Summary
Cell division in prokaryotes initiates with assembly of the Z-ring at midcell, which, in Escherichia coli, is tethered to the inner leaflet of the cytoplasmic membrane through a direct interaction with FtsA, a widely conserved actin homolog. The Z-ring is comprised of polymers of tubulin-like FtsZ and has been suggested to provide the force for constriction. Here, we demonstrate that FtsA exerts force on membranes causing redistribution of membrane architecture, robustly hydrolyzes ATP and directly engages FtsZ polymers in a reconstituted system. Phospholipid reorganization by FtsA occurs rapidly and is mediated by insertion of a C-terminal membrane targeting sequence (MTS) into the bilayer and further promoted by a nucleotide-dependent conformational change relayed to the MTS. FtsA also recruits FtsZ to phospholipid vesicles via a direct interaction with the FtsZ C-terminus and regulates FtsZ assembly kinetics. These results implicate the actin homolog FtsA in establishment of a Z-ring scaffold, while directly remodeling the membrane, and provide mechanistic insight into localized cell wall remodeling, invagination and constriction at the onset of division.
Graphical abstract
Abbreviated Summary
FtsA is an actin-like ATPase involved in bacterial cell division that binds to phospholipid vesicles, inserts the C-terminus into the phospholipid bilayer and rapidly reconfigures vesicle architecture in the presence of ATP. Recruitment of FtsZ polymers to the FtsA proteo-liposomes in vitro establishes a large scaffold. Our results suggest that during division, FtsA polymerizes at the membrane surface and exerts force on membrane, leading to localized membrane reorganization and cell wall remodeling at the Z-ring.

Introduction
Bacterial cell division is a highly regulated process, which, in E. coli, proceeds through a septal constriction event executed by a large dynamic protein complex, called the divisome. At the core of the divisome are two highly conserved proteins, FtsZ and FtsA (Haeusser & Margolin, 2016). FtsZ, a homolog of the eukaryotic cytoskeletal protein tubulin, assembles into a ring containing staggered FtsZ polymers, called the Z-ring (Lutkenhaus et al., 2012). FtsA, which shares structural homology with the actin family of proteins, binds directly to the FtsZ C-terminal region and tethers the Z-ring to the inner face of the cytoplasmic membrane (Szwedziak et al., 2012, van den Ent & Lowe, 2000). Ordered recruitment of proteins to the divisome is essential for rapid division, yet how the divisome promotes septal constriction remains unclear. In E. coli, FtsA and FtsZ assemble first at the division site as a proto-ring and then sequentially recruit at least 10 additional cell division proteins (Pichoff & Lutkenhaus, 2002, Hale & de Boer, 1997, Hale & de Boer, 1999, Pogliano et al., 1997, Wang et al., 1997, Goehring & Beckwith, 2005, Aarsman et al., 2005, Soderstrom et al., 2016, Alexeeva et al., 2010). The inner membrane protein ZipA is also recruited early in division and has been reported to function as a static tether for the Z-ring (Pichoff & Lutkenhaus, 2002). In addition to FtsZ, FtsA interacts with FtsEX, an ATP-binding cassette transporter-like complex that may modulate the FtsA multimeric state, recruits FtsI, a murein transpeptidase involved in septal wall synthesis, and binds FtsN, a bitopic membrane protein that activates peptidoglycan synthesis (Du et al., 2016, Dai et al., 1996, Bernard et al., 2007, Busiek et al., 2012, Corbin et al., 2007, Corbin et al., 2004, Shiomi & Margolin, 2008). After recruitment of FtsN, late septal proteins arrive to insert additional peptidoglycan at the division site and the Z-ring is disassembled following constriction (Rico et al., 2010).
FtsA contains the canonical actin-fold and is a member of the actin/Hsc70/hexokinase superfamily (Bork et al., 1992, van den Ent & Lowe, 2000). The actin-fold contains two discrete domains, which are each further divided into two subdomains. FtsA has structural homology with actin and the bacterial actin homolog MreB in subdomains ‘1A’ and ‘2A’, and despite less homology, subdomain ‘2B’ is positioned in both proteins to coordinate nucleotide. A major structural difference is the presence of the ‘1C switch-domain’ of FtsA. In comparison to the prototypical ‘1B’ subdomain from the actin-like protein MreB, the ‘1C’ subdomain is positioned on the alternate side of domain ‘1A’ (Fig. 1A) (Szwedziak et al., 2012, van den Ent & Lowe, 2000). MreB is important for maintaining cell morphology in rod-shaped bacteria, binds to the membrane, and, like FtsA, has been crystallized as an actin-like protofilament (Errington, 2015, van den Ent et al., 2014, Szwedziak et al., 2012). In higher eukaryotes, bundled actin fibers generate constrictive force in collaboration with other proteins (Llinas et al., 2015). In lower eukaryotes, actin forms the core of the cytokinetic contractile ring localizing to the plasma membrane where it may provide the constrictive force for septation (Pollard & Wu, 2010, Pelham & Chang, 2002). Despite FtsA and actin being essential for cell division in many organisms, a major distinction is that FtsA binds directly to phospholipids using a highly conserved C-terminal membrane targeting sequence (MTS) whereas actin requires accessory proteins to associate with the membrane. The MTS is predicted to form an amphipathic helix critical for anchoring the Z-ring to the membrane through an FtsZ-FtsA interaction (Pichoff & Lutkenhaus, 2005). FtsA membrane association is critical for FtsA function during division in vivo (Szwedziak et al., 2012, Pichoff & Lutkenhaus, 2005). Replacement of the FtsA MTS with an analogous motif from MinD or the transmembrane domain from MalF supports essential FtsA functions in vivo, suggesting that the FtsA MTS is a generic targeting motif. However, overexpression of an FtsA mutant protein truncated at the C-terminus produces a curved cell phenotype (Gayda et al., 1992, Shiomi & Margolin, 2008, Yim et al., 2000). The role of self-association and the influence of phospholipids on oligomerization is even less clear. Several mutations that map to the FtsA protomer interface in the polymer model, including R286W, do not abrogate function in vivo (Pichoff et al., 2012, Geissler et al., 2003). Moreover, cells expressing mutant FtsA deficient for self-interaction require less FtsN and bypass the requirements for ZipA and FtsEX (Du et al., 2016, Reddy, 2007, Pichoff et al., 2012, Geissler et al., 2007, Geissler et al., 2003). Protein interaction analyses of yeast and bacterial two-hybrid systems expressing truncated FtsA, without the C-terminal 15 amino acid MTS, generated conflicting reports of self-interaction (Pichoff & Lutkenhaus, 2007, Pichoff & Lutkenhaus, 2005, Yim et al., 2000).
Fig. 1. FtsA hydrolyzes ATP rapidly in the presence of liposomes.

A. E. coli FtsA (residues 13-381) modeled onto T. maritima FtsA (pdb: 4A2B) (Szwedziak et al., 2012). Subdomains 1A (blue, 13-88, 156-196, 359-381), 1C (gold, 89-155), 2A (purple, 197-232) and 2B (green, 233-303) are shown, where indicated. Amino acids Arg 286 (green) and Lys 86 (light blue) are shown as Corey-Pauling-Koltun (CPK) models. ATPγS is shown as a stick model.
B. Hydrolysis of ATP by FtsA (0.25, 0.50 and 1.00 μM) was monitored with time as described in Experimental Procedures. Data is an average of three replicates represented as mean ± SEM.
C. FtsA (4 μM) was prepared as described in Experimental Procedures and visualized by negative stain TEM.
D. FtsA (4 μM) was incubated with ATP (4 mM), prepared and visualized by negative stain TEM. Size bars in C and D are 50 nm.
E. FtsA (4 μM) in the absence or presence of ATP (4 mM) was stained with FM 4-64 and then injected into a flow chamber and visualized by confocal fluorescence microscopy. Size bars are 5 μm.
FtsA used in B-E contains copurifying PLs.
The role of ATP hydrolysis for FtsA function is unknown. ATP hydrolysis activity has been shown to be essential for FtsA function in vivo as several mutations mapping near the ATP-binding pocket cause thermosensitive growth defects (Herricks et al., 2014). FtsA from E. coli, Thermotoga maritima, Streptococcus pneumoniae, Pseudomonas aeruginosa, Deinococcus radiodurans and Bacillus subtilis hydrolyze ATP slowly, with rates ranging from undetectable to a few ATP molecules per minute (Feucht et al., 2001, Herricks et al., 2014, Loose & Mitchison, 2014, Paradis-Bleau et al., 2005, Lara et al., 2005, Szwedziak et al., 2012, Modi & Misra, 2014). In another study of E. coli FtsA, ATP was important for FtsA to recruit FtsZ on a lipid monolayer and to stimulate FtsZ polymerization dynamics (Loose & Mitchison, 2014).
Here, we provide insight into the molecular events that occur at the interface between the Z-ring and the membrane during early division. We show that ATP-binding by phospholipid-associated FtsA is sufficient to remodel membrane architecture, whereby phospholipid binding promotes both affinity of FtsA to ATP and subsequent ATP hydrolysis via communication between the MTS and the ATP-binding site. Finally, we show that FtsA recruits FtsZ to phospholipids to form a large scaffolded network. We propose a model for FtsA function in vivo during cell division that suggests FtsA directly remodels the cell membrane and the Z-ring to promote constriction.
Results
Phospholipid association regulates FtsA ATP hydrolysis
The domain organization of FtsA is similar to actin; however, FtsA contains an additional subdomain (‘1C’) in lieu of subdomain ‘1B’ of MreB (Fig. 1A) (Szwedziak et al., 2012, van den Ent & Lowe, 2000). Also, FtsA engages the membrane directly through a C-terminal MTS, which is critical for FtsA function in vivo (Szwedziak et al., 2012, Pichoff & Lutkenhaus, 2005). FtsA from T. maritima forms linear actin-like polymers, which are thought to assemble adjacent to the membrane surface (Szwedziak et al., 2014, Szwedziak et al., 2012). Currently, it is unknown if binding to the phospholipid membrane regulates FtsA assembly, ATP hydrolysis, or direct protein interactions. To investigate these questions further, we induced expression of FtsA from a high copy plasmid under the control of an IPTG-inducible promoter in E. coli for 20 hours at 16 °C, lysed the cells and purified native FtsA using ammonium sulfate precipitation, ion exchange chromatography and size exclusion chromatography. Then we assayed fractions containing FtsA for ATP hydrolysis using the malachite green phosphate detection assay and observed that the FtsA protein elution fractions correspond to fractions with robust ATPase activity (Supporting Information Fig. S1A). Next we compared ATP hydrolysis at several concentrations of FtsA; all concentrations of FtsA tested showed robust ATPase activity, with an ATP hydrolysis rate of 20.9 ± 0.5 pmol Pi min−1 pmol−1 of FtsA for reactions containing 1 μM FtsA (Fig. 1B). The rate of ATP hydrolysis by the native protein is >10-fold faster than previous reports of E. coli FtsA with an N-terminal hexahistidine tag (Herricks et al., 2014). Finally, we also measured ATP hydrolysis using a secondary assay, an NADH enzyme-coupled assay, and calculated the rate to be 36.8 ± 0.5 pmol Pi min−1 pmol−1 (Supporting Information Fig. S1B). This rate is 1.8-fold faster than we detected with malachite green reagent; the difference is due to replenishment of ATP through the activity of pyruvate kinase, preventing ADP accumulation.
