role of genes in depression’s etiology. This strategy was designed to take advantage of insights from a long line of theory and research demonstrating that people’s mindsets can powerfully shape how they respond to adversity. Specifically, people who believe that their own personal characteristics are predetermined and unchanging (a “fixed mindset”) may respond to negative experiences with feelings of resignation or helplessness, whereas those who view their characteristics as malleable (a “growth mindset”) are more likely to be resilient (Dweck, 2012). In particular, there is considerable evidence that teaching people about the malleability of an individual’s social and psychological state can help them to view adversity as temporary and surmountable, leading to a variety of benefits, including more hopeful outlooks and significantly improved motivation, mental health, and wellbeing (Blackwell, Trzesniewski, & Dweck, 2007; Miu & Yeager, 2014; Walton & Cohen, 2011).
In the present study, we tested an intervention designed to modify individuals’ beliefs about the malleability of depressive symptoms by teaching them that even in the context of biological vulnerability, depression is not immutable (Lebowitz & Ahn, 2015; Lebowitz et al., 2013). We tested the ability of such an educational intervention, focused on various ways in which genes’ effects on depression can be moderated, to mitigate negative effects of receiving test results purportedly indicating an elevated genetic risk of major depressive disorder. We reasoned that if this intervention was found to be effective, such a finding would be of particular relevance to situations in which people receive genetic test results related to their health risk and are provided with education to help them interpret this feedback, such as in genetic counseling settings.
We did not test the effect of this intervention among participants who were told that they were not genetically predisposed to depression because we did not hypothesize that such feedback would have negative consequences, and therefore there does not appear to be a strong need for an intervention. Given that the current experiment involves deception, which needs to be offset by potential scientific benefits, we chose not to test the effect of the intervention after telling people that they did not have elevated genetic susceptibility, as there does not appear to be much benefit to be gained.
To summarize, the first aim of the present study was to examine whether, for people with elevated levels of depressive symptomatology, receiving purported biological test results indicating heightened genetic susceptibility to major depression would yield decreased optimism about overcoming their symptoms, compared to test results indicating the absence of such heightened genetic susceptibility. We hypothesized that such an effect would, in fact, emerge, due to genetic essentialism. The second aim, which was the principal goal of the research, was to test the effectiveness of our educational intervention in combatting such harmful consequences by undermining genetic essentialism. Among the participants led to believe they tested positive for increased genetic susceptibility to depression, some received the intervention, while others did not. We hypothesized that the intervention would effectively mitigate the negative effects of genetic predisposition feedback.
Use of Deception
The present research, which was approved by the Institutional Review Board, involved leading participants to believe they were receiving personalized information about whether or not they were genetically predisposed to depression. Providing this kind of misleading information to participants raises important ethical issues, so we sought to minimize the deception’s duration and to avoid harming participants.
According to the Ethical Principles of Psychologists and Code of Conduct, deception may be used in research of substantial prospective value when “effective nondeceptive alternative procedures are not feasible,” the work is not “reasonably expected to cause physical pain or severe emotional distress,” and the deception is revealed to participants “as early as is feasible, preferably at the conclusion of their participation” (American Psychological Association, 2017, p. 11). As discussed above (as well as in “Discussion and Conclusions”), we aimed to examine the impact of receiving individualized genetic feedback per se, without true genomic differences among participants as a potential confounding variable, so it was essential to determine what kind of “genetic feedback” participants received through random assignment (i.e., some deception was unavoidable). That is, in order to study the effects of personalized information about genetic susceptibility to depression, it was necessary to provide such information to participants and gauge their responses, but using true information about genetic susceptibility would have meant that participants in the different conditions would have had different mood-related genetic predispositions. For instance, those who are indeed genetically susceptible to depression might be more likely to be pessimistic in general than those who are not genetically susceptible to depression. In such a case, it would have been impossible to discern whether any differences between the conditions in how individuals responded to the genetic information stemmed from the genetic feedback itself, or merely the pre-existing differences between the individuals in each condition. As a result, the only way to test the effects of genetic feedback, independent of true genetic differences, was to randomly assign participants to receive a certain type of genetic feedback, regardless of their true genetic makeup, resulting in deception. Because genomic testing is expected to become increasingly widespread in healthcare, we judged the benefit of investigating its impact and devising intervention strategies to be significant enough to justify brief deception.
Given the unique advantages of experimental designs for drawing causal conclusions about the effects of personally relevant information on health-related perceptions, study designs in which participants are briefly misled about their own health status have been used in psychology for more than 30 years (Jemmott, Ditto, & Croyle, 1986). The costs and benefits of using deception in empirical research in general have received considerable attention in the literature. Some objections to the use of deception are based on the notion that deception harms participants by decreasing their ability to trust “fiduciaries” (e.g., psychologists; p. 169) and depriving them of a right to self-determination, as they may choose to participate in an experiment without fully understanding what the experiment entails (Baumrind, 1985). Some authors have also argued that deceiving research participants can trigger suspicion that in turn can alter their responses in a way that impairs experimental control, although there is disagreement about whether such effects are substantial or only negligible (Hertwig & Ortmann, 2008; Ortmann & Hertwig, 2002). Notably, empirical research has shown that when participants are told (after completing an experiment) that they were deceived during the experimental procedures, they typically do not report negative psychological reactions to the deception, and participants often feel positively about research that involves deception (Uz & Kemmelmeier, 2016). In particular, participants may find participating in deception-based research to be more enjoyable and interesting, and to have more educational value, than participating in nondeceptive research (Uz & Kemmelmeier, 2016). Factors such as the content of the false information provided to participants (rather than the fact that it was false) or the level of professionalism of an experimenter (rather than whether or not she engaged in deception) appear to be more potent determinants of participants’ reactions than deception itself (Boynton, Portnoy, & Johnson, 2013; Epley & Huff, 1998).
Nonetheless, to protect our participants from potential harms stemming from the deception involved in the present study, we provided a full debriefing after their participation, to dispel any false beliefs that could have been established as a result of the false genetic feedback, in a process that employed special procedures that are unusual for a psychology experiment (see below). Participants were free to discontinue their participation at any time, with no risk of missing out on compensation, yet the completion rate was 99.5% (as detailed below), and no adverse events were reported.
Methods
Recruitment
U.S. adults were recruited via Amazon.com’s Mechanical Turk (MTurk) service, which allows individuals to complete short tasks in exchange for payment (Buhrmester, Kwang, & Gosling, 2011). Participants were recruited via a two-stage process. First, they had the opportunity to provide a mailing address in exchange for $1 and were told that they would receive, by mail, materials necessary to complete the full study and would be paid an additional $10 if they did so. Then, we sent the instructions for completing the study online and the necessary materials (see Participants and Procedures) to all of the mailing addresses provided (N=1000). Twelve parcels were returned due to invalid mailing addresses, and we received a total of 790 responses (i.e., individuals who provided informed consent to participate), of whom 786 (99.5%) continued to the end of the study (see Participants and Procedures for information about how the final sample was culled from these).
