Abstract
Cognitive neuroscience models suggest both reward valuation and cognitive control contribute to reward-based decision-making. The current study examined the relationship between cognitive control and delay discounting (i.e., choosing smaller, immediate over larger, delayed rewards) in a large sample of boys and girls diagnosed with attention-deficit/hyperactivity disorder (ADHD; N = 95) and typically developing control children (TD; N = 59). Specifically, we examined performance on multiple measures of cognitive control (i.e., Go/No-Go task, Stop Signal task, and Spatial Span task) and delay discounting (i.e., Classic Delay Discounting and Real-Time Delay Discounting tasks), as well as the relationship between these measures. Results indicated that sex moderated the effects of group on task performance. Specifically, girls with ADHD, but not boys with the disorder, exhibited atypical delay discounting of real-time rewards. Results from correlational analyses indicated that delay discounting and cognitive control were not significantly correlated in the overall sample. Multiple regression analyses demonstrated that among girls with ADHD poorer spatial working memory and inhibitory control predicted greater real-time discounting. Collectively, findings provide support for distinct patterns of cognitive control and delay discounting among school-aged girls and boys with ADHD. Additionally, findings suggest that among girls with ADHD, those who exhibit relatively poor working memory and inhibitory control might be a particularly vulnerable subgroup with the greatest propensity to exhibit maladaptive decision-making.
Keywords: ADHD, decision-making, delay discounting, cognitive control, executive function
Attention-deficit/hyperactivity disorder (ADHD) is a chronic neurodevelopmental condition characterized by impairing inattention and/or hyperactivity/impulsivity (American Psychiatric Association, 2013) affecting approximately 5% of children worldwide (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007). In particular, the impulsive response style observed in many individuals with ADHD (particularly hyper-active/impulsive and combined presentations) is associated with increased risk for conduct problems (Grizenko, Paci, & Joober, 2010), substance abuse (Verdejo-Garcia, Lawrence, & Clark, 2008), and incarceration (Retz et al., 2004). Therefore, the explicit examination of ADHD-related impulsivity is crucial given its association with particularly maladaptive behaviors and subsequent pejorative outcomes.
Impulsivity is a multi-faceted construct (Whiteside, Lynam, Miller, & Reynolds, 2005) that is operationalized and assessed in a variety of ways. Factor-analytic research has identified several forms of impulsivity, including rapid-response impulsivity/impulsive disinhibition (i.e., the inability to withhold a response or prevent an ongoing response) and choice-impulsivity/delay aversion (i.e., a preference for smaller, immediate rewards over larger, delayed rewards) (Reynolds, Ortengren, Richards, & de Wit, 2006). The current study examines the relationship between choice-impulsivity (in the form of reward-based decision-making) and rapid-response impulsivity (as one of several measures of cognitive control), both of which are emphasized in ADHD theory and research (Luman, Tripp, & Scheres, 2010; Sagvolden, Johansen, Aase, & Russell, 2005; Sonuga-Barke, 2002).
Within the ADHD literature, choice-impulsivity has been measured using a variety of reward-based decision-making paradigms including choice-delay tasks and delay discounting tasks, both of which involve choices between smaller-sooner and larger-later rewards (see meta-analysis by Patros et al., 2016). It is noted that the primary discrepancies between choice-delay and delay discounting tasks are the dependent variables obtained (i.e., cumulative reward vs. indifference point, respectively) and the inclusion of fixed versus variable pre-reinforcement delays. Nevertheless, across these tasks, children with ADHD generally exhibit a stronger preference for immediate reward (Patros et al., 2016). However, the majority of studies examining choice-impulsivity in children with ADHD have used choice-delay tasks (Patros et al., 2016) limiting our understanding of how children with ADHD perform on delay discounting tasks which tend to be more commonly used in the broader cognitive neuroscience literature and in studies of adult clinical populations (see reviews by Hamilton et al., 2015; Peters & Buchel, 2011). Furthermore, recent findings from a study involving two delay discounting tasks demonstrated that reward-based decision-making in children with ADHD may depend on characteristics of the task (e.g., type of reward, task duration) and/or the participant (e.g., sex; Rosch & Mostofsky, 2016), emphasizing the need for further research in this area.
While many prominent theoretical models of ADHD propose that underlying motivational and/or cognitive deficits lead to impulsive behavior (Barkley, 1997; Nigg & Casey, 2005; Sagvolden et al., 2005; Sonuga-Barke, Bitsakou, & Thompson, 2010; Tripp & Wickens, 2008), these models differ in whether cognitive dysfunction is the “core deficit” (Barkley, 1997) or whether cognitive and motivational deficits represent independent processes implicated in ADHD (Sonuga-Barke, 2003). Often in the ADHD literature, a heightened preference for immediate reward assessed during reward-based decision-making is considered to be a motivational deficit. In contrast, cognitive neuroscience models propose that reward-based decision-making is governed by cognitive control (i.e., cognitive processes required for goal-oriented behavior) and reward valuation processes. This perspective is supported by neuroimaging research demonstrating that reward-based decision-making involves a cognitive control neural network (e.g., lateral prefrontal cortex and parietal cortex) and a reward valuation neural network (e.g., ventromedial prefrontal cortex, orbitofrontal cortex, and striatum; Peters & Buchel, 2011). Therefore, deficient cognitive control or atypical reward processing, both of which are implicated in ADHD (Castellanos & Tannock, 2002; Nigg & Casey, 2005; Sagvolden et al., 2005; Sonuga-Barke, 2002; Tripp & Wickens, 2008), may contribute to a stronger preference for immediate reward among individuals with ADHD. In particular, impairments in cognitive control may lead to behavioral disinhibition, poor attention regulation, and deficient working memory processes (e.g., storage/rehearsal) all of which are subsumed under the concept of executive functions, which are generally impaired in children with ADHD (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Several theories of ADHD have also emphasized atypical reinforcement sensitivity as an explanatory factor of reward-based decision-making (see review by Luman et al., 2010). Given the multifactorial etiology of ADHD likely involving motivational and cognitive deficits, both of which may contribute to greater delay discounting often observed in ADHD. However, we do not yet fully understand how ADHD-related reward-based decision-making is related to cognitive deficits implicated in this disorder. The current study aims to test predictions from cognitive neuroscience models by examining the relationship between cognitive control and delay discounting in children with ADHD.