Native FtsA rapidly hydrolyzes ATP; therefore, we hypothesized that it may also be competent for assembly into actin-like polymers. To visualize FtsA protein assemblies directly and determine if polymers are present, we analyzed FtsA in the presence and absence of ATP by negative staining with uranyl acetate and transmission electron microscopy (TEM) (Fig. 1C and 1D). In reactions containing FtsA, we observed large spherical structures of various sizes, ranging from 20 to 500 nm in diameter. Many of the large structures have smooth, rounded edges with collapsed surface folds and resemble liposomes (Fig. 1C) (Supporting Information Fig. S1C). When FtsA was incubated with ATP and then visualized by TEM, we observed more spherical structures and a heterogeneous population of clustered, spherical vesicles containing long tubulated regions (Fig. 1D) (Supporting Information Fig. S1D). To confirm that the liposome-like structures contain lipids, we stained the reaction mixtures with the lipophilic dye FM 4-64, and then visualized the structures by confocal fluorescence microscopy. FM 4-64 emits fluorescence when bound to lipids and is commonly used to stain membranes of whole cells. As expected, incubation of FtsA with FM4-64 produces fluorescent liposome-like structures consistent with the presence of phospholipids (Fig. 1E).
FtsA localizes to the interior membrane surface in vivo. Therefore, lipids present in the FtsA preparation are most likely derived from the cytoplasmic membrane of E. coli. To determine if the copurifying lipids are critical for ATPase activity, we used the nonionic detergent Triton X-100 (TX100) to disrupt the liposomes and then measured the rate of ATP hydrolysis. Increasing TX100 concentration in reactions containing FtsA and lipids results in a concentration-dependent decrease in the rate of ATP hydrolysis (Fig. 2A). At 2% TX100, FtsA hydrolyzes ATP at a rate that is approximately 90% slower than FtsA without detergent (2.3 ± 3.9 min−1 and 19.8 ± 3.7 min−1, respectively). To confirm that addition of TX100 disrupts liposomes, FtsA was incubated with TX100 (2%) and then fractionated by size exclusion chromatography. In the absence of detergent, FtsA elutes at a position corresponding to the void volume, indicating that it is present as one or more species larger than 250 kDa, which is consistent with the presence of large structures or liposomes (Fig. 2B). After treatment with detergent, FtsA elutes later, with a calculated size of 94.7 kDa, which could either correspond to an FtsA dimer or an FtsA-detergent micelle (Fig. 2B).
Fig. 2. Phospholipids are essential for rapid ATP hydrolysis by FtsA.

A. ATP hydrolysis by FtsA was measured over time as described in Experimental Procedures in the presence of increasing concentrations of TX100 detergent (0, 0.25, 0.5, 1, 2%), which was included in the reaction buffer. FtsA used in A contains copurifying PLs and ATP hydrolysis is prevented with increasing TX100.
B. Elution profile of FtsA (solid line) and FtsA with 0.5% TX100 (dashed line) by size exclusion chromatography. Fractions were analyzed by SDS-PAGE and densitometry. VO denotes the void volume. FtsA used in B contains copurifying PLs and the elution profile shifts in the presence of TX100, which solubilizes lipid vesicles.
C. Detection of phosphate released in reactions of untreated FtsA containing copurifying PLs (1 μM), or FtsA (1 μM) extracted with 2% TX100 (FtsAextracted) and then treated with a detergent removal spin column (FtsAextracted-DR), or with lipid removal agent (250 μg ml−1) (FtsAextracted-LR) and reconstituted with SUVs (500 μg ml−1), where indicated. Phosphate was detected at 0, 5 and 10 minutes by malachite green reagent.
D. ATP hydrolysis of FtsA (1 μM) or FtsA(ΔMTS) (1 μM), where indicated, was monitored over time. FtsA used in D contains copurifying PLs.
E. FtsA (2 μM) was extracted from copurifying PLs and then exchanged into buffer without detergent and incubated with mant-ATP (1 to 250 μM) in the presence (open circles) and absence (closed circles) of SUVs (500 μg ml−1). Mant-ATP fluorescence was monitored as described in Experimental Procedures. Data from A, C and D are an average of three replicates represented as mean ± SEM.
Next, we attempted to restore fast ATP hydrolysis by reconstitution of FtsA into small unilamellar vesicles (SUVs) prepared from E. coli phospholipids. As described previously, treatment of FtsA with detergent resulted in slow ATP hydrolysis (Fig. 2A and 2C) (Supporting Information Fig. S1E). After addition of TX100, residual phospholipids and detergent were then reduced or removed from the FtsA-containing reaction by buffer exchange, where indicated, followed by treatment with detergent- or lipid-removal resin. None of the treatments restores FtsA ATPase activity to the level of untreated FtsA (Fig. 2C); however, after treatment, FtsA was supplemented with SUVs, and ATP hydrolysis by FtsA was stimulated (Fig. 2C). These results indicate that rapid ATP hydrolysis by FtsA is promoted by the interaction with phospholipids. As a control, to confirm this, we constructed and purified a truncated FtsA mutant protein, FtsA(ΔMTS), which does not contain the 15 amino acid MTS at the C-terminus. As expected, FtsA(ΔMTS) hydrolyzes ATP at a rate of 0.7 ± 0.3 pmol Pi min−1 pmol−1, which is <5% of the activity of wild type FtsA (Fig. 2D). Together, these results indicate that phospholipid engagement by FtsA is essential for rapid ATP hydrolysis. To further identify the lipids that copurify with FtsA, we compared lipid composition profiles of FtsA to FtsA(ΔMTS) and determined that majority of the lipid signal associated with wild type FtsA is attributable to phosphatidylethanolamine and cardiolipin (Supporting Information Table S1).
Phospholipids stimulate ATP hydrolysis by FtsA suggesting that phospholipid binding shifts the conformation of FtsA to one that has an altered affinity for nucleotide. To test this, we measured binding of FtsA to a fluorescent ATP analog, 3′-O-(N-methylanthraniloyl)-ATP (mant-ATP), in the presence and absence of phospholipids. Mant-ATP was previously shown to interact with E. coli FtsA purified from inclusion bodies (Martos et al., 2012). We observed that phospholipid-free FtsA, prepared by incubation of FtsA with TX100 and subsequent removal of detergent and phospholipids, binds to mant-ATP with a dissociation constant (KD) of 26.2 ± 3.0 μM (Fig. 2E). However, in the presence of phospholipids the interaction between FtsA and mant-ATP is 2-fold stronger with a KD of 12.8 ± 0.9 μM (Fig. 2E). These results demonstrate that interaction between FtsA and lipids enhances affinity of FtsA for ATP, which could be due to an allosteric change in conformation that is dependent on engagement of the MTS with phospholipids or due to increasing the effective concentration on the vesicle surface. Finally, we also purified His-SUMO-FtsA, which was previously reported to recruit FtsZ polymers to a lipid surface in vitro yet was defective for ATP hydrolysis (Loose & Mitchison, 2014). Using the expression and purification strategy described here, we observed that His-SUMO-FtsA, purified using the procedure developed for native FtsA, is active for ATP hydrolysis, with a rate that is 58% slower than native FtsA (8.6 ± 0.5 pmol Pi min−1 pmol−1) (Supporting Information Fig. S2A and S2B). For comparison, we also purified His-SUMO-FtsA by metal affinity chromatography as described (Loose & Mitchison, 2014) and determined that, with this method, His-SUMO-FtsA did not copurify with phospholipids and ATP hydrolysis was not restored by supplementation with E. coli SUVs (data not shown). Together, these results suggest that the interaction of FtsA with phospholipids preserves ATP hydrolysis activity.
FtsA directly remodels liposome architecture
Since ATP hydrolysis assays showed that prepared E. coli phospholipids support rapid ATP hydrolysis and the MTS is predicted to insert into a phospholipid bilayer, we investigated if the addition of FtsA modifies the architecture of SUVs. Therefore, we added phospholipid-free FtsA to purified vesicles and examined protein-lipid architecture by negative stain TEM. We visualized untreated SUVs alone and after incubation with phospholipid-free FtsA (Fig. 3A). There were no observable structures in samples containing phospholipid-free FtsA (Fig. 3Aiii). Also, we observed that prior to incubation with FtsA, the SUVs are small and uniform, with an average diameter of 31.4 ± 8.4 nm (Fig. 3Aiv). After incubation with FtsA, we observed a striking reconfiguration of observable structures. Instead of homogeneous spherical vesicles, we observed many large vesicle clusters and tubulated regions (Fig. 3Ai 3Aii and inset).
Fig. 3. The C-terminal MTS of FtsA inserts into SUVs and is modified by ATP.

A. TEM of FtsA (3 μM) extracted from copurifying PLs and supplemented with SUVs (500 μg ml−1) and ATP (4 mM) (i and ii). Prior to the addition of SUVs and ATP, FtsA was extracted from copurifying PLs with 2% TX100, then exchanged into buffer without detergent (i) and also treated with a detergent removal spin column (FtsAextracted-DR) (ii and inset). In control reactions, extracted FtsA (FtsAextracted-DR) shows no apparent structures (iii) and exogenous SUVs (500 μg ml−1) alone (iv) are uniform and approximately 50 nm. Size bars are 50 nm.
B. Dynamic light scatter of FtsA (2 μM), as described in Experimental Procedures, alone or incubated with ATP (4 mM), ADP (2mM) or ATPγS (2mM) for 30 min at 23 °C and scanned 11 times. Plots shown are an average of 3 independent experiments, each with 11 scans. FtsA used in B contains copurifying PLs.