Participants and Procedures
All procedures involving human participants were approved by the institutional review board and were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments.
For each participant, we created a “saliva testing kit” (see Figure 1) containing a test strip and a small plastic container full of mouthwash. The test strip and the mouthwash cup were both enclosed in sealed plastic bags, which were enclosed within a hinged plastic container affixed with the label depicted in Figure 1.
Figure 1.
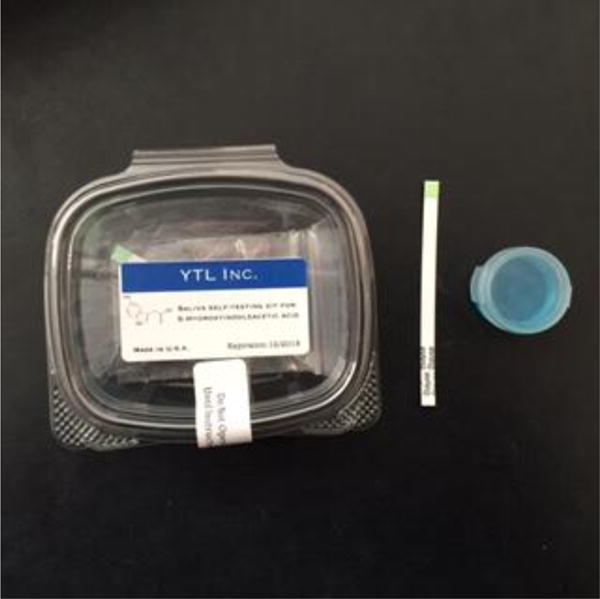
Photograph of a fully assembled “saliva testing kit” (left), as well as the test strip (middle) and mouthwash container included within (right).
All study procedures were administered using Qualtrics.com online data-collection software. Participants received their saliva testing kit in an envelope accompanied by a letter explaining how to access the study procedures online from their home computers and instructing them to do so when they had sufficient time to spare and the ability to complete the study in a quiet and private location. Participants were instructed not to open the saliva testing kit until prompted by onscreen instructions.
When participants accessed the online procedures, they first provided informed consent via a consent form displayed onscreen (informed consent was obtained from all individual participants included in the study). The consent form stated: “The purpose of the study is to learn about people’s beliefs, feelings, and attitudes related to genetic testing. You will answer some questions about your mood and how you have been feeling lately. You will then be provided with some information and answer more questions about what you think and feel. Since we are interested in your natural impressions, there are no right or wrong answers to the questions we are asking.” The consent form did not mention that participants would undergo a “genetic test” as part of the study because it would not have been ethical to include deceptive statements in the consent form. That is, if we had mentioned genetic testing in the consent form that participants would undergo genetic testing (or any biomedical test), some participants could have been persuaded to consent to participate in the study specifically because they were under the false impression that they would receive valuable medical information as part of their participation. To avoid the clear ethical problems inherent in such a possibility, the consent form contained no mention of any biomedical testing.
Participants were also told, “You have the right to withdraw from this study at any time or skip any part of the procedure that makes you uncomfortable. Doing so will not affect your ability to be compensated for agreeing to participate.” Except for the informed consent, participants were not required to provide responses in any part of the experiment, and thus upon learning about the “saliva test,” participants were free to skip to the end of the experiment if they did not wish to carry out the saliva test.
The study itself necessarily involved some deception of participants, as disclosing to participants (before the end of their participation) that the genetic feedback they were receiving was generated at random would likely have rendered our results invalid. Participants were therefore not informed of the fact that the test results were false until the debriefing phase (described below), which occurred after the study was complete. In light of this fact, the consent form informed participants that “full information about the study will be provided to you after your participation.” The consent form included an email address for one of the researchers and encouraged participants to contact the experimenter with any questions or concerns. At the bottom of the online consent form was an “Agreement to Participate” that stated “Agreement to Participate: I have read the above information, have had the opportunity to ask any questions about this study I may have, and I agree to participate in this study.” Below was a check-box accompanied by the words “I agree.” Participants could not proceed to participate in the study unless they checked this box.
Because the current study examines effects of genetic feedback on people with elevated levels of depressive symptomatology, we first measured their levels of depressive symptoms. Immediately after providing informed consent, all participants completed a well validated and widely used measure of depressive symptomatology, the Beck Depression Inventory-II (BDI-II), on which higher scores indicate greater symptom severity (Dozois, 2010). We omitted one item, “Suicidal Thoughts or Wishes,” because the online administration of our procedures precluded appropriate responses to reports of suicidality.1 We wished to minimize the potential that this omission would reduce the sensitivity of the instrument, so we used 13 as our cutoff for elevated depressive symptomatology, rather than 14, which is the standard cutoff for at least “mild” levels of depression (Dozois, 2010). Out of the 786 MTurk respondents who completed the study, 267 (or 34.0%) met this criterion. These participants were told: “Based on your answers to the preceding questions, it seems that you are feeling sad, blue, or depressed. Please keep this in mind as you answer the questions on the following screens.” This was done because we wanted participants’ ratings to reflect their judgments about their current depressive symptoms. Participants who scored below 13 on the BDI-II did not receive the above feedback and completed the rest of the study in essentially the same way. (See Supplemental Materials for details of the procedure followed by these participants, as well as their demographics and results.)
Next, participants were told that recent scientific research had shown that some genes can influence a person’s risk of developing depression, and that as part of their participation in the study, they would undergo a test to determine whether they have genes that play a role in causing “Major Depressive Disorder.” Then, the data-collection software randomly assigned each participant, automatically, to one of three conditions — the Gene-Absent condition, the Gene-Present condition, or the Gene-Present/Intervention condition — which are explained in detail below and summarized in Figure 2.
Figure 2.
