Abstract
Immunotherapy is revolutionizing the management of multiple solid tumors, and early data have revealed the clinical activity of PD-1/PD-L1 antagonists in small numbers of metastatic breast cancer patients. Clinical activity appears more likely if the tumor is triple negative, PD-L1+, and/or harbors higher levels of TILs. Responses to atezolizumab and pembrolizumab appear to be durable in metastatic triple negative breast cancer (TNBC), suggesting these agents may transform the lives of responding patients. Current clinical efforts are focused on developing immunotherapy combinations that convert non-responders to responders, deepen those responses that do occur, and surmount acquired resistance to immunotherapy. Identifying biomarkers that can predict the potential for response to single agent immunotherapy, identify the best immunotherapy combinations for a particular patient, and guide salvage immunotherapy in patients with progressive disease are high priorities for clinical development. Smart clinical trials testing rational immunotherapy combinations that include robust biomarker evaluations will accelerate clinical progress, moving us closer to effective immunotherapy for almost all breast cancer patients.
Keywords: immunotherapy, breast cancer, immune checkpoint blockade, vaccines, PD-1/PD-L1
Introduction (5277)
Breast cancer remains a significant threat to the health and wellness of women in the United States, accounting for 30% of all new cancer diagnoses and almost 41,000 deaths annually (1). Although advances in early detection and therapy have resulted in a 38% decrease in the breast cancer death rate, almost all patients who develop metastatic disease will succumb to it. These sobering data illustrate a critical need for innovative approaches to breast cancer therapy that reduce relapse and death due to this disease. In recent years, accumulating data support a key role for the immune system in determining both response to standard therapy and long-term survival in breast cancer patients (2). Both these data and the striking clinical success of immune checkpoint antagonists across multiple solid tumors (3, 4) have re-ignited interest in immune-based strategies for breast cancer treatment and prevention (5, 6).
Setting the Stage for Modern Breast Cancer Immunotherapy
The dualistic role of the immune system in breast cancer
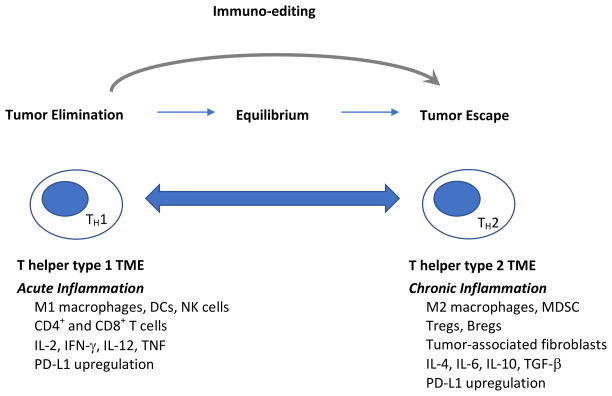
The immune system plays an active role in breast cancer development, progression, and control (5). The evolving interactions between mammary tumors and host immunity is characterized by immunoediting (7). Early in mammary tumorigenesis, acute inflammation activates innate immunity, resulting in both tumor cell death and the maturation of dendritic cells (DCs) that prime the tumor-specific T cell response. At this stage, either immune-mediated rejection of incipient tumors or the selection of tumor cell variants that can escape the immune response occurs. Ultimately there is a shift from acute to chronic inflammation, establishing a complex tumor microenvironment (TME) consisting of suppressive immune cells (regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and B cells) and stromal cells (fibroblasts and endothelial cells) that allow overt immune escape and tumor progression to occur (8, 9). During this shift, the CD4 T cell response is skewed from T helper type 1 to T helper type 2 (10), immune checkpoint molecules are upregulated on tumor cells and immune cells in response to early immune activation (11), and immune suppressive metabolic pathways are activated in multiple immune cell types (12, 13). Together, these forces establish a formidable network of immune suppression within the breast TME. This microenvironment, and other factors described in this review, impinge on the immune system to sculpt antitumor immunity (Figure 1).
Figure 1. The immune system plays a role in breast tumor growth and progression, and also in breast tumor elimination.
Early in breast tumor development, the acute inflammatory response results in the production of interleukin-12 (IL-12) and interferon-γ (IFN-γ), establishing a T helper type 1 environment at the tumor site. During this phase, DCs mature, process tumor-associated antigens, and migrate to the tumor-draining lymph nodes to present antigen to naïve CD4+ and CD8+ T cells, resulting in an immune response that ultimately lyses tumor cells. This immune response initially results in complete tumor rejection. However, the pressure it imposes leads to the selection of tumor cell variants that escape the immune response. This process of immuno-editing establishes a state of equilibrium, or dormancy. As inflammation at the tumor site shifts from acute to chronic, the tumor microenvironment evolves to a T helper type 2 profile. A suppressive tumor microenvironment comprised of a complex community of tumor cells, immune cells and host stromal cells is established, and breast tumors grow and metastasize unchecked by the immune system. Abbreviations: TH1= T helper type 1; TH2=T helper type 2; TME= tumor microenvironment; DCs=dendritic cells, NK=natural killer, IL-2=interleukin-2; IFN-γ=interferon-γ; IL-12=interleukin-12; TNF=tumor necrosis factor; PD-L1=programmed death ligand-1; MDSC=myeloid-derived suppressor cells; IL-4=interleukin-4; IL-6=interleukin-6; IL-10=interleukin-10; TGF-β=transforming growth factor-β.
Immune biomarkers are both prognostic and predictive in breast cancer
Breast cancer is a heterogeneous disease. It can be classified into three major clinically relevant subtypes that are managed differently: luminal (expressing the estrogen receptor (ER) and/or progesterone receptor (PR)), human epidermal growth factor receptor-2+ (HER-2+), and triple-negative, lacking expression of ER, PR, and HER-2 (TNBC) (14). Of these subtypes, HER-2+ breast cancers and TNBCs are more likely than luminal breast cancers to harbor stromal infiltrating immune cells (TILs) at diagnosis, with a linear relationship between stromal TIL content and clinical outcomes (2, 15). HER-2+ breast cancers and TNBCs are also more likely to express the programmed death ligand-1 (PD-L1) in the TME than luminal breast cancers (15, 16). Higher levels of TILs at diagnosis predict benefit from adjuvant and neoadjuvant chemotherapy, with longer progression-free survival (PFS) and overall survival (OS) (2). A higher CD8+ T cell/Treg ratio is also associated with a greater likelihood of cPR to neoadjuvant chemotherapy (17, 18). Some solid tumors that harbor TILs and express PD-L1 are more likely to respond to PD-1/PD-L1 blockade (19, 20), suggesting this may also be the case for breast cancers.