C. Reaction mixtures containing FtsA (2 μM) were monitored by 90° light scatter as described in Experimental Procedures. A baseline was collected for 5 min, then ATP (4 mM) with and without EDTA (15 mM), ADP (2 mM) or buffer was added, and signal monitored for an additional 25 min. FtsA used in C contains copurifying PLs.
D. Dose-dependent FRET efficiency from the FtsA W408 and W415 tryptophan donors, both in the MTS, to DPH incorporated into liposomes (100 μg ml−1) (1:500 w/w) was examined by emission of DPH fluorescence as described in Experimental Procedures. Traces show increasing FtsA concentrations (0, 0.5, 1.0, 2.0 and 3.0 μM). FtsA used in D contains copurifying PLs and DPH-treated SUVs were added.
E. Box and whiskers plot of FRET efficiency of several FtsA concentrations (0.5, 1.0 and 2.0 μM) in the presence (red) or absence (black) of ATP (4 mM). The box encompasses the maximum and minimum values. The line within each box represents the median (n=3).
F. FRET efficiency of increasing concentrations of FtsA(ΔMTS) (0.5, 1.0 and 2.0 μM) to DPH incorporated in liposomes (100 μg ml−1). Data in B-D and F are representative of three trials.
To further investigate the role of ATP in remodeling vesicle architecture, we monitored ATP-dependent reorganization by two independent light scattering methods. First, we used dynamic light scattering (DLS) to probe heterogeneity and relative particle sizes of purified FtsA, with copurifying lipids, in the presence and absence of ATP (Fig. 3B). We observed that the majority of particles in the preparation of FtsA correspond to approximately 50 nm (Fig. 3B). However, after incubation with ATP, we observed the presence of a large, broad peak centered near 300 nm, which represents approximately half of the volume distribution of all particles present (Fig. 3B). The peak was not observed in reactions of FtsA incubated with ADP or ATPγS. The detection of this additional large peak in the presence of ATP is consistent with the large structures observed by TEM.
Next, we employed a real-time vesicle remodeling assay using 90° angle light scatter to detect physical changes when ATP is added. In this assay, we measured the background light scatter of FtsA and copurifying liposomes, then added nucleotide and monitored the change in light scatter over time. The addition of ATP induces a large light scatter increase, which occurs rapidly and plateaus approximately 15 min after the addition of ATP (Fig. 3C). The increase in light scatter induced by ATP is stable for 15 min, and then the signal falls by approximately 50% over the next 60 min (Supporting Information Fig. S3A). To determine whether ATP binding or hydrolysis is associated with the rapid increase in light scatter, we repeated the 90° angle light scatter assay with ADP and ATP in the presence of EDTA to prevent hydrolysis. In the presence of EDTA, ATP is sufficient to scatter light, although the maximal signal is approximately 20% lower than without EDTA (Fig. 3C). We confirmed that FtsA binds similarly to mant-ATP in both the presence and absence of EDTA (Supporting Information Fig. S3B). When ADP was added to the reaction, the signal change after 30 min was 74% lower than with ATP (Fig. 3C), suggesting that ADP weakly promotes reorganization. This is further supported by TEM of FtsA in the presence of ADP, which shows isolated, intact vesicles, in contrast to the tubulated vesicles with ATP (Supporting Information Fig. S3C). These results are consistent with ATP promoting lipid reorganization. Furthermore, ATPγS was unable to promote robust light scatter, consistent with DLS results (Fig. 3B) (Supporting Information Fig. S4A), and GTP was also unable to stimulate light scatter (Fig. S4B). In the control experiment, we tested if FtsA(ΔMTS) scatters light when ATP is added, but detected no change, suggesting that the increase in scatter requires engagement of the membrane (Supporting Information Fig. S3D). In addition, TX100 prevents ATP-dependent reorganization by wild type FtsA in 90° light scattering assays and the formation of large 300 nm structures assembled with ATP by DLS (Supporting Information Fig. S3E and S4C). As expected, supplementation of FtsA with E. coli SUVs after treatment with detergent restores ATP-dependent light scatter (Supporting Information Fig. S3F). Finally, when we tested His-SUMO-FtsA, we also observed that His-SUMO-FtsA scatters light upon addition of ATP and copurifies with phospholipid vesicles (Supporting Information Fig. S2C and S2D).
Next, we determined if ATP binding changes the orientation of the MTS in the interior of the lipid bilayer, leading to reorientation of the lipid plane relative to FtsA. We used the lipophilic probe diphenyl hexatriene (DPH) as an acceptor in fluorescence resonance energy transfer (FRET) assays, with the donor fluorescence emitted by excitation of two naturally occurring tryptophan residues in the MTS (W408 and W415). In reactions containing FtsA and SUVs pre-incubated with DPH, excitation of the tryptophan residues at 285 nm resulted in a DPH emission signal, which peaked at 430 nm, and the intensity was dependent on FtsA concentration (0 to 3 μM) (Fig. 3D). As a control, we measured DPH fluorescence at several phospholipid concentrations and determined that phospholipid concentration does not alter the amplitude at 430 nm under the range of concentrations tested and in the absence of protein (Supporting Information Fig. S3G). To determine if ATP changes FRET efficiency between the FtsA tryptophans and DPH, we compared DPH emission in the presence and absence of ATP. We detected a decrease in the maximum emission amplitude at all FtsA concentrations in the presence of ATP, resulting in a 23% loss of emission at 3 μM FtsA (Fig. 3E). This suggests that when FtsA binds ATP, the MTS repositions within the plane of the lipid bilayer altering the FRET efficiency by limiting access of the DPH acceptor. In the control experiment, we examined the FRET efficiency of FtsA(ΔMTS) and DPH, which does not contain either tryptophan residue near the C-terminus. As expected, we detect no emission of DPH in the presence of FtsA(ΔMTS) (Fig. 3F). These results indicate that the FtsA C-terminal MTS inserts into the lipid bilayer, insertion does not require ATP and the MTS repositions within the membrane when FtsA binds ATP.
A mutation in a loop proximal to the FtsA ATP-binding site impairs function in vitro
To further investigate how nucleotide binding and hydrolysis is coordinated with other regions of the protein, we performed site-directed mutagenesis in a loop near the nucleotide-binding region of FtsA (Fig. 1A, Supporting Information Fig. S5A and Fig. S5B). This loop in the structural model of E. coli FtsA (residues 84-89) occupies the space similar to the sensor loop in Saccharomyces cerevisiae actin (Sc residues 70-78) (Supporting Information Fig. S5C and Fig. S5D). We identified a lysine residue (K86) in FtsA in this loop near the binding region of the ATP γ-phosphate and constructed and purified the FtsA mutant protein FtsA(K86A). FtsA(K86A) is defective for ATP hydrolysis, compared to wild type FtsA, and no stimulation is observed with addition of prepared phospholipids (Fig. 4A). We performed the 90° angle light scatter assay with FtsA(K86A) to detect reorganization; however, the mutant protein failed to scatter light upon addition of ATP (Fig. 4B). To determine if this defect is due to an inability of FtsA(K86A) to bind ATP, we measured binding to mant-ATP and calculated a KD for the interaction of 27.9 ± 0.5 μM (Fig. 4C), which is similar to the KD of wild type FtsA with mant-ATP (Fig. 2E). Next, we tested if the MTS of FtsA(K86A) intercalates into the lipid bilayer using the Trp-DPH FRET assay, but failed to detect a concentration-dependent FRET signal for FtsA(K86A) (Fig. 4D). These results suggest that residue K86 near the ATP-binding site may participate in communication between the nucleotide binding site and the membrane. To determine if FtsA(K86A) localizes to the Z-ring in vivo, we expressed GFP-FtsA(K86A) in cells and monitored localization in live dividing cells. Cells expressing GFP-FtsA exhibited fluorescence at the septa and were determined to be 8.5 ± 0.5 μm (n=222) in length. In contrast, cells expressing GFP-FtsA(K86A) were significantly different in length, 5.6 ± 0.1 μm (n=297) (p<0.0001), which is 34% shorter than cells expressing GFP-FtsA (Fig. 4E). These results suggest that expression of GFP-FtsA interferes with cell division, but expression of GFP-FtsA(K86A) is less toxic. We also observed that GFP-FtsA(K86A) localizes to the division ring, although it is impaired for ATP-hydrolysis and membrane insertion in vitro (Fig. 4E).
Fig. 4. FtsA(K86A), which contains a mutation in a loop near the active site, is defective for function.

A. ATP hydrolysis by FtsA (1 μM), FtsA(K86A) (1 μM) or FtsA(R286W) (1 μM), with and without supplemental SUVs (500 μg ml−1), where indicated. Data is an average of three replicates represented as mean ± SEM. FtsA wild type and mutant protein used in A contain copurifying PLs.
B. Phospholipid reorganization by FtsA (2 μM), FtsA(K86A) (2 μM) or FtsA(R286W) (2 μM) were monitored by 90° angle light scatter. A baseline signal was measured for 5 min then ATP was added and monitored for 60 min. Data is representative of three replicates. FtsA wild type and mutant protein used in B contain copurifying PLs.
C. Binding of FtsA(K86A) to mant-ATP monitored by measuring fluorescence emission at increasing mant-ATP concentrations as described in Experimental Procedures.
D. FRET efficiency of increasing concentrations of the FtsA(K86A) MTS to DPH incorporated in liposomes as described in Experimental Procedures.
E. Confocal fluorescence and differential interference contrast (DIC) microscopy of wild type cells (MG1655) expressing GFP-FtsA or GFP-FtsA(K86A) induced under growth conditions described in Experimental Procedures. Size bars are 5 μm.
Lastly, to determine if self-interaction is critical for phospholipid reorganization in vitro, we constructed and purified FtsA(R286W), which was previously reported to be defective for self-interaction and capable of bypassing the requirement for ZipA during division (Geissler et al., 2003, Pichoff et al., 2012). In this study, FtsA(R286W) is defective for rapid ATP hydrolysis compared to wild type FtsA (Fig. 4A). By size exclusion chromatography, FtsA(R286W) elutes as a monomer with a calculated size of 50.6 kDa; however the peak is very broad with a shoulder overlaying both the dimer position (~80 kDa) and the void volume position (Supporting Information Fig. S6A). In contrast, elution of FtsA(ΔMTS) peaks at 108 kDa, which is most consistent with a dimer (Supporting Information Fig. S6B). Confocal fluorescence microscopy of FtsA(R286W) stained with FM 4-64 indicates the presence of a few fluorescent vesicles (Supporting Information Fig. S6C); however, in quantitative assays we determined that there is 89% less copurifying phospholipid associated with FtsA(R286W) than with wild type FtsA (Supporting Information Fig. S6D). Next, we performed the 90° angle light scatter assay and determined that although FtsA(R286W) has an ATP hydrolysis rate that is 81% slower than wild type FtsA, FtsA(R286W) produces a modest change in light scatter when ATP is added (Fig. 4A and 4B); however, the amplitude increase develops at a slower rate than for wild type FtsA (Fig. 4B). These results indicate that FtsA(R286W) may be capable of forming large phospholipid complexes, but to a much lesser extent than wild type FtsA. These results further suggest that fast ATP hydrolysis and self-association may be important for promoting robust lipid engagement and reorganization.