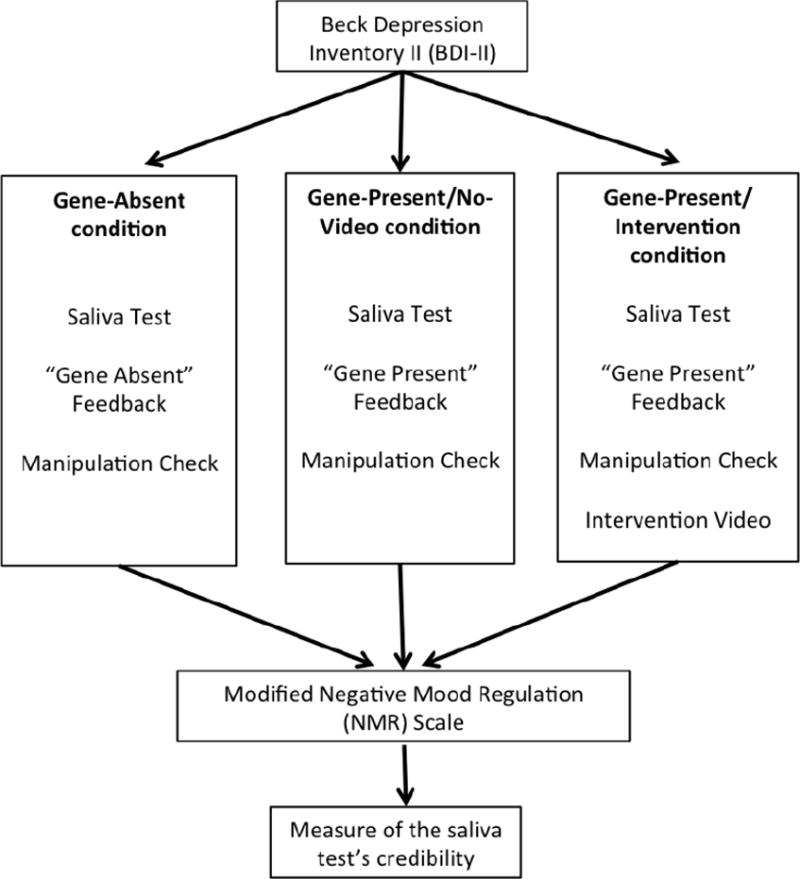
Summary of study design and procedures.
All participants were instructed to open their saliva testing kit, rinse their mouths with the enclosed mouthwash, and then insert the test-strip below their tongues for several seconds. Unbeknownst to the participants, the mouthwash had been mixed with glucose powder. The test-strip was actually sensitive to glucose (Jemmott et al., 1986), but participants were told that it was sensitive to the serotonin metabolite 5-Hydroxyindoleacetic acid, which participants were led to believe can be detected in saliva as an indicator of one’s genetic risk for major depression. The glucose contained in the mouthwash caused the glucose-sensitive area of the test-strip to turn from blue to brownish green for all participants. However, the data-collection software asked participants to indicate whether the strip had turned “Brown or Green,” “Red or Pink,” or “White” to suggest to participants that their saliva test could have yielded different results, depending on their genetic risk for depression. If a participant chose a response other than “Brown or Green”, reflecting either inattentiveness or equipment failure, their data were removed from our analyses. Of the 267 participants with elevated BDI-II scores, 8 participants’ data were removed for this reason, leaving 259 participants.
Upon indicating that their test strip had turned “brown or green,” participants in the Gene-Present and Gene-Present/Intervention conditions were told that this revealed “abnormally low levels of 5-Hydroxyindoleacetic acid” in their saliva, signifying that “your DNA contains a gene that has been shown to significantly increase a person’s risk of developing Major Depression.” They were further told that their test results indicated that they carried the “short” form of the serotonin transporter gene, which “can cause a chemical imbalance in the brain involving the neurotransmitter serotonin, which is important in mood” and “is associated with changes in brain structure, especially in areas of the brain important for emotion, such as the amygdala.” Participants in the Gene-Absent condition received the same background information but were told that their test result showed “normal low levels of 5-Hydroxyindoleacetic acid” and that this indicated they did not carry the susceptibility gene.
Next, all participants were told that they would be asked questions involving “Major Depressive Disorder” and received a brief explanation of what the disorder is, including its 16.5% lifetime prevalence and average age of onset of 32 (in the U.S.)
All participants then completed two manipulation-check items; “What do you think the odds are (from 0% to 100%) that you are currently experiencing an episode of Major Depression or will experience such an episode at some point in the future?” and “What do you think the odds are (from 0% to 100%) that your child or children will suffer from Major Depression at some point? (If you do not currently have children, please answer this question imagining that you have one or more children at some point in the future.)” Among participants who were told that their DNA increased their risk of depression, we reasoned that those who actually believed this should have provided higher ratings than those who were told the opposite.
Next, participants in the Gene-Present/Intervention condition watched a short audiovisual presentation (7 minutes and 53 seconds in length) providing education about the malleability of biological factors involved in causing depression, with a special emphasis on the non-deterministic nature of genes. For example, it explained that “genetics alone can never make someone depressed” and that “even if a person has a genetically identical twin with depression, most of the time that person will not become depressed.” It also included a primer on epigenetics, informing participants that “even if a person has depression-related genes, these genes may not be active” and presenting information about how epigenetic tags can result in genes being “turned ‘on’ or ‘off.” Additionally, the video discussed gene-by-environment and gene-by-gene interactions, focusing on how even genes that appear to increase susceptibility to depression may have no such effects if the individual possessing them is exposed to positive environments and experiences or carries other genes that reduce vulnerability. Thus, the intervention shared some characteristics of approaches that have been recommended for psychiatric genetic counseling, such as the jar model (in which genetic and environmental factors are represented as different kinds of objects that combine to fill a jar, and a disorder is said to occur when the jar becomes full) (Meiser et al., 2016). Our intervention’s discussion of the interactive (rather than merely additive) effects of genetic and environmental factors, as well as its emphasis on how environments and experiences can be chosen specifically to counteract genetic susceptibility—were particularly novel. (The intervention video is available at www.youtube-nocookie.com/embed/hupQ_kkJXrg).
After watching the video, participants in the Gene-Present/Intervention condition were asked to “write a few sentences about the information you learned from the video,” and were told, “we hope that what you write can be used or quoted in information given to others receiving genetic test results similar to the results you just received.” They were asked to “summarize the information in the video and give at least a few examples of how you (or somebody else) might use the information you learned to prevent or overcome depression.” This part of the procedure took advantage of the so-called “Saying is Believing” effect, in which people come to internalize a point of view and believe it more strongly when they have advocated it themselves (Higgins, 1999; Lebowitz et al., 2013; Walton & Cohen, 2011)
Participants in the Gene-Absent and Gene-Present conditions were not presented with any audiovisual information. While the provision of genetic test results to healthcare consumers without accompanying education and professional support is not standard practice in clinical settings currently, one major concern in the genetic counseling and human genetics fields is the possibility that direct-to-consumer genetic testing could lead to such situations becoming more commonplace (Harris, Kelly, & Wyatt, 2013; Hudson, Javitt, Burke, Byers, & Committee, 2007). In this way, the Gene-Absent condition simulated potential real-life cases wherein people might receive genetic test results unaccompanied by any educational video. (See Figure 2 for the summary of differences in procedures among the three conditions.)