While the relationship between reward-based decision-making and cognitive control has been examined within the context of ADHD (Karalunas & Huang-Pollock, 2011; Patros et al., 2015; Solanto et al., 2007; Sonuga-Barke et al., 2010; Wahlstedt, Thorell, & Bohlin, 2009), findings across studies have been equivocal and studies have predominantly utilized choice-delay tasks rather than delay discounting tasks. Given potentially important differences between delay-related decision-making paradigms (Rosch & Mostofsky, 2016; Scheres, Lee, & Sumiya, 2008), findings from choice-delay task studies may not generalize to delay discounting tasks. Furthermore, previous studies have typically examined a single metric of cognitive control despite evidence of heterogeneity of cognitive deficits in ADHD (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Willcutt et al., 2005) thereby limiting our understanding of how various cognitive deficits relate to reward-based decision-making in ADHD.
Beyond limitations related to methodological design, examination of underlying gender differences is often neglected due to difficulty obtaining adequate samples of girls with ADHD. Evidence of ADHD-related sex differences across behavioral and neural domains is mounting, while the proportion of males to females diagnosed with the disorder has fallen to approximately 2:1 (Ramtekkar, Reiersen, Todorov, & Todd, 2010). In recent years, studies have generally shown evidence of greater motor deficits among boys with ADHD both in terms of behavior (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008; Hasson & Fine, 2012; Seymour, Mostofsky, & Rosch, 2016) and the associated neural circuitry (Dirlikov et al., 2015; Jacobson et al., 2015; Mahone et al., 2011; Qiu et al., 2009; Seymour et al., 2017). In contrast, girls with ADHD tend to display equivalent or greater executive dysfunction both in terms of behavior (Rucklidge, 2010; Seymour et al., 2016) and the associated neural circuitry (Dirlikov et al., 2015; Jacobson et al., 2015). Moreover, recent evidence suggests that girls with ADHD show greater delay discounting relative to typically developing (TD) girls and to boys with ADHD (Rosch & Mostofsky, 2016). Collectively, while previous studies have demonstrated important relationships between variables leading to impulsive behavior, several limitations warrant hesitation with regard to generalizability of such findings.
The current study is the first to explicitly examine the relationship between cognitive control and delay discounting across a sample of girls and boys diagnosed with ADHD using multiple delay discounting tasks and measures of cognitive control (e.g., response inhibition, response variability). Consequently, this study provides a unique contribution to the literature with regard to examining an understudied and therefore less understood question regarding the neuropsychological processes associated with variability in delay discounting task performance among girls and boys with ADHD. Furthermore, this study aimed to replicate gender-difference findings from previous work (Rosch & Mostofsky, 2016), which is essential given the relative dearth of literature explicating gender differences in reward-based decision-making in children with ADHD. It was hypothesized that (a) children with ADHD, relative to TD children, would exhibit greater delay discounting and weaker cognitive control as assessed using spatial span backwards, attention regulation, and inhibitory control tasks, (b) poor cognitive control would be related to greater delay discounting, and (c) the pattern of neuropsychological deficits and associations with delay discounting may differ for girls and boys with ADHD.
Method
Participants
A total of 154 8–12-year-old children participated in this study: 95 children with ADHD (29 girls) and 59 TD children (20 girls). Participants were primarily recruited through local schools, with additional resources including community-wide advertisement, volunteer organizations, medical institutions, and word of mouth. This study was approved by the Johns Hopkins Institutional Review Board, and all data was obtained in compliance with their regulations. After providing a complete description of the study to the participants, oral informed consent was obtained from a parent/guardian prior to the initial phone screening and written informed consent and assent was obtained from the parent/guardian and the child upon arrival at the initial visit to the laboratory.
An initial screening was conducted through a telephone interview with a parent. Children with a history of intellectual disability, seizures, traumatic brain injury, or other neurological illnesses were excluded from participation. Eligible participants and their parents attended one to three laboratory sessions lasting from 8:30 am to 3:30 pm. The number of days of participation and daily schedule varied depending on the studies in which children were enrolled. The delay discounting and cognitive control tasks described below were administered as part of a broader battery of neuropsychological tests and experimental paradigms in addition to structural and functional magnetic resonance imaging. The cognitive control tasks were typically administered in the morning of the first testing day whereas the delay discounting tasks were typically administered in the afternoon of the second testing day, although the task order could change across participants depending on other scheduling constraints. Children taking psychotropic medications other than stimulants were excluded from participation and all children taking stimulants were asked to withhold medication the days prior to and of testing. Intellectual ability was assessed during the first visit using the Wechsler Intelligence Scale for Children, Fourth Edition (n = 112, WISC-IV; Wechsler, 2003) or Fifth Edition (n = 42, Wechsler, 2014) and participants with full-scale IQ (FSIQ) scores below 80 were excluded. In addition to inquiring about a history of a learning disability, children were also administered the Word Reading subtest from the Wechsler Individual Achievement Test, Second Edition (WIAT-II; Wechsler, 2002) to further screen for a reading disorder and were excluded if their Word Reading scores fell below a standard score of 85.
Diagnostic status was established through administration of either the Diagnostic Interview for Children and Adolescents, Fourth Edition (n = 97, DICA-IV; Reich, Welner, & Herjanic, 1997) or the Kiddie Schedule for Affective Disorders and Schizophrenia for School Aged Children Present Lifetime version (n = 57, KSADS-PL; Kaufman et al., 2013). Both semi-structured clinical interviews allow for the broad assessment/screening of diagnostic rule-outs. Children meeting criteria for diagnosis of conduct, mood, generalized anxiety, separation anxiety, or obsessive–compulsive disorders on either interview were excluded. A comorbid diagnosis of oppositional defiant disorder (ODD) was permitted given the high base rate comorbidity between ADHD and ODD. Parents (n = 93) and teachers (when available; n = 47) also completed the Conners Parent and Teacher Rating Scales-Revised Long Version or the Conners-3 (CPRS and CTRS; Conners, 2002, 2008) and/ or the ADHD Rating Scale-IV, home and school versions (ADHD-RS; DuPaul, Power, Anastopoulos, & Reid, 1998).