Tumor antigens and vaccines in breast cancer
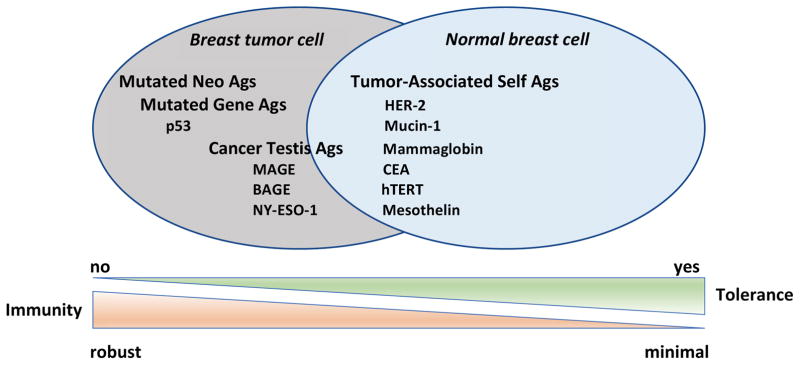
The immune system eliminates tumors by recognizing tumor antigens processed and presented in the context of major histocompatibility complex (MHC) Class I (for CD8+ T cells) and MHC Class II (for CD4+ T cells), resulting in the cross-priming and activation of T cells and tumor rejection. Breast cancer vaccines are designed to induce or amplify a population of tumor-specific T cells that can recognize and lyse breast tumors. Historically, breast cancer vaccines have incorporated shared tumor antigens that are overexpressed in tumors relative to normal tissues, or are restricted to mammary tissue (Figure 2). Shared tumor antigens are typically recognized as self by the immune system. To avoid autoimmunity, thymic selection results in the deletion of high avidity T cells specific for these tumor antigens, leaving in place a population of lower avidity (weaker) T cells for recruitment to the tumor-specific immune response.
Figure 2. Breast tumor antigens and immune recognition.
Tumor antigens expressed by normal cells and tumors partially overlap. Historically, tumor antigens targeted by immunotherapy have been proteins shared by normal host tissue and tumor cells for which there is established immune tolerance. Specific mutations in these proteins and/or their over-expression by tumors relative to normal tissue facilitates the preferential recognition of tumor cells relative to normal cells by the immune system. More recently, the importance of tumor neoantigens unique to a given tumor relative to other tumors or normal tissue has emerged. These neoantigens result from the genomic instability of tumors. Tumors with a high mutational load (more neoantigens) tend to have a higher response rate to immune checkpoint blockade, and are thought to be more readily recognized by the immune system due to lack of antigen-specific immune tolerance to the expressed neoantigens. Abbreviations: Ags=antigens; MAGE=melanoma associated antigen; BAGE=B melanoma antigen; HER-2=human epidermal growth factor receptor-2; CEA=carcinoembryonic antigen; hTERT=human telomerase reverse transcriptase.
Breast cancer vaccines that deliver shared tumor antigens have been evaluated in multiple trials that have tested peptide and/or protein vaccines specific for HER-2 or the carbohydrate antigen Mucin-1, DC-based vaccines specific for HER-2, cell-based (poly-antigen) vaccines that secrete granulocyte-macrophage colony stimulating factor (GM-CSF), and viral vector vaccines that deliver carcinoembryonic antigen (CEA), Mucin-1, and a triad of molecules that stimulate T cell activation (TRICOM) (5–6). These trials together have demonstrated that shared tumor antigen vaccines are safe, and can induce an antigen-specific immune response. However, the magnitude of the T cell response induced is usually low, and vaccine-induced immunity has not typically correlated with clinical benefit or response. One major limitation of the vaccines tested thus far is their potency due to both the shared tumor antigens targeted, the vaccine platforms used, and the advanced disease settings in which the vaccines were tested. As an alternative, vaccines that include mutation-specific antigens are tumor-specific and perceived by the immune system as foreign. The activated T cell repertoire would thus include high avidity (stronger) T cells that can be effectively by activated by vaccines to lyse tumor cells presenting these neoantigens (21). Consistent with this concept, tumors with a high mutational load are more likely to respond to immune checkpoint blockade (22, 23). Efforts to profile the mutational landscape of an individual patient’s tumor in order to generate a personalized breast cancer vaccine with high potency are underway, but the extent to which mutational load influences clinically meaningful breast tumor immunity remains to be determined (24). A major limitation to the potency of both generalized and personalized tumor vaccines thus far is the activity of immune checkpoint pathways and other mechanisms of immune suppression that keep vaccine-induced T cells in check (5). Several clinical trials have tested breast cancer vaccines in combination with low dose chemotherapy (to mitigate Treg-mediated suppression) (25, 26), or full dose chemotherapy (to reduce tumor burden, induce antigen release to support T cell priming, and reduce immune suppressive mechanisms in the TME) (27). Other combination vaccine trials have tested peptide- and cell-based vaccines that deliver HER-2 with trastuzumab alone (which can augment immune priming, enhance effector CD8+ T cell activity, and promote immune memory) (28, 29) or trastuzumab and low dose cyclophosphamide (to abrogate Treg activity) (30). Data to date are consistent with augmented vaccine-induced immunity induced by combination therapy. An area of high interest is combining breast cancer vaccines with antagonists of CTLA-4 or PD-1/PD-L1 to abrogate the signaling that shuts vaccine-induced T cells down at the tumor site.
Recent Clinical Advances in Breast Cancer Immunotherapy
Immune checkpoint antagonists specific for CTLA-4, PD-1, and PD-L1 have revolutionized cancer therapy, inducing durable objective responses that sometimes translate into an overall survival (OS) benefit in multiple cancer types (3, 4). Although several drugs are now FDA-approved for multiple cancers, none has yet been approved for breast cancer. Even so, the CTLA-4 antagonists tremelimumab and ipilimumab have been tested in small breast cancer trials, with evidence of immune modulation. Furthermore, accumulating data suggest that antagonists of PD-1/PD-L1 signaling can induce durable clinical responses in some patients with metastatic TNBC, and likely have meaningful clinical activity in rare patients with ER+ HER-2- breast cancer as well. These data are summarized in Table 1.
Table 1.