All participants then completed a modified version of the Negative Mood Regulation (NMR) scale (Catanzaro & Mearns, 1990; Kemp, Lickel, & Deacon, 2014). which was the main dependent measure of the study. The NMR scale measures how well one expects to be able to regulate (i.e., control) one’s negative mood states in the future. We used it to gauge a potential determinant of prognostic pessimism: the extent to which participants in the various conditions differed in expectations about their ability or inability to overcome future experiences of depression. While the manipulation-check items might be considered a more direct measure of participants’ prognostic expectations, as they explicitly asked participants to rate their perceptions of likelihood of future episodes of depression, we reasoned that the NMR scale would be less susceptible to demand characteristics. That is, after telling some participants that they had an elevated likelihood of experiencing depression and others that they did not have such an elevated likelihood, the finding that the former group rated themselves as more likely to experience depression in the future than the latter group could be considered merely an indication that participants understood the test results provided to them. The NMR, by contrast, provides insight into a psychological mechanism by which test results could engender prognostic pessimism.
The original NMR scale consists of a sentence stem (“When I’m upset, I believe that…”) and 30 items, each of which asks respondents to rate their agreement with a potential clause to complete the stem (e.g. “I can do something to feel better”). The scale is intended to measure one’s agency to regulate their negative moods in general, so we adapted it for the current study in order to use it as a measure of participants’ feelings of agency regarding their depressive symptoms. For example, because the study was focused on participants with elevated BDI-II scores reflecting on their current symptoms, we removed “When I’m upset” from the stem and simply used “I believe that…”. We also removed items involving actions whose effectiveness in combating depression may not be obvious to laypeople (e.g. “Doing something nice for someone else”), as we intended participants’ responses to reflect their expectations about their own agency in reacting to depression, rather than their belief in the effectiveness of particular behaviors in regulating negative moods generally. Finally, some items were modified to make them more relevant for depression (e.g., from “I can find a way to relax” to “Reducing my stress will help cheer me up”). The adapted version contained 17 items (Cronbach α=.90 in our sample), which are reproduced in the Supplemental Materials.
After completing the modified NMR scale and before completing optional demographic questions, participants rated their perceptions of the saliva test’s credibility by rating their agreement with the following statement: “The test I underwent as part of today’s study gave accurate and reliable information about my genetic makeup.” Response options were “Strongly Disagree,” “Disagree,” “Neither Agree nor Disagree,” “Agree,” and “Strongly Agree.” Among participants who scored at least 13 on BDI-II and correctly rated the color on the test strip as having changed to “brown or green” after the saliva test (N=259, as noted above), a majority (N=165; 63.71%) selected “Agree” or “Strongly Agree.”
For the final sample, we utilized only these 165 participants. This sample was 51.5% male and had a mean age of 31.79 years (SD=9.79). See Supplemental Materials for further details and analyses of demographic data, as well as analyses of data from participants who were excluded due to low BDI-II scores or low ratings of the saliva test’s credibility.
The decision to exclude participants who did not rate the saliva test as accurate and reliable was based on a desire to approximate the effects of actual genetic testing. In the present study, participants were aware that they were participating in a psychological experiment, and a significant proportion may therefore have harbored suspicion about the possibility of deception in our methods (Ortmann & Hertwig, 2002), including possible suspicion about the authenticity of the saliva test. By contrast, the likelihood that recipients of real genetic testing would similarly suspect that the testing methods or results were not real is presumably much smaller.2 Thus, including participants who discredited the saliva test could have attenuated our experimental manipulations, potentially resulting in underestimation of genetic feedback’s effects (see Supplemental Materials for supporting evidence).
In addition, there were no demographic variables that distinguished participants who found the saliva test credible from those who did not (nor any that significantly predicted scores on our main dependent measure; see Supplemental Materials for details). As such, our primary analyses, reported here, focused only on those participants who had correctly rated the color on the test strip as having changed to “brown or green” after the saliva test, scored at least 13 on the BDI-II, and rated the saliva test results as credible.
Debriefing
At the end of the study, participants underwent a thorough debriefing process, in which they were fully debriefed as to the factitious nature of the saliva test they had undergone and were informed about treatment options for depression (the debriefing included a link to an online directory for finding mental-health treatment). The debriefing also included contact information for the researchers and for the Institutional Review Board. This debriefing information was delivered onscreen to participants. Because the study was conducted online, meaning that there was no opportunity for an experimenter to debrief participants in person and gauge their understanding of the debriefing’s contents, we added a special extra step to the debriefing process. Specifically, at the end of the debriefing, participants were required to correctly rate three statements as “true or false,” to ensure that they had understood the debriefing. All three statements were true, in order to be sure that the debriefing did not contain any untrue or deceptive language. The three statements were: “No actual genetic testing was performed on me as part of today’s study,” “No actual information about my genetic make-up or my risk for depression (or any other illness) has been uncovered as part of today’s study,” and “Treatment is available for depression and other psychological difficulties.” To ensure that participants would not skip this step, we programmed the procedure so that participants could not receive compensation for their participation until they had answered the “true or false” questions correctly. After they correctly answered these questions, a unique “completion code” was displayed by the online data-collection software; participants had to input this completion code into the MTurk system in order to receive compensation. Displayed along with the completion code was a text-only version of the educational intervention about the non-deterministic role of genes in depression. All participants therefore eventually received the intervention in some form.
Data Analysis
Our primary analyses—which were conducted among the 165 participants who scored at least 13 on the BDI-II, correctly indicated that the test trip changed to “brown or green” after coming in contact with their saliva, and selected “Agree” or “Strongly Agree” in response to the statement about the saliva test’s credibility—are reported in the Results section below. These include t-tests confirming that the genetic-feedback manipulation successfully affected participants’ beliefs about their susceptibility to depression (and that of their genetic descendants), as well as a univariate ANOVA (and follow-up pairwise comparisons with independent-samples t-tests as well as more conservative Dunnet t-tests) examining the effect of condition on NMR scores.
We report additional analyses in the Supplemental Materials. These include an analysis of the effect of condition among BDI-II high-scorers (i.e., participants scoring at least 13 on the BDI-II) who did not rate the saliva test as credible, analyses of credibility ratings and BDI-II scores by condition among BDI-II high-scorers, and the relationship between credibility ratings and demographic factors among BDI-II high-scorers. Other analyses reported in the Supplemental Materials concern the effect of condition on NMR scores among BDI-II low-scorers (i.e., participants scoring below 13 on the BDI-II) who did and did not rate the saliva test as credible, as well as whether demographic factors could have moderated the effects of condition on NMR scores.