ADHD diagnosis was established based on the following criteria: (1) T-score of 60 or higher on the ADHD Inattentive or Hyperactive/Impulsive scales on the CPRS or CTRS, when available, or a score of 2 or 3 on at least 6/9 items on the Inattentive or Hyperactivity/ Impulsivity scales of the ADHD-RS and (2) an ADHD diagnosis on the DICA-IV or KSADS-PL, which considered information provided by the parent about functioning at home and at school, in addition to onset, course, duration, and frequency of symptoms. It is noted that CTRS data was available for 43 children in the ADHD group. In the absence of teacher ratings, a child would only meet diagnostic criteria for ADHD if the primary caregiver reported ADHD symptoms and associated impairment at home and at school or in other settings during the diagnostic interview. This information was then reviewed and the diagnosis was confirmed by a child neurologist or psychologist.
Inclusion in the TD group required scores below clinical cutoffs on the parent and teacher rating scales (CPRS, CTRS, and/or ADHD-RS). It is noted that CTRS scores were available for 20 control participants. Control participants did not meet diagnostic criteria for any psychiatric disorder based on DICA-IV or KSADS-PL nor could they have history of neurological disorder or be taking psychotropic medication. TD participants were also required to have WIAT-II Word Reading standard scores of 85 or above and WISC FSIQ scores of 80 or above. Children included in the TD group also could not have an immediate family member diagnosed with ADHD.
Procedures
Parent-report measures
Conners Parent Rating Scale (CPRS)
Parents completed the Conners Parent Rating Scale-Revised Long Version (n = 22) or the Conners-3 (n = 128) (Conners, 2002, 2008). The Conners-3 Parent (CPRS) Rating Scale is a narrow band measure that specifically assesses ADHD symptomatology including inattention and hyperactivity/impulsivity, and concurrently gathers information pertaining to present comorbid behavioral/emotional disturbances (e.g., ODD, conduct disorder; Conners, Sitarenios, Parker, & Epstein, 1998). Both versions of the CPRS have demonstrated strong psychometric properties indicated by good internal consistency and test–retest reliability in addition to strong criterion validity (Conners, 2008). The CPRS was missing for four participants (two TD and two ADHD).
Delay discounting measures
Classic Delay Discounting task
Participants completed a computer-based delay discounting task involving 91 choices between a varying amount of money now ($0–$10.50 in $0.50 increments) or $10.00 after a varying delay (1, 7, 30, or 90 days) (Rosch & Mostofsky, 2016; Wilson, Mitchell, Musser, Schmitt, & Nigg, 2011). Participants were instructed to indicate whether they preferred the immediate or delayed option using a computer mouse. They were also told that some of the choices were real, and they would actually receive the amount of money at the specified delay that they chose for some of the items in the form of gift cards or prizes (two choices semi-randomly selected). This was intended to encourage children to carefully consider their choices rather than making all of the choices purely hypothetical in an attempt to improve the validity of their decision-making. This task took 10–15 min to complete. As in prior studies (Rosch & Mostofsky, 2016; Wilson et al., 2011), an indifference point (i.e., the point at which the subjective value of the immediate reward is equivalent to the delayed reward) was identified for each delay in order to calculate area under the curve (AUC; Myerson, Green, & Warusawitharana, 2001) in Excel (Reed, Kaplan, & Brewer, 2012). Smaller AUC values indicate greater delay discounting thought to reflect greater impulsivity.
Real-time Delay Discounting task
The Real-Time Delay Discounting task involved immediately consumable rewards and variable reward and delay amounts (Rosch & Mostofsky, 2016). This task has been used previously to differentiate children with ADHD from their TD peers (Rosch & Mostofsky, 2016) and demonstrating the validity of this task as a measure of delay discounting in that the subjective value of the delayed reward decreased as the delay increased among children with and without ADHD. During this task, participants made nine choices between playing a preferred game for a shorter amount of time (either 15, 30, or 45 s) immediately or for a fixed longer amount of time (60 s) after waiting (either 25, 50, or 100 s). After making a choice, participants experienced the delays and rewards associated with that choice in real time prior to making their next choice. Participants could bring their own game and were offered several game options (handheld video game, tablet games, coloring, Legos, etc.) to maximize the rewarding value for each individual. Their preferred game was placed in a clear box in front of them when they made their choices and while waiting to play. This task involved two practice choices, during which participants experienced both the immediate and delayed options, followed by nine test choices and took ~40 min to complete. The immediate reward values were presented in ascending order within each delay and the order of the delays was fully counterbalanced across subjects. The indifference point, defined as the lowest immediate value selected for each delay, was used to calculate the AUC as described above.
Cognitive control measures
Go/No-Go (GNG) task
Participants completed a computer-based GNG task (e.g., Shiels Rosch, Dirlikov, & Mostofsky, 2013). Task stimuli consisted of green spaceships for go trials and red spaceships for no-go trials (20% of trials) presented for 300 ms with an interstimulus interval of 2000 ms. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships only. There were 11 practice trials followed by 217 experimental trials presented in a pseudorandom order. Reaction times (RTs) were recorded during the entire trial length (2300 ms). Trials with responses faster than 200 ms were excluded from all analyses. Participants were excluded if the proportion of go trials with RTs <200 ms exceeded .30 (n = 1), if the omission error rate exceeded .50 (n = 0), or if the ex-Gaussian goodness-of-fit value (generated using the “eglike” function in the DISTRIB toolbox referenced below) was poor (i.e., values >2000, n = 0) on either task (final n = 154). The goodness-of-fit value was examined to determine how well each participant’s RT data fit the ex-Gaussian model and to exclude data that does not fit an ex-Gaussian distribution. Measures of cognitive control obtained during this task include: (1) the proportion of commission errors for no-go stimuli reflect-ing inhibitory control and (2) RT variability based on tau, an ex-Gaussian estimate of speed and variability of the exponential component of the RT distribution (Castellanos, Sonuga-Barke, Milham, & Tannock, 2006). Ex-Gaussian indicators were computed in MATLAB version 7.1 (The Mathworks, Inc., Natick, MA) using the DISTRIB toolbox (Lacouture & Cousineau, 2008).
