Abstract
Background
To examine the prospective association between multivitamin supplementation during pregnancy and biomarker measures of maternal plasma folate and vitamin B12 levels at birth and child's Autism Spectrum Disorder (ASD) risk.
Methods
This report included 1257 mother-child pairs, who were recruited at birth and prospectively followed through childhood at the Boston Medical Center. ASD was defined from diagnostic codes in electronic medical records. Maternal multivitamin supplementation was assessed via questionnaire interview; maternal plasma folate and B12 were measured from samples taken 2-3 days after birth.
Results
Moderate (3-5 times/week) self-reported supplementation during pregnancy was associated with decreased risk of ASD, consistent with previous findings. Using this as the reference group, low (≤2 times/week) and high (>5 times/week) supplementation was associated with increased risk of ASD. Very high levels of maternal plasma folate at birth (≥60.3 nmol/L) had 2.5 times increased risk of ASD (95% confidence interval [CI] 1.3, 4.6) compared to folate levels in the middle 80th percentile, after adjusting for covariates including MTHFR genotype. Similarly, very high B12 (≥536.8 pmol/L) showed 2.5 times increased risk (95% CI 1.4, 4.5).
Conclusion
There was a “U” shaped relationship between maternal multivitamin supplementation frequency and ASD risk. Extremely high maternal plasma folate and B12 levels at birth were associated with ASD risk. This hypothesis-generating study does not question the importance of consuming adequate folic acid and vitamin B12 during pregnancy; rather, raises new questions about the impact of extremely elevated levels of plasma folate and B12 exposure in-utero on early brain development.
Keywords: Autism, folate, vitamin B12, prenatal supplement intake
Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous group of neurodevelopmental conditions characterized by impaired social reciprocity, abnormal communication and repetitive or unusual behaviour. 1, 2 The prevalence of ASD was about five per 10,000 individuals in 1980s, but recent estimates in the U.S. suggest that it is now one in 68 individuals. 3, 4 The aetiology of ASD is complex, and includes the interplay of genetic and environmental factors. 5, 6 Folate is an essential B vitamin involved in nucleic acid synthesis, DNA methylation, and repair. 7 Folic acid is a synthetic form of folate that is commonly used to fortify foods, and consumed as nutritional supplements. 8 In light of substantial evidence that folic acid supplementation reduces the risk of neural tube defects (NTD) in offspring, the US Public Health Service recommended in 1992 that women of reproductive age consume 400 μg/d before and during pregnancy. Subsequently, mandatory fortification of cereal grain products at a suggested level of 140 μg folic acid/100 g was implemented in the US in 1998. 9
In the post-fortification era, serum folate levels in the US have increased 2.5 times across all life stages, including among pregnant women. 10 A recent NHANES study showed that unmetabolized folic acid (either from folic acid supplementation or fortified grain products) has been detected in most of the U.S. population. 11 Furthermore, data from the Boston Birth Cohort showed a wide range of individual variation in plasma folate levels ranging from insufficient to excessive levels. 12 The association between folic acid intake during pregnancy and ASD risk in offspring has been equivocal. Several studies suggest that mothers who use a periconceptional multivitamin or folic acid supplementation are less likely to have offspring with ASD, 2, 13 yet, others have hypothesized an opposite relationship. 14-16 Preliminary studies that included both women's report of prenatal vitamin use and maternal biomarker data found a protective effect of prenatal vitamins intake on ASD based on report, but this relationship could not be confirmed using biomarker data. 17, 18 Given this background, in this hypothesis-generating study, we evaluated the relationship between self-reported pregnancy multivitamin intake, and plasma folate levels in mothers at birth and ASD in offspring. Since both vitamin B12 and folate are intricately involved in one-carbon metabolism and there are very few studies on B12 status on human brain, 19 we also explored the association between maternal B12 biomarker levels and ASD risk in offspring.
Methods
Participants and data collection procedure
The study included mother-infant pairs who were recruited at the Boston Medical Center (BMC) at the time of birth from 1998 to 2013 and followed up prospectively from 2003 to 2015 (supplemental Figure 1), as described elsewhere. 12, 20 Children who were not intending to receive paediatric care at the BMC were excluded. Mothers who had a post-birth blood sample for analysing plasma folate and B12 and who had data on maternal multivitamin supplement intake during at least the third trimester were included in the analysis.
Mothers of newborns were approached 24-72 hours postpartum to participate in the study. After obtaining informed consent, a standard questionnaire was used to collect relevant maternal data including supplement intake. Maternal and infant medical records were reviewed using standardized abstraction forms to collect data on pre-pregnancy weight and pregnancy related complications. Maternal blood samples collected 24-72 hours post-delivery were later analysed for maternal plasma folate, B12 and homocysteine levels. Children were followed from birth through both study visits and clinical paediatric visits at the BMC. Electronic Medical Records (EMR) containing clinicians' primary and secondary diagnoses using ICD-9 codes were obtained for every postnatal clinical visit starting in 2003. The study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health and Boston University Medical Center.
Identification of children with ASD
Based on EMR, children who were ever diagnosed with autism (ICD-9 code 299.00), Asperger syndrome (299.80) and/or pervasive developmental disorder not otherwise specified (299.90) were categorized as having ASD, as described elsewhere. 21 Children who concomitantly had ASD and ADHD, ASD and other developmental disabilities, or ASD and intellectual disabilities were classified as having ASD. Children without ASD, ADHD (314.0-314.9), other developmental (315.0 - 315.9), or intellectual disabilities (317 – 319) constituted the ‘neurotypical’ group. Children diagnosed with ADHD or other developmental or intellectual disabilities without concurrent ASD were excluded from the analysis. One subject who had Ventricular Septal Defect (VSD), but was miscoded as having ASD, was identified and excluded. For sensitivity analyses, children with ASD were restricted to those with an ASD code for at least 2 visits and where at least one visit was with a specialist (developmental behavioural paediatrician, paediatric neurologist or child psychologist). Sensitivity analyses were also implemented restricting neurotypical children to only those that did not have other competing diagnoses, including congenital anomalies and psychiatric or behavioural disorders (see supplemental Table 1).