Results
We first used independent-samples t-tests to examine whether participants’ ratings on the manipulation-check items differed as a function of whether they were told they did or did not carry a gene heightening their susceptibility to depression. That is, we pooled participants in the Gene-Present (N=54) and Gene-Present/Intervention (N=53) conditions and compared them to participants in the Gene-Absent condition (N=58). This revealed that participants in the two Gene-Present conditions rated the odds that they were currently experiencing major depression or would in the future (M=78.52%, SD=25.33) as significantly higher than did those in the Gene-Absent condition (M=54.69%, SD=32.36), t(163)=5.22, p<.001, d=.82 (95% C.I. [.49, 1.15]). This difference emerged despite the fact that all participants in the sample had scored at least 13 on the BDI-II. In addition, participants in the two Gene-Present conditions rated the odds that their (extant or future) children would someday suffer from major depression (M=63.41%, SD=23.93) as significantly higher than did those in the Gene-Absent condition (M=43.41%, SD=25.97), t(163)=4.97, p<.001, d=.80 (95% C.I. [.47, 1.13]). These results suggest that our manipulations were successful.
Next, we examined effects on our dependent variable. A univariate ANOVA revealed a significant effect of condition on NMR scores, F(2,162)=9.07, p<.001 (see Figure 3), ηp2 =.10, observed power = .973. This effect remained significant when controlling for BDI-II scores as a covariate, F(2, 161)=8.00, p<.001, ηp2 =.09, observed power = .953.
Figure 3.
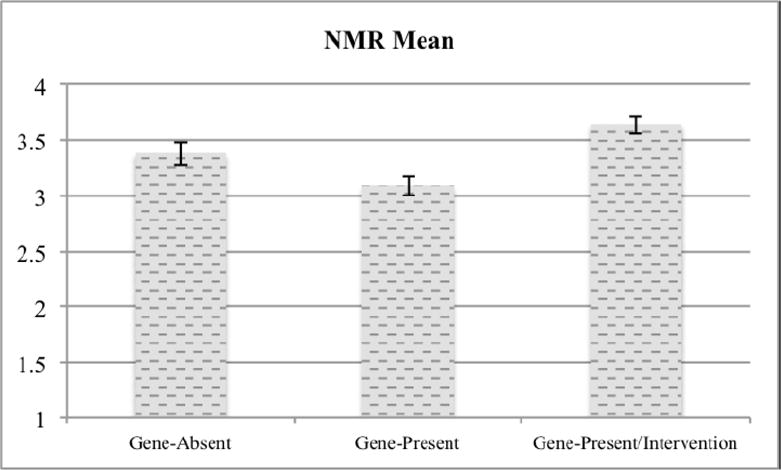
Mean NMR scale scores by condition.
Pairwise comparisons showed that NMR scores were higher in the Gene-Absent condition (M=3.38, SD=.77) than in the Gene-Present condition (M=3.09, SD=.64), t(110)=2.16, p=.03, d=.41 (95% C.I. [.03, .79]). In other words, among people with elevated levels of depressive symptomatology who were provided with (and convinced by) genetic test results regarding their risk of major depressive disorder, the contents of those test results appeared to alter feelings of agency in regulating their own moods. Compared to people told that they did not carry an allele increasing their risk of developing major depression, those told they did carry such a gene expressed reduced confidence in their own mood-regulation abilities.
However, a short audiovisual intervention demonstrated effectiveness in mitigating these negative effects. That is, NMR scores were significantly higher in the Gene-Present/Intervention condition (M=3.65, SD=.59) than in the Gene-Present condition, t(105)=4.67, p<.001, d=.90 (95% C.I. [.50, 1.31]).
To assess whether the pairwise comparisons of NMR scores remained significant when using more conservative statistical methods that adjust for multiple comparisons, we also conducted two-sided Dunnet t-tests comparing the Gene-Present condition to each of the other conditions. Even this more conservative approach found that mean NMR scores were significantly lower in the Gene-Present condition compared to both the Gene-Absent condition (p=.045) and the Gene-Present/Intervention condition (p=.045).
Discussion and Conclusions
Depression and other mental disorders are increasingly conceptualized as stemming from genetic and other biological causes (Pescosolido et al., 2010). Increased understanding of psychiatric genetics may necessitate new approaches to genetic counseling for mental disorders (Austin & Honer, 2007; Gershon & Alliey-Rodriguez, 2013). Additionally, direct-to-consumer genetic testing services purporting to offer information about risk for psychiatric disorders are expected to become increasingly common (Couzin, 2008). Given these shifts, the possibility that personalized genetic test results indicating increased risk for depression can deleteriously affect people’s confidence in their ability to respond adaptively to depressive symptoms—as our results suggest, consistent with our hypothesis—creates cause for concern. This notion is especially alarming because believing in one’s likelihood of overcoming depression can be a self-fulfilling prophecy (B. R. Rutherford et al., 2010), which highlights the potential negative clinical consequences of abandoning such beliefs.
However, our results suggest—also as hypothesized—that a brief psychoeducation intervention about the non-deterministic nature of genes can help to overcome the potential harms of learning that one’s genes entail increased risk of major depression. Notably, the intervention used in the present study was successful in increasing participants’ confidence in their ability to respond effectively to depressive symptoms, whereas existing evidence suggests that it may be difficult to increase individuals’ sense of control over their psychiatric symptoms using standard genetic counseling (Hippman et al., 2016). Our results provide evidentiary support for the recommendation that genetic counseling for mental disorders emphasize the potential of choice and environmental factors to decrease risk even when genes are involved in a disorder’s etiology (Austin & Honer, 2005). These findings complement existing research that suggests that genetic counseling interventions, including those delivered with genetic test results, can enhance patients’ adherence to medical recommendations, presumably (at least in part) because such interventions help people to understand how choosing to follow these recommendations can help to mitigate their genetic risks (Aspinwall, Taber, Leaf, Kohlmann, & Leachman, 2013; Madlensky et al., 2017; S. Rutherford, Zhang, Atzinger, Ruschman, & Myers, 2014; Taber et al., 2015). Our results also add to the existing literature demonstrating the psychological benefits of brief interventions framing negative experiences and difficulties as temporary and surmountable (Blackwell et al., 2007; Walton & Cohen, 2011).
The present research has several important strengths. First, we used a true experimental design, randomly assigning participants as to what their purported genetic test results would say, as well as whether they would view the intervention. This random assignment allowed more powerful causal conclusions to be drawn from the results, because the “test results” received by participants were less likely to be confounded with their actual levels of symptomatology than they might be in true genetic testing. That is, if people whose actual genetic test results indicated increased risk of depression were found to be less confident in their ability to regulate their moods and levels of depression than others not found to carry such elevated genetic risk, it would be unclear whether such a pattern was due to the test results or to a psychological difference stemming from the actual genetic difference between the groups. Our experimental design, by contrast, allows us to conclude more confidently that the between-group differences we observed were actually caused directly by the saliva test results (and the accompanying intervention) themselves.