FtsA recruits FtsZ to phospholipids and destabilizes steady-state FtsZ polymers through a direct interaction with the FtsZ C-terminus
FtsA functions as a membrane tether for recruitment of FtsZ polymers to the cytoplasmic membrane (Pichoff & Lutkenhaus, 2005). Previous reports also indicate that FtsA recruits FtsZ to a lipid monolayer (Loose & Mitchison, 2014) and TmFtsA recruits TmFtsZ inside liposomes and induces negative curvature (Szwedziak et al., 2014). In low-speed sedimentation assays, approximately 30% of FtsA associates with the pellet fraction due to the presence of copurifying phospholipids, and this amount increases to 84% when the reaction is supplemented with SUVs (Supporting Information Fig. S7A). To identify determinants for the interaction between FtsZ, FtsA and phospholipid vesicles, we performed a low-speed sedimentation assay to collect vesicles and vesicle-associated protein under different nucleotide conditions (Fig. 5A and 5B). We incubated FtsA with SUVs and ATP and then determined if FtsZ fractionates with the SUV pellet. In the presence of GTP or GMPCPP, conditions that promote FtsZ polymerization, the majority of FtsZ is pellet-associated (62.8% and 76.1%, respectively) in reactions containing FtsA, SUVs and ATP (Fig. 5A); however, with GDP, which does not support FtsZ polymerization, only 18.3 % of FtsZ is detected in the pellet in reactions containing FtsA, SUVs and ATP. In the presence of ATPγS, less FtsZ was detected in the pellet under all conditions (GDP, GTP and GMPCPP) in reactions containing FtsA and SUVs, suggesting that ATP promotes the interaction between FtsA and FtsZ better than ATPγS (Fig. 5A). In a control experiment, we observed that when FtsA was omitted, FtsZ polymers fractionated with the supernatant in the presence and absence of SUVs since FtsZ polymers do not bind SUVs directly (Supporting Information Fig. S7B); however, when FtsA was included in the reaction, the majority of FtsZ fractionated with the pellet, indicating that FtsA recruits FtsZ polymers to SUVs (Supporting Information Fig. S7B), and this percentage is higher with ATP (Fig. 5A and 5B) (Supporting Information Fig. S7B). Finally, we also observed that in the presence of ADP, FtsA can recruit FtsZ polymers to SUVs, but to a lesser extent than without ADP or with ATP (Fig. 5A and 5B). Together, these results show that FtsA recruits GTP- and GMPCPP-stabilized FtsZ polymers to SUVs, but that non-polymerized FtsZ is not efficiently recruited. Moreover, the presence of ATP likely stabilizes this interaction.
Fig. 5. FtsA recruits FtsZ to phospholipids and destabilizes steady state FtsZ polymers.

A. Phospholipid recruitment assays with reaction mixtures of FtsZ (6 μM) with GTP, GDP or GMPCPP, as indicated, were pre-assembled and added to pre-incubated mixtures of FtsA (2 μM) and SUVs (500 μg ml−1) with ATP or ATPγS then fractionated as described in Experimental Procedures. Supernatants and resuspended pellets were visualized by SDS-PAGE and quantified by densitometry. The ratio of copurifying to exogenous lipids was 1:2.
B. Phospholipid recruitment assay, as in A, but reaction mixtures of FtsZ (6 μM) with GTP, GDP or GMPCPP, as indicated, were pre-assembled and added to pre-incubated mixtures of FtsA (2 μM) and SUVs with or without ADP then fractionated as previously.
C. Confocal fluorescence microscopy was performed in a chamber slide containing FL-FtsZ polymerized with GTP (green) (i) or FtsA, with copurifying PLs stained with FM 4-64 (red) and ATP (ii). Reactions containing FtsA, with copurifying FM 4-64 stained vesicles, ATP, GTP and FL-FtsZ (iii) or FL-FtsZ(R379E) (iv) were assembled and visualized as described in Experimental Procedures. Size bars indicate 5 μm.
D. Reactions of FtsA (0 to 6 μM) and, where indicated, FtsZ or FtsZ(R379E) (4 μM), with GTP (2 mM) and ATP (4 mM) were incubated for 5 min, then fractionated by ultracentrifugation. Pellets were visualized by SDS-PAGE and quantified by densitometry. FtsA used in D contains copurifying PLs.
E. GTP hydrolysis assays of FtsZ (6 μM) incubated with GTP (1 mM) and increasing concentrations of FtsA (0 to 6 μM) were monitored over time and assayed for free phosphate as described in Experimental Procedures. Data from D and E are an average of three replicates represented as mean ± SEM.
To directly visualize recruitment of FtsZ to FtsA-associated phospholipid vesicles, we used confocal fluorescence microscopy. Active fluorescent FtsZ, labeled with Alexa fluor 488 (FL-FtsZ), was incubated with GTP, added to a channel slide with an 80 μm thick flow chamber and visualized. We observed large, fluorescent FtsZ sheets and bundles, likely composed of long, networked FtsZ polymers (Fig. 5Ci), which have been described previously (Camberg et al., 2009). FtsA-decorated liposomes were stained with FM 4-64 (Fig. 5Cii) and then incubated with FL-FtsZ in the presence of GTP and ATP, and both fluorophores were visualized (Fig. 5Ciii). We observed overlapping fluorescence between FL-FtsZ and FtsA-decorated liposomes, consistent with our observations of direct recruitment of FtsZ to phospholipids by FtsA.
After incubation of GTP-induced FtsZ polymers with FtsA and phospholipids, the FL-FtsZ structures observed by fluorescence microscopy resembled vesicles rather than the networked, polymer bundles (Fig. 5Ciii), suggesting the interaction with FtsZ may destabilize FtsZ polymers. To test this directly and determine if FtsA affects the abundance of FtsZ polymers under steady state conditions, we assembled FtsZ with GTP and a bifunctional GTP/ATP-regenerating system in the presence of increasing FtsA, and collected FtsZ polymers by ultracentrifugation. We observed that with the addition of GTP, FtsZ fractionates predominantly with the high-speed centrifugation pellet indicating that FtsZ polymerizes (Fig. 5D). However, when FtsA is added to the reaction, there is a concentration-dependent decrease in the amount of FtsZ present in the pellet (Fig. 5D). This suggests that as FtsA approaches equimolar stoichiometry with FtsZ, FtsA is effective at destabilizing FtsZ polymers.
If an FtsZ-interacting protein prevents GTP-dependent assembly, then this protein should also reduce the rate of FtsZ GTP hydrolysis. This has been reported for the cell division inhibitor SulA, which binds to the FtsZ protofilament interface and reduces the rate of GTP hydrolysis by FtsZ (Mukherjee et al., 1998, Cordell et al., 2003). Regarding FtsA, previous studies have produced conflicting results; purified FtsA from Staphylococcus aureus and D. radiodurans stimulates the GTPase activity of cognate FtsZ, which could lead to destabilization of FtsZ filaments (Fujita et al., 2014, Modi & Misra, 2014). By contrast, FtsA from D. radiodurans decreases GTPase activity of E. coli FtsZ (Modi & Misra, 2014). We performed GTP hydrolysis assays with EcFtsZ and observed a rate of 4.8 ± .0.1 pmol Pi min−1 pmol−1, which agrees with previous reports (Camberg et al., 2014). In control experiments, we detected no GTP hydrolysis activity by FtsA (Supporting Information Fig. S8A). When FtsA was added to reactions containing FtsZ, the rate of GTP hydrolysis decreased by 55%, suggesting that the FtsA-FtsZ interaction decreases FtsZ GTP hydrolysis (Fig. 5E). Reciprocally, we tested if FtsZ inhibits FtsA ATP hydrolysis but observed no effect (Supporting Information Fig. S8B).
In the crystal structure model of TmFtsZ by Szwediak et al., Arg 301 forms a salt bridge with Asp 338 of TmFtsA (Szwedziak et al., 2012). Therefore, we constructed a mutation at the analogous residue in EcFtsA, Arg 379, to disrupt the interaction between FtsZ and FtsA. This mutant was previously reported to have a GTP hydrolysis rate similar to wild type FtsZ and is competent for GTP-dependent polymerization (Camberg et al., 2014, Viola et al., 2017). FtsA does not inhibit polymerization of FtsZ(R379E), which is in agreement with residues in the FtsZ C-terminus mediating the interaction with FtsA (Fig. 5D). To confirm impaired interaction, we performed fluorescence microscopy to observe if FtsA can recruit FL-FtsZ(R379E) to phospholipid vesicles. Although FL-FtsZ(R379E) polymers were visible and appear similar to wildtype, they do not overlay with fluorescent FtsA-associated liposomes (Fig. 5Civ), indicating that Arg 379 of FtsZ is a critical residue for interacting with FtsA. We also tested if FtsA recruits GTP- or GMPCPP-stabilized FtsZ(R379E) polymers to SUVs in a low-speed sedimentation assay, but detected only 35.6% of total FtsZ(R379E) in the pellet fraction with GTP and ATP suggesting that FtsZ(R379E) is impaired for the interaction with FtsA (Supporting Information Fig. S7C).