Exposures
Plasma folate was measured using chemiluminescent immunoassay with diagnostic kits (Shenzhen New Industries Biomedical Engineering Co., Ltd. China) and plasma B12 was measured using the Beckman Coulter ACCESS Immunoassay System (Beckman-Coulter Canada, Mississauga, Canada) using a MAGLUMI 2000 Analyzer. The interassay coefficient of variation was less than 4%. 12 Mothers with the lowest and highest deciles (top and bottom 10th percentiles) of the plasma folate (<14.7 and ≥60.3 nmol/L) and B12 (<247.0 and ≥536.8 pmol/L) distributions were compared against the middle 80th percentile.
Preconception multivitamin intake was dichotomized (no vs. yes) and prenatal multivitamin supplement intake was coded as a categorical variable (supplement ≤2 times/week, 3-5 times/week and >5 times/week). Mothers ever diagnosed with diabetes (250.00-250.93) were identified as pregestational diabetes cases and those ever diagnosed with diabetes mellitus complicating pregnancy (648.00 and 648.03) comprised gestational diabetes cases.
Covariates
Plasma homocysteine levels were measured using automatic clinical analyzers (Beckman-Coulter). Homocysteine was considered as a binary variable with the top 10th percentile of the distribution compared against the bottom 90th percentile (<11.7 μmol/L). Other covariates were chosen a priori based on previous studies looking at maternal nutritional status and the risk of ASD. 13, 22 Neonates who were delivered at or after 37 completed weeks of gestation were considered full term; those delivered <34 weeks, and ≥34 but <37 weeks of gestation were considered early and late preterm, respectively. Race-ethnicity was categorized into black, white, Hispanic and Other. Other covariates assessed were: child sex (female vs. male), maternal age at delivery, smoking during pregnancy (“ever smoked” 3 months before pregnancy/during pregnancy vs. “no smoking” during preconception/ pregnancy), parity (not including the index pregnancy), maternal education (high school or less vs. some college or more), year of the baby's birth (1998-2006 vs. 2007-2013) and Methylene tetrahydrofolate reductase (MTHFR) C677T genotypes (CC vs. CT vs. TT).
Statistical Analyses
The primary outcome variable was ASD and exposures were maternal vitamin supplementation during preconception, 1st, 2nd and 3rd trimesters or maternal plasma folate and B12 levels in the days after birth. Preliminary data analysis was performed to compare neurotypical children and those with ASD using chi-squared tests for categorical variables and ANOVA for continuous variables. We fitted a Cox proportional hazard regression model to estimate hazard ratios and account for the variability in length of follow-up. Birth of the child was defined as the time of origin and the child's first postnatal visit recorded in the EMR was defined as the time of entry. The child exited the ASD risk pool if he/she had the event (ASD diagnosis) or was censored after his/her recorded last postnatal visit. In addition, the role of maternal MTHFR C677T genotype was examined via stratification and cross-product proportional hazard regression analyses. We tested the interaction of maternal folate (as a binary variable) and 1) B12 levels (as a binary variable) and 2) MTHFR C677T (as a categorical variable) on the risk of ASD.
Results
A total of 1257 mother-infant pairs were included in the analysis, of which 86 were ASD cases and 1171 were children with neurotypical development (supplemental Figure 1). Children with ASD had co-morbidities including ADHD (n=25), intellectual disabilities (n=39) and other developmental disabilities (n=76), that were not mutually exclusive. Table 1 describes the characteristics of mothers and children in each group. The frequency of multivitamin supplement intake during third trimester differed between mothers whose children had ASD when compared to those who had children with neurotypical development. They were also more likely to have higher pre-pregnancy BMI, pre-gestational/gestational diabetes and very high maternal B12 (≥90th percentile). The distribution of folate and B12 levels in our study population is consistent with the NHANES data for women of reproductive age (supplemental Figure2) and detailed comparisons are provided in supplemental Tables 2 and 3. To understand the impact of differential follow-up, analyses were conducted on this cohort comparing baseline characteristics of the children included in the analyses and those excluded from the analyses.
Table 1. Maternal and offspring characteristics by offspring case status (neurotypical vs. ASD) in the Boston Birth Cohorta.
| Characteristics | Neurotypical (n=1,171) | ASD (n=86) |
|---|---|---|
| Mothers | ||
| Age at birth (years), mean (SD) | 28.3 (6.6) | 30.9 (6.5) |
| Parity (%) | ||
| 0 | 501 (42.8) | 31 (36.1) |
| 1 | 337 (28.8) | 37 (43.0) |
| 2 or more | 326 (27.8) | 18 (20.9) |
| Missing | 7 (0.6) | 0 (0.0) |
| Mother's education (%) | ||
| High School or less | 754 (64.4) | 48 (55.8) |
| Some college or more | 415 (35.3) | 37 (43.0) |
