Abstract
Objective
Pertussis can cause life-threatening illness in infants. Data regarding neurodevelopment after pertussis remains scant. The aim of this study was to assess cognitive development of infants with critical pertussis 1 year after pediatric intensive care unit (PICU) discharge.
Design
Prospective cohort study.
Setting
Eight hospitals comprising the Eunice Kennedy Shriver National Institute for Child Health and Human Development Collaborative Pediatric Critical Care Research Network and 18 additional sites across the United States.
Patients
Eligible patients had laboratory confirmation of pertussis infection, were < 1 year of age, and were admitted to the PICU for at least 24 hours.
Interventions
The Mullen Scales of Early Learning was administered at a 1 year follow up visit. Functional status was determined by examination and parental interview.
Measurements and Main Results
Of 196 eligible patients, 111 (57%) completed the Mullen Scales of Early Learning. The mean scores for visual reception, receptive language, and expressive language domains were significantly lower than the norms (p<0.001), but not fine and gross motor domains. Forty one patients (37%) had abnormal scores in at least 1 domain and 10 (9%) had an Early Learning Composite score 2 or more standard deviations below the population norms. Older age (p<0.003) and Hispanic ethnicity (p<0.008) were associated with lower mean Early Learning composite score, but presenting symptoms and PICU course were not.
Conclusions
Infants who survive critical pertussis often have neurodevelopmental deficits. These infants may benefit from routine neurodevelopmental screening.
Keywords: Pertussis, Child Development, Outcome Assessment, Intensive Care, Neurologic Complications, Multicenter Study
Introduction
Pertussis outbreaks, with over 28,000 cases in 2014, occur in the United States despite high vaccine rates. [1] Critical pertussis, defined as infections with sufficient severity to merit Pediatric Intensive Care Unit (PICU) admission, primarily affects infants less than 3 months old and is associated with hospital mortality rates ranging between 5.5% and 14%.[2–5] Factors associated with mortality include pulmonary hypertension and hyper-leukocytosis. [5, 6] Acute neurologic complications of critical pertussis include seizures and encephalopathy.
Long-term outcome data for infants with critical pertussis are scant. A few small series reported neurodevelopmental problems including lower IQ scores, developmental delay, seizures and school performance problems; these were associated with lymphocytosis, apnea, seizures and encephalopathy during the acute illness. [2, 7, 8] Other studies, however, have not found cognitive impairment, even in children with acute neurological complications during the acute illness. [9, 10]
The Mullen Scales of Early Learning (MSEL) was a developed as specific measure of early development for children ages birth to 68 months. [11] The test was designed for administration by professionals who have experience working with infants and has a short administration time. Interscorer reliability coefficients range from 0.91 to 0.99. [11] The MSEL assesses multiple domains of cognitive and motor function, making it useful for conditions which may have differential effects on development. The MSEL has good internal validity and has been use in infant follow-up studies including premature infants and children with congenital heart disease.[11–17]
The aim of this study was to assess cognitive development of infants with critical pertussis 1 year after PICU discharge. The cohort consisted of those infants with laboratory confirmed pertussis hospitalized in a PICU from 2008 to 2013 as part of a prospective cohort study conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network (CPCCRN). We hypothesized that these infants were at risk for lower developmental scores compared to age adjusted normalized scores.
Methods
The study was conducted at the 7 sites (one site had 2 hospitals) in the NICHD CPCCRN network and 18 additional PICUs across the United States. Details of screening, enrollment, patient inclusion, and the acute illness have been published [5] and the study protocol is available (http://www.cpccrn.org/documents/PertussisProtocolVersion4.0109Aug12.pdf). Briefly, children 0 to 18 years were enrolled if they had laboratory-confirmed evidence of pertussis infection (polymerase chain reaction (PCR) and/or positive culture) and were hospitalized in the PICU for at least 24 hours or died. Patients were enrolled from June 2008 to May 2013. For analyses of the one-year outcomes, additional inclusion criteria were age less than 1 year when hospitalized and completion of the Mullen Scales of Early Learning at one year after PICU discharge. The protocol was approved by the IRB at each participating institution and written parental consent was obtained prior to enrollment.
Clinical data were collected through chart abstraction and parental and staff interviews. Data included demographics, prematurity, pertussis vaccinations, chronic conditions, illness history, and aspects of the PICU course including apnea, bradycardia, seizures, cardiac arrest, central nervous system (CNS) hemorrhage, pulmonary hypertension, extracorporeal membrane oxygenation (ECMO) use, and white blood cell (WBC) count. Severity of illness was assessed using the Pediatric Risk of Mortality III score.[18] Functional status of feeding, arousal, communication, motor strength, and motor tone, and use of anticonvulsants was assessed on PICU discharge and at the 1-year follow-up visit. Clinical and laboratory data were collected from admission until PICU discharge, 28 days, or death, whichever occurred earlier.