Spatial Span task
Visual–spatial storage/rehearsal was assessed via a computerized adaptation (Shiels et al., 2008) of the spatial span subtest from the WISC-IV or WISC-V Integrated that incorporated features of the Spatial Span task from the CANTAB (Luciana, 2003). In this task, an array of 10 white squares on a black background is presented on the computer screen. On each trial, a yellow smiley face appears in two to eight of the squares at a rate of one square per second. For forward span, assessing short-term storage or maintenance of visual–spatial information, children were instructed to use a computer mouse to click on the squares in the same order in which the smiley face appeared. For backward span, participants were asked to click on the squares in the reverse order in which the smiley face appeared measuring the manipulation of visual–spatial information, requiring participants to update and reorder the stimuli. The task terminated when both trials within a difficulty level were incorrect beginning with two-location sequences and advancing to a maximum of eight-location sequences. The measure of cognitive control obtained from this task is the total number of trials completed correctly for the backward span condition with a higher score suggesting better visual–spatial storage/rehearsal (e.g., O’Brien, Dowell, Mostofsky, Denckla, & Mahone, 2010).
Stop Signal task
The Stop Signal task measured children’s ability to inhibit responding once a prepotent go response had been established. Task stimuli consisted of arrows pointing left or right, and the child was instructed to press the left button for left-facing arrows and the right button for right-facing arrows (Rosch et al., 2016). A 32-trial go practice was followed by a 32-trial stop practice, for which children were instructed to inhibit responding whenever the go stimulus presentation was followed by the auditory stop signal (25% of trials). The stop signal was a 1000 Hz tone presented for 100 ms. On the first stop trial, the tone onset was 250 ms after the onset of the go stimulus and adjusted dynamically. If a participant correctly inhibited, the latency between stimulus presentation and stop signal increased by 50 ms (i.e., became more difficult). If the participant failed to inhibit, the latency decreased by 50ms (i.e., became easier). After the practice trials, four test blocks of 64 trials each were administered (256 test trials total). The measure of cognitive control obtained from this task is the stop signal reaction time (SSRT) calculated as the mean go RT – mean stop signal delay (SSD), such that higher SSRTs indicate worse inhibitory control. Consistent with a previous meta-analysis examining Stop Signal task metrics in children with ADHD, SSRT was planned to be selected as the primary metric of behavioral inhibition if statistically significant between-group differences in SSD were present (Alderson, Rapport, & Koffler, 2007). Alternatively, SSD was planned to be selected as the secondary metric of behavioral inhibition if no statistically significant between-group differences in SSD were present (Alderson et al., 2007). We recognize that both metrics may be of interest and therefore present SSRT as the primary metric (in light of group differences in SSD reported below) as well as SSD (in footnotes).
Data analysis
Data analysis was accomplished using SPSS Statistics Version 24 (IBM, Chicago). In order to illustrate the pattern of cognitive and motivational deficits in the current sample relative to the existing ADHD literature, we initially tested for diagnostic group differences on the battery of delay discounting and cognitive control tasks. Data were screened for outliers defined as values exceeding three standard deviations above or below the group mean. Participants included in these analyses did not have outlying data points for any of the task measures. Importantly, sex was included as a factor in the model given accumulating evidence of sex differences in neuropsychological deficits among children with ADHD. These analyses were conducted using multivariate analysis of variance (MANOVA) with the between-subjects factors of diagnosis (ADHD vs. TD) and sex (girls vs. boys) and the six task metrics as the dependent variables (i.e., classic discounting AUC, real-time discounting AUC, GNG commission error rate, RT variability, spatial span backward, and SSRT). Pairwise comparisons were examined to follow up statistically significant Diagnosis × Sex interactions.
Next, we examined the relationship between performance on the delay discounting and cognitive control measures to determine whether these neuropsychological deficits are distinct or overlapping. The cognitive control measures include RT variability, spatial span backward performance, and inhibitory control (GNG commission error rate and SSRT). Correlations were examined in the full sample and among girls with ADHD only to elucidate the relationship between delay discounting and cognitive control deficits given previous research suggesting maladaptive delay discounting may be specific to girls and task-dependent (Rosch & Mostofsky, 2016). Partial correlations with age as a covariate were also conducted and any change in results is reported. For all correlation analyses, a false discovery rate (FDR) of .95 was applied to correct for multiple comparisons (Benjamini & Hochberg, 1995). Finally, a linear regression was conducted to determine whether diagnostic group differences in delay discounting were accounted for by cognitive control performance.
Results
Sample characteristics
Demographic information for the sample is provided in Table 1, along with inferential statistics regarding diagnostic group differences and sex differences within the ADHD sample. Diagnostic groups did not differ in several important demographics including age, sex, race, and socioeconomic status (SES). The ADHD group had lower FSIQ, as is often seen in the childhood ADHD literature (Frazier, Demaree, & Youngstrom, 2004). Girls and boys with ADHD also did not significantly differ in age, race, or SES, nor did they differ in ADHD subtype, or frequency of comorbid ODD. However, fewer girls with ADHD were prescribed stimulant medication (p = .034) and girls with ADHD had higher T-scores on the CPRS Inattention Scale (p = .036), although they did not differ in ADHD-RS raw scores for the Inattention (p = .439) and Hyperactive/Impulsive (p = .344) scales. To determine whether heightened inattention symptom severity among girls with ADHD contributed to the findings, effects involving Diagnosis × Sex interactions were also conducted with a subset of boys with ADHD matched on inattention symptom severity (p = .844) and the pattern of findings did not change. Therefore, results are reported with the full sample.
Table 1.