| Missing | 4 (0.3) | 1 (1.2) |
| Maternal BMI (SD) | 26.3 (6.2) | 28.1 (7.6) |
| Diabetes (%) | ||
| No | 1056 (90.2) | 70 (81.4) |
| Gestational diabetes mellitus | 67 (5.7) | 8 (9.3) |
| Diabetes mellitus | 48 (4.1) | 7 (8.1) |
| Missing | 0 (0.0) | 1 (1.2) |
| Smoking during & 3 months prior to pregnancy (%) | ||
| No | 1000 (85.4) | 69 (80.2) |
| Yes | 164 (14.0) | 15 (17.4) |
| Missing | 7 (0.6) | 2 (2.3) |
| Maternal plasma folate (%) | ||
| <14.7 nmol/L (<10th percentile) | 118 (10.1) | 7 (8.1) |
| ≥14.7 to <60.3 nmol/L (10-90 percentile) | 942 (80.4) | 65 (75.6) |
| ≥60.3 nmol/L (≥90th percentile) | 111 (9.5) | 14 (16.3) |
| Maternal plasma vitamin B12 (%) | ||
| <247.0 pmol/L (<10th percentile) | 119 (10.2) | 6 (7.0) |
| ≥247.0 to <536.8 pmol/L 10-90 percentile) | 945 (80.7) | 62 (72.1) |
| ≥536.8 pmol/L (≥90th percentile) | 107 (9.1) | 18 (20.9) |
| Maternal plasma homocysteine (%) | ||
| <11.7 μmol/L (<90th percentile) | 1050 (89.7) | 80 (93.0) |
| ≥11.7 μmol/L (≥90th percentile) | 121 (10.3) | 6 (7.0) |
| Maternal supplement intake (3rd trimester) (%) | ||
| <2 times/week | 116 (9.9) | 15 (17.4) |
| 3 – 5 times/week | 447 (38.2) | 19 (22.1) |
| >5 times/week | 608 (51.9) | 50 (60.5) |
| Maternal MTHFR genotype | ||
| CC | 742 (63.4) | 52 (60.5) |
| CT | 323 (27.6) | 24 (27.9) |
| TT | 65 (5.6) | 7 (8.1) |
| Missing | 41 (3.5) | 3 (3.5) |
| Offspring | ||
| Gender (%) | ||
| Male | 525 (44.8) | 63 (73.3) |
| Female | 646 (55.2) | 23 (26.7) |
| Gestational age (%) | ||
| Term | 911 (77.8) | 57 (66.3) |
| Late preterm (34-36 weeks) | 161 (13.8) | 13 (15.1) |
| Early preterm (<34 weeks) | 99 (8.5) | 16 (18.6) |
| Year of birth (%) | ||
| 1998-2006 | 526 (44.9) | 38 (44.2) |
| 2007-2013 | 645 (55.1) | 48 (55.8) |
The Boston Birth Cohort uses a rolling enrolment and the study sample were enrolled between 1998 and 2013, and have been followed up from birth up to the last visit recorded in the EMR
BMI, body mass index; MTHFR, methylene tetrahydrofolate reductase
Maternal multivitamin supplement intake preconception was not statistically significantly associated with the risk of ASD in children (Table 2). Consistent with previous research, 13 1st trimester supplement intake (≥ 3 times/week) was protective against ASD risk when compared to those who reported taking a supplement <3 times/week; or conversely, low levels of multivitamin supplement intake were associated with increased ASD risk. When supplement intake was stratified by intake frequency, a “U” shaped relationship was observed, with maternal supplement intake ≤2 times/week and >5 times/week during both demonstrating statistically significantly increased risk for ASD (Table 2 and Figure 1). This “U” shaped relationship was consistent across all trimesters and became stronger after adjusting for potential confounders.
Table 2. Maternal self-reported multivitamin intake during preconception and 1st, 2nd and 3rd trimesters and ASD risk in offspring in the Boston Birth Cohort (N=1,257).
| Unadjusted | Adjusteda | ||
|---|---|---|---|
| Maternal Supplement intake | Nb | HR (95% CI) | HR (95% CI) |
| Preconception | |||
| Yes | 54 | 0.4 (0.1, 1.8) | 0.5 (0.1, 2.1) |
| No | 1087 | 1.0 (Reference) | 1.0 (Reference) |
| First Trimester | |||
| ≤2 times/week | 164 | 2.1 (1.1, 4.0) | 3.4 (1.6, 7.2) |
| 3-5 times/week | 457 | 1.0 (Reference) | 1.0 (Reference) |
| >5 times/week | 628 | 1.9 (1.1, 3.1) | 2.3 (1.2, 3.9) |
| Second Trimester | |||
| ≤2 times/week | 115 | 2.6 (1.3, 5.3) | 3.8 (1.8, 8.0) |
| 3-5 times/week | 466 | 1.0 (Reference) | 1.0 (Reference) |
| >5 times/week | 674 | 1.8 (1.1, 3.0) | 2.1 (1.2, 3.6) |
| Third Trimester | |||
| ≤2 times/week | 131 | 2.7 (1.4, 5.3) | 3.5 (1.7, 7.4) |
| 3-5 times/week | 466 | 1.0 (Reference) | 1.0 (Reference) |
| >5 times/week | 660 | 1.8 (1.1, 3.1) | 2.1 (1.2, 3.6) |
Adjusted for maternal characteristics: age, education, parity, BMI, smoking status, diabetes status, race and MTHFR genotype; offspring characteristics: gestational age, sex and year of birth
N may be different for preconception and each trimester periods due to missing data
BMI, body mass index; MTHFR, methylene tetrahydrofolate reductase
Figure 1. Maternal self-reported multivitamin supplement intake and ASD risk in offspring in the Boston Birth Cohort (N=1,257).
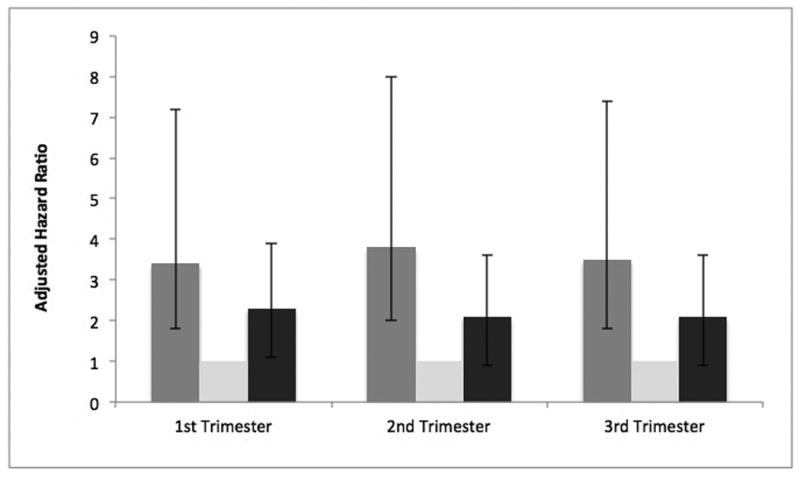
Adjusted association between maternal multivitamin supplement intake during 1st, 2nd and 3rd trimesters and risk of ASD in offspring (Ref category: supplement intake 3-5 times/week); Adjusted for maternal characteristics: age, education, parity, BMI, smoking status, diabetes status, race and MTHFR genotype; offspring characteristics: gestational age, sex and year of birth
Elevated maternal plasma B12 (>600 pmol/L) compared to non-elevated (≥200 – ≤600 pmol/L) levels was associated with increase in risk of ASD in offspring in both unadjusted and adjusted models (Table 3). 23 Deficient B12 (<200 pmol/L) compared to normal levels was not associated with ASD risk (Table 3).