PD-1/PD-L1 Blockade in Metastatic Breast Cancer
| Antibody | Target | Combination | Breast Cancer Subtype | # Patients | ORR | DCR |
|---|---|---|---|---|---|---|
|
| ||||||
| Avelumab | PD-L1 | Single agent | All | 168 | 4.8% | 28% |
|
| ||||||
| PD-L1+ All | 12 | 33.3% | NR | |||
|
| ||||||
| TNBC | 58 | 8.6% | 31% | |||
|
| ||||||
| PD-L1+ TNBC | 9 | 44.4% | NR | |||
|
| ||||||
| PD-L1− TNBC | 39 | 2.6% | NR | |||
|
| ||||||
| Pembrolizumab | PD-1 | Single agent | PD-L1+ TNBC | 27 | 18.5% | 26% |
|
| ||||||
| Single agent | TNBC | 170 | 4.7% | 7.6% | ||
| PD-L1+ TNBC | 105 | 4.8% | 9.5% | |||
| PD-L1− TNBC | 64 | 4.7% | 4.7% | |||
|
| ||||||
| Single agent | PD-L1+ TNBC, 1st line | 52 | 23.1% | NR | ||
|
| ||||||
| PD-L1+ ER+ HER-2- BC | 25 | 12% | 20% | |||
|
| ||||||
| Atezolizumab | PD-L1 | Single agent | TNBC | 112 | 10% | 23% |
|
| ||||||
| PD-L1+ TNBC | 71 | 13% | 27% | |||
|
| ||||||
| PD-L1− TNBC | 37 | 5% | 16% | |||
|
| ||||||
| Atezolizumab | PD-L1 | Nab-paclitaxel | TNBC | 32 | 38% | NR |
|
| ||||||
| Pembrolizumab | PD-1 | Eribulin | TNBC | 39 | 33.3% | 41% |
Abbreviations: PD-1=programmed death-1; PD-L1=programmed death ligand-1; ORR=overall response rate; DCR=disease control rate; NR=not reported; TNBC=triple negative breast cancer; ER=estrogen receptor; HER-2=human epidermal growth factor receptor-2.
CTLA-4 Blockade in Breast Cancer
CTLA-4 is upregulated shortly after T cell activation, binding CD80/CD86 to provide negative feedback to CD28 co-stimulation and limiting T cell activation during the priming phase of the immune response (31). This helps to prevent uncontrolled immunity. Two humanized monoclonal antibodies specific for CTLA-4 are in the clinic.
Tremelimumab
The anti-CTLA-4 agent tremelimumab remains investigational in every tumor type. Escalating doses of tremelimumab have been tested with concurrent exemestane in 26 patients with ER+ HER-2- breast cancer (32). Five patients had dose limiting toxicity (DLT), which included diarrhea (4 counts) and elevated serum transaminase levels (1 count). The maximum tolerated dose (MTD) was 6 mg/kg every 90 days. The best response was stable disease (SD) for ≥12 weeks in 42% of patients, and a significant increase in the ratio of ICOS+/FoxP3+ CD4+ T cells was observed in most patients.
Ipilimumab
Ipilimumab is currently approved as a single agent for early and late stage melanoma, and is under investigation in multiple other tumor types both as a single agent, and in combination with PD-1/PD-L1 blockade (4). A study in breast cancer evaluated a single dose of neoadjuvant ipilimumab alone or given with cryoablation in 12 patients with early breast cancer prior to mastectomy; 6 additional patients received pre-operative cryoablation alone (33). Combination immunotherapy induced circulating T helper type 1 cytokines, ICOS+Ki67+CD4+ and ICOS+Ki67+CD8+ T cells, and an increased CD8+ T cell/FoxP3+ T reg ratio within the tumor. Clonally expanded TILs (detected by deep sequencing of TCR DNA) correlated with the TIL score by H&E (34). Based on these promising results, a follow up study is evaluating cryoablation combined with CTLA-4 (ipilimumab 1 mg/kg) and PD-1 blockade (nivolumab 3 mg/kg) (NCT02833233).
PD-1/PD-L1 Blockade in Breast Cancer
The programmed cell death-1 (PD-1) receptor is upregulated on activated T cells and binds two known ligands, PD-L1 and PD-L2. Through interactions with PD-L1 on the surface of tumor cells and immune cells, PD-1 signaling counters T cell activation during the effector phase of the immune response (31). Metastatic breast cancer responds to treatment with humanized monoclonal antibodies that target PD-L1 (avelumab and atezolizumab) and PD-1 (pembrolizumab) (35). Side effects associated with the use of these agents in breast cancer to date have been consistent with those expected for the drug class.
Avelumab
Avelumab was evaluated in multiple tumor types in the Phase Ia/1b JAVELIN study (36). The Phase 1b portion of this trial enrolled 168 patients in a breast cancer-specific expansion cohort regardless of either disease subtype or PD-L1 expression (37). The subtype distribution was 42.9% ER+/PR+/HER-2- disease, 34.5% TNBC, and 15.5% HER-2+ breast cancer; the disease subtype was unknown in 7.1% of patients. Over half of the patients had ≥3 prior lines of therapy for metastatic disease. The overall response rate (ORR) for the entire cohort was 4.8%, and included 1 complete response (CR), 7 partial responses (PRs), and 39 patients with stable disease (SD) for a disease control rate (DCR) of 28%. Responses were observed in all breast cancer subtypes, but appeared to be higher in TNBC. 58 patients showed an ORR of 8.6%, with 0 CRs, 5 PRs, and 13 patients with SD, and a DCR of 31%; PD-L1 expression was evaluable in 136 patients; 12 patients had ≥10% PD-L1+ immune cells in the TME, and 124 had <10%; the PR rates in these two groups were 33.3% and 2.4% respectively. In 48 TNBC patients evaluable for PD-L1 expression, 9 were PD-L1+ and 39 were PD-L1−, with ORRs of 44.4% and 2.6% respectively. A Phase 3 trial is testing the addition of one year of avelumab to curative therapy for high-risk early stage TNBC, with primary and secondary outcomes of disease-free survival (DFS) and OS, respectively (NCT02926196). Furthermore, a Phase Ib/2 study is also evaluating avelumab in combination with the immune modulator anti-41BB in multiple advanced cancers, including TNBC (NCT02554812). 41BB (CD137) is an inducible co-stimulatory receptor, and agonist antibodies specific for 41BB may potentiate antitumor immunity (38).
Atezolizumab
A Phase 1a study evaluated single agent atezolizumab in multiple tumor types (39), enrolling 115 patients with TNBC (40, 41). PD-L1 expression was assessed using the SP142 antibody, where tumors were positive if they had ≥5% PD-L1+ tumor-infiltrating immune cells. Enrollment was initially restricted to PD-L1+ patients, and subsequently opened to patients with any level of PD-L1 expression. Ultimately, 63% of patients were PD-L1+, 33% of patients were PD-L1−, and 4% of patients had unknown PD-L1 status. Patients were generally heavily pretreated, with a median of 7 prior lines of therapy; 17% of enrolled patients received atezolizumab as their first line therapy for metastatic disease. The ORR in 112 evaluable patients was 10%, with an ORR of 13% in PD-L1+ patients and 5% in PD-L1− patients. Although numbers are small, the ORR in patients treated first line was 26%, whereas the ORR in patients treated second and third line was 4–8%. The DCR was 23% in all patients, 27% in PD-L1+ patients, and 16% in PD-L1− patients. At a median follow up of 15.2 months, the median OS was 9.3 months. The OS rate at 1-year was 41%; notably, the survival rate of patients who had a CR or PR was 100% at up to 2–3 years. About 11% of patients who had PD by standard RECIST had atypical responses/pseudoprogression and also enjoyed long-term survival.