Reconstruction of synthetic proto-ring complexes containing phospholipids, FtsA and polymerized FtsZ
To investigate the detailed architecture of FtsA-decorated liposomes in association with FtsZ polymers, we visualized complexes of FtsA, FtsZ and SUVs in the presence of ATP and GMPCPP by TEM. FtsA, ATP and SUVs, without FtsZ, form distorted and tubulated liposomes. Tubulated liposomes were frequently observed with a smooth surface and an opposing surface lined with projections approximately 50 Å from the lipid surface (Fig. 6A). These projections, observed in images of reactions containing FtsA, ATP and either copurifying lipids or supplemental SUVs, are consistent in size with FtsA cytoplasmic domains aligned on the phospholipid surface (Fig. 6Aii, box) (Supporting Information Fig. S9). The majority of aligned projections are present at regions of neutral or negative curvature (Supporting Information Fig. S9). Next, FtsZ was added to a mixture of FtsA, ATP and SUVs, but did not noticeably change the architecture of the proteo-lipid structures (Fig. 6B). Finally, we constructed synthetic proto-ring complexes by incubating stable FtsZ polymers, assembled with the GTP analog GMPCPP, FtsA, ATP and SUVs. In the presence of FtsZ polymers, long bundled fibers reaching 3.5 μm in length and 50 to 80 nm in width were observed (Fig. 6Ci-v). The bundles appear to contain FtsZ polymers coated in phospholipids, recruited by the direct interaction with FtsA. Bundles assembled into a twisted helical network, periodically containing branches or bifurcations (Fig. 6Cii and 6Cv), with several bundles appearing frayed at the ends (Fig. 6Civ). In contrast, FtsZ polymers alone, without FtsA and SUVs, appeared as single stranded and laterally associated fibers reaching 450 nm in length, with single protofilaments ~ 5 nm thick (Fig. 6D). Addition of SUVs to FtsZ polymers had no effect on the dimensions of the FtsZ polymers (Fig. 6E). These results shows that lipid-associated FtsA forms a large structural scaffold with FtsZ polymers in vitro.
Fig. 6. Reconstruction of synthetic proto-ring complexes.

A. FtsA (4 μM) visualized by negative stain TEM with SUVs (500 μg ml−1) and ATP (4 mM). Size bars are 50 nm in Ai and 20 nm in Aii. Yellow line denotes region of negative membrane curvature with FtsA aligned on the surface; yellow box encompasses FtsA aligned on the membrane surface in a region of neutral curvature. The ratio of copurifying to exogenous lipids is 1:1.
B. FtsA (2 μM) was visualized by TEM, as in A, in the presence of FtsZ (4 μM). The ratio of copurifying to exogenous lipids is 1:2.
C. Synthetic proto-ring complexes were assembled by incubation of FtsA (2 μM), FtsZ (4 μM), ATP (4 mM), GMPCPP (0.2 mM) and SUVs (500 μg ml−1), then visualized by TEM (i-v). Arrows indicate regions of helical twists and/or branches. The ratio of copurifying to exogenous lipids in is 1:2.
D. FtsZ (4 μM) with GMPCPP was visualized by TEM.
E. TEM, as in D, but FtsZ was incubated with GMPCPP and SUVs (500 μg ml−1).
TEM in A-E was performed as described in Experimental Procedures.
Accumulation of membrane in cells overexpressing FtsA
To investigate if overexpression of FtsA in cells leads to morphological defects or regions of enhanced curvature at the septum, E. coli BL21(λde3) cells overexpressing FtsA from pET-ftsA (Fig. 7A) or containing the control vector (Fig. 7B) were fixed, embedded, prepared as thin sections and visualized by negative stain TEM. Cells expressing FtsA at 16 °C for 20 hours remained viable and were on average 15% longer than cells containing the control vector (2.69 ± 0.07 μm and 2.33 ± 0.04 μm, respectively) (Supporting Information Fig. S10A, S10B and S10C). In cells expressing FtsA, we observed an extensive network of membranes at the septum (Fig. 7Aii). We also observed clusters of membrane-lined regions and a large number of intracellular vesicles that appeared empty and had accumulated at the cell poles (Fig. 7Aiii and inset), similar to intracellular membranes and vesicles observed in cells overexpressing the Lipid A disaccharide synthase (LpxB) or other membrane glycosyltransferases (Metzger & Raetz, 2009, Eriksson et al., 2009). In contrast, in cells containing the control vector, membrane was observed along the edge of the cell at the site of cell wall invagination and polar vesicles were only occasionally observed (Fig. 7Bi and 7Bii). These results suggest that overexpression of FtsA in vivo leads to the formation of intracellular vesicles and increased membrane surface area throughout the cell and at the division site.
Fig. 7. Membrane distortion and intracellular vesicle formation in cells overexpressing FtsA.
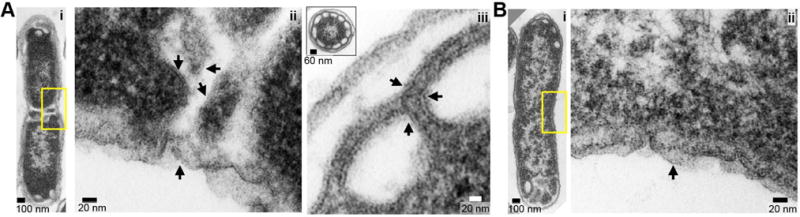
A, B. In A, E. coli BL21(λde3) cells overexpressing FtsA from pET-ftsA (A) or empty vector (B) were grown, fixed, prepared as thin sections and visualized by negative stain TEM as described in Experimental Procedures. Yellow box highlights a region of the septum viewed at higher magnification (Aii, Bii); arrows indicated discernible membranes. In Aiii, clusters of membrane-lined regions accumulated at the cell poles (inset, polar cross-section) of cells overexpressing FtsA, but were rarely observed in cells containing the control vector (Bi).
Discussion
Here we report that FtsA, the membrane-associated actin homolog from E. coli, hydrolyzes ATP robustly, directly reorganizes phospholipid membranes and recruits FtsZ to the membrane. The native FtsA preparation used in this study copurifies with lipid vesicles and efficiently inserts into prepared lipid vesicles composed of purified E. coli membrane phospholipids. Extraction of FtsA from phospholipids abolishes rapid ATP hydrolysis, but this activity is restored by reconstitution of the reaction with prepared phospholipid vesicles (Fig. 2C). Our results show that interaction between phospholipids and FtsA is essential for rapid ATP hydrolysis, which is consistent with our finding that FtsA(ΔMTS) hydrolyzes ATP at <5% of the wild type FtsA hydrolysis rate (Fig. 2D).
Although ATP is not required for the direct interaction of FtsA with phospholipids, ATP binding promotes remodeling of lipid vesicle architecture of reconstituted complexes (Fig. 3A, 3B and 3C). Direct membrane remodeling was also previously described for S. pneumoniae FtsA in a report suggesting that SpFtsA could bind to phospholipids in the presence of ATP and induce local changes in membrane architecture (Krupka et al., 2014), and ATP dependent lipid tubulation and/or remodeling has also been described for several other membrane-associated proteins including MinD, amphiphysin and epsin (Hu et al., 2002, Peter et al., 2004, Ford et al., 2002). Notably, we observed that when phospholipid-free FtsA was added to purified vesicles, the vesicles adopted large, curving tubulated clusters (Fig. 3A). The C-terminal helix of FtsA inserts directly into the phospholipid bilayer in the absence and presence of ATP, as determined by FRET; however, the FRET efficiency is modified in the presence of ATP indicating that the position of the MTS and/or the accessibility of the MTS within the membrane changes between nucleotide occupancy states (Fig. 3D and 3E).
How then does FtsA modify membrane architecture? Membrane bending likely occurs as a result of insertion of the C-terminal helix into the bilayer and the additional collaboration among adjacent domains on the surface of the membrane, which may be regulated by nucleotide cycling and, in vivo, by other cell division proteins. Our results support that there is communication between the active site where ATP binds and the C-terminal helix of FtsA. First, the ATP analog mant-ATP binds FtsA with higher affinity when phospholipids are present, indicating that phospholipid engagement either directly modifies the conformation of FtsA to promote tighter nucleotide binding (Fig. 2E) or, alternatively, may concentrate FtsA on the vesicle surface, increasing the effective concentration. Second, activation of DPH fluorescence by tryptophan residues in the C-terminal helix is reduced in the presence of ATP (23% lower FRET signal when ATP is present, Fig. 3E), which suggests that although the MTS remains inserted, its relative position in the membrane changes to limit access of the acceptor probe. Consistent with this, a previous report indicated that FtsA(R286W) and a fluorescent FtsZ chimera, FtsZ-YFP, incorporate into liposomes and produce concave membrane perturbations, which occasionally produced liposome scission events, suggesting FtsA may be critical for membrane scission (Osawa & Erickson, 2013). The oligomeric state of FtsA likely plays an additional role, but key details remain unclear. It was reported that FtsA is capable of polymerization with ATP and further suggested that FtsA may assemble more robustly when the MTS has been removed (Szwedziak et al., 2012, Krupka et al., 2014). In this study, we observed FtsA as tightly arranged subunits of FtsA aligned on the surface of the membrane consistent with the presence of FtsA polymers; however these were observed in both the presence and absence of ATP (Fig. 6A). In light scatter experiments, ATP-dependent reorganization is supported by FtsA(R286W) (Fig. 4B); however, FtsA(R286W) is defective for rapid ATP hydrolysis and self-interaction indicating that oligomerization is not the primary driver of membrane remodeling. FtsA self-interaction could, however, modulate cycles of ATP binding, hydrolysis and/or nucleotide release. Consistent with this hypothesis, although FtsA(R286W) is capable of ATP-dependent membrane reorganization, the amplitude change is lower and occurs more slowly than wild type FtsA (Fig. 4B), which may be attributable to slow nucleotide hydrolysis and/or exchange as well as fewer phospholipids. Several reports obtained from in vivo genetic studies have suggested that interactions between FtsA and other division proteins, including ZipA, FtsN, and FtsEX, may prevent FtsA self-association and promote monomerization (Pichoff et al., 2012, Du et al., 2016). Therefore, FtsA-interacting proteins may function to inhibit polymerization, thereby regulating cycles of ATP binding and release and cycles of membrane reorganization. Further study will be required to determine the precise roles of FtsA-interacting proteins with respect to membrane remodeling by FtsA and if there is equilibrium between FtsA monomers and polymers associated with the membrane.
The structural model of actin from S. cerevisiae shows several key functional regions at the nucleotide binding site, including P loop 1 and P loop 2, as well as the sensor loop (amino acids 70-78), which has been suggested to play a critical role in phosphate release after ATP hydrolysis (Supporting Information Fig. S5C and Fig. S5D) (Kudryashov & Reisler, 2013, Murakami et al., 2010, Rould et al., 2006). However, in the E. coli FtsA structural model showing a repositioned ‘1C’ subdomain, FtsA appears to be missing an analogous sensor loop. However, two loops are situated in a similar location as the actin sensor loop, one of which contains K86 (Supporting Information Fig. S5A and Fig. S5B). FtsA(K86A) is capable of nucleotide binding, yet is defective for ATP hydrolysis and membrane insertion (Fig. 4A-D). Lys 86 and the surrounding loop regions may serve as a sensor to regulate γ-phosphate occupancy and/or relay to nearby regions and the C-terminal helix. However, further study will be required to determine the conformational steps involved in regulating FtsA function.