Cognitive development was assessed with the Mullen Scales of Early Learning (MSEL) 1 year after PICU discharge. The MSEL evaluates gross motor, fine motor, visual reception, and receptive and expressive language based on a nationally representative sample of U.S. children. Raw scores from each are scale are converted to a T score based on the patient’s age and has a population mean of 50 and a standard deviation (SD) of 10. [11] A summary measure termed the Early Learning Composite score is derived from the visual reception, fine motor, receptive language and expressive language T scores and has mean of 100 and a SD of 15. Scores that were 2 standard deviations or more below the mean were defined as abnormal. The patient’s chronologic age used for MSEL testing was adjusted for those who were premature at birth (n = 24) as described in MSEL administration instructions. [11] The MSEL was administered in person by trained neuropsychologists, developmental pediatricians, developmental psychologists, or neurologists. The MSEL is available only in English. Twenty-five MSELs were administered by a bilingual examiner (24 Spanish, 1 other language), and 3 with an interpreter (2 Spanish, 1 other language) when the parent did not speak English (n=28).
Statistical Analysis
We descriptively characterized the acute illness and initial PICU course for all patients eligible for one year follow up, and compared those who completed the MSEL to those who did not. Continuous data were summarized as median and interquartile range (IQR, 25th and 75th percentiles). Associations between demographic and clinical variables and whether or not follow up was completed were evaluated using the Pearson chi-square or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
We evaluated change in functional assessment status (from PICU discharge to one year) using the McNemar test for paired data. MSEL scale T scores were summarized graphically using a box-and-whisker plot, and the observed mean scores were compared to the population mean (from the normative sample) using a one-sample t-test. The Early Learning Composite score was summarized overall and within demographic and clinical subgroups as mean ± SD. The overall mean was compared to the population mean using a one-sample t-test. Univariable associations with the Early Learning Composite outcome were evaluated based on t-test or ANOVA for categorical variables and simple linear regression for continuous measures. Clinical events that occurred in fewer than 5 patients were not included in the univariable analyses including: cardiac arrest, evidence of a CNS hemorrhage, pulmonary hypertension, and ECMO use. A multivariable linear regression model was constructed including any variable with p < 0.15 in univariable analyses. A sensitivity analysis was conducted excluding non-English speaking patients. A significance level of 0.05 was used for all analyses. Analyses were performed in SAS® version 9.4 (SAS Institute, Cary, NC).
RESULTS
A total of 225 patients with critical pertussis were enrolled based on their PICU admission and positive culture or PCR. The enrollment diagram is shown in Figure 1. Nineteen patients died, 17 during the acute hospitalization, and 2 prior to the one-year follow-up (1 from blunt force trauma and 1 from respiratory syncytial viral pneumonitis). Seven patients were greater than one year of age on enrollment, parental permission was withdrawn in 3, and 85 were lost to follow up or did not complete the MSEL. Of the 111 patients who completed the MSEL, 2 had a cardiac arrest, 1 had CNS hemorrhage, 4 had pulmonary hypertension, and 3 received ECMO during the acute hospitalization.
Figure 1.
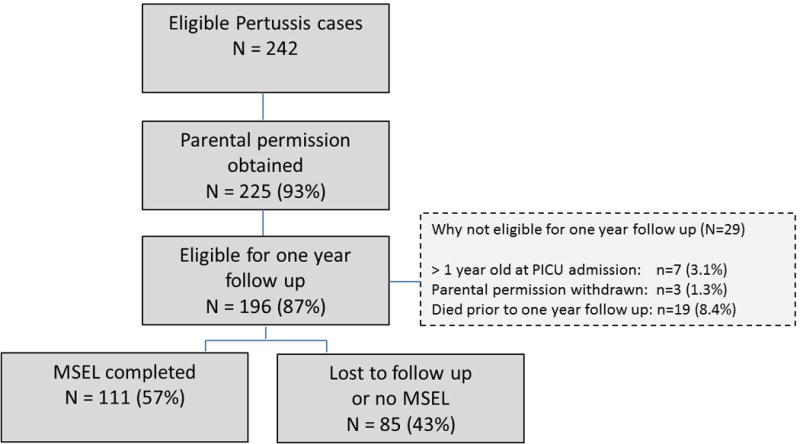
Enrollment and Follow-up Flow Diagram.
Patient demographics and clinical information are shown in Table 1. There were no statistically significant differences between those lost to follow-up and those who completed the MSEL. Of those who completed the MSEL, 86% were less than 3 months of age on presentation, 23% had a history of prematurity, and 19% had received one or more vaccinations. Apnea occurred in 60%, bradycardia in 69%, seizures in 7%, 35% received mechanical ventilation, and 7% received a therapy to reduce their WBC count.
Table 1.
Demographic and Clinical Data.