Table 3. Maternal plasma folate, vitamin B12 concentrations in samples obtained 24-72 hours after delivery and risk of ASD in offspring in the Boston Birth Cohort (N=1,257).
| Unadjusted | Adjusteda | ||
|---|---|---|---|
| Maternal folate - WHO cutpoints | n | HR (95% CI) | HR (95% CI) |
| <13.5 nmol/L | 103 | 0.7 (0.3, 1.6) | 1.1 (0.5, 2.8) |
| ≥13.5 nmol/L to ≤45.3 nmol/L | 852 | 1.0 (Reference) | 1.0 (Reference) |
| >45.3 nmol/L (corresponds to 76th percentile) | 302 | 1.2 (0.8, 2.0) | 1.5 (0.9, 2.5) |
| Maternal vitamin B12 | |||
| <200 pmol/L | 35 | 1.7 (0.6, 4.8) | 1.9 (0.7, 5.3) |
| ≥200 pmol/L to ≤600 pmol/L | 1136 | 1.0 (Reference) | 1.0 (Reference) |
| >600 pmol/L (corresponds to 93.1th percentile) | 86 | 2.7 (1.4, 4.9) | 3.0 (1.6, 5.7) |
| Maternal Folate – Extreme Deciles | |||
| <14.7 nmol/L (<10th percentile) | 125 | 0.7 (0.3, 1.5) | 1.2 (0.5, 2.8) |
| >14.7 to <60.3 (≥10th to <90th, middle 80th percentile) | 1007 | 1.0 (Reference) | 1.0 (Reference) |
| ≥60.3 nmol/L (≥90th percentile) | 125 | 1.8 (1.0, 3.2) | 2.5 (1.3, 4.6) |
| Vitamin B12 – Extreme Deciles | |||
| <247.0pmol/L (<10th percentile) | 125 | 0.7 (0.3, 1.6) | 0.7 (0.3, 1.7) |
| ≥247.0 to <536.8 (≥10th to <90th, middle 80th percentile) | 1007 | 1.0 (Reference) | 1.0 (Reference) |
| ≥536.8pmol/L (≥90th percentile) | 125 | 2.6 (1.6, 4.5) | 2.5 (1.4, 4.5) |
| Joint effects of folate & vitamin B12 Percentilesb | |||
| Folate & vitamin B12 (10-90 percentile) | 815 | 1.0 (Reference) | 1.0 (Reference) |
| Folate (<10th percentile) & vitamin B12 (10-90 percentile) | 104 | 0.6 (0.2, 1.5) | 0.8 (0.3, 2.2) |
| Folate (≥90th percentile) & vitamin B12 (10-90 percentile) | 88 | 0.6 (0.2, 1.8) | 0.8 (0.2, 2.6) |
| Vitamin B12 (<10th percentile) & Folate (10-90 percentile) | 94 | 0.6 (0.2, 1.6) | 0.5 (0.2, 1.6) |
| Vitamin B12 (≥90th percentile) & Folate (10-90 percentile) | 98 | 1.2 (0.6, 2.7) | 1.1 (0.5, 2.4) |
| Either Folate & vitamin B12 (<10 percentile)c | 229 | 0.6 (0.3, 1.1) | 0.8 (0.4, 1.5) |
| Both Folate & vitamin B12 (<10 percentile) | 21 | 1.1 (0.3, 4.5) | 2.4 (0.5, 10.4) |
| Either Folate or vitamin B12 (≥90 percentile)d | 223 | 1.6 (0.9, 2.5) | 1.8 (1.1, 3.1) |
| Both Folate & vitamin B12 (≥90 percentile) | 27 | 6.3 (3.3, 12.1) | 13.7 (6.5, 28.9) |
| Maternal Homocysteine | |||
| ≥11.7 μmol/L (≥90 percentile) | 127 | 0.5 (0.2, 1.1) | 0.5 (0.2, 1.4) |
Adjusted for maternal characteristics: age, education, parity, BMI, smoking status, diabetes status, race and MTHFR genotype; offspring characteristics: gestational age, sex and year of birth
There was interaction between maternal plasma folate and vitamin B12 (P<0.01)
Either folate or vitamin B12 <10th percentile compares the risk of having a ASD child in mothers who had at least one of the biomarkers <10th percentile versus those who had both of these biomarkers in the middle 80th percentile
Either folate or vitamin B12 ≥90th percentile compares the risk of having a ASD child in mothers who had at least one of the biomarkers ≥90th percentile versus those who had both of these biomarkers in the middle 80th percentile
The risk of ASD in children along the continuum of plasma folate and B12 is presented in Figure 2 and supplemental Tables 4 and 5. Using the WHO suggested threshold, the risk of ASD was not significantly different between children when their mothers had possibly deficient (<13.5 nmol/L) or excess (>45.3 nmol/L) plasma folate levels after birth, compared to mothers who had normal levels (≥13.5 to ≤45.3) (Table 3). However, the risk of ASD among mother-child pairs with higher levels of plasma folate suggests association at increasing folate levels.
Figure 2. Association between maternal folate and vitamin B12 concentrations and risk of ASD in offspring in the Boston Birth Cohort (N=1,257).