Our use of individuals with actual symptoms of depression represents another important strength of the present research. The advent of direct-to-consumer genetic testing may increase the frequency with which people learn that they are genetically predisposed to disorders of which they do not have symptoms, but compared to the average person, individuals with symptoms of a disorder are nonetheless presumably more likely to undergo genetic testing related to their risk for that disorder, which increases the real-world applicability of our findings. In addition, in the case of depression, there is particular clinical importance in knowing the psychological impact of such genetic test results and of an intervention aiming to counteract their potential harms. Indeed, even among people with elevated levels of depressive symptoms—that is, people whose outlooks are particularly likely to be characterized by pessimism and hopelessness—our intervention was effective.
Study limitations
One limitation of the present research is that it did not include a baseline condition in which participants receive no genetic feedback. Therefore, our demonstration of negative effects of the gene-present feedback on NMR scores are limited to the observation that NMR scores of participants who received the “gene-present” feedback were lower relative to those of participants who received the “gene-absent” feedback. Our data also do not permit us to determine whether the gene-absent feedback had positive effects on NMR scores, as we did not include a baseline condition for comparison. Additionally, the study did not include a condition in which participants viewed the intervention video after receiving the gene-absent feedback, so our analyses cannot fully isolate the effects of the intervention from the effects of the test results.
The present study also examined the impact of genetic test results, and of the intervention, only immediately after participants received them. A potential consequence of this limitation is that the extent of the clinical significance of these effects is not entirely clear from our data. For example, longer-term follow-up with clinical measures would allow for a more robust understanding of the significance and durability of any negative effects that might stem from receiving personalized genetic susceptibility information. This was not ethically permissible in the current study because the genetic feedback we presented to participants was not authentic, but future research could take up this important question. Some recent research does suggest that the benefits of an intervention like the one we used can last beyond the immediate term (Lebowitz & Ahn, 2015). However, an important unanswered question is how genetic test results indicating susceptibility for depression, as well psychoeducation interventions like the one used in the present study, might affect people’s responsiveness to treatment for depression.
Furthermore, the present research evaluated the effects of delivering the intervention after participants received their saliva test results. It may be possible to achieve significant benefits by delivering such an intervention—or providing other forms of psychiatric genetic counseling—before individuals receive personalized genetic feedback, so that their initial interpretation of the test result’s meaning can be formed in the context of knowledge about the non-deterministic role of genes in the etiology of mental disorders.
Additionally, the present studies dealt only with depression and tested only one type of educational intervention. However, mental disorders vary widely in the extent to which they are conceptualized as stemming from genetic and other biological causes (Ahn, Proctor, & Flanagan, 2009). This could mean that the consequences of genetic test results could differ in important ways as a function of the disorder for which genetic risk is being assessed. For example, the negative psychological impact of genetic test results may be greater for mental disorders that are seen as more biologically determined (e.g., schizophrenia), and effectively intervening in such cases could prove especially challenging. Future research could shed light on this important issue. Also, major depression is a complex disorder influenced by many genetic and environmental factors, whereas the present study framed the “genetic test results” as specifying the presence or absence of one gene that purportedly has a significant effect on a person’s susceptibility to depression. Awaiting future research is the question of whether people might respond differently to more realistic personalized feedback about their genetic susceptibility (e.g., incorporating multiple genes to more comprehensively inform probabilistic assumptions about susceptibility, or conveying the fact that information about a single gene is of limited utility). Moreover, future research could test the effectiveness of other kinds of educational information or other approaches to delivering such interventions and how it compares to the effects observed in the present study. Additionally, genetic testing in psychiatric care may be used for purposes other than elucidating individual differences in overall susceptibility to particular disorders. In particular, there has been significant interest in using genetic information and other biomarkers to find the best antidepressant medication for individual patients (Simon & Perlis, 2010; Uher et al., 2010; Williams et al., 2011). While it is not yet possible to reliably use genetic test results for this purpose, future studies could examine whether the effects of receiving results from pharmacogenetics-oriented testing differ from those of susceptibility testing as simulated in the present study. Such differences could arise because pharmacogenetics-oriented testing may be more likely to be clinician-mediated (i.e., less likely to be available in direct-to-consumer form) or because such testing explicitly aims to identify effective treatments, which might help to counteract essentialist assumptions.
Practice Implications
Our results suggest that personalized test results that inform people about their genetic risk for depression could have important negative consequences. However, they also indicate that educating people about the non-deterministic nature of risk-conferring genes has the potential to mitigate the negative impact of learning that one has elevated genetic susceptibility to depression. This suggests that purveyors of psychiatrically informative genetic test results may wish to include such psychoeducation as part of the process of returning test results to consumers—a role already played by genetic counselors in clinical settings where their services are available. Our results indicate that focusing on the malleability of genetic effects on depression are likely to be particularly helpful, including in a genetic counseling context, and that a brief audiovisual intervention is an effective means of delivering this kind of information. Given the importance of expectancies in mental health, doing so could result in important clinical benefits by increasing patients’ positive outcome expectancies, which can lead to more beneficial treatment results.
Supplementary Material
Acknowledgments
This work was supported (via grant number R01-HG007653) by the National Institutes of Health. The first author also received support from National Institutes of Health grant P50-HG007257. The funding agency had no role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript.
Footnotes
Our modified version of the BDI-II demonstrated high internal consistency (reliability), Cronbach alpha = .94.
Participants could have provided low ratings of the saliva test’s accuracy and reliability for reasons other than suspicion of the test being fake—such as people’s tendency toward defensive processing of threatening health information (Etchegary & Perrier, 2007), which may lead individuals to reject personalized messages that suggest they may be at risk for health problems. If this had been the reason some participants did not rate the saliva test as credible, though, there would likely have been more participants endorsing the credibility of the saliva test in the Gene-Absent condition than in the Gene-Present conditions (because the gene-absent feedback did not contain threatening information). On the contrary, among participants who scored at least 13 on the BDI-II there was no significant difference by condition in credibility ratings, F(2, 256)=.65, p=.53. Nonetheless, the present study did not directly measure the reasons people might have discredited the saliva test, and future research examining the causes of such reactions in depth would be highly valuable.
Conflict of Interest Statements:
Matthew S. Lebowitz declares no conflict of interest.
Woo-kyoung Ahn declares no conflict of interest.
“Human Studies and Informed Consent” Statement. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
References
- Ahn W, Proctor CC, Flanagan EH. Mental Health Clinicians’ Beliefs About the Biological, Psychological, and Environmental Bases of Mental Disorders. Cognitive Science. 2009;33(2):147–182. doi: 10.1111/J.1551-6709.2009.01008.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Ethical Principles of Psychologists and Code of Conduct. 2017. Retrieved from http://www.apa.org/ethics/code/ethics-code-2017.pdf.