Pembrolizumab
Pembrolizumab has been evaluated in several clinical trials that demonstrated its safety and clinical activity in multiple tumor types (3, 4). KEYNOTE-012 was a Phase Ib study that evaluated pembrolizumab monotherapy in advanced PD-L1+ TNBC (42, 43). Tumor PD-L1 expression was evaluated by the 22C3 antibody, with staining in ≥1% of tumor cells or any PD-L1 expression by immune cells defined as positive. Of 111 patients pre-screened for PD-L1 expression, 58.6% (65 patients) were PD-L1+; 32 patients were enrolled and treated. Patients had a median of 2 prior lines of chemotherapy for metastatic disease, with 25% having ≥5 prior lines of therapy. In 27 patients evaluable for efficacy, the ORR was 18.5%, with 1 CR, 4 PRs, and 7 patients with SD; the DCR was 25.9%. The median OS was 10.2 months, and the OS rate at 1-year was 41.1%. The median duration of response (DOR) had not yet been reached. The KEYNOTE-086 Phase 2 trial evaluated pembrolizumab monotherapy both as salvage treatment for previously treated patients with metastatic TNBC expressing any level of PD-L1 (Cohort A), and as first-line therapy for patients with metastatic PD-L1+ TNBC (Cohort B) (44, 45). In Cohort A, 170 patients were enrolled and treated; about 62% (105 patients) had PD-L1+ TNBC, and over 40% of patients had been treated with ≥3 prior lines of therapy. Clinical activity was modest, with no apparent impact of PD-L1 expression level on clinical benefit (ORR of 4.7%-4.8%, PFS of 1.9–2.0 months, and OS of 8.3–10.0 months). Early analyses suggest longer OS at 9 months for patients with a CR or PR (100%) relative to patients with SD (89.6%) or PD (39%), similar to atezolizumab. Notably, the ORR for the 52 patients in Cohort B was higher than for patients overall, at 23.1%. This is also consistent with the atezolizumab data, where responses in metastatic TNBC appear to be highest when given first-line, and/or in PD-L1-selected patients (41). The KEYNOTE-119 Phase 3 trial, comparing pembroblizumab with chemotherapy of physician’s choice (NCT02555657), continues to accrue patients.
The utility of PD-1/PD-L1 blockade for TNBC in the adjuvant and neoadjuvant settings is under intensive investigation. Early data from the I-SPY trial revealed that adding pembrolizumab to neoadjuvant paclitaxel results in an estimated pCR rate of 46% versus 16% in HER-2- patients, 60% versus 20% in TNBC patients, and 34% versus 13% in ER+/PR+/HER-2- patients (46). The probability of a successful Phase 3 trial demonstrating that the addition of pembrolizumab to paclitaxel results in a superior cPR rate was estimated at >99% for all HER-2- patient subgroups. Treatment emergent adverse events included higher rates of thyroid dysfunction and adrenal insufficiency. KEYNOTE-173, a trial investigating pembrolizumab combined with chemotherapy for the neoadjuvant therapy of TNBC, also suggests that PD-L1+ TNBC might be more likely to respond (47).
The evaluation of PD-1/PD-L1 agents in other breast cancer subtypes has been limited, but single agent pembrolizumab was evaluated in a small cohort of advanced ER+ HER-2- breast cancer as part of the KEYNOTE-028 study (48). Of 248 patients prescreened for PD-L1 expression, 19.4% (48 patients) had PD-L1+ tumors. Of these, 25 patients were enrolled and treated. All had received at least one prior line of therapy for metastatic disease, and 44% had received ≥5 lines of prior therapy. The ORR was 12%, with 0 CRs, 3 PRs and 4 patients with SD; the clinical benefit rate (CBR) was 20%. All responding patients had been on study for at least 26 weeks at the data cut-off.
Expanding the Clinical Success of Breast Cancer Immunotherapy
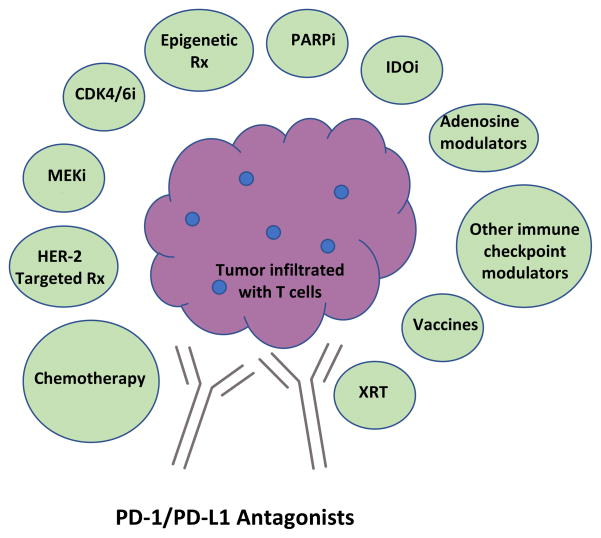
Early data with antagonists of PD-1/PD-L1 illustrate that some breast cancers are inherently immunogenic, with durable clinical responses in some responding patients. However, single agent activity occurs in less than 10% of patients with metastatic disease. Current efforts focus on developing immunotherapy combinations that can convert non-responders to responders, deepen responses that do occur, and surmount acquired resistance to immunotherapy. Promising combinations are illustrated in Figure 3 and discussed below.
Figure 3. Summary of selected immunotherapy combinations for breast cancer treatment with strong mechanistic rationale.
Because blockade of the PD-1/PD-L1 pathway has clear activity in multiple cancer, many regard it as a fundamental component of future cancer immunotherapies. Current clinical efforts are focused on developing immunotherapy combinations, many based on PD-1/PD-L1 blockade, that convert nonresponders to responders, deepen responses that do occur, and surmount acquired immunotherapy resistance. Other combinations, some of which include PD-1/PD-L1 blockade, are also in development. Abbreviations: PD-1=programmed death receptor-1; PD-L1=programmed death ligand-1; chemo=chemotherapy; HER-2=human epidermal growth factor receptor-2; Rx=therapy; MEKi=mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor; CDK4/6i=cyclin-dependent kinase 4/6 inhibitor; PARPi=poly (adenosine diphosphate (ADP)-ribose polymerase inhibitor; IDOi=indoleamine 2,3 dioxygenase inhibitor, XRT=radiotherapy.