Demographic and clinical characteristics of ADHD and TD groups overall and within sex.
| TD | ADHD | Group comparisons | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||
| Girls (n = 20) | Boys (n = 39) | All (n = 59) | Girls (n = 29) | Boys (n = 66) | All (n = 95) | Girls TD vs. ADHD |
Boys TD vs. ADHD |
All TD vs. ADHD |
ADHD boys vs. girls | |||||||
|
| ||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p-values | ||||
| Age (years) | 10.0 | 1.1 | 10.1 | 1.3 | 10.1 | 1.2 | 9.9 | 1.1 | 10.2 | 1.4 | 10.1 | 1.3 | .772 | .720 | .831 | .206 |
| % Male | n/a | n/a | 34% | n/a | n/a | 31% | n/a | n/a | .662 | n/a | ||||||
| % Minority | 45% | 44% | 44% | 38% | 39% | 39% | .621 | .673 | .530 | .893 | ||||||
| SESa | 55.7 | 9.0 | 55.2 | 10.3 | 55.4 | 9.8 | 55.8 | 8.1 | 53.6 | 9.9 | 54.2 | 9.4 | .989 | .446 | .485 | .315 |
| WISCb FSIQ | 113.3 | 10.4 | 117.0 | 14.6 | 115.8 | 13.4 | 110.5 | 10.0 | 107.0 | 11.3 | 108.1 | 11.0 | .358 | <.001 | <.001 | .157 |
| WISC GAI | 113.5 | 11.5 | 119.4 | 15.3 | 117.4 | 14.3 | 112.1 | 11.4 | 109.8 | 12.5 | 110.5 | 12.1 | .698 | .001 | .002 | .399 |
| CPRSc IA | 48.2 | 5.9 | 44.8 | 6.4 | 46.0 | 6.4 | 78.9 | 10.7 | 73.1 | 9.2 | 74.9 | 10.0 | <.001 | <.001 | <.001 | .009 |
| CPRS HI | 45.9 | 6.1 | 46.2 | 5.6 | 46.1 | 5.7 | 70.0 | 16.5 | 73.3 | 13.3 | 72.2 | 14.4 | <.001 | <.001 | <.001 | .310 |
| ADHD-RSd IA | 3.4 | 2.7 | 3.3 | 2.8 | 3.4 | 2.8 | 19.3 | 4.6 | 18.5 | 4.1 | 18.7 | 4.2 | <.001 | <.001 | <.001 | .439 |
| ADHD-RS HI | 2.7 | 2.8 | 2.4 | 2.5 | 2.5 | 2.6 | 12.3 | 6.8 | 13.8 | 6.5 | 13.4 | 6.6 | <.001 | <.001 | <.001 | .344 |
| ADHD presentation | n/a | n/a | n/a | 21:7 | 52:14 | 73:21 | n/a | n/a | n/a | .687 | ||||||
| CO:IA (count) | ||||||||||||||||
| % Stim Med | 0 | 0 | 0 | 39% | 63% | 55% | n/a | n/a | n/a | .034 | ||||||
| % ODD | 0 | 0 | 0 | 34% | 38% | 37% | n/a | n/a | n/a | .752 | ||||||
% Minority = percentage of subjects with a self-reported race of African-American, Asian, Hispanic, or biracial; SES = Hollingshead Four-Factor Index of Socioeconomic Status; WISC = Wechsler Intelligence Scale for Children; FSIQ = Full-scale IQ; GAI = General Ability Index; CPRS IA T = Conners Parent Rating Scales DSM Inattention Scale T-score; CPRS HI T = Conners Parent Rating Scales DSM Hyperactivity/Impulsivity Scale T-score; ADHD-RS HI = ADHD Rating Scale Hyperactivity/Impulsivity raw score; ADHD-RS IA = ADHD Rating Scale Inattention raw score; CO = combined presentation; IA = predominantly inattentive presentation; % Stim Med = percentage of subjects taking stimulant medication at the time of the study (all subjects discontinued medication the day prior to and day of study participation); % ODD = percentage of subjects diagnosed with comorbid oppositional defiant disorder.
SES missing for seven kids (two TD, five ADHD).
112 participants completed the WISC-IV, 42 participants completed the WISC-V.
CPRS missing for four participants (two TD, two ADHD).
ADHD-RS missing for eight participants (two TD, six ADHD).
Diagnostic group differences in neuropsychological task performance
Between-group differences in SSD were examined to determine the validity of SSRT as the primary metric of inhibitory control. Results from the 2 Diagnosis × 2 Sex ANOVA indicated a significant main effect of diagnosis, F(1,150) = 9.3, p = .003, supporting the use of SSRT as the metric of inhibitory control. The MANOVA resulted in a significant multivariate effect of diagnosis, F(6, 145) = 4.2, p = .001.1 Examination of univariate tests indicated several main effects of diagnosis (see Table 2), such that children with ADHD displayed greater real-time discounting, poorer spatial span backward performance, higher RT variability, and higher SSRT. The main effect of diagnosis for real-time discounting was qualified by a significant Diagnosis × Sex interaction. Examination of post-hoc comparisons for the real-time discounting task indicated that girls with ADHD exhibited greater discounting than TD girls (p = .005, d = .79) and ADHD boys (p = .016, d = .52), whereas boys with ADHD did not significantly differ from TD boys (p = .940, d = .02). A Diagnosis × Sex interaction was also observed for GNG commission error rate, such that boys with ADHD made more commission errors than TD boys (p = .003, d = .60) and ADHD girls (p = .002, d = .69), whereas commission error rate did not significantly differ among girls with ADHD and TD girls (p = .731, d = .10).
Table 2.