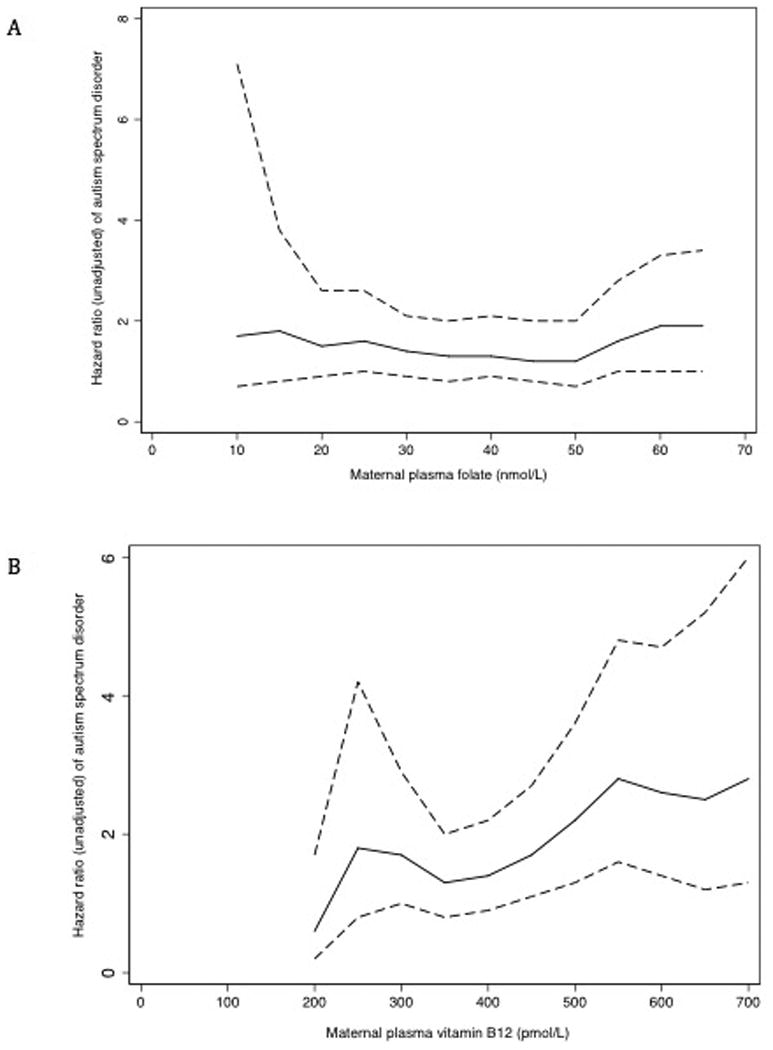
Unadjusted association between maternal plasma folate (panel A) and plasma vitamin B12 (panel B) levels at different cut-off points and risk of ASD in offspring. The unadjusted HR for plasma folate (panel A) was truncated at 65 nmol/L due to the small sample size beyond the specified cutoff point (unadjusted HR for mothers whose plasma folate ≥65 nmol/L was 1.9, 95% CI 1.0, 3.4; n=113). The unadjusted HR for plasma vitamin B12 (panel B) was truncated at 700 pmol/L due to declining sample sizes beyond the specified cutoff point (unadjusted HR for mothers whose plasma vitamin B12 ≥700 pmol/L was 2.8, 95% CI, 1.3, 6.0; n=45).
We categorized and compared the lowest and highest deciles with the middle 80th percentiles for maternal folate (<14.7 and ≥60.3 nmol/L) and B12 levels (<247.0 and ≥536.8 pmol/L). Mothers with plasma folate levels in the highest 10th percentile, when compared with the middle 80th percentile, had a significant increase in the risk of children's ASD in both unadjusted and adjusted models (Table 3). Mothers who had plasma folate levels in the lowest decile did not have increased ASD risk in children in the adjusted model (Table 3). Plasma B12 levels in the top decile, when compared to the middle 80%, was associated with about two and half times increased risk (Table 3). However, the risk of ASD in children whose mothers had lowest levels of plasma B12 was not significantly different than the referent group in both models. Similarly, elevated maternal plasma homocysteine when compared to non-elevated levels did not alter ASD risk even after accounting for confounders (Table 3).
We compared self-reported maternal multivitamin supplement intake to measured biomarker levels after birth. Nearly all mothers took supplements during pregnancy; the frequencies of use for 1st, 2nd and 3rd trimesters were 86.2%, 90.2% and 89.1% respectively. Within each supplement intake category, there was a range in maternal plasma folate and B12 levels (supplemental Table 6, supplemental Figures 3 and 4). There were no differences in the mean plasma folate levels between non-supplement users and different levels of supplement users, except those who had supplements 3-5 times/week. The percentage of mothers with elevated plasma folate and B12 did not vary between different levels of supplement intake. When supplement intake was stratified by parity, a greater percentage of nulliparous women were likely to consume supplements >5 times/week in the 1st and 3rd trimesters (supplemental Table 7).
We assessed the joint effects of maternal plasma folate and B12 levels on the risk of ASD in children by considering mothers who had both biomarkers in the middle 80th percentile at delivery as the referent category (Table 3). The risk of ASD in children was not different between mothers with only one or the other biomarker in an extreme decile compared to mothers who had both biomarkers in the middle 80th percentile, after adjusting for confounders. For mothers who had at least one biomarker level (plasma folate and/or B12) in the lowest decile, the risk of ASD in children was not different, nor was it for both in the lowest decile (Table 3). Mothers who had at least one or both biomarkers in the top decile did have an increased risk for ASD in their offspring in adjusted models (Table 3). There was an interaction between maternal folate and B12 (P <0.01).
MTHFR genotype was available for 96.5% of the mothers (n=1213) of which 794 (65.9%), 347 (28.6%) and 72 (5.9%) had CC, CT and TT genotype respectively. In this sample, maternal MTHFR genotype did not differ between children with neurotypical development and those with ASD (supplemental Table 8). No differences in genotype were observed by maternal folate and B12 levels (supplemental Table 9). The geometric means of plasma folate were also not significantly different across different genotypes (supplemental Table 10) and there was no interaction between maternal folate and MTHFR genotype status on ASD risk.
To assess the influence of EMR misclassification of ASD or neurotypical development on these findings, a sensitivity analysis (supplemental Table 11) was conducted applying stringent criteria for case diagnosis (ASD code for at least 2 visits, including a specialist) and for children with neurotypical development (excluding potential developmental disability indications) (supplemental Table 1). The results demonstrate slightly stronger associations in this smaller sample; mothers who had plasma folate in the top decile had more than two-fold greater ASD risk (HR 2.4, 95% CI 1.1, 5.0) and with plasma B12 in the top decile had almost four times increased risk (HR 3.9, 95% CI 2.0, 7.7), after adjusting for confounders. Consistent with results in the full sample, the risk of ASD was highest when mothers had elevated levels of both plasma folate and B12 (HR 16.4, 95% CI 6.5, 41.7).
Comment
Main findings
The results show that moderate intake (3-5 times/week) of multivitamin supplements during pregnancy is associated with decreased risk of ASD in offspring, consistent with the previous literature. 2, 13 Upon further examination of the risk at the low and high ends of intake, the study results further suggest that while infrequent intake (≤2 times/week) of multivitamin supplements is associated with increased risk of ASD, as has been reported previously, high frequency of multivitamin supplement intake (>5 times/week) is also associated with increased risk of ASD in children in this cohort, compared to moderate intake. This is the first study to report on the prospective association between measured maternal plasma folate and B12 biomarkers at birth and risk of ASD in offspring in a large, prospective US birth cohort. Consistent with the “U-shaped” finding for supplement intake, the biomarker analyses showed that very high levels of maternal plasma folate and B12 (≥90th percentile) at birth were also associated with increased risk of ASD in offspring.