Although FtsA conformations during division remain insufficiently clear, recent work has suggested that FtsZ polymers treadmill around the circumference of the cell in association with FtsA during division (Bisson-Filho et al., 2017). We investigated the function of FtsA to directly recruit FtsZ to the membrane and observed that FtsA at substoichometric ratios recruits FtsZ to phospholipid vesicles in vitro through a direct interaction with Arg 379 in the FtsZ C-terminal conserved region (Fig. 5C and 5D). Under conditions when FtsA is in excess over FtsZ, FtsA destabilizes steady state FtsZ-GTP polymers in sedimentation assays and reduces the overall rate of GTP hydrolysis by FtsZ, suggesting that protofilament assembly is also disrupted (Fig. 5E). FtsA was recently reported to form minirings on a lipid surface and destabilize FtsZ polymers through preventing FtsZ polymer lateral interactions (Krupka et al., 2017). When FtsA and phospholipids are incubated with GMPCPP-stabilized FtsZ polymers in vitro, large twisted bundles are observed. These bundles are likely composed on the interior of FtsZ polymers, and phospholipids wrap around the exterior. We propose that FtsA-mediated membrane reorganization occurs in vivo at the septum where FtsZ polymers undergo active remodeling. In our current model of FtsA during early division (Fig. 8): (1) FtsA directly interacts with the cytoplasmic leaflet of the inner membrane and inserts the C-terminus into the phospholipid bilayer, (2) in the phospholipid bound conformation, ATP binding induces a conformational reorientation of FtsA, promoting membrane distortion and (3) FtsZ polymers provide a scaffold to direct the FtsA-mediated remodeling of the membrane. FtsA then recruits cell wall synthesis and remodeling enzymes, including FtsI and FtsN, adjacent to the distorted region of the membrane on the periplasmic side to add new peptidoglycan. In this model local membrane deformations by FtsA at multiple locations around the circumference of the cell serve as sites of peptidoglycan synthesis, which is consistent with recent work suggesting that interactions of FtsZ polymers with FtsA at the division site guides peptidoglycan insertion (Bisson-Filho et al., 2017, Yang et al., 2017). Creation of a membrane adjacent protein scaffold may ultimately be a key driver of division, as protein crowding has recently been reported as sufficient to promote membrane fission events in vitro (Snead et al., 2017). Altogether, this study provides direct evidence that FtsA self-organizes, forms a scaffold with FtsZ and restructures membranes to direct cell wall remodeling at the Z-ring during early constriction.
Fig. 8. Model of FtsA function during E. coli cell division at the septum.

Model of FtsA function during E. coli cell division at the septum in a cross-sectioned cell: (1) FtsA binds the cytoplasmic leaflet of the inner membrane and inserts the C-terminus into the phospholipid bilayer. (2) In the phospholipid bound conformation, ATP binding promotes a conformational reorientation of FtsA, leading to membrane distortion. FtsA recruits FtsZ polymers to the membrane proximal site through a direct interaction with the FtsZ C-terminal region. (3) Membrane remodeling activity occurs at the interface of FtsZ polymers and FtsA. FtsA then recruits additional cell wall remodeling enzymes to insert peptidoglycan on the periplasmic side of the site of membrane invagination. (CM, cytoplasmic membrane; P, periplasm; OM, outer membrane).
Experimental Procedures
Bacterial strains and plasmid construction
E. coli strains and plasmids used in this study are listed in Table 1. The ftsA gene was amplified from E. coli MG1655 and cloned into pET-24b using NdeI and EcoRI restriction sites. Site-specific mutants in ftsA were constructed by site-directed mutagenesis of ftsA expression plasmids using the QuikChange mutagenesis system (Agilent) and confirmed by sequencing. Plasmid pSEB293, containing GFP-FtsA, was mutagenized to create GFP-FtsA(K86A); pET-ftsA was mutagenized to create FtsA(K86A), FtsA(R286W) and truncated FtsA, FtsAΔMTS, by inserting a TAA stop codon at amino acid position 405 in ftsA. Where indicated, cell viability was measured by serial dilution of liquid cultures and spread plating onto solid media. Plates were incubated at 37 °C for 20 hours to count single colonies.
Table 1.
Strains and plasmids used in this study.
| Strain | Relevant genotype | Source, Reference or Construction |
|---|---|---|
|
| ||
| MG1655 | LAM- rph-1 | (Blattner et al., 1997) |
| BL21(λde3) |
F- ompT gal dcm lon hsdSB(rB- mB-) λ(de3[lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) |
EMD Millipore, USA |
| JC0390 | MG1655 (ΔaraEp∷kan) | (Viola et al., 2017) |
| Plasmids | ||
|
| ||
| pSEB293 | amp Para∷gfp-ftsA | (Pichoff & Lutkenhaus, 2007) |
| pBAD-gfp-ftsA(K86A) | amp Para∷gfp-ftsA(K86A) | This study |
| pET-24b | kan, PT7 promoter | EMD Millipore, USA |
| pET-ftsA | kan | This study |
| pET-ftsA(K86A) | kan | This study |
| pET-ftsA(R286W) | kan | This study |
| pET-ftsA(ΔMTS) | kan | This study |
| pET-ftsZ | kan | (Camberg et al., 2009) |
| pET-ftsZ(R379E) | kan | (Camberg et al., 2014) |
| pML29 | amp PT7∷his-sumo-ftsA | (Loose & Mitchison, 2014) |
Protein purification
FtsZ and FtsZ(R379E) were purified as previously reported for wild type FtsZ (Camberg et al., 2014, Camberg et al., 2009). FtsA, FtsA mutant proteins and, where indicated, His-SUMO-FtsA (Loose & Mitchison, 2014) were expressed in BL21 (λde3) carrying pET vectors encoding wild type or mutant FtsA. The cells were grown in in LB lennox broth supplemented with 50 μg ml−1 kanamycin at 37 °C to an OD600 of 1.2. Expression was induced with 0.5 mM isopropyl-β-D-thiogalactoside (IPTG) for 20 hours at 16 °C. Cells were harvested by centrifugation for 30 min at 6,000 × g at 4 °C and resuspended in 25 ml of cold lysis buffer [25 mM Tris-HCl pH 8.5, 50 mM KCl, 10 mM MgCl2, 10% glycerol, 1 mM EDTA, 1 mM tris(2-carboxyethyl)phosphine (TCEP)]. Cells were lysed by French press and soluble cell extracts were obtained by centrifugation at 30,000 × g for 30 min. Extracts were fractionated with 25% ammonium sulfate, and precipitant was resuspended in cold lysis buffer, applied to Q sepharose fast flow (GE Healthcare, Piscataway, NJ) column equilibrated with lysis buffer and eluted using a linear KCl gradient (50 to 1000 mM). The peak fractions were pooled, precipitated with 50% ammonium sulfate, resuspended in buffer containing [25 mM Tris-HCl pH 7.5, 200 mM KCl, 10 mM MgCl2, 10% glycerol, 1 mM EDTA, 1 mM tris(2-carboxyethyl)phosphine (TCEP)] and fractionated on a Sephacryl S-200 column (50 ml) (GE Healthcare, Piscataway, NJ) at 0.5 ml min−1. Unless otherwise indicated all FtsA used in assays contained copurified phospholipid. For experiments with FtsA that was extracted from PLs, as indicated, FtsA was removed from copurifying phospholipid vesicles by adding TX100 (2%), incubating for 15 minutes on ice, and then exchanging the buffer on G25 Sephadex resin and removing residual detergent with a detergent removal spin column (ThermoFisher). Where indicated, residual PLs were removed by treatment of sample with calcium silicate lipid removal agent (250 mg added per 50 μg of protein) (Sigma-Aldrich). After treatment protein was used immediately. Protein concentrations were determined by bradford assay with comparison to a bovine serum albumin standard curve using Bio-Rad Protein Determination Reagent and are reported as FtsZ monomers and FtsA monomers.
Analytical size exclusion chromatography of FtsA, FtsA(K86A), FtsA(ΔMTS) and FtsA(R286W) was performed in the presence or absence of TX100 using a Sephacryl S-200 (45 ml) column equilibrated with buffer containing 25 mM Tris-HCl (pH 7.5), 200 mM KCl, 10 mM MgCl2, 1 mM EDTA and 1 mM TCEP. Where indicated, FtsA was incubated with 2% TX100 for 10 min and applied to a column equilibrated with buffer containing 0.5% TX100. Eluting fractions were analyzed for FtsA content by SDS-PAGE and coomassie staining and intensity was measured by densitometry using NIH Image J.
Nucleotide binding and hydrolysis assays
To detect ATP binding, reaction mixtures (75 μl) of FtsA (2 μM) with and without SUV (0.5 mg ml−1) were incubated for 5 minutes (23 °C) in reaction buffer containing 25 mM Tris-HCl (pH 7.5), 200 mM KCl and 10 mM MgCl2, with increasing concentrations of mant-ATP (1 to 250 μM) (Life Technologies). Fluorescence intensity was measured with an Agilent Eclipse fluorescence spectrophotometer using an excitation wavelength of 350 nm and an emission wavelength of 400 nm and 5 nm slit widths. Binding curves were fit to a one site specific binding model in GraphPad Prism (version 4.0b) to calculate Kd values using the following equation F = Fmax × [L]n/(Kdn + [L]n); where F is fluorescence intensity, L is ligand concentration and n is unconstrained.
ATP and GTP hydrolysis was measured by monitoring the amount of inorganic phosphate released at indicated times (23 °C) in reactions (25 μl) using malachite green reagent for phosphate detection. ATP hydrolysis assays were performed in reaction buffer with ATP (1 mM) and FtsA (1 μM). Where indicated, E. coli phospholipid vesicles were added (0.5 mg ml−1). E. coli total phospholipid extracts (Avanti Polar Lipids) were used to prepare large unilamellar vesicles (LUVs) as previously described (Camberg et al., 2007). LUVs were extruded 15 times through a 100-nm polycarbonate membrane at 65 °C to produce SUVs. Small aliquots were frozen at −80 °C until use. GTP hydrolysis assays were performed in buffer containing 50 mM MES (pH 6.5), 100 mM KCl and 10 mM MgCl2 with FtsZ (6 μM) and GTP (1 mM). In all ATPase and GTPase assays, unless otherwise stated, total phosphate content was determined at 0, 5 and 10 min using Biomol Green (Enzo Life Sciences) by comparison to a phosphate standard curve. Where indicated, an NADH enzyme-coupled assay was used to measure ATP hydrolysis by FtsA by monitoring absorbance at 340 nm (Graf et al., 2009).