Univariate analysis of variance testing for effects of diagnosis and interactions with sex on task measures.
| TD | ADHD | ANOVA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||
| Girls (n = 20) | Boys (n = 39) | All (n = 59) | Girls (n = 29) | Boys (n = 66) | All (n = 95) | Diagnosis | Diagnosis × Sex | |||||||||
|
| ||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | d | p | d | |
| Real-time DD | 0.59 | 0.14 | 0.56 | 0.14 | 0.57 | 0.14 | 0.48 | 0.13 | 0.56 | 0.16 | 0.54 | 0.16 | .028 | .25 | .035 | .74 |
| Classic DD | 0.45 | 0.27 | 0.43 | 0.26 | 0.43 | 0.26 | 0.39 | 0.26 | 0.47 | 0.30 | 0.44 | 0.29 | .883 | .04 | .356 | .33 |
| SS backward | 6.65 | 1.69 | 6.79 | 2.36 | 6.75 | 2.15 | 6.00 | 1.56 | 5.70 | 2.22 | 5.79 | 2.04 | .019 | .45 | .550 | .21 |
| GNG Com | 0.40 | 0.19 | 0.40 | 0.18 | 0.40 | 0.18 | 0.38 | 0.19 | 0.51 | 0.18 | 0.47 | 0.19 | .160 | .37 | .046 | .68 |
| GNG tau | 91.86 | 36.08 | 98.46 | 48.76 | 96.22 | 44.66 | 120.62 | 49.46 | 116.01 | 55.28 | 117.42 | 53.35 | .010 | .41 | .531 | .22 |
| SST SSRTa | 231.65 | 95.39 | 214.81 | 110.21 | 220.52 | 104.90 | 317.61 | 129.85 | 321.83 | 122.49 | 320.54 | 124.10 | <.001 | .79 | .614 | .16 |
ADHD = attention-deficit/hyperactivity disorder; Com = commission error rate; DD = delay discounting area under the curve; GNG = Go/No-Go; SS = spatial span; SSRT = stop signal reaction time; SST = Stop Signal task; TD = typically developing controls.
Results for SSD are very similar to SSRT such that there is a significant main effect of Diagnosis (p = .003) and no evidence of a Diagnosis × Sex interaction (p = .928).
Correlations between delay discounting and cognitive control measures
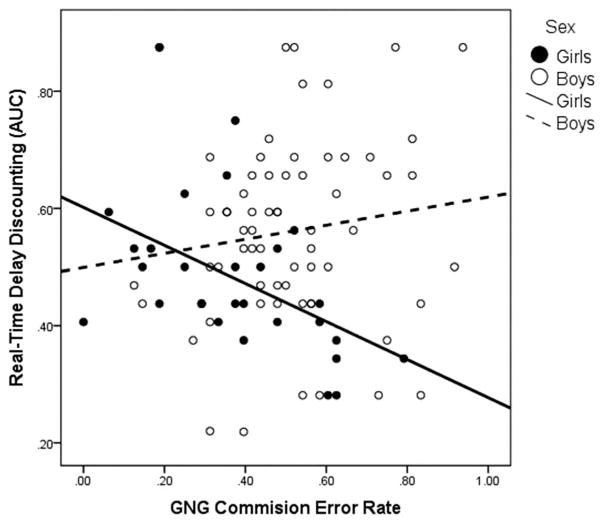
Examination of bivariate correlations between the discounting and cognitive control tasks in the full sample, including children with ADHD and TD controls, suggested that performance on the delay discounting tasks was correlated, r(154) = .300, p < .001, and performance across all cognitive control measures was correlated, all ps <.01 (see Table 3). These correlations remained significant after applying an FDR correction of .95. However, no significant correlations were observed between delay discounting on either task and any of the cognitive control measures in the full sample, rs(154) <.15, ps >.07 (see Table 3). Given evidence of anomalous delay discounting among girls, but not boys, with ADHD, the relationship between discounting and cognitive control was also assessed separately for girls and boys with ADHD. Results indicated that real-time discounting AUC was significantly negatively correlated with greater commission errors among girls with ADHD (p = .008; Figure 1, correlation remained significant after applying an FDR correction), while none of the remaining correlations were statistically significant. Among boys with ADHD, no statistically significant correlations emerged (Table 4). Furthermore, the difference in the correlation between real-time discounting and GNG commission errors among girls and boys with ADHD was significant (Fisher’s r-to-z two-tailed t-test, p = .005).
Table 3.
Task correlations among children with ADHD and typically developing (TD) controls*.
| Full sample (n = 154) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Classic discounting AUC | 1 | |||||
| 2. Real-time discounting AUC | .300** | 1 | ||||
| 3. Spatial span backward | .123 | .065 | 1 | |||
| 4. GNG tau | −.115 | −.146 | −.306** | 1 | ||
| 5. GNG commission error | .068 | .041 | −.256** | .294** | 1 | |
| 6. Stop signal SSRTa | −.033 | −.134 | −.362** | .441** | .353** | 1 |
Values reported are the zero-order Pearson correlation. ADHD = attention-deficit/hyperactivity disorder; TD = typically developing controls; AUC = area under the curve (more AUC = less discounting).
Correlation coefficients for SSD are as follows for the six variables listed in Table 3: 1 (r = .007), 2 (r = .079), 3 (r = .210**), 4 (r = −.205*), 5 (r = −.389**), and 6 (r = −.750**).
p < .05;
p < .01.
Figure 1.
Relationship between the commission error rate during the Go/No-Go task and real-time delay discounting among girls and boys with ADHD.
Table 4.
Correlations between delay discounting and performance on measures of cognitive control among girls with ADHD*.
| Real-time discounting AUC | ||
|---|---|---|
|
| ||
| ADHD girls n = 29 |
ADHD boys n = 64 |
|
| 1. Spatial span backward | −.137 | .173 |
| 2. GNG tau | −.315 | −.165 |
| 3. GNG commission error | −.482** | .133 |
| 4. Stop signal SSRTa | −.225 | −.171 |
Values reported are the zero-order Pearson correlation. ADHD = attention-deficit/hyperactivity disorder; AUC = area under the curve (more AUC = less discounting).
Correlation coefficients for the relationship between SSD and real-time discounting AUC are as follows for ADHD girls (r = .221) and ADHD boys (r = −.004).
p < .05;
p < .01.