| Clinical Characteristics | Lost to follow up | MSEL completed(1) | P-value |
|---|---|---|---|
| N = 85 | N = 111 | ||
| Demographics | |||
| Age < 3 months at PICU admission | 70 (82%) | 96 (86%) | 0.43 |
| Female | 43 (51%) | 66 (59%) | 0.22 |
| Race | 0.10 | ||
| Black or African American | 14 (16%) | 13 (12%) | |
| White | 48 (56%) | 81 (73%) | |
| Other | 8 (9%) | 7 (6%) | |
| Recorded as Unknown | 15(18%) | 10 (9%) | |
| Hispanic Ethnicity | 48 (57%) | 57 (52%) | 0.50 |
| Prematurity (< 37 weeks gestation) | 21 (25%) | 25 (23%) | 0.69 |
| Pertussis vaccinations | 21 (25%) | 21 (19%) | 0.33 |
| Chronic medical condition | 19 (22%) | 16 (14%) | 0.15 |
| History of Apnea | 55 (65%) | 61 (55%) | 0.17 |
| History of Seizures | 5 (6%) | 6 (5%) | 1.00 |
| History of Bradycardia | 25 (29%) | 36 (32%) | 0.65 |
| PICU Course | |||
| PRISM III Score, median (IQR) | 0 (0, 3) | 0 (0, 3) | 0.67 |
| WBC (×10ˆ3/uL), median (IQR) | 23 (15, 36) | 22 (16, 34) | 0.60 |
| Apnea | 48 (56%) | 67 (60%) | 0.58 |
| Seizure | 5 (6%) | 8 (7%) | 0.71 |
| Bradycardia | 52 (61%) | 77 (69%) | 0.23 |
| Mechanical ventilation | 35 (41%) | 39 (35%) | 0.39 |
| Exchange transfusion or leukopheresis | 4 (5%) | 8 (7%) | 0.47 |
| PICU length of stay, median (IQR) | 6 (3, 12) | 6 (3,12) | 0.99 |
MSEL = Mullen Scales of Early Learning
The functional status at PICU discharge and 1 year after discharge are shown in Table 2 for feeding, arousal, communication, and motor assessments. There were no statistically significant differences in PICU discharge assessments in those with and without 1 year follow up. For the MSEL cohort at PICU discharge, 35% of patients were receiving some or all of their feeds from a nasogastric, nasojejunal, or gastrostomy tube. Very few patients (<5%) had dysfunction in the other areas of functional assessment. By the one-year follow-up, 98% of patients were feeding without a device, but a significantly larger number were demonstrating decreased communication function (p=0.002). Four percent (n=4) were receiving anticonvulsants on PICU discharge compared to <1% (n=1) at one-year follow-up. Hearing assessments were performed after hospital discharge in 26 patients of which 24 were judged as normal.
Table 2.
Functional Status at PICU Discharge and at One-Year Follow-up
| Functional Domain | PICU Discharge, (n=110)(1) | Status at 1 Year, (N=110)(1) | P-value (PICU d/c vs. 1 year)(2) |
|---|---|---|---|
| n (%) | n (%) | ||
| Feeding assessment | < 0.001 | ||
| Oral feeding only | 72 (65%) | 108 (98%) | |
| Oral plus tube feeding (NG, NJ, or gastrostomy) | 15 (14%) | 0 (0%) | |
| NG, NJ, or gastrostomy tube feeding only | 23 (21%) | 2 (2%) | |
| Arousal assessment | 0.56 | ||
| Awake, alert, or looks to clapping | 108 (98%) | 109 (99%) | |
| Arouses but does not localize sound | 2 (2%) | 1 (1%) | |
| Communication assessment | 0.002 | ||
| Age-appropriate vocalization, facial expressiveness, gestures | 106 (96%) | 92 (84%) | |
| Decreased vocalization or non-verbal interaction | 4 (4%) | 18 (16%) | |
| Motor strength assessment | 0.08 | ||
| Normal activity for age | 109 (99%) | 106 (96%) | |
| Asymmetric limb activity or withdrawal to stimulation | 1 (1%) | 4 (4%) | |
| Withdraws from pain | 0 (0%) | 0 (0%) | |
| Motor tone assessment | 0.56 | ||
| Normal tone for age | 107 (97%) | 108 (98%) | |
| Increased or decreased tone for age | 3 (3%) | 2 (2%) |
One patient had incomplete one year data and is not included.
P-value based on McNemar test for paired data (normal vs. abnormal).
The Mullen domain scores obtained at one-year follow-up are shown in figure 2. The mean scores for visual reception, receptive language, and expressive language domains were significantly lower than the norms (p<0.001), but the motor domains were not. Forty one patients (37%) had abnormal scores (≤2 standard deviations below the mean) in at least 1 domain. The most common area of impairment was in one of the two language development domains. Twenty-three (21%) patients had expressive language delay and 16 (14%) had delays in the receptive language domain. The Mullen Early Learning Composite Score is shown in Figure 3. Consistent with the impairment in the domain scores, the composite score was significantly lower than the norm (mean 90.3 +/− 16.8, p<0.001). The range of composite scores was 49 to 134 with 10 patients (9%) having a composite score 2 or more standard deviations below the population mean.
Figure 2. Mullen Domain Scores.
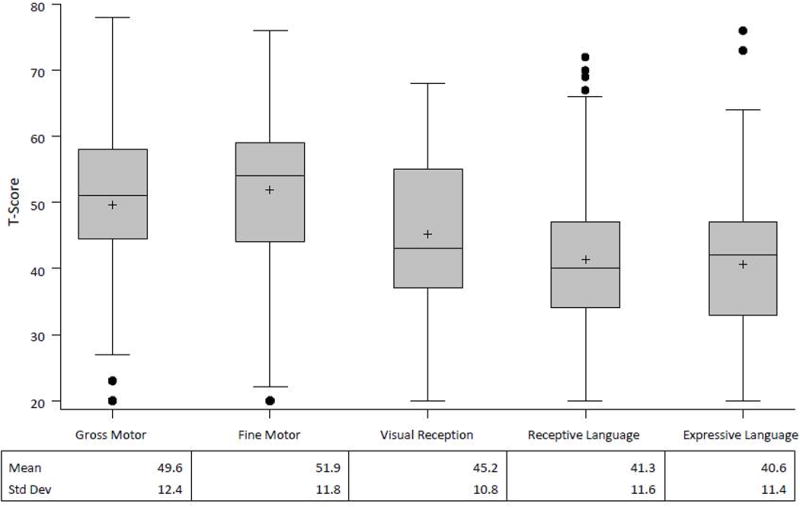
Box-and-whisker plots show summary statistics as min (with outliers depicted by filled circles), 25th percentile, median, 75th percentile, max, and mean (+). The T score is a standardized score with a population mean of 50 and standard deviation of 10. The scores for visual reception, receptive and expressive language (p<0.001) but not motor were significantly lower than the population norms.