With regard to post-delivery biomarkers, which are reasonably consistent with third trimester pregnancy levels, 24, 25 maternal plasma folate and B12 in the highest decile (≥90th percentile) were each associated with increased risk of ASD in children. A moderate level of self-reported supplement intake (3-5 times/week) is protective against ASD, but that an increased risk is observed with higher and lower intake of multivitamin supplements, and with very high concentrations of plasma folate (≥90th percentile) and B12 (≥90th percentile) biomarkers at birth. This observation suggests that while deficiency is detrimental, excess nutrient status might also be associated with elevated risk.
Interpretation
For over a century, it has been known that the dose-response relationship for many micronutrients is non-monotonic: at low levels benefits increase with intake until plateauing at optimal concentrations, with toxicity at higher levels, as regulatory mechanisms become overwhelmed. 26, 27 In a landmark paper, Daly et al. showed that maximum NTD risk was observed in mothers who had folate deficiency (0-4.4 nmol/L). There was a dose response relationship with risk plateauing beyond maternal folate levels of 11.3 nmol/L and no apparent additional benefits beyond levels of 15.9 nmol/L. 28 At the other end of the spectrum, this study observed an increased ASD risk when mothers had plasma folate levels in the top decile during the third trimester (corresponding to 60.3 nmol/L), which is well beyond the highest level recommended by WHO (45.3 nmol/L).
This study is not able to directly attribute the source of these high levels for the top decile.
It is possible that adverse birth outcomes such as NTD, spontaneous abortions, stillbirths and developmental disabilities, 29, 30,30,31 in a previous delivery might have prompted some pregnant women to consume higher dosages of prenatal vitamins, which could explain elevated biomarker levels. However, there were no significant difference in previous adverse pregnancy outcomes between mothers with ASD and children with neurotypical development in this sample. Upon further examination of EMR-based medication history for mothers of children with ASD, none of them were prescribed megadoses of prenatal vitamins.
Mandatory folic acid fortification was instituted in the U.S. in January 1998 and the BBC began enrolling mothers in October 1998. Considering that BBC is a post-fortification study, it is possible that women with high folic acid intake also consumed folic acid from multiple sources including fortified foods, possibly creating an accumulation of folic acid, as observed in a recent NHANES study. 32 In this sample, mothers who consumed more supplements were also more likely to have consumed fortified foods (e.g. pasta, bread, cereal), suggesting that the combination of dietary folic acid along with supplement intake might have potentially resulted in elevated levels, although correlations between supplement intake and biomarker levels were not extremely high.
This study notes that the highest ASD risk (HR 13.7, 95% CI 6.5, 28.9) was observed in children of mothers with both plasma folate and B12 elevated (≥90th percentile). In addition, a significant interaction was observed between plasma folate and B12 (p<0.001) suggesting possible perturbation in one-carbon metabolism, which intimately involves both micronutrients.
One possible theory suggests that elevated maternal folate levels in the past few decades have altered natural selection, increasing the survival rates of those with the MTHFR C677T polymorphism by reducing miscarriage rates. 15 It is also possible that genetic variation may interfere with folic acid absorption or metabolism to folate precluding the benefits of folic acid and promoting accumulation in maternal blood. In this study, there was no relationship between the MTHFR C677T variant, plasma folate and ASD risk. However, the study did not exhaustively examine variation in this gene, or other genes in the one-carbon cycle. Also, due to the small number of mothers with TT genotype in this sample, the study might be underpowered to address this question.
It is not surprising that some nutrients are tightly regulated within a narrow range, given that both deficiency and excess can induce abnormal brain development. 33 The concentration of nutrients can have an impact on the brain development with a nutrient that promotes normal development in one concentration may be toxic at another. 33 Similarly, the timing of supplementation or deficiency can also have a role to play in brain function. 33, 34 Pregnant women have superior absorption of folic acid and B12 compared to non-pregnant counterparts 35 and excrete minimal folate in urine during the third trimester 36 – all of which could have lead to increased plasma levels. Considering the elevated maternal levels in addition to foetus's ability to actively absorb micronutrients, it is likely that some offspring may have accumulated high levels of these micronutrients. The foetal brain is vulnerable to nutritional insults especially during the third trimester, when several neurological processes including synaptogenesis increase in cortical volume and cortical connectivity between different regions, myelination and pruning are occurring at a rapid rate. 33, 37-40
Human and animal studies have shown that sub-optimal intake of folic acid during pregnancy can induce persistent changes in the offspring's genome, thereby influencing physiological outcomes. 41-45 With regards to neurocognitive development, increase in maternal folate during gestation in animal models alters gene expression in cerebral and cerebellar hemispheres. 41, 42 Specifically, key developmental genes involved in neural pathways, gamma-Aminobutyic acid (GABA), dopamine-serotonin and synaptic plasticity demonstrated altered expression and impairment in many of the pathways are linked to ASD. 42, 46, 47 Prolonged exposure to high folic acid has been shown to alter offspring's behaviour, including greater anxiety-like behaviour, ultrasonic vocalizations in pups (linked to autism in mouse models) and hyperactivity. 42 B12 plays an important role in DNA methylation, in addition to being integrally involved in myelination, 48 cellular growth and differentiation. 49 Yet, there is a dearth of research on the role of B12 status on developing brains. 19 A cross-sectional study showed that maternal B12 measured at parturition was inversely associated with DNA methylation of insulin-like growth factor (IGF-2) in cord blood. 50
Limitations and Strengths of the study
Case and neurotypical development classification was based on EMR, rather than using adjudication based on research-reliable gold standard diagnostic assessments such as the Autism Diagnostic Interview-Revised (ADI-R) or the Autism Diagnostic Observation Schedule (ADOS). 51 This approach enables consideration of all children with available administrative data, but may misclassify some children as ASD who have other developmental or behavioural problems. The study findings were consistent even when restricting cases to those with multiple visits indicating an ASD diagnostic code, including by a specialist. Further, if such misclassification of atypical, but non-ASD children exists in the sample, the results would imply that elevated maternal plasma folate and B12 levels could have implications in other developmental disabilities beyond ASD.