Regression analyses
To further clarify the relationship between real-time discounting and cognitive control among girls with ADHD, the only group displaying anomalous delay discounting, linear regression was conducted to determine which measure of cognitive control best predicted real-time discounting among girls with ADHD. This regression model included all four cognitive control measures as predictors of real-time discounting among girls with ADHD. The overall model was significant, F(4, 24) = 4.5, p = .007, and both spatial span backward (p = .007) and GNG commission error rate (p = .003) were unique predictors of discounting, whereas GNG tau and stop signal SSRT did not account for unique variance (see Table 5).2
Table 5.
Linear regression model predicting real-time delay discounting among girls with ADHD from neuropsychological task performance.
| Real-time discounting AUC | ||
|---|---|---|
|
| ||
| Variable | β | 95% CI |
| Constant | .938 | [.67, 1.2] |
| Spatial span backward | −.033* | [−.06, −.004] |
| GNG tau | −.001 | [−.002, .000] |
| Stop signal SSRTa | .000 | [.000, .000] |
| GNG commission | −.363** | [−.59, −.14] |
| R2 | .429 | |
| F | 4.5** | |
n = 29. β = Unstandardized regression coefficient; AUC = area under the curve; CI = confidence interval.
Results for SSD are as follows: R2 = .427, F = 4.5**, β = .000, 95% CI = [.000, .000].
p < .05,
p < .01.
In a final set of analyses, we examined whether diagnostic group differences in real-time discounting were accounted for by cognitive control task performance given that diagnostic groups differed on real-time delay discounting and cognitive control measures. Specifically, we included diagnostic group, sex, and their interaction as predictors of real-time discounting along with the cognitive control variables in a linear regression model to determine the degree to which the diagnostic group effect accounted for variance that was unique versus shared with neuropsychological performance. This model was intended to parse the overlapping variance between diagnostic group and cognitive control performance in relation to delay discounting. When the cognitive control measures were simultaneously included in the model with diagnostic group, sex, and their interaction, the main effect of diagnosis remained significant, β = −.095, p = .035,3 whereas the Diagnosis × Sex interaction was no longer significant, β = .103, p = .056, although the change in p-value was negligible (previously p = .035). Furthermore, none of the measures of cognitive control were unique predictors of real-time discounting (all ps >.26). Thus, diagnostic group differences in real-time delay discounting do not appear to be due to diagnostic group differences in the included metrics of cognitive control.
Discussion
This study was the first to examine the unique relationship between delay discounting and several theoretically relevant neuropsychological measures of cognitive control (e.g., response variability, short-term memory, and inhibitory control) across a large sample of boys and girls with and without ADHD. We hypothesized that (a) children with ADHD, relative to TD children, would exhibit greater delay discounting and perform more poorly on tasks involving cognitive control, (b) poor cognitive control would be related to greater delay discounting, and (c) the pattern of neuropsychological deficits and associations with delay discounting may differ for girls and boys with ADHD. Overall, performance across cognitive control and delay discounting tasks differed among children with and without ADHD, and in some instances, among boys and girls with ADHD. Additionally, results demonstrated that cognitive control was generally not related to delay discounting in the overall sample, although cognitive control was related to heightened delay discounting among girls with ADHD. Results are discussed in further detail below.
ADHD-related sex differences in cognitive control and delay discounting
The direction and magnitudes of statistically significant diagnostic group effects were generally consistent with hypotheses based on findings from previous studies (see meta-analysis by Willcutt et al., 2005) suggesting that ADHD is characterized by a broad array of executive dysfunction (Nigg et al., 2005). A particularly novel finding in the current study is that several sex differences emerged when comparing task performance across diagnostic groups. For example, while girls and boys with ADHD displayed impaired inhibitory control on the Stop Signal task, only boys with ADHD showed impaired inhibitory control on the GNG task. As some current literature suggests that SSRT and GNG commission error rate are differentially influenced by underlying cognitive processes (i.e., working memory and behavioral inhibition, respectively; Tarle, Alderson, Patros, Lea, & Arrington, Under Review), the current finding appears to suggest that girls with ADHD may not exhibit behavioral disinhibition to the same degree as boys with the disorder.
Further evidence of sex differences was apparent on the real-time discounting task such that girls, but not boys, with ADHD exhibited increased delay discounting relative to TD children. In addition, neither girls nor boys with ADHD differed from controls on the classic monetary discounting task. These results replicate our previous findings (Rosch & Mostofsky, 2016) and may suggest that choosing between immediately consumable rewards in real time may be a more sensitive measure of reward-based decision-making in girls with ADHD than is a classic monetary discounting task. Increased delay discounting exhibited by girls with ADHD, relative to boys, might appear surprising on the surface given that girls with ADHD often exhibit decreased impulsive behavior (Hasson & Fine, 2012) and are therefore characterized as generally less impulsive than boys. The current study was unique, however, with regard to its inclusion of a relatively large number of girls diagnosed with the combined presentation of ADHD which allowed for the examination of sex differences in impulsive behavior across an otherwise equivalent group of girls and boys with the disorder. Furthermore, our sample consists primarily of non-referred participants recruited through public schools and therefore these gender differences are likely not an artifact of referral bias as previously shown (Biederman et al., 2005). These methodological strengths allowed for a more confident examination of discrepant neuropsychological profiles of girls and boys with ADHD although additional experimental and meta-analytic studies are required.
Relationship between cognitive control and delay discounting
There was no evidence of significant correlations between delay discounting and cognitive control in the overall sample of children with ADHD, nor did accounting for cognitive control performance eliminate the effects of diagnosis or the diagnosis by sex interaction in delay discounting. Findings contradict results from a recent experimental examination of the influence of visuospatial working memory on choice-impulsivity (Patros et al., 2015); however, this discrepancy is likely due to the current study’s examination of visuospatial storage/rehearsal rather than working memory/central executive processes. It is also noted that Patros and colleagues’ (2015) use of a choice-delay task likely placed relatively more demands on visuospatial processing when compared to the discounting tasks utilized in the current study, thus providing an alternative explanation for this discrepancy across studies. Additionally, it is of note that spatial span backward performance predicted delay discounting when examined specifically within girls with ADHD, suggesting that the inclusion of male participants might have suppressed overall effects. This finding was also contrary to Barkley’s (1997) behavioral disinhibition model of ADHD postulating that core inhibitory deficits underlie secondary or tertiary outcomes (e.g., maladaptive decision-making) and are, instead, consistent with Sonuga-Barke and colleagues (2002, 2010) theory suggesting that executive dysfunction and atypical motivation may be independent characteristics of ADHD. It is noted, however, that because the current study did not directly test competing predictions from Barkley’s (1997) or Sonuga-Barke’s (2010) models of ADHD, the literature would benefit from this examination in the future.