Figure 3. Distribution of the Mullen Early Learning Composite Score.
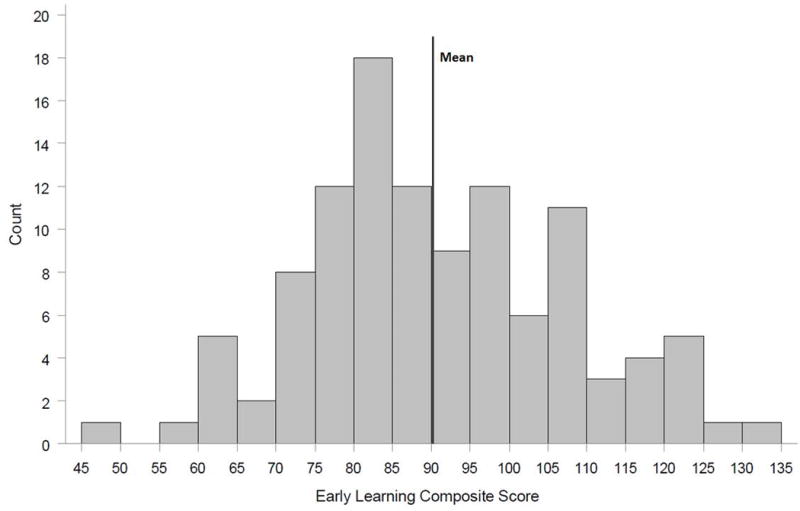
The Early Learning Composite is derived from the four cognitive scales (fine motor, visual reception, receptive language, and expressive language) and is normalized to have a mean of 100 and a standard deviation of 15. The mean observed in the study cohort is significantly different from the population mean (p<0.001).
Table 3 shows the univariable associations of the demographic and clinical factors with the Mullen Early Learning Composite Score. Older age (3 – 11 months), Hispanic or Latino ethnicity, and previous pertussis vaccinations were significantly associated with decreased performance. Because age and documented pertussis vaccinations were highly collinear, we included age but not vaccination in the multivariable model as this was the stronger predictor. Otherwise, the multivariable model included all variables in table 3 with p<0.15 from univariable analyses. In this model, only older age (p=0.003) and Hispanic ethnicity (p=0.008) were significantly associated with decreased Mullen Early Learning Composite scores when corrected for these co-variates.
Table 3.
Univariable Associations with Mullen Early Learning Composite Score
| Clinical Characteristic | Mullen Early Learning Composite Score(1) | ||
|---|---|---|---|
| Mean ± SD | p-value | ||
| Demographics | |||
| Chronologic Age at PICU admission | 0.003 | ||
| < 3 months | 92.1 ± 16.1 | ||
| 3–11 months | 78.4 ± 16.9 | ||
| Sex | 0.08 | ||
| Female | 92.5 ± 16.9 | ||
| Male | 86.9 ± 16.4 | ||
| Race | 0.75 | ||
| Black or African American | 88.8 ± 15.8 | ||
| White | 91.2 ± 15.8 | ||
| Other | 87.4 ± 18.1 | ||
| Ethnicity | 0.004 | ||
| Hispanic or Latino | 86.1 ± 15.4 | ||
| Not Hispanic or Latino | 95.3 ± 17.3 | ||
| Prematurity (< 37 weeks gestation) | 0.47 | ||
| Yes | 92.4 ± 17.1 | ||
| No | 89.6 ± 16.8 | ||
| Pertussis vaccinations | 0.006 | ||
| Yes | 81.3 ± 16.0 | ||
| No | 92.3 ± 16.4 | ||
| Chronic medical condition | 0.50 | ||
| Yes | 87.6 ± 14.6 | ||
| No | 90.7 ± 17.2 | ||
| History of apnea | 0.83 | ||
| Yes | 90.6 ± 18.1 | ||
| No | 89.9 ± 15.3 | ||
| History of seizures | 0.31 | ||
| Yes | 83.5 ± 19.6 | ||
| No | 90.6 ± 16.7 | ||
| History of Bradycardia | 0.11 | ||
| Yes | 93.9 ± 18.6 | ||
| No | 88.5 ± 15.8 | ||
| PICU Course | |||
| PRISM-III score, correlation (r) | −0.075 | 0.44 | |
| WBC (×10ˆ3/uL), correlation (r) | −0.13 | 0.20 | |
| Apnea | 0.69 | ||
| Yes | 90.8 ± 16.6 | ||
| No | 89.5 ± 17.4 | ||
| Seizure | 0.13 | ||
| Yes | 81.5 ± 18.2 | ||
| No | 90.9 ± 16.6 | ||
| Bradycardia | 0.52 | ||
| Yes | 89.6 ± 16.6 | ||
| No | 91.8 ± 17.5 | ||
| Mechanical ventilation | 0.26 | ||
| Yes | 87.8 ± 16.3 | ||
| No | 91.6 ± 17.1 | ||
| Exchange transfusion or leukopheresis | 0.11 | ||
| Yes | 81.1 ± 12.5 | ||
| No | 91.0 ± 17.0 | ||
Data are mean +/− SD except for the PRISM III scores and WBC counts, which are Pearson correlations (r).