Combining Chemotherapy with Immune-Based Therapy
Chemotherapy has a variable impact on the immune response depending on the drug, the dose, and the timing of chemotherapy in relation to immune-based therapy (49). A number of trials are looking at the addition of chemotherapy to PD-1/PD-L1 blockade with the goal of enhancing immune priming through antigen release and DC modulation, or augmenting immunity through relieving immune suppressive signals in the TME. Nab-paclitaxel and eribulin are two drugs that can modulate immunity in these ways, and early data from two small trials has already been reported. Atezolizumab (840 mg every 2 weeks) has been tested with nab-paclitaxel (125 mg/m2 days 1, 8, and 15 every 28 days) in a Phase 1b study that enrolled patients with metastatic TNBC regardless of PD-L1 status (50). Thirty-two patients were evaluable for safety and response. Grade 3–4 hematologic toxicity occurred in over half the patients, but was manageable. At a median follow up of >5 months, the ORR was 38%, with 1 CR, 11 PRs, and 2 additional patients demonstrating a nonclassical response. Responses occurred in patients with both PD-L1+ and PD-L1− disease, and there was a trend for higher response rate in patients treated first line relative to later line. A Phase 3 global, randomized, double-blind, placebo-controlled study is currently underway evaluating the addition of atezolizumab to nab-paclitaxel for the first line therapy of metastatic TNBC (NCT02425891). Pembrolizumab (200 mg IV every 3 weeks) has been tested with eribulin (1.4 mg/m2 days 1 and 8 every 21 days) in an ongoing trial designed to enroll 95 patients with metastatic TNBC of any PD-L1 status treated with ≤2 prior lines of chemotherapy (51). An interim analysis of 39 patients demonstrated the safety of the combination, with the most common side effects of fatigue, alopecia, nausea, neutropenia, and peripheral neuropathy. The ORR for 17 patients treated first line was 41.2%, and for those treated second or third line was 27.3%; this included 1 CR and 12 PRs. The CBR was 41%. PD-L1 status did not appear to impact the likelihood of response; the ORR and CBR for PD-L1+ patients were 29.4% and 35.3%, and for PD-L1− patients were 33.3% and 44.4%. This trial is ongoing; a second trial designed to enroll patients with metastatic ER+HER-2- breast cancer is planned. The KEYNOTE-355 Phase 3 trial is evaluating pembrolizumab with chemotherapy relative to various chemotherapy regimens alone as first-line therapy for incurable TNBC (NCT02819518).
HER-2-Directed Therapy and Immunotherapy
Trastuzumab (H), a humanized monoclonal antibody that specifically binds to HER-2 homodimers, is the cornerstone of therapy for both early and late stage HER-2-overexpressing breast cancer (52, 53). Added to standard chemotherapy, it prolongs survival in metastatic disease, and decreases the risk of relapse in early stage disease by about 50%. Pertuzumab (P), a second humanized monoclonal antibody specific for HER-2, prevents the formation of HER-2/HER-3 heterodimers. The addition of P to H and taxotere (THP) for the first line therapy of metastatic disease further prolongs survival (54), and neoadjuvant therapy with HP added to taxotere and carboplatin (TCHP) gives the highest reported complete pathologic response (cPR) rate reported to date (55). Ado-trastuzumab emtansine (TDM1) is composed of trastuzumab conjugated to a chemotherapeutic emtansine moiety, and improves both PFS and OS relative to lapatinib and capecitabine in patients with HER-2+ breast cancer who have progressed on a taxane and trastuzumab (56). Trastuzumab itself has intrinsic immune modulating activity, with the capacity to both mediate ADCC (57) and promote a HER-2-specific T cell response (58). The emtansine moiety of TDM1 may further augment immune priming by modulating DC activity (59). Our group has found that the preclinical equivalent of trastuzumab or trastuzumab itself can augment the activity of a cell-based vaccine in preclinical models (60, 61) and in patients (30). Exploration of the mechanism in HER-2 transgenic mice demonstrated enhanced Fc-mediated immune priming by DCs, augmented effector T cell activity, and a durable memory T cell response (61). Others have shown that combined therapy with a trastuzumab-like antibody plus a PD-1 antibody markedly augments the clearance of HER-2+ tumors in mice (62), and that combining TDM1 with both CTLA-4 and PD-1 antibodies prolongs survival in over 90% of mice bearing HER-2+ tumors relative to TDM1 or immunotherapy alone (63). Multiple clinical trials are underway testing the addition of PD-1/PD-L1 blockade to HER-2-based therapies for both locally advanced and metastatic HER-2+ breast cancer. A Phase 2 global, randomized, double-blind, placebo-controlled study is currently underway evaluating whether the addition of atezolizumab to TDM1 can further improve clinical outcomes in patients with metastatic HER-2+ breast cancer previously treated with trastuzumab and a taxane (NCT02924883).
MEK Inhibitors and Immunotherapy Combinations
Inhibitors of mitogen-activated protein kinase (MAPK) kinase (MEK) can cause the regression of tumors with activating mutations in the Ras signaling pathway. Although MEK inhibitors have been shown to block naïve T cell priming in tumor-bearing mice, they can increase the number of effector CD8+ T cells by preventing activation-induced cell death, leaving effector T cell activity intact (64). Combining MEK inhibitors with PD-L1 antibodies resulted in synergistic, durable antitumor activity. In breast cancer, an analysis of the residual disease of TNBC treated with neoadjuvant chemotherapy showed that TILs are associated with better prognosis, and that alterations in Ras-MAPK signaling were associated with lower levels of TILs (65). MEK inhibition upregulated cell surface expression of MHC Class I and II as well as PD-L1 on TNBC in vitro and in vivo. In mouse models of breast cancer, combined treatment with MEK inhibitors and PD-1 pathway antagonists resulted in enhanced tumor-specific immune responses and augmented tumor control. Clinical trials testing the combination of MEK inhibition and blockade of the PD-1 pathway for breast cancer are in development or underway.
CDK4/6 Inhibitors and Immune-Based Therapy
Several targeted agents that block CDK4/6 signaling have demonstrated clinical activity in combination with an aromatase inhibitor and faslodex for the first and second line therapy of metastatic ER+ breast cancer, respectively (66). Notably, data are beginning to emerge that these agents can induce TILs (67). As discussed previously, ER+HER-2- breast cancers are unlikely to contain TILs or respond to monotherapy with agents that target the PD-1 pathways (48). These observations together suggest that these agents might be one strategy for transforming a cold ER+ breast cancer into an inflamed tumor poised to respond to immune checkpoint blockade. It will be interesting to evaluate the addition of PD-1/PD-L1 blockade to the combination of endocrine therapy and CDK4/6 antagonists in relevant models.