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma Genetic Counseling and Test Reporting Improve Screening Adherence Among Unaffected Carriers 2 Years Later. Cancer Epidemiology Biomarkers & Prevention. 2013;22(10):1687. doi: 10.1158/1055-9965.EPI-13-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JC, Honer WG. The potential impact of genetic counseling for mental illness. Clinical Genetics. 2005;67(2):134–142. doi: 10.1111/j.1399-0004.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- Austin JC, Honer WG. The Genomic Era and Serious Mental Illness: A Potential Application for Psychiatric Genetic Counseling. Psychiatric Services. 2007;58(2):254–261. doi: 10.1176/ps.2007.58.2.254. [DOI] [PubMed] [Google Scholar]
- Baumrind D. Research using intentional deception: Ethical issues revisited. American Psychologist. 1985;40(2):165–174. doi: 10.1037/0003-066X.40.2.165. [DOI] [PubMed] [Google Scholar]
- Beck AT, Clark DA. Anxiety and depression: An information processing perspective. Anxiety Research. 1988;1(1):23–36. doi: 10.1080/10615808808248218. [DOI] [Google Scholar]
- Blackwell LS, Trzesniewski KH, Dweck CS. Implicit theories of intelligence predict achievement across an adolescent transition: A longitudinal study and an intervention. Child development. 2007;78(1):246–263. doi: 10.1111/j.1467-8624.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Boynton MH, Portnoy DB, Johnson BT. Exploring the Ethics and Psychological Impact of Deception in Psychological Research. IRB. 2013;35(2):7–13. [PMC free article] [PubMed] [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A New Source of Inexpensive, Yet High-Quality, Data? Perspectives on Psychological Science. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Catanzaro SJ, Mearns J. Measuring generalized expectancies for negative mood regulation: Initial scale development and implications. Journal of personality assessment. 1990;54:546–563. doi: 10.1080/00223891.1990.9674019. [DOI] [PubMed] [Google Scholar]
- Caulfield T, Chandrasekharan S, Joly Y, Cook-Deegan R. Harm, hype and evidence: ELSI research and policy guidance. Genome Medicine. 2013;5(3):21–26. doi: 10.1186/gm425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RE, Wright AJ, Marteau TM. Impact of communicating personalized genetic risk information on perceived control over the risk: A systematic review. Genetics in Medicine. 2011;13(4):273–277. doi: 10.1097/GIM.0b013e3181f710ca. [DOI] [PubMed] [Google Scholar]
- Couzin J. Gene Tests for Psychiatric Risk Polarize Researchers. Science. 2008;319(5861):274–277. doi: 10.1126/science.319.5861.274. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Heine SJ. Genetic essentialism: On the deceptive determinism of DNA. Psychological Bulletin. 2011;137(5):800–818. doi: 10.1037/a0021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar-Nimrod I, Zuckerman M, Duberstein PR. The effects of learning about one’s own genetic susceptibility to alcoholism: a randomized experiment. Genetics in Medicine. 2013;15(2):132–138. doi: 10.1038/gim.2012.111. [DOI] [PubMed] [Google Scholar]
- Dozois DJA. Beck Depression Inventory-II. In: Weiner IB, Craighead WE, editors. The Corsini Encyclopedia of Psychology. 4th. New York, NY: Wiley; 2010. [Google Scholar]
- Drmanac R. The advent of personal genome sequencing. Genetics in Medicine. 2011;13(3):188–190. doi: 10.1097/GIM.0b013e31820f16e6. [DOI] [PubMed] [Google Scholar]
- Dweck CS. Mindsets and human nature: Promoting change in the Middle East, the schoolyard, the racial divide, and willpower. The American psychologist. 2012;67(8):614–622. doi: 10.1037/a0029783. [DOI] [PubMed] [Google Scholar]
- Epley N, Huff C. Suspicion, Affective Response, and Educational Benefit as a Result of Deception in Psychology Research. Personality and Social Psychology Bulletin. 1998;24(7):759–768. doi: 10.1177/0146167298247008. [DOI] [Google Scholar]
- Etchegary H, Perrier C. Information Processing in the Context of Genetic Risk: Implications for Genetic-Risk Communication. Journal of Genetic Counseling. 2007;16(4):419–432. doi: 10.1007/s10897-006-9082-z. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Mello P, Lerman C. Behavioral Consequences of Testing for Obesity Risk. Cancer Epidemiology Biomarkers & Prevention. 2005;14(6):1485–1489. doi: 10.1158/1055-9965.epi-04-0913. [DOI] [PubMed] [Google Scholar]
- Gershon ES, Alliey-Rodriguez N. New Ethical Issues for Genetic Counseling in Common Mental Disorders. American Journal of Psychiatry. 2013;170(9):968–976. doi: 10.1176/appi.ajp.2013.12121558. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and Depression: Current Status and Future Directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Kelly SE, Wyatt S. Counseling Customers: Emerging Roles for Genetic Counselors in the Direct-to-Consumer Genetic Testing Market. Journal of Genetic Counseling. 2013;22(2):277–288. doi: 10.1007/s10897-012-9548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam N. Genetic essentialism, neuroessentialism, and stigma: Commentary on Dar-Nimrod and Heine (2011) Psychological Bulletin. 2011;137(5):819–824. doi: 10.1037/a0022386. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Ortmann A. Deception in Experiments: Revisiting the Arguments in Its Defense. Ethics & behavior. 2008;18(1):59–92. doi: 10.1080/10508420701712990. [DOI] [Google Scholar]
- Higgins ET. ‘Saying is believing’ effects: When sharing reality about something biases knowledge and evaluations. In: Levine JM, Messick DM, Thompson LL, editors. Shared cognition in organizations: The management of knowledge. 1. Mahwah, NJ: Lawrence Erlbaum Associates Inc; 1999. pp. 33–49. [Google Scholar]
- Hippman C, Ringrose A, Inglis A, Cheek J, Albert AYK, Remick R, Austin JC. A pilot randomized clinical trial evaluating the impact of genetic counseling for serious mental illnesses. Journal of Clinical Psychiatry. 2016;77(2):e190–e198. doi: 10.4088/JCP.14m09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth S, Javitt G, Melzer D. The Current Landscape for Direct-to-Consumer Genetic Testing: Legal, Ethical, and Policy Issues. Annual Review of Genomics and Human Genetics. 2008;9(1):161–182. doi: 10.1146/annurev.genom.9.081307.164319. [DOI] [PubMed] [Google Scholar]
- Hudson K, Javitt G, Burke W, Byers P, Committee ASI. ASHG Statement on Direct-to-Consumer Genetic Testing in the United States. American Journal of Human Genetics. 2007;81(3):635–637. doi: 10.1097/01.AOG.0000292086.98514.8b. [DOI] [PubMed] [Google Scholar]