We performed a sensitivity analysis by repeating the analysis after excluding the 28 patients whose families did not speak English. The mean scores for scores for visual reception, receptive language, and expressive language domains were still significantly lower than the norms (p ≤0.001). On univariable analysis, older age, Hispanic ethnicity, and previous pertussis vaccinations remained significantly associated with decreased Mullen Early Learning Composite Scores (p<0.05) as seen in supplement table 1. In a revised multivariable model that included age, sex, ethnicity, and history of bradycardia, only older age (p =0.02) and ethnicity (p=0.04) remained significantly associated with lower Mullen Early Learning composite scores.
Discussion
In this report of the developmental status of 111 survivors of critical pertussis, 37% of patients had abnormal MSEL scores in at least 1 domain and 9% had an Early Learning Composite score 2 or more standard deviations below the population norm one year after PICU discharge. The results of testing can be described as an age equivalent (i.e. the age at which the child’s raw score is the median score). [11] For example, a 15 month old child who has an expressive language score 2 standard deviations below the norm has the age equivalent performance of a 10–11 month old in that domain and gross motor domain the equivalent age would be 12–13 months. The most frequent abnormal scores occurred in the language domains and communication was the most frequent abnormal functional assessment. Survivors of critical pertussis who were 3–12 months old when they presented had worse neurodevelopmental outcomes than those who were 0–3 months old. Clinical factors such as presenting symptoms, occurrence of pulmonary hypertension or shock, and peripheral white blood cell counts, were not associated with worse neurodevelopmental scores.
The frequency of neurodevelopmental dysfunction in this cohort of patients with critical pertussis is comparable to that reported in critically ill patients with sepsis or meningoencephalitis. [19–21] While this study did not investigate the pathophysiological processes associated with neurodevelopment dysfunction, clinical pertussis often includes inflammation as well as other processes more specific to pertussis including primary injury from pertussis toxin or other neurotoxins,[22, 23] hypoxia, hemorrhage, and vascular occlusion. In 2 case series, MRI studies have demonstrated the occurrence of acute demyelination in pertussis. [24, 25] In the United States, seizures are reported in 2.2% of patients hospitalized with pertussis [26] and in 4–18 % with critical pertussis. [4–6, 9] Acute seizures have been associated with mortality and an increased incidence of epilepsy. [5, 6, 8]
Older infants were at higher risk for lower MSEL scores. Older infants may have had a single dose of pertussis vaccine which appears to reduce the risk of death, hospitalization and pneumonia but not encephalopathy and seizures during the acute hospitalization [27] and may not protect against long-term neurodevelopmental problems.
Previously identified associations of mortality including pulmonary hypertension, shock, or elevated WBC count were not associated with the developmental outcome in this cohort. This contrasts with previously reported associations of seizures and apnea with developmental outcomes. [2, 7] The difference may be due to differences in study design. In a retrospective study of critical pertussis, 10% of survivors had neurodevelopmental problems identified through medical record review. All of the patients were abnormal during the acute illness. A composite outcome that included death and respiratory disability as well as neurological disability was used to test for possible associations. Additionally, if formal developmental testing was not used, more subtle forms of dysfunction may not have been detected. [2] The second case-control study compared patients with apnea or seizures to uncomplicated pertussis who may not have required critical care. [7] In this cohort, 48% of the patients with complicated pertussis required remedial help during school compared to 20% without complicated pertussis. A case series for 14 patients with acute seizure or encephalopathy did not find any abnormalities on long term follow up, but formal developmental testing was not performed. [9] Our data is suggests that a significant proportion of patients with critical pertussis regardless of specific symptoms appear to be at risk for neurodevelopmental problems which may only be evident with formal screening.
Our follow-up assessments were completed after only 1 year in infants so further developmental problems could present or some problems may resolve. For instance, most patients no longer required artificial feeding methods at the 1 year follow up visit while communication dysfunction was more frequently recognized. We speculate that this is due to recovery from critical illness and/or ability to recognize dysfunction rather than pertussis illness. Longitudinal developmental studies of critically ill patients have shown changes in the incidence and types of abnormalities. For example, in patients with repaired transposition of the great arteries, the incidence of detected developmental abnormalities was substantially higher at school age compared to age 1–5 years. [29, 30] Determining the frequency and length of developmental surveillance after pertussis and other major pediatric critical illness remains a research need. The lack of factors associated with developmental dysfunction suggests that all survivors of critical pertussis should undergo neurodevelopmental surveillance and screening.
Hispanic ethnicity which has been associated with mortality in some pertussis studies and decreased functional status after sepsis was associated with lower MSEL scores. [19, 27, 28] The association is likely multifactorial. Although the MSEL has been used in non-English speaking populations, we found lower scores in the language domains which may reflect the effects of using translated materials and interpreters. [31–34] Alternatively, true language delays are seen in multilingual children when compared to monolingual children that may also explain lower scores. [34, 35] Finally, there may be cultural or socioeconomic factors accounting for the association. The MSEL was chosen for its ease of administration in a multicenter study, short administration time, and ability to assess multiple domains in the age range. Other common tests of infant development such as the Bayley Scales of Infant and Toddler Development and the Battelle Development Inventory are likewise not validated in non-English speakers. While language differences may account for the association of Hispanic ethnicity and lower MSEL scores, the results from univariable and multivariable analysis were not substantially changed after excluding non-English speaking patients.