Maternal dietary intake data during preconception and pregnancy might have provided additional perspectives on the study results and lack of this information is a limitation. Maternal plasma folate and B12 were measured 24-72 hours after delivery; this may reflect maternal folate and B12 levels only during the third trimester and may not reflect early pregnancy status. Despite plasma folate being a marker of recent intake and more susceptible to variations in diet, it is well correlated with red blood cell folate and thus is a reliable marker. 52 Although we had information on the frequency of maternal multivitamin supplement intake, lack of information on specific dosage is a limitation. A study based on NHANES data suggests that a majority of pregnant women consume ≥800 μg of folic acid when using supplements and we assume this to be true of women in this cohort as well. 53 We adjusted for well-recognized ASD risk factors, but there is a possibility of residual confounding. Finally, the study population consists mainly of urban low-income minority women and extrapolation of study findings to other populations should be made with caution.
While the study suggests that very low or very high maternal folate and B12 measured at delivery may be associated with ASD in offspring, further research in the following areas will be beneficial, beginning with replication of these findings in independent cohorts with more vigorous ASD phenotyping, understanding the mechanism connecting elevated folate, B12 to neurocognitive development and examining the source of elevated biomarkers. Further, more research is needed to understand if the window of exposure affects the impact of folic acid supplementation.
Conclusion
The aetiology of ASD is complex and this study provides a new perspective but not a definitive explanation. This hypothesis-generating study does not question the importance of consuming adequate amounts of folic acid and B12 during pregnancy; the results confirm the protective effects of adequate vitamin supplementation for ASD risk, as observed in previous studies. Rather, it raises questions about whether excessive amounts of maternal folate and B12 may be harmful if a “U-shaped” risk curve is confirmed, as has been observed for other health-related risk.54 This result suggests further investigation of this potentially U-shaped risk in other cohorts and underscores the critical importance of identifying optimum maternal levels of folate and B12 for foetal/child neurodevelopment.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants and the field staff without whom this study would not have been possible.
Funding source: This work was supported in part by the Maternal and Child Health Bureau (R40MC27443 to XW and MDF). The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes PERI grants (20-FY02-56, #21-FY07-605 to XW); and the National Institutes of Health (NIH) grants (R21ES011666, R01HD041702, R21HD066471, U01AI090727, R21AI079872, and R01HD086013 to XW). Ramkripa Raghavan is supported by a student grant award by the Wendy Klag Center for Autism and Developmental Disorders, JHBSPH and by John and Alice Chenoweth-Pate Fellowship in her current training.
Footnotes
Conflict of interest: None of the authors have a conflict of interest pertaining to this work
References
- 1.Liptak GS, Benzoni LB, Mruzek DW, Nolan KW, Thingvoll MA, Wade CM, et al. Disparities in diagnosis and access to health services for children with autism: data from the National Survey of Children's Health. Journal of Developmental and Behavioral Pediatrics. 2008;29:152–160. doi: 10.1097/DBP.0b013e318165c7a0. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology. 2011;22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeVilbiss EA, Gardner RM, Newschaffer CJ, Lee BK. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br J Nutr. 2015:1–10. doi: 10.1017/S0007114515002470. [DOI] [PubMed] [Google Scholar]
- 4.Developmental Disabilities Monitoring Network Surveillance Year Principal I, Centers for Disease C, Prevention. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR: Surveillance Summaries. 2014;63:1–21. [PubMed] [Google Scholar]
- 5.Xu G, Jing J, Bowers K, Liu B, Bao W. Maternal diabetes and the risk of autism spectrum disorders in the offspring: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders. 2014;44:766–775. doi: 10.1007/s10803-013-1928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. British Journal of Psychiatry. 2009;195:7–14. doi: 10.1192/bjp.bp.108.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role. Advances in Nutrition. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen KE, Mikael LG, Leung KY, Levesque N, Deng L, Wu Q, et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. American Journal of Clinical Nutrition. 2015;101:646–658. doi: 10.3945/ajcn.114.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. Journal of Nutrition. 2002;132:2792–2798. doi: 10.1093/jn/132.9.2792. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. Journal of Nutrition. 2012;142:886–893. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, et al. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. Journal of Nutrition. 2015;145:520–531. doi: 10.3945/jn.114.201210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, et al. Association Between Maternal Prepregnancy Body Mass Index and Plasma Folate Concentrations With Child Metabolic Health. JAMA Pediatrics. 2016;170:e160845. doi: 10.1001/jamapediatrics.2016.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suren P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570–577. doi: 10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beard CM, Panser LA, Katusic SK. Is excess folic acid supplementation a risk factor for autism? Medical Hypotheses. 2011;77:15–17. doi: 10.1016/j.mehy.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Rogers EJ. Has enhanced folate status during pregnancy altered natural selection and possibly Autism prevalence? A closer look at a possible link. Medical Hypotheses. 2008;71:406–410. doi: 10.1016/j.mehy.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 16.King CR. A novel embryological theory of autism causation involving endogenous biochemicals capable of initiating cellular gene transcription: a possible link between twelve autism risk factors and the autism ‘epidemic’. Medical Hypotheses. 2011;76:653–660. doi: 10.1016/j.mehy.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Braun JM, Froehlich T, Kalkbrenner A, Pfeiffer CM, Fazili Z, Yolton K, et al. Brief report: are autistic-behaviors in children related to prenatal vitamin use and maternal whole blood folate concentrations? Journal of Autism and Developmental Disorders. 2014;44:2602–2607. doi: 10.1007/s10803-014-2114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steenweg-de Graaff J, Ghassabian A, Jaddoe VW, Tiemeier H, Roza SJ. Folate concentrations during pregnancy and autistic traits in the offspring. The Generation R Study. European Journal of Public Health. 2015;25:431–433. doi: 10.1093/eurpub/cku126. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hodgson NW, Trivedi MS, Abdolmaleky HM, Fournier M, Cuenod M, et al. Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia. PloS One. 2016;11:e0146797. doi: 10.1371/journal.pone.0146797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics. 2016;137:1–10. doi: 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. American Journal of Clinical Nutrition. 2012;96:80–89. doi: 10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendt JF, Nexo E. Unexpected high plasma cobalamin : proposal for a diagnostic strategy. Clinical Chemistry and Laboratory Medicine. 2013;51:489–496. doi: 10.1515/cclm-2012-0545. [DOI] [PubMed] [Google Scholar]