Light scattering assays
Dynamic light scattering (DLS) measurements were made using Zetasizer Nano ZS with a detector angle of 173° and a 4 mW, 633 nm He–Ne laser (Malvern Instruments). To determine size distribution, FtsA (2 μM) alone or with ATP (4 mM), ADP (2 mM), ATPγS (2 mM) or TX-100 (2%), where indicated, were incubated for 30 min, added to a polystyrene cuvette and scanned at 23 °C. The intensity-weighted hydrodynamic diameter profiles are reported as the average of three replicates of 11 scans per replicate.
To monitor nucleotide-dependent phospholipid reorganization by FtsA, His-SUMO-FtsA and FtsA mutant proteins, 90° angle light scattering was performed. Reaction mixtures containing FtsA, His-SUMO-FtsA or FtsA mutant protein (2 μM) with copurified PLs, or detergent-extraced FtsA supplemented with SUVs (100 μg ml−1), where indicated, were monitored for light scatter with time after the addition of 4 mM ATP using an Agilent Eclipse fluorescence spectrophotometer with both excitation and emission wavelengths set to 450 nm with 5 nm slit widths. Baseline readings were collected for at least 3 minutes, ATP (4 mM), and where indicated EDTA (15 mM), ATPγS (2 mM) or ADP (2 mM) was added and light scattering was measured for up to 120 min.
Assembly and recruitment assays
To measure FtsZ polymer formation by ultracentrifugation, reaction mixtures (25 μl) with FtsZ or FtsZ(R379E) (4 μM), were prepared in buffer containing 50 mM MES (pH 6.5), 100 mM KCl, 10 mM MgCl2, 2 mM GTP and a nucleotide regenerating system containing acetate kinase (25 μg ml−1) and acetyl phosphate (15 mM). Where indicated, FtsA (0 to 4 μM) and ATP (4 mM) were added. Polymerization reactions were incubated for 5 min at 23 °C and then centrifuged at 129,000 ×g for 30 min. Pellets were resuspended in equal volume as supernatants and analyzed by SDS-PAGE and coomassie staining.
Phospholipid recruitment assays with FtsA and FtsZ were performed by incubating FtsZ or FtsZ(R379E) (6 μM) with GMPCPP (0.2 mM), GDP (1 mM) or GTP (2 mM), where indicated, in reaction buffer for 3 min, and then added to a reaction containing FtsA (2 μM) pre-assembled with SUVs (500 μg ml−1) and ADP (2 mM), ATP (4 mM) or ATPγS (2 mM), and then incubated for an additional 10 min at 30 °C. Phospholipid vesicles were collected by low speed centrifugation at 12,000 × g for 12 min. Supernatants and pellets were analyzed by SDS-PAGE and coomassie staining. Percent of protein in the pellet fraction was determined by densitometry using NIH ImageJ.
Trp-DPH FRET assay was performed by incorporation of 1,6-Diphenyl-1,3,5-hexatriene (DPH) (Sigma-Aldrich) into liposomes at 1:500 DPH to lipid (w/w) unless otherwise indicated as described (Masuda et al., 2006). The fluorescence of tryptophan was excited at 285 nm (5 nm slit widths) and emission spectra by FtsA wild type and mutant protein (0 to 3 μM), after incubation with DPH-liposomes (100 μg ml−1) for 6 minutes at 30 °C and, where indicated, ATP (0.1 mM). DPH emission was scanned from 400 to 500 nm.
Confocal Fluorescence Microscopy
Cultures of MG1655 containing plasmid pSEB293 (Pichoff & Lutkenhaus, 2005) encoding GFP-FtsA, or mutagenized to contain GFP-FtsA(K86A), were grown overnight on solid LB lennox media containing ampicillin (100 μg ml−1), and then restreaked onto plates containing arabinose (0.7 nM) and grown for 5 hours at 37°C. Cells were applied to a 4% agarose pad containing MOPS [3-(N-morpholino) propanesulfonic acid] minimal media with 0.5% glycerol and a coverslip was added. Samples were visualized with a Zeiss LSM 700 confocal fluorescence microscope with excitation at 488nm and 555nm. Where indicated, a Nomarski prism was used to acquire differential interference contrast (DIC) images. All images were captured on an AxioCam digital camera with ZEN 2012 software. Cell lengths were measured using NIH ImageJ.
To monitor assembly of protein and lipid complexes, FtsZ and FtsZ(R379E) were fluorescently labeled with Alexa Fluor 488 (FL-FtsZ) and cycles of polymerization and depolymerization were performed to collect active, labeled protein as described (Gonzalez et al., 2003, Camberg et al., 2014). FtsA samples containing copurifying phospholipid vesicles were labeled using FM 4-64EX at 250 μg ml−1. Reactions of, where indicated, FtsA-FM4-64EX (1 μM) with FtsA (3 μM) and FL-FtsZ or FL-FtsZ(R379E) (1 μM) with FtsZ or FtsZ(R379E) (3 μM) were mixed in the presence of 4 mM ATP, 2 mM GTP and a regenerating system, applied to a flow chamber. We used a 10 μL flow chamber (thickness 0.08 mm) as described (Bailey et al., 2015) and visualized reactions by confocal fluorescence microscopy. Lipid concentrations of FtsA fractions were determined by incubation of FtsA with FM 4-64 (1.25 μg ml−1). Fluorescence intensity was measured with an Agilent Eclipse fluorescence spectrophotometer using an excitation wavelength of 565 nm and an emission wavelength of 745 nm and 5 nm slit widths and compared to a phospholipid standard curve.
Electron Microscopy
Reactions containing assembly buffer mixtures of 4 μM FtsA, 4 mM ATP, 4 μM FtsZ, 0.2 mM GMPCPP and 0.5 mg ml−1 SUVs, where indicated, were incubated for 5 min at 23 °C, applied to a mesh carbon/formvar coated grid, fixed with glutaraldehyde (1%) and negative stained with uranyl acetate (2%). For whole cell thin sections, bacterial cell pellets were fixed with 2.5% glutaraldehyde in 0.15 M sodium cacodylate buffer (pH 7.4) at 4 °C for several days. Samples were rinsed in 0.15 M sodium cacodylate buffer and post-fixed in 1% osmium tetroxide for 1 hour at 4°C. Following distilled water rinses, samples were stained en bloc with 2% aqueous uranyl acetate. Pellets were encased in 2% agar, and dehydrated through a graded acetone series and infiltrated and embedded in Spurr’s epoxy resin (Ladd Research Industries, Burlington, VT). Semi-thin sections (1μm) were prepared using a Reichert-Jung Ultracut E ultramicrotome (Leica Biosystems, Buffalo Grove, IL), and stained with methylene blue and azure II. Ultra-thin sections were prepared and retrieved onto 300-mesh thin bar copper grids, and contrasted with uranyl acetate and lead citrate. Sections were examined using a CM-10 electron microscope (FEI, Hillsboro, OR). Images were collected with a model 785 Erlangshen ES1000W CCD camera (Gatan, Pleasanton, CA).
Supplementary Material
Acknowledgments
We thank Chris LaBreck, Shannon May, Eric DiBiasio and Cathy Trebino for helpful discussions and critical reading of the manuscript, Carol Ayala at the Rhode Island Hospital for expert assistance with EM, Joe Lutkenhaus for pSEB293 and Martin Loose for pML29. We also thank Cal Vary at Maine Medical Center Research Institute for lipid profiling and identification and Marc Llaguno and Xinran Liu at the Center for Cellular and Molecular Imaging at Yale School of Medicine. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM118927 to J. Camberg. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This material is based upon work conducted at a Rhode Island NSF EPSCoR research facility, the Genomics and Sequencing Center, supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057.