Epigenetic Therapy integrated with Immunotherapy
There is great interest in the potential of epigenetic therapy to prime for response to immunotherapy in breast cancer. Studies have shown epigenetic modulation can promote an type I interferon response and restore production of T helper type 1 cytokines and chemokines (68–69). Another preclinical study showed that treating tumor-bearing mice (including the breast tumor 4T1) with entinostat combined with CTLA-4 and PD-1 antibodies could eradicate both primary tumors and metastases by reducing granulocytic MDSCs (70). A Phase II clinical trial showed that the addition of entinostat to exemestane for patients with advanced ER+ breast cancer resulted in an 8.3-month improvement in median OS relative to patients treated with exemestane alone (71). Exploratory studies of blood samples from 34 patients showed both lower numbers of MDSCs and decreased MDSC CD40 expression as well as increased MHC Class II expression on CD14+ monocytes two weeks after initiating therapy; no alterations of T cell phenotypes were observed. Multiple clinical trials evaluating the combination of epigenetic modulation with PD-1/PD-L1 blockade or the combination of CTLA-4 and PD-1 blockade are underway.
PARP Inhibition and Immunotherapy
Poly (ADP-ribose) polymerase 1 (PARP) inhibitors have recently been reported to modulate the immune microenvironment by upregulating PD-L1 expression in breast cancer cell lines and animal models (72). Antibodies that block PD-L1 restored the sensitivity of PARP inhibitor treated cells to T cell-mediated killing. In addition, polymeric adenosine diphosphate ribose (poly (ADP-ribose) or PAR) and PD-L1 expression were shown to be inversely correlated in human breast tumors. The combination of a PARP inhibitor and PD-L1 blockade significantly delayed tumor outgrowth relative to either agent alone in mouse models of breast cancer. Studies have shown synergy between CTLA-4 blockade and PARP inhibition in BRCA-deficient ovarian cancer models (73). In breast and ovarian cancer patients, the combination of PD-L1 blockade and PARP inhibition or VEGF inhibition have both shown promise (74). These data support trials exploring the combination of PARP inhibitors and/or anti-angiogenic therapies on a backbone of PD-1/PD-L1 blockade in BRCA-mutated breast and ovarian cancer.
Integrating IDO Inhibitors and Immune Checkpoint Blockade
Indoleamine 2,3-dioxygenase (IDO) is an enzyme that converts tryptophan to kynurenine, thereby suppressing immunity in the TME (75). Like PD-L1, IDO is up-regulated by interferon-γ-secreting T cells in the TME as a means of immune escape, and these two pathways are potentially redundant pathways of immune suppression in breast cancers that have TILs. The combination of the oral IDO inhibitor indoximod and taxotere has been tested in solid tumors (including breast cancer), with evidence of safety and clinical activity (2 PRs and 2 minor regressions in breast cancer) (76). Several clinical trials are evaluating the activity of combined inhibition of IDO and the PD-1 pathway in multiple tumor types, including breast cancer. Promising activity was recently reported with the combination of indoximod and pembrolizumab in melanoma (77).
Inhibiting Adenosine Signaling and PD-1/PD-L1 Blockade
Adenosine is another metabolite that creates a network of immune suppression in the TME (78). Nucleotides released by tumor cells are hydrolyzed by CD39 from ATP to AMP, and then by CD73 from AMP to adenosine. This creates a cloud of adenosine in the TME that binds to adenosine receptors (particularly the adenosine A2a receptor) on the surface of immune cells, skewing the TME to a state of immune suppression. Agents that target this pathway to reverse immune suppression include therapeutic antibodies specific for the cell surface ectonucleases CD39 and CD73, and small molecule inhibitors of adenosine receptor signaling. CPI-444 is a small molecule antagonist of the A2aR currently in testing alone and with atezolizumab in a multi-cohort study of a range of advanced, treatment refractory cancers, including TNBC (79). Preliminary data reveal a favorable safety profile to date, with evidence of clinical activity both as a single agent and in combination in multiple tumor types, including in PD-1/PD-L1 resistant or refractory patients. This and other trials evaluating agents that target the adenosine network are ongoing.
Integrating Radiotherapy with Immune Checkpoint Blockade
Modulation of tumor immunity underlies the abscopal effect, where irradiation of an index lesion is associated with regression of distant, un-irradatiated tumor lesions. Accumulating data demonstrate that radiotherapy can enhance tumor immunity in multiple ways (80, 81). It releases tumor antigens, and facilitates their processing and cross-presentation by dendritic cells, thus promoting T cell priming. Furthermore, radiotherapy upregulates chemokines and vascular adhesion molecules to support trafficking of T cells to tumors, and further augments tumor immunogenicity by upregulating the expression of MHC molecules, stress-induced ligands, and death receptors on cancer cells themselves. However, the impact of radiotherapy dose and schedule on these mechanisms remains unclear. Hypofractionated radiotherapy sequenced with ipilimumab did produce significant abscopal effects in melanoma and lung cancer (81). There are several ongoing clinical trials testing radiotherapy with distinct immunotherapies, including PD-1/PD-L1 blockade.
Vaccination Strategies with Immune Checkpoint Blockade
As reviewed here earlier, vaccines for breast cancer therapy have shown evidence of modest immunity but limited clinical activity when given to patients with advanced disease as a single agent or in combination with standard chemotherapy. This is likely due in large part to dominant repression of tumor immunity by immune checkpoint signaling, particularly through the PD-1 pathway. Giving vaccines as a single agent may be much like stepping on the immune accelerator while the parking brake is engaged. With the advent of immune checkpoint modulators that have clear clinical activity, we are poised to bring vaccination strategies back to the clinic to accelerate T cell priming and activation while simultaneously releasing the brakes from tumor immunity with immune checkpoint blockade. Combining DC-based vaccines that deliver multiple tumor antigens, genetically engineered poly-antigen vaccines like TRICOM, or whole cell tumor vaccines with agents that abrogate PD-1 signaling or other dominant pathways of immune suppression have a much greater chance of success than past strategies using vaccines alone. Even these vaccine platforms may be limited, however, by the fact that they are based on known tumor antigens that tend to be recognized as self by the immune system. The correlation of clinical responses induced by immune checkpoint blockade with endogenous immune responses specific for the neo-antigens present in highly mutated tumors has created intense interest in generating personalized vaccines based on the unique neo-antigens present in a given patient’s tumor (21). Platforms for neo-antigen-based breast cancer vaccines include peptides, genetically engineered bacterial or viral vectors, or nucleic acid-based vaccines (5). The cryoablation strategy described earlier is also a unique strategy for inducing immunity tailored to an individual’s tumor, and has already been tested with CTLA-4 blockade in breast cancer patients (33, 34). Another novel approach to personalized vaccination is the intratumoral delivery of agonists that activate innate immunity, for example through the stimulator of interferon genes (STING) pathway. The intratumoral delivery of the STING agonist ADU-S100 induces interferon-β production and DC maturation, and establishes a gradient of T cell-recruiting chemokines that promotes effective T cell priming and trafficking to the tumor site (82). Furthermore, sequencing ADU-S100 with PD-L1 blockade and modulation of the OX40 receptor can break antigen-specific tolerance to mediate tumor regression in a tolerized mouse breast cancer model (82). Single agent ADU-S100 is currently in clinical trials (NCT02675439).