There are some limitations to this study. First, a substantial portion of patients were lost to follow up over the one year. However, demographics, illness course, nor discharge assessments appeared to be different between the groups. Second, we did not collect information regarding other confounding variables such as exposure to sedative medications in the PICU or parental socioeconomic status; therefore, we could not adjust for this in our associations with outcome. Additionally it is not possible to separate the effects of pertussis from general critical illness. Third, the lack of association with clinical features may have been, in part, due to inadequate power. For example, only 4 patients tested one year after PICU discharge had pulmonary hypertension and 2 had a cardiac arrest.
Conclusion
In this prospective cohort study of critical pertussis, over one-third of survivors had significantly abnormal neurodevelopmental scores in 1 or more domains. Neither illness characteristics nor PICU course was associated with lower developmental scores. Our results suggest that all survivors of critical pertussis like survivors of sepsis and other major critical illness may benefit from routine neurodevelopmental screening.
Supplementary Material
Acknowledgments
Members of the CPCCRN participating in this study – University of Utah (Data Coordinating Center), Salt Lake City, UT: Linda Herrera, MPH; Children’s Hospital of Pittsburgh, Pittsburgh, PA: Michael Bell, MD, Alan Abraham, BA, CCRC; Children’s National Medical Center, Washington, DC: Jean Reardon, MA, BSN, RN; Elyse Tomanio, BSN, RN; Children’s Hospital of Michigan, Detroit, MI: Ann Pawluszka, BSN, RN; Children’s Hospital Los Angeles, Los Angeles, CA: Jennifer Yuen Kwok, JD; Arkansas Children’s Hospital, Little Rock, AR: Glenda C. Hefley, MNSc, CCRP; Seattle Children’s Hospital, Seattle, WA: Stephanie Hamilton, BSN, CCRP; Children’s Hospital of Philadelphia, Philadelphia, PA: Athena F. Zuppa, MD, MSCE, Mary Ann DiLiberto, BS, RN, CCRC, Carolann Twelves, RN, BSN, CCRC; Children’s Hospital of Phoenix, Phoenix, AZ: Aimee La Bell, MS, RN; Mott Children’s Hospital, Ann Arbor, MI: Frank W. Moler, MD, Monica S. Weber, RN, BSN, CCRP, and Lauren Conlin, BSN, RN.
Funding Source: This work was supported, in part, by cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (U10HD05009, U10HD049945, U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063106, U10HD063114, U01HD049934). Initial support was provided by the National Vaccine Program Office at the United States Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Copyright form disclosure: Dr. Berger’s institution received funding from Eunice Kennedy Shriver National Institute of Child Health and Development and Association for Pediatric Pulmonary Hypertension. He received support for article research from the National Institutes of Health (NIH). Dr. Clark’s institution received funding from the NIH. She received support for article research from the NIH. Dr. Holubkov’s institution received funding from the NIH. He disclosed other support from: DSMB member for Fibrocell Inc (past), American Burn Association (past), and Armaron Bio (current). He received support for article research from the NIH. He received funding from St. Jude Medical (biostatistical consulting), Pfizer Inc (DSMB), DURECT Corporation (biostatistical consulting). Dr. Pollack’s institution received funding from the NIH. He received support for article research from the NIH. Dr. Berg’s institution received funding from the National Institute for Child Health and Human Development (NICHD). He received support for article research from the NIH. Dr. Carcillo’s institution received funding from the NICHD. He received support for article research from the NIH. Dr. Dalton’s insitution received funding from the NIH. She received funding from Innovative ECMO Concepts, Inc. and Maquet. She received support for article research from the NIH. Dr. Harrison’s institution received funding from the NIH. He received support for article research from the NIH. Dr. Meert’s institution received funding from the NIH. She received support for article research from the NIH. Dr. Newth’s institution received funding from the NICHD. He received support for article research from the NIH. Dr. Shanley’s institution received funding from the NIH. He received support for article research from the NIH. He received funding from IPRF. Dr. Wessel’s institution received funding from the NIH. He received support for article research from the NIH. Dr. Anand received support for article research from the NIH. Dr. Zimmerman’s institution received funding from the NIH and Immunexpress, Seattle (research funding). He received support for article research from the NIH. He received funding from Society of Critical Care Medicine (travel reimbursement to attend board meetings) and from Elsevier Publishing (royalties for co-editing the textbook, Pediatric Critical Care). Dr. Sanders Jr received support for article research from the NIH. Dr. Liu’s institution received funding from the NIH/NICHD. She received support for article research from the NIH. Dr. Burr’s institution received funding from the NICHD. He received support for article research from the NIH. Dr. Doctor received support for article research from the NIH. His institution received funding from the NIH, DoD, and Children’s Discovery Institute. Dr. Dean’s institution received funding from the NICHD. He received support for article research from the NIH. Dr. Jenkins disclosed government work: I completed this work as part of official duties as a Federal Employee of the National Institutes of Health. Dr. Nicholson disclosed government work. She received support for article research from the NIH.