Footnotes
Author contributions
J.C. and M.G.V. designed and performed experiments, interpreted data and wrote manuscript. J.L.C. designed experiments, interpreted data and wrote manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Disteche M, den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol. 2005;55:1631–1645. doi: 10.1111/j.1365-2958.2005.04502.x. [DOI] [PubMed] [Google Scholar]
- Alexeeva S, Gadella TW, Jr, Verheul J, Verhoeven GS, den Blaauwen T. Direct interactions of early and late assembling division proteins in Escherichia coli cells resolved by FRET. Mol Microbiol. 2010;77:384–398. doi: 10.1111/j.1365-2958.2010.07211.x. [DOI] [PubMed] [Google Scholar]
- Bailey ME, Sackett DL, Ross JL. Katanin Severing and Binding Microtubules Are Inhibited by Tubulin Carboxy Tails. Biophys J. 2015;109:2546–2561. doi: 10.1016/j.bpj.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard CS, Sadasivam M, Shiomi D, Margolin W. An altered FtsA can compensate for the loss of essential cell division protein FtsN in Escherichia coli. Mol Microbiol. 2007;64:1289–1305. doi: 10.1111/j.1365-2958.2007.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson-Filho AW, Hsu YP, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, Brun YV, Garner EC. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci USA. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busiek KK, Eraso JM, Wang Y, Margolin W. The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. J Bacteriol. 2012;194:1989–2000. doi: 10.1128/JB.06683-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberg JL, Hoskins JR, Wickner S. ClpXP protease degrades the cytoskeletal protein, FtsZ, and modulates FtsZ polymer dynamics. Proc Natl Acad Sci USA. 2009;106:10614–10619. doi: 10.1073/pnas.0904886106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberg JL, Johnson TL, Patrick M, Abendroth J, Hol WG, Sandkvist M. Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J. 2007;26:19–27. doi: 10.1038/sj.emboj.7601481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camberg JL, Viola MG, Rea L, Hoskins JR, Wickner S. Location of dual sites in E. coli FtsZ important for degradation by ClpXP; one at the C-terminus and one in the disordered linker. PloS one. 2014;9:e94964. doi: 10.1371/journal.pone.0094964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Geissler B, Sadasivam M, Margolin W. Z-ring-independent interaction between a subdomain of FtsA and late septation proteins as revealed by a polar recruitment assay. J Bacteriol. 2004;186:7736–7744. doi: 10.1128/JB.186.22.7736-7744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Wang Y, Beuria TK, Margolin W. Interaction between cell division proteins FtsE and FtsZ. J Bacteriol. 2007;189:3026–3035. doi: 10.1128/JB.01581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci USA. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai K, Xu Y, Lutkenhaus J. Topological characterization of the essential Escherichia coli cell division protein FtsN. J Bacteriol. 1996;178:1328–1334. doi: 10.1128/jb.178.5.1328-1334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Pichoff S, Lutkenhaus J. FtsEX acts on FtsA to regulate divisome assembly and activity. Proc Natl Acad Sci USA. 2016;113:E5052–5061. doi: 10.1073/pnas.1606656113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson HM, Wessman P, Ge C, Edwards K, Wieslander A. Massive formation of intracellular membrane vesicles in Escherichia coli by a monotopic membrane-bound lipid glycosyltransferase. J Biol Chem. 2009;284:33904–33914. doi: 10.1074/jbc.M109.021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Bacterial morphogenesis and the enigmatic MreB helix. Nat Rev Microbiol. 2015;13:241–248. doi: 10.1038/nrmicro3398. [DOI] [PubMed] [Google Scholar]
- Feucht A, Lucet I, Yudkin MD, Errington J. Cytological and biochemical characterization of the FtsA cell division protein of Bacillus subtilis. Mol Microbiol. 2001;40:115–125. doi: 10.1046/j.1365-2958.2001.02356.x. [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Fujita J, Maeda Y, Nagao C, Tsuchiya Y, Miyazaki Y, Hirose M, Mizohata E, Matsumoto Y, Inoue T, Mizuguchi K, Matsumura H. Crystal structure of FtsA from Staphylococcus aureus. FEBS Lett. 2014;588:1879–1885. doi: 10.1016/j.febslet.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Gayda RC, Henk MC, Leong D. C-shaped cells caused by expression of an ftsA mutation in Escherichia coli. J Bacteriol. 1992;174:5362–5370. doi: 10.1128/jb.174.16.5362-5370.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Elraheb D, Margolin W. A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc Natl Acad Sci USA. 2003;100:4197–4202. doi: 10.1073/pnas.0635003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler B, Shiomi D, Margolin W. The ftsA* gain-of-function allele of Escherichia coli and its effects on the stability and dynamics of the Z ring. Microbiology. 2007;153:814–825. doi: 10.1099/mic.0.2006/001834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring NW, Beckwith J. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol. 2005;15:R514–526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Jimenez M, Velez M, Mingorance J, Andreu JM, Vicente M, Rivas G. Essential cell division protein FtsZ assembles into one monomer-thick ribbons under conditions resembling the crowded intracellular environment. J Biol Chem. 2003;278:37664–37671. doi: 10.1074/jbc.M305230200. [DOI] [PubMed] [Google Scholar]
- Graf C, Stankiewicz M, Kramer G, Mayer MP. Spatially and kinetically resolved changes in the conformational dynamics of the Hsp90 chaperone machine. EMBO J. 2009;28:602–613. doi: 10.1038/emboj.2008.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusser DP, Margolin W. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol. 2016;14:305–319. doi: 10.1038/nrmicro.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- Hale CA, de Boer PA. Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J Bacteriol. 1999;181:167–176. doi: 10.1128/jb.181.1.167-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herricks JR, Nguyen D, Margolin W. A thermosensitive defect in the ATP binding pocket of FtsA can be suppressed by allosteric changes in the dimer interface. Mol Microbiol. 2014;94:713–727. doi: 10.1111/mmi.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci USA. 2002;99:6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka M, Cabre EJ, Jimenez M, Rivas G, Rico AI, Vicente M. Role of the FtsA C terminus as a switch for polymerization and membrane association. mBio. 2014;5:e02221. doi: 10.1128/mBio.02221-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka M, Rowlett VW, Morado D, Vitrac H, Schoenemann K, Liu J, Margolin W. Escherichia coli FtsA forms lipid-bound minirings that antagonize lateral interactions between FtsZ protofilaments. Nature communications. 2017;8:15957. doi: 10.1038/ncomms15957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashov DS, Reisler E. ATP and ADP actin states. Biopolymers. 2013;99:245–256. doi: 10.1002/bip.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara B, Rico AI, Petruzzelli S, Santona A, Dumas J, Biton J, Vicente M, Mingorance J, Massidda O. Cell division in cocci: localization and properties of the Streptococcus pneumoniae FtsA protein. Mol Microbiol. 2005;55:699–711. doi: 10.1111/j.1365-2958.2004.04432.x. [DOI] [PubMed] [Google Scholar]
- Llinas P, Isabet T, Song L, Ropars V, Zong B, Benisty H, Sirigu S, Morris C, Kikuti C, Safer D, Sweeney HL, Houdusse A. How actin initiates the motor activity of Myosin. Developmental Cell. 2015;33:401–412. doi: 10.1016/j.devcel.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol. 2014;16:38–46. doi: 10.1038/ncb2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton. 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martos A, Monterroso B, Zorrilla S, Reija B, Alfonso C, Mingorance J, Rivas G, Jimenez M. Isolation, characterization and lipid-binding properties of the recalcitrant FtsA division protein from Escherichia coli. PloS one. 2012;7:e39829. doi: 10.1371/journal.pone.0039829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Takeda S, Sone M, Ohki T, Mori H, Kamioka Y, Mochizuki N. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J. 2006;25:2889–2897. doi: 10.1038/sj.emboj.7601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger LET, Raetz CR. Purification and characterization of the lipid A disaccharide synthase (LpxB) from Escherichia coli, a peripheral membrane protein. Biochemistry. 2009;48:11559–11571. doi: 10.1021/bi901750f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi K, Misra HS. Dr-FtsA, an actin homologue in Deinococcus radiodurans differentially affects Dr-FtsZ and Ec-FtsZ functions in vitro. PloS one. 2014;9:e115918. doi: 10.1371/journal.pone.0115918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Cao C, Lutkenhaus J. Inhibition of FtsZ polymerization by SulA, an inhibitor of septation in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2885–2890. doi: 10.1073/pnas.95.6.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Yasunaga T, Noguchi TQ, Gomibuchi Y, Ngo KX, Uyeda TQ, Wakabayashi T. Structural basis for actin assembly, activation of ATP hydrolysis, and delayed phosphate release. Cell. 2010;143:275–287. doi: 10.1016/j.cell.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Liposome division by a simple bacterial division machinery. Proc Natl Acad Sci USA. 2013;110:11000–11004. doi: 10.1073/pnas.1222254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis-Bleau C, Sanschagrin F, Levesque RC. Peptide inhibitors of the essential cell division protein FtsA. Prot Engin Des Sel. 2005;18:85–91. doi: 10.1093/protein/gzi008. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Chang F. Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature. 2002;419:82–86. doi: 10.1038/nature00999. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 2002;21:685–693. doi: 10.1093/emboj/21.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol. 2005;55:1722–1734. doi: 10.1111/j.1365-2958.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Identification of a region of FtsA required for interaction with FtsZ. Mol Microbiol. 2007;64:1129–1138. doi: 10.1111/j.1365-2958.2007.05735.x. [DOI] [PubMed] [Google Scholar]
- Pichoff S, Shen B, Sullivan B, Lutkenhaus J. FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Mol Microbiol. 2012;83:151–167. doi: 10.1111/j.1365-2958.2011.07923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Pogliano K, Weiss DS, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Wu JQ. Understanding cytokinesis: lessons from fission yeast. Nat Rev Mol Cell Biol. 2010;11:149–155. doi: 10.1038/nrm2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. Role of FtsEX in cell division of Escherichia coli: viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. J Bacteriol. 2007;189:98–108. doi: 10.1128/JB.01347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AI, Garcia-Ovalle M, Palacios P, Casanova M, Vicente M. Role of Escherichia coli FtsN protein in the assembly and stability of the cell division ring. Mol Microbiol. 2010;76:760–771. doi: 10.1111/j.1365-2958.2010.07134.x. [DOI] [PubMed] [Google Scholar]
- Rould MA, Wan Q, Joel PB, Lowey S, Trybus KM. Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J Biol Chem. 2006;281:31909–31919. doi: 10.1074/jbc.M601973200. [DOI] [PubMed] [Google Scholar]
- Shiomi D, Margolin W. Compensation for the loss of the conserved membrane targeting sequence of FtsA provides new insights into its function. Mol Microbiol. 2008;67:558–569. doi: 10.1111/j.1365-2958.2007.06085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead WT, Hayden CC, Gadok AK, Zhao C, Lafer EM, Rangamani P, Stachowiak JC. Membrane fission by protein crowding. Proc Natl Acad Sci USA. 2017;114:E3258–E3267. doi: 10.1073/pnas.1616199114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom B, Mirzadeh K, Toddo S, von Heijne G, Skoglund U, Daley DO. Coordinated disassembly of the divisome complex in Escherichia coli. Mol Microbiol. 2016;101:425–438. doi: 10.1111/mmi.13400. [DOI] [PubMed] [Google Scholar]
- Szwedziak P, Wang Q, Bharat TA, Tsim M, Lowe J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. eLife. 2014;3:e04601. doi: 10.7554/eLife.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwedziak P, Wang Q, Freund SM, Lowe J. FtsA forms actin-like protofilaments. EMBO J. 2012;31:2249–2260. doi: 10.1038/emboj.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Izore T, Bharat TA, Johnson CM, Lowe J. Bacterial actin MreB forms antiparallel double filaments. eLife. 2014;3:e02634. doi: 10.7554/eLife.02634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Lowe J. Crystal structure of the cell division protein FtsA from Thermotoga maritima. EMBO J. 2000;19:5300–5307. doi: 10.1093/emboj/19.20.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola MG, LaBreck CJ, Conti J, Camberg JL. Proteolysis-Dependent Remodeling of the Tubulin Homolog FtsZ at the Division Septum in Escherichia coli. PloS one. 2017;12:e0170505. doi: 10.1371/journal.pone.0170505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang J, Mukherjee A, Cao C, Lutkenhaus J. Analysis of the interaction of FtsZ with itself, GTP, and FtsA. J Bacteriol. 1997;179:5551–5559. doi: 10.1128/jb.179.17.5551-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lyu Z, Miguel A, McQuillen R, Huang KC, Xiao J. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science. 2017;355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim L, Vandenbussche G, Mingorance J, Rueda S, Casanova M, Ruysschaert JM, Vicente M. Role of the carboxy terminus of Escherichia coli FtsA in self-interaction and cell division. J Bacteriol. 2000;182:6366–6373. doi: 10.1128/jb.182.22.6366-6373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