Instead, the current study tested cognitive neuroscience models of delay discounting, suggesting that cognitive control and reward valuation processes contribute to delay discounting. Thus, the lack of relationship between cognitive control and delay discounting in the full sample may suggest that reward valuation contributes more strongly to immediate reward preference than do cognitive control processes among school-age children, as it is the interaction between cognitive control and reward valuation processes that is thought to govern decision-making (Peters & Buchel, 2011). Consequently, future examination of the relationship between reward valuation metrics (both rating scale and experimental) and discounting would shed light on the relative contributions of cognitive and motivational processes to reward-based decision-making. Nevertheless, it is recommended that future studies include both cognitive control and reward valuation measures to more comprehensively examine cognitive and motivational mechanisms of delay discounting. Additionally, inclusion of multiple neurocognitive tasks measuring similar constructs will help ensure that processes of interest are being captured.
Although cognitive control measures and delay discounting were not related in the overall sample, there was evidence that inhibitory control during the GNG task was strongly correlated with delay discounting among girls with ADHD, the only group displaying atypical delay discounting. The regression analyses indicated that cognitive control (specifically, storage/rehearsal of visual–spatial information and GNG inhibitory control) was uniquely associated with discounting among girls with ADHD. This pattern of findings suggests that among girls with ADHD those who exhibit relatively poor visual–spatial storage/rehearsal and poor inhibitory control show the greatest delay discounting in the context of real rewards and delays. This is particularly interesting given that inhibitory control was not generally impaired among girls with ADHD at the group level, but those girls that show weaker inhibitory control tend to show greater delay discounting suggesting a potentially vulnerable subgroup of girls with ADHD.
Limitations and future directions
While the current study provided valuable information regarding the relationship between neuropsychological processes and discounting in children with ADHD, it is not without limitations. For example, the current study only considered the relationship between cognitive control measures and delay discounting, but did not include measures of reward valuation, frustration tolerance, or reward learning. Future studies should consider including these variables to determine if perhaps these motivational constructs are more strongly related to delay discounting in children. In addition, the current study included a relatively limited age range of children, making findings only representative of school-age children, and a relatively small sample of girls with ADHD. Replication of findings in preschool, adolescent, and adult populations and larger samples of girls will provide valuable information regarding sex differences and the developmental trajectory of reward-based decision-making in ADHD. Lastly, because children with ADHD included in the current study were diagnosed with limited comorbid conditions (i.e., ODD) findings might not generalize to a more severe/clinical ADHD population. Inclusion of additional, lower base rate comorbidities (e.g., major depressive disorder, anxiety disorders) in future studies will serve to increase generalizability of findings to clinical populations of children with ADHD.
Conclusion
The current study successfully examined the relationship between a variety of theoretically relevant neuropsychological measures of cognitive control and delay discounting in a large sample of children with and without ADHD. Primary findings indicated ADHD-related sex differences in cognitive control that inform theoretical models of ADHD tailored specifically to girls and boys with ADHD. Findings strongly suggest that there is a particularly vulnerable subgroup of girls with ADHD that appears to be at a higher likelihood, even compared to boys diagnosed with the disorder, to exhibit maladaptive decision-making. Longitudinal studies will be important to better understand the relationship between developmental changes in cognitive control and delay discounting and their relationship to ADHD. The continued acquisition of knowledge in this area will eventually allow for the development of treatment protocols that address secondary or tertiary outcomes associated with poor decision-making commonly implicated in children with ADHD.
Acknowledgments
Funding
This work was supported in part by grants from the National Institute of Mental Health (R01 MH078160; R01 MH085328, K23 MH101322, U54 HD079123) and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award program UL1 TR 000424-06
Footnotes
With SSD in the model, this result remains significant: F(6, 145) = 3.2, p = .006.
With SSD in the model, results essentially remain the same such that the overall model was significant, F(4, 24) = 4.5, p = .008, and both spatial span backward (p = .027) and GNG commission error rate (p = .004) were unique predictors of discounting, whereas stop signal SSD did not account for unique variance (p = .906). One notable change is that GNG tau was also significant in the model with SSD (p = .043), but not in the model with SSRT (p = .092). This is likely due to greater shared variance between SSRT and GNG tau, which are both reaction time measures that are more highly correlated (r = .441) than SSD and GNG tau (r = −.205).
Results with SSD in the model are essentially the same such that the main effect of diagnosis remained significant, β = −.097, p = .031, whereas the Diagnosis × Sex interaction was no longer significant, β = .101, p = .063, although the change in p-value was negligible (previously p = .035). Furthermore, none of the measures of cognitive control were unique predictors of real-time discounting (all ps >.16).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alderson RM, Rapport MD, Koffler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35(5):745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: Author; 2013. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Biederman J, Kwon A, Aleardi M, Chouinard VA, Marino T, Cole H, … Faraone SV. Absence of gender effects on attention deficit hyperactivity disorder: Findings in nonreferred subjects. The American Journal of Psychiatry. 2005;162(6):1083–1089. doi: 10.1176/appi.ajp.162.6.1083. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71(19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales-Revised. Toronto: Multi-Health Systems; 2002. [Google Scholar]
- Conners CK. Conners 3. North Tonawanda, NY: Multi-Health Systems; 2008. [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26(4):257–268. doi: 10.1023/A:1022602400621. [DOI] [PubMed] [Google Scholar]
- Dirlikov B, Rosch KS, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH. Distinct frontal lobe morphology in girls and boys with ADHD. Neuroimage: Clinical. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD rating scale—IV. New York, NY: Guilford Press; 1998. [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Grizenko N, Paci M, Joober