Footnotes
Reprints will not be ordered.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Collaborating Sites contributing to this study – Centers for Disease Control and Prevention, Atlanta, GA: Fatima Coronado, MD, MPH; Children’s Healthcare of Atlanta at Egleston, Atlanta, GA: James Fortenberry, MD, Richard Toney, RN, Cheryl L. Stone, RN; Miller Children’s Hospital Long Beach, Long Beach, CA: Kevin C. O’Brien, MD, FAAP; Children’s Hospital of Orange County, Orange, CA: Patricia P. Liao, MD, Tiffany Patterson, BS, CRC; Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN; Children’s Hospital and Research Center Oakland, Oakland, CA: Heidi R. Flori, MD, Julie Simon, BSN, RN; Children’s Hospital of Wisconsin, Milwaukee, WI: Rainer G. Gedeit, MD, Melissa Christensen, BS; Dell Children’s Medical Center of Central Austin, Austin, TX: Renee A. Higgerson, MD, LeeAnn Christie, MSN, RN; Dartmouth-Hitchcock Medical Center, Lebanon, NH: Daniel L. Levin, MD, J. Dean Jarvis, BSN, MBA, CCRN; Miami Children’s Hospital, Miami, FL: Balagangadhar R. Totapally, MBBS, DCH, MD, MRCP; New York Presbyterian Hospital – Cornell University, New York, NY: Chani S. Traube, MD, Charlene S. Carlo; Penn State Hershey Children’s Hospital, Hershey, PA: Neal J. Thomas, MD, MSc, Debbie Spear, RN, CCRN; St. Louis Children’s Hospital, St. Louis, MO: Jose A. Pineda, MD, Tina Day, CCRC; Stollery Children’s Hospital, University of Alberta, Alberta, Canada; Texas Children’s Hospital and Baylor College of Medicine, Houston, TX: Moushumi S. Sur, MD, Nancy Jaimon, BSN, RN; University of Nebraska Medical Center/Children’s Hospital, Omaha, NE: Edward J. Truemper, MD, Machelle A. Zink, MEd, BSN, RN, CCRC; University of Virginia, Charlottesville, VA: Robin L. Kelly, RN.
References
- 1.Pertussis Cases by Year (1922–2014)[http://www.cdc.gov/pertussis/surv-reporting/cases-by-year.html]
- 2.Surridge J, Segedin ER, Grant CC. Pertussis requiring intensive care. Arch Dis Child. 2007;92(11):970–975. doi: 10.1136/adc.2006.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillis J, Grattan-Smith T, Kilham H. Artificial ventilation in severe pertussis. Arch Dis Child. 1988;63(4):364–367. doi: 10.1136/adc.63.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namachivayam P, Shimizu K, Butt W. Pertussis: severe clinical presentation in pediatric intensive care and its relation to outcome. Pediatr Crit Care Med. 2007;8(3):207–211. doi: 10.1097/01.PCC.0000265499.50592.37. [DOI] [PubMed] [Google Scholar]
- 5.Berger JTCJ, Shanley TP, Wessel DL, Clark A, Holubkov R, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Collaborative Pediatric Critical Care Research Network CPCCRN: Critical Pertussis Ilness in Children: A Multicenter Prospective Cohort Study. Pediatr Crit Care Med. 2013;14:354–365. [Google Scholar]
- 6.Murray EL, NIeves D, Bradley JS, Gargas J, Mason WH, Lehman D, Harriman K, C JD. Characteristics of Severe Bordetella Pertussis Infection among Infants ≤ 90 Days of Age Admitted to PEdiatric Intensive Care Units - Southern California, September 2009–June 2011. Journal of the Pediatric Infectious Dieseases Society. 2013:1–6. doi: 10.1093/jpids/pis105. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Study of intellectual performance of children in ordinary schools after certain serious complications of whooping cough. Swansea Research Unit of the Royal College of General Practitioners. British Medical Journal Clinical Research Ed. 1987;295(6605):1044–1047. doi: 10.1136/bmj.295.6605.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen M, Thygesen SK, Ostergaard JR, Nielsen H, Henderson VW, Ehrenstein V, Norgaard M, Sorensen HT. Hospital-Diagnosed Pertussis Infection in Children and Long-term Risk of Epilepsy. JAMA. 2015;314(17):1844–1849. doi: 10.1001/jama.2015.13971. [DOI] [PubMed] [Google Scholar]
- 9.Incorpora G, Pavone L, Parano E, Cocuzza M, Catalano F, Trifiletti R. Neurological complications in hospitalized patients with pertussis: a 15-year Sicilian experience. Childs Nerv Syst. 1996;12(6):332–335. doi: 10.1007/BF00301022. [DOI] [PubMed] [Google Scholar]
- 10.de Greeeff SC, van Buul LW, Westerhof A, Wijga AH, van de Kassteele J, Oostvogels B, van der Maas NAT, Mooi FR, de Melker HE. Pertussis in infancy and the association with respiratory and cognitive disorders at toddler age. Vaccine. 2011;29(46):8275–8278. doi: 10.1016/j.vaccine.2011.08.112. [DOI] [PubMed] [Google Scholar]
- 11.Mullen EM. Mullen Scales of Early Learning. Minneapolis, MN: NCS Pearson, Inc; 1995. AGS Edition. [Google Scholar]
- 12.Bassan H, Limperopoulos C, Visconti K, Mayer DL, Feldman HA, Avery L, Benson CB, Stewart J, Ringer SA, Soul JS, et al. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120(4):785–792. doi: 10.1542/peds.2007-0211. [DOI] [PubMed] [Google Scholar]