- Jemmott JB, Ditto PH, Croyle RT. Judging health status: Effects of perceived prevalence and personal relevance. Journal of personality and social psychology. 1986;50(5):899–905. doi: 10.1037/0022-3514.50.5.899. [DOI] [PubMed] [Google Scholar]
- Kemp JJ, Lickel JJ, Deacon BJ. Effects of a chemical imbalance causal explanation on individuals’ perceptions of their depressive symptoms. Behaviour research and therapy. 2014;56:47–52. doi: 10.1016/j.brat.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Krell HV, Leuchter AF, Morgan M, Cook IA, Abrams M. Subject Expectations of Treatment Effectiveness and Outcome of Treatment With an Experimental Antidepressant. Journal of Clinical Psychiatry. 2004;65(9):1174–1179. doi: 10.4088/JCP.v65n0904. [DOI] [PubMed] [Google Scholar]
- Kvaale EP, Haslam N, Gottdiener WH. The ‘side effects’ of medicalization: a meta-analytic review of how biogenetic explanations affect stigma. Clinical psychology review. 2013;33(6):782–794. doi: 10.1016/j.cpr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Lebowitz MS, Ahn W. Emphasizing malleability in the biology of depression: Durable effects on perceived agency and prognostic pessimism. Behaviour research and therapy. 2015;71:125–130. doi: 10.1016/j.brat.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MS, Ahn WK, Nolen-Hoeksema S. Fixable or fate? Perceptions of the biology of depression. Journal of consulting and clinical psychology. 2013;81(3):518–527. doi: 10.1037/a0031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlensky L, Trepanier AM, Cragun D, Lerner B, Shannon KM, Zierhut H. A Rapid Systematic Review of Outcomes Studies in Genetic Counseling. Journal of Genetic Counseling. 2017:1–18. doi: 10.1007/s10897-017-0067-x. [DOI] [PubMed] [Google Scholar]
- Marteau T, Senior V, Humphries SE, Bobrow M, Cranston T, Crook MA, Wray R. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: A randomized controlled trial. American Journal of Medical Genetics Part A. 2004;128A(3):285–293. doi: 10.1002/ajmg.a.30102. [DOI] [PubMed] [Google Scholar]
- McBride CM, Koehly LM, Sanderson SC, Kaphingst KA. The Behavioral Response to Personalized Genetic Information: Will Genetic Risk Profiles Motivate Individuals and Families to Choose More Healthful Behaviors? Annual Review of Public Health. 2010;31(1):89–103. doi: 10.1146/annurev.publhealth.012809.103532. [DOI] [PubMed] [Google Scholar]
- Meiser B, Peate M, Levitan C, Mitchell PB, Trevena L, Barlow-Stewart K, Schofield PR. A Psycho-Educational Intervention for People with a Family History of Depression: Pilot Results. Journal of Genetic Counseling. 2016:1–10. doi: 10.1007/s10897-016-0011-5. [DOI] [PubMed] [Google Scholar]
- Meyer B, Pilkonis PA, Krupnick JL, Egan MK, Simmens SJ, Sotsky SM. Treatment expectancies, patient alliance and outcome: Further analyses from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Journal of consulting and clinical psychology. 2002;70(4):1051–1055. doi: 10.1037//0022-006x.70.4.1051. [DOI] [PubMed] [Google Scholar]
- Miu AS, Yeager DS. Preventing Symptoms of Depression by Teaching Adolescents That People Can Change: Effects of a Brief Incremental Theory of Personality Intervention at 9-Month Follow-Up. Clinical psychological science. 2014 doi: 10.1177/2167702614548317. [DOI] [Google Scholar]
- Ortmann A, Hertwig R. The costs of deception: Evidence from psychology. Experimental Economics. 2002;5(2):111–131. doi: 10.1023/A:1020365204768. [DOI] [Google Scholar]
- Persky S, Kaphingst KA, Condit CM, McBride CM. Assessing hypothetical scenario methodology in genetic susceptibility testing analog studies: a quantitative review. Genetics in Medicine. 2007;9(11):727–738. doi: 10.1097/gim.0b013e318159a344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. “A disease like any other”? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. The American Journal of Psychiatry. 2010;167(11):1321–1330. doi: 10.1176/appi.ajp.2010.09121743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC, Yang LH, Cruz-Rojas R. Effects of Attributing Serious Mental Illnesses to Genetic Causes on Orientations to Treatment. Psychiatric Services. 2006;57(3):382–387. doi: 10.1176/appi.ps.57.3.382. doi: [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Wager TD, Roose SP. Expectancy and the treatment of depression: A review of experimental methodology and effects on patient outcome. Current Psychiatry Reviews. 2010;6(1):1–10. doi: 10.2174/157340010790596571. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S, Zhang X, Atzinger C, Ruschman J, Myers MF. Medical management adherence as an outcome of genetic counseling in a pediatric setting. Genetics in Medicine. 2014;16(2):157–163. doi: 10.1038/gim.2013.90. [DOI] [PubMed] [Google Scholar]
- Sanderson SC, Persky S, Michie S. Psychological and Behavioral Responses to Genetic Test Results Indicating Increased Risk of Obesity: Does the Causal Pathway from Gene to Obesity Matter? Public Health Genomics. 2010;13(1):34–47. doi: 10.1159/000217794. [DOI] [PubMed] [Google Scholar]
- Simon GE, Perlis RH. Personalized medicine for depression: Can we match patients with treatments? American Journal of Psychiatry. 2010;167(12):1445–1455. doi: 10.1176/appi.ajp.2010.09111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber JM, Aspinwall LG, Stump TK, Kohlmann W, Champine M, Leachman SA. Genetic test reporting enhances understanding of risk information and acceptance of prevention recommendations compared to family history-based counseling alone. Journal of Behavioral Medicine. 2015;38(5):740–753. doi: 10.1007/s10865-015-9648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perroud N, Ng MYM, Hauser J, Henigsberg N, Maier W, McGuffin P. Genome-Wide Pharmacogenetics of Antidepressant Response in the GENDEP Project. American Journal of Psychiatry. 2010;167(5):555–564. doi: 10.1176/appi.ajp.2009.09070932. [DOI] [PubMed] [Google Scholar]
- Uz I, Kemmelmeier M. Can deception be desirable? Social Science Information. 2016;56(1):98–106. doi: 10.1177/0539018416675070. [DOI] [Google Scholar]
- Walton GM, Cohen GL. A brief social-belonging intervention improves academic and health outcomes of minority students. Science. 2011;331(6023):1447. doi: 10.1126/science.1198364. [DOI] [PubMed] [Google Scholar]
- Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, Gordon E. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12(1):4. doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