Conclusions and Future Directions
Immune checkpoint blockade has shown promise for breast cancer treatment, illustrating the potential of harnessing the immune system for clinical benefit in this disease. Antagonists of the PD-1/PD-L1 pathway can induce durable clinical responses in some breast cancer patients with metastatic TNBC. Both validation of these early findings and efforts to extend immunotherapy to patients with HER-2+ and luminal breast cancer are underway. In addition, clinical trials evaluating the integration of immunotherapy into the adjuvant and neoadjuvant settings have already begun. The near-term future will see the development of combination immunotherapies that can convert breast cancers from immunologically cold lesions to immune-activated tumors poised for response to immunotherapy. Personalized immunotherapy strategies that utilize vaccines that deliver tumor-specific neo-antigens and/or immune modulating agents chosen based on the immunologic milieu of a given tumor are under rapid development. Developing biomarkers that predict response and resistance to therapy, and identifying environmental modifiers of immunity (the microbiome, metabolic and hormonal parameters, and concurrent drug therapy) are areas of growing investigation (83). Applying vaccination approaches integrated with the lessons learned from modern breast cancer immunotherapy will undoubtedly also bring us closer to the ultimate goal of immune-based breast cancer prevention.
Acknowledgments
Funding support: L. A. Emens is supported by NIH R25CA171973, the Breast Cancer Research Foundation, and the Bloomberg Foundation.
Footnotes
Conflict of Interest
Dr. Emens reports receiving research support from Genentech, Roche, EMD Serono, Astrazeneca, Aduro Biotech, Corvus, and Merck. She has served as a consultant to Astrazeneca, Syndax, Peregrine, Bayer, Molecuvax, Celgene, Vaccinex, eTHeRNA, Amgen, and Gritstone. Under a licensing agreement between Johns Hopkins University and Aduro Biotech, Dr. Emens and the University are entitled to milestone payments and royalty on the sales of the GM-CSF-secreting breast cancer vaccine. The terms of these arrangements are being managed by the Johns Hopkins university in accordance with its conflict of interest policies.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, et al. Clinical relevance of host immunity in breast cancer: from TILs to clinic. Nat Rev Clin Oncol. 2016;13:228–41. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 3.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SM. Antagonists of PD-1 and PD-L1 in Cancer Treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond RL, Seliger B, Marincola FM. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017 doi: 10.1016/j.ejca.2017.01.035. in press. [DOI] [PubMed] [Google Scholar]
- 5.Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12:1597–1611. doi: 10.1586/era.12.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park) 2015;29:375–85. [PubMed] [Google Scholar]
- 7.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3:a003285. doi: 10.1101/cshperspect.a003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas T. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5:915–9. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho P-C, Kaech SM. Re-energizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. 2017;46:38–44. doi: 10.1016/j.coi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN Guidelines Insights: Breast Cancer, Version 1. 2017. J Natl Compr Canc Netw. 2017;15:433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 15.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, et al. PD-L1 (B7-H1) expression and the immune microenvironment in primary and metastatic breast carcinomas. Human Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Li M, Lian Z, Zhu H, Kong L, Wang P, et al. Prognostic role of programmed death ligand-1 expression in breast cancer: a systematic review and meta-analysis. Target Oncol. 2016;11:753–761. doi: 10.1007/s11523-016-0451-8. [DOI] [PubMed] [Google Scholar]
- 17.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating FOXP3+ regulatory T cells. Clin Cancer Res. 2008;24:13–20. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 18.Asano Y, Kashiwagi S, Goto W, Kurata K, Noda S, Takashima T, et al. tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br J Surg. 2016;103:845–54. doi: 10.1002/bjs.10127. [DOI] [PubMed] [Google Scholar]
- 19.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yarchoan M, Johnson BA, 3rd, Lutz ER, Laheru D, Jaffee EM. Targeting neoantigens to augment antitumor immunity. Nat Rev Cancer. 2017;17:209–22. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizvi NA, Hellman MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–50. doi: 10.1016/j.breast.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Miles D, Roche H, Martin M, Perren TJ, Cameron DA, Glaspy J, et al. Phase III multicenter clinical trial of sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist. 2011;16:1092–100. doi: 10.1634/theoncologist.2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emens LA, Asquith JA, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27:5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heery CR, Ibrahim NK, Arlen PM, Mohebtash M, Murray JL, Koenig K, et al. Docetaxel alone or in combination with a therapeutic cancer vaccine (PANVAC) in patients with metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2015;1:1087–95. doi: 10.1001/jamaoncol.2015.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disis ML, Wallace DR, Gooley TA, Dany Y, Slota M, Lu H, et al. Concurrent trastuzumab and HER-2neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27:4685–92. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travis CG, Litton JK, Arrington K, Ponniah S, Ibrahim NK, Gail V, et al. Results of a phase 1b trial of combination immunotherapy with a CD8+ T cell eliciting vaccine and trastuzumab in breast cancer patients. Ann Surg Oncol. 2017 doi: 10.1245/s10434-017-5844-0. doi:10.1245s10434-017-5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Gupta R, Petrik S, Laiko M, Leatherman JM, Asquith JM, et al. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER-2+ metastatic breast cancer. Cancer Immunol Res. 2014;2:949–61. doi: 10.1158/2326-6066.CIR-14-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonderheide RH, LoRusso PM, Khalli M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulatory expression on patient T cells. Clin Cancer Res. 2010;16:3485–94. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 33.McArthur HL, Diab A, Page DB, Yuan J, Solomon SB, Sacchini V, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res. 2016;22:5729–37. doi: 10.1158/1078-0432.CCR-16-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page DB, Yuan J, Redmond D, Wen YH, Durack JC, Emerson R, et al. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res. 2016;4:835–44. doi: 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emens LA, Kok M, Ojalvo LS. Targeting the programmed cell death-1 pathway in breast and ovarian cancer. Curr Opin Obstet Gynecol. 2016;28:142–7. doi: 10.1097/GCO.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 36.Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18:587–98. doi: 10.1016/S1470-2045(17)30239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dirix L, Takacs I, Nikolinakos P, Jerusalem G, Arkenau H-T, Hamilton E, et al. Avelumab (MSB0010718C), and anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor trial. Cancer Res. 2015;76(Suppl 4) doi: 10.1007/s10549-017-4537-5. abstr S1–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilkueta A, Etxeberria I, Bolanos E, et al. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46:513–22. doi: 10.1002/eji.201445388. [DOI] [PubMed] [Google Scholar]
- 39.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emens LA, Braiteh FS, Cassier P, Delord JP, Eder JP, Shen X, et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. 2015;75(Suppl 9) abstr PD1–6. [Google Scholar]