- 13.Bolduc M-E, Du Plessis AJ, Sullivan N, Khwaja OS, Zhang X, Barnes K, Robertson RL, Limperopoulos C. Spectrum of neurodevelopmental disabilities in children with cerebellar malformations. Dev Med Child Neurol. 2011;53(5):409–416. doi: 10.1111/j.1469-8749.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 14.Caudle SE, Katzenstein JM, Karpen S, McLin V. Developmental assessment of infants with biliary atresia: differences between boys and girls. J Pediatr Gastroenterol Nutr. 2012;55(4):384–389. doi: 10.1097/MPG.0b013e318259ed20. [DOI] [PubMed] [Google Scholar]
- 15.Ungerleider RM, Shen I, Yeh T, Schultz J, Butler R, Silberbach M, Giacomuzzi C, Heller E, Studenberg L, Mejak B, et al. Routine mechanical ventricular assist following the Norwood procedure–improved neurologic outcome and excellent hospital survival. Ann Thorac Surg. 2004;77(1):18–22. doi: 10.1016/s0003-4975(03)01365-1. [DOI] [PubMed] [Google Scholar]
- 16.Wachtel EV, Zaccario M, Mally P. Impact of Respiratory Morbidities on Neurodevelopmental Outcome of Late Preterm Infants. Am J Perinatol. 2015;32(12):1164–1168. doi: 10.1055/s-0035-1551673. [DOI] [PubMed] [Google Scholar]
- 17.Schraeder BD. Assessment of measures to detect preschool academic risk in very-low-birth-weight children. Nurs Res. 1993;42(1):17–21. [PubMed] [Google Scholar]
- 18.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Farris RWD, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med. 2013;14(9):835–842. doi: 10.1097/PCC.0b013e3182a551c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41(4):1094–1103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]
- 21.Bronner MB, Knoester H, Sol JJ, Bos AP, Heymans HSA, Grootenhuis MA. An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med. 2009;10(6):636–642. doi: 10.1097/PCC.0b013e3181ae5c1a. [DOI] [PubMed] [Google Scholar]
- 22.Davis LE, Burstyn DG, Manclark CR. Pertussis encephalopathy with a normal brain biopsy and elevated lymphocytosis-promoting factor antibodies. Pediatr Infect Dis. 1984;3(5):448–451. doi: 10.1097/00006454-198409000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Grant CC, McKay EJ, Simpson A, Buckley D. Pertussis encephalopathy with high cerebrospinal fluid antibody titers to pertussis toxin and filamentous hemagglutinin. Pediatrics. 1998;102(4 Pt 1):986–990. doi: 10.1542/peds.102.4.986. [DOI] [PubMed] [Google Scholar]
- 24.Budan B, Ekici B, Tatli B, Somer A. Acute disseminated encephalomyelitis (ADEM) after pertussis infection. Ann Trop Paediatr. 2011;31(3):269–272. doi: 10.1179/1465328111Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 25.Hiraiwa-Sofue A, Ito Y, Mori H, Ichiyama T, Okumura A. Pertussis-associated encephalitis/encephalopathy with marked demyelination in an unimmunized child. J Neurol Sci. 2012;320(1–2):145–148. doi: 10.1016/j.jns.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Farizo KM, Cochi SL, Zell ER, Brink EW, Wassilak SG, Patriarca PA. Epidemiological features of pertussis in the United States, 1980–1989. Clin Infect Dis. 1992;14(3):708–719. doi: 10.1093/clinids/14.3.708. [DOI] [PubMed] [Google Scholar]
- 27.Tiwari TSP, Baughman AL, Clark TA. First pertussis vaccine dose and prevention of infant mortality. Pediatrics. 2015;135(6):990–999. doi: 10.1542/peds.2014-2291. [DOI] [PubMed] [Google Scholar]
- 28.Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009;28(3):194–198. doi: 10.1097/INF.0b013e31818c9032. [DOI] [PubMed] [Google Scholar]
- 29.Hovels-Gurich HH, Seghaye M-C, Schnitker R, Wiesner M, Huber W, Minkenberg R, Kotlarek F, Messmer BJ, Von Bernuth G. Long-term neurodevelopmental outcomes in school-aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2002;124(3):448–458. doi: 10.1067/mtc.2002.122307. [DOI] [PubMed] [Google Scholar]
- 30.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114(5):e572–576. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]
- 31.Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. Jama, Pediatr. 2015;169(2):154–162. doi: 10.1001/jamapediatrics.2014.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koura KG, Boivin MJ, Davidson LL, Ouedraogo S, Zoumenou R, Alao MJ, Garcia A, Massougbodji A, Cot M, Bodeau-Livinec F. Usefulness of child development assessments for low-resource settings in francophone Africa. J Dev Behav Pediatr. 2013;34(7):486–493. doi: 10.1097/DBP.0b013e31829d211c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaidez V, Hansen RL, Hertz-Picciotto I. Autism spectrum disorders in Hispanics and non-Hispanics. Autism. 2012;16(4):381–397. doi: 10.1177/1362361311434787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlson SM, Meltzoff AN. Bilingual experience and executive functioning in young children. Dev. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