- 24.Tamura T, Picciano MF. Folate and human reproduction. American Journal of Clinical Nutrition. 2006;83:993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- 25.Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM. Cobalamin status during normal pregnancy and postpartum: a longitudinal study comprising 406 Danish women. European Journal of Haematology. 2006;76:521–525. doi: 10.1111/j.0902-4441.2006.t01-1-EJH2550.x. [DOI] [PubMed] [Google Scholar]
- 26.Raubenheimer D, Lee KP, Simpson SJ. Does Bertrand's rule apply to macronutrients? Proceedings: Biological Sciences. 2005;272:2429–2434. doi: 10.1098/rspb.2005.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barua S, Kuizon S, Ted Brown W, Junaid MA. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. Journal of Molecular Neuroscience. 2015 doi: 10.1007/s12031-015-0673-8. [DOI] [PubMed] [Google Scholar]
- 28.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA. 1995;274:1698–1702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RD, Genetics C, Wilson RD, Audibert F, Brock JA, Carroll J, et al. Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies. Journal d'Obstétrique et Gynécologie du CanadaJournal of Obstetrics and Gynaecology Canada. 2015;37:534–552. doi: 10.1016/s1701-2163(15)30230-9. [DOI] [PubMed] [Google Scholar]
- 30.Zahran KM, Adb Elaal DEM, Kamel HS, Samy EI, Ismail AM, Abbas AM. A combination treatment of folic acid, aspirin, doxycycline and progesterone for women with recurrent early pregnancy loss; hospital based study. Middle East Fertility Society Journal. 2016;21:22–26. [Google Scholar]
- 31.Czeizel AE, Puho E. Maternal use of nutritional supplements during the first month of pregnancy and decreased risk of Down's syndrome: case-control study. Nutrition. 2005;21:698–704. doi: 10.1016/j.nut.2004.10.017. discussion 774. [DOI] [PubMed] [Google Scholar]
- 32.Orozco AM, Yeung LF, Guo J, Carriquiry A, Berry RJ. Characteristics of U.S. Adults with Usual Daily Folic Acid Intake above the Tolerable Upper Intake Level: National Health and Nutrition Examination Survey, 2003-2010. Nutrients. 2016;8:195. doi: 10.3390/nu8040195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 34.Fuglestad AJ, Rao R, Georgieff MK. The role of nutrition in cognitive development. Available from: http://hirezz.com/satvenandmer.com/pdf/dha/Nutrition and Cognitive Development.pdf.
- 35.Hellegers A, Okuda K, Nesbitt RE, Jr, Smith DW, Chow BF. Vitamin B12 absorption in pregnancy and in the newborn. American Journal of Clinical Nutrition. 1957;5:327–331. doi: 10.1093/ajcn/5.3.327. [DOI] [PubMed] [Google Scholar]
- 36.West AA, Yan J, Perry CA, Jiang X, Malysheva OV, Caudill MA. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. American Journal of Clinical Nutrition. 2012;96:789–800. doi: 10.3945/ajcn.112.037523. [DOI] [PubMed] [Google Scholar]
- 37.Mahoney AD, Minter B, Burch K, Stapel-Wax J. Autism spectrum disorders and prematurity: a review across gestational age subgroups. Advances in Neonatal Care. 2013;13:247–251. doi: 10.1097/ANC.0b013e31828d02a1. [DOI] [PubMed] [Google Scholar]
- 38.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusick SE, Georgieff MK. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. Journal of Pediatrics. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Development and Psychopathology. 2015;27:411–423. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barua S, Kuizon S, Brown WT, Junaid MA. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Frontiers in Neuroscience. 2016;10:168. doi: 10.3389/fnins.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barua S, Chadman KK, Kuizon S, Buenaventura D, Stapley NW, Ruocco F, et al. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PloS One. 2014;9:e101674. doi: 10.1371/journal.pone.0101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ly A, Ishiguro L, Kim D, Im D, Kim SE, Sohn KJ, et al. Maternal folic acid supplementation modulates DNA methylation and gene expression in the rat offspring in a gestation period-dependent and organ-specific manner. The Journal of Nutritional Biochemistry. 2016;33:103–110. doi: 10.1016/j.jnutbio.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 44.Friso S, Choi SW. Gene-nutrient interactions and DNA methylation. Journal of Nutrition. 2002;132:2382S–2387S. doi: 10.1093/jn/132.8.2382S. [DOI] [PubMed] [Google Scholar]
- 45.Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. American Journal of Clinical Nutrition. 2013;97:94–99. doi: 10.3945/ajcn.112.042572. [DOI] [PubMed] [Google Scholar]
- 46.Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nature Reviews: Neuroscience. 2015;16:551–563. doi: 10.1038/nrn3992. [DOI] [PubMed] [Google Scholar]
- 47.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Current Biology. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food and Nutrition Bulletin. 2008;29:S126–131. doi: 10.1177/15648265080292S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCullough LE, Miller EE, Mendez MA, Murtha AP, Murphy SK, Hoyo C. Maternal B vitamins: effects on offspring weight and DNA methylation at genomically imprinted domains. Clinical Epigenetics. 2016;8:8. doi: 10.1186/s13148-016-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ba Y, Yu H, Liu F, Geng X, Zhu C, Zhu Q, et al. Relationship of folate, vitamin B12 and methylation of insulin-like growth factor-II in maternal and cord blood. European Journal of Clinical Nutrition. 2011;65:480–485. doi: 10.1038/ejcn.2010.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falkmer T, Anderson K, Falkmer M, Horlin C. Diagnostic procedures in autism spectrum disorders: a systematic literature review. European Child and Adolescent Psychiatry. 2013;22:329–340. doi: 10.1007/s00787-013-0375-0. [DOI] [PubMed] [Google Scholar]
- 52.Galloway M, Rushworth L. Red cell or serum folate? Results from the National Pathology Alliance benchmarking review. Journal of Clinical Pathology. 2003;56:924–926. doi: 10.1136/jcp.56.12.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Branum AM, Bailey R, Singer BJ. Dietary supplement use and folate status during pregnancy in the United States. Journal of Nutrition. 2013;143:486–492. doi: 10.3945/jn.112.169987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aaltonen J, Ojala T, Laitinen K, Piirainen TJ, Poussa TA, Isolauri E. Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics. 2008;152:79–84. 84 e71–72. doi: 10.1016/j.jpeds.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 55.Heavner K, Burstyn I. A Simulation Study of Categorizing Continuous Exposure Variables Measured with Error in Autism Research: Small Changes with Large Effects. International Journal of Environmental Research and Public Health. 2015;12:10198–10234. doi: 10.3390/ijerph120810198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