- 41.Schmid P, Cruz C, Braiteh FS, Eder JP, Tolaney S, Kuter I, et al. Atezolizumab in metastatic triple-negative breast cancer: long-term clinical outcomes and biomarker analyses. Presented at the 2017 Meeting of the American Association for Cancer Research; April 1–5, 2017. Washington DC; Abstr 2986. [Google Scholar]
- 42.Nanda R, Chow LQ, Dees EC, Berger R, Gupta R, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase 1b KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–7. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanda R, Specht J, Dees EC, Berger R, Gupta S, Geva R, et al. KEYNOTE-012: Long-lasting responses in a phase 1b study of pembrolizumab for metastatic triple-negative breast cancer (mTNBC). Presented at the 2016 San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. Abstr P6-10-03. [Google Scholar]
- 44.Adams S, Schmid P, Rugo H, Winer EP, Loriat D, Awada A, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple negative triple negative breast cancer: KEYNOTE-086 Cohort A. Journal of Clinical Oncology. 2017;35(Suppl) abstr 1008. [Google Scholar]
- 45.Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentis M, Nanda R, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1+ metastatic triple-negative breast cancer (mTNBC): preliminary data from Keynote 086 Cohort B. Journal of Clinical Oncology. 2017;35(Suppl) abstr 1088. [Google Scholar]
- 46.Nanda R, Liu MC, Yau C, Asare S, Hilton N, Van’t Veer L, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer: results from I-SPY-2. Journal of Clinical Oncology. 2017;35(Suppl) abstr 506. [Google Scholar]
- 47.Schmid P, Park YH, Couselo E-M, Park SB, Sohn J, Im SB, et al. Pembrolizumab (Pembro) plus chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): preliminary results from KEYNOTE-173. Journal of Clinical Oncology. 2017;35(Suppl) abstr 156. [Google Scholar]
- 48.Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, et al. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, estrogen receptor-positive (ER+)/HER-2-negative advanced breast cancer enrolled in KEYNOTE-028. Cancer Res. 2015;76(Suppl 4) abstr S5–07. [Google Scholar]
- 49.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:43–43. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams S, Diamond JR, Hamilton EP, Pohlmann PR, Tolaney SM, Molinero L, et al. A Phase 1b trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC) J Clin Oncol. 2016;34(Suppl) abstr 1009. [Google Scholar]
- 51.Tolaney S, Savulsky C, Aktan G, Xing D, Almonte A, Karantza V, et al. Phase 1b/2 study to evaluated eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Presented at the 2016 San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. abstr P5-15-02. [Google Scholar]
- 52.Loibl S, Gianni L. HER-2-positive breast cancer. Lancet. 2016 doi: 10.1016/S0140-6736(16)32417-5. pii: S0140-8736(16)32147-5. [DOI] [PubMed] [Google Scholar]
- 53.Jelovac D, Emens LA. HER-2-directed therapy for metastatic breast cancer. Oncology (Williston Park) 2013;27:168–75. [PubMed] [Google Scholar]
- 54.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER-2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumb in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER-2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–84. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 56.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER-2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER-2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S, et al. Improved natural killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER-2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med. 2015;13:2014. doi: 10.1186/s12967-015-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muller P, Martin K, Theurich S, Schreiner J, Savic S, Terszowski G, et al. Microtubule-depolymerizing agents used in antibody-drug conjugates induce antitumor immunity by stimulation of dendritic cells. Cancer Immunol Res. 2014;2:741–55. doi: 10.1158/2326-6066.CIR-13-0198. [DOI] [PubMed] [Google Scholar]
- 60.Wolpoe ME, Lutz ER, Ercolini AM, Murata S, Ivie SE, Garrett ES, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte-macrophage colony-stimluting factor secreting whole cell vaccination to augment CD8+ effector T cell function and tumor-free survival in HER-2/neu transgenic mice. J Immunol. 2003;171:2161–9. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 61.Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8 T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest. 2008;118:1700–11. doi: 10.1172/JCI34333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-erbB2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 2011;108:7142–7. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, et al. Trastuzumab emtansin (T-DM1) render HER-2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 64.Ebert PJ, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity. 2016;44:609–21. doi: 10.1016/j.immuni.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 65.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 checkpoint inhibitors. Clin Cancer Res. 2016;22:1499–509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:17. doi: 10.1186/s13058-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurvitz S, Martin M, Fernandez Abad M, Chan D, Rostorfer R, Petru E, et al. Biological effects of abemaciclib in a phase 2 neoadjuvant study for postmenopausal patients with HR+ HER-2- breast cancer. Presented at the 2016 San Antonio Breast Cancer Symposium; December 6–10, 2016; San Antonio, TX. abstr S4–06. [Google Scholar]
- 68.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–86. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumor immunity and immunotherapy. Nature. 2015;527:249–53. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci USA. 2014;111:11774–9. doi: 10.1073/pnas.1410626111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomita Y, Lee MJ, Lee S, Tomita S, Chumsri S, Cruickshank S, et al. The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: correlative analysis of ENCORE 201, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. Oncoimmunology. 2016;5:e1219008. doi: 10.1080/2162402X.2016.1219008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257–68. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J-M, Mathews A-C, Peer A, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and cinical activity of the programmed death-ligand-1 inhibitor durvaluamab in combination with poly [ADP-ribose polymerase inhibitor olaparib or vascular endothelial growth factor receptor-1–3 inhibitor 1–3 cedarinib in women’s cancers: a dose escalation phase I study. J Clin Oncol. 2017;35:2193–2202. doi: 10.1200/JCO.2016.72.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. 2017;76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–46. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zakharia Y, McWilliams R, Shaheen M, Grossman K, Drabick J, Milhem M, et al. Interim analysis of the phase 2 clinical trial of the IDO pathway inhibitor indoximod in combination with pembrolzumab for patients with advanced melanoma. Presented at the 2017 Meeting of the American Association for Cancer Research; April 1–5, 2017; Washington DC. Abstr CT117. [Google Scholar]
- 78.Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nature Rev Cancer. 2013;13:642–57. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 79.Emens LA, Powderly J, Fong L, Brody J, Forde P, Hellmann M, et al. CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. Presented at the 2017 Meeting of the American Association for Cancer Research; April 1–5, 2017; Washington DC. Abstr CT119. [Google Scholar]
- 80.Demaria S, Golden EB, Formenti S. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–52. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 81.Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29. doi: 10.1016/j.ejca.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 82.Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol Res. 2017;5:468–79. doi: 10.1158/2326-6066.CIR-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kroemer G, Senovilla L, Galluzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–38. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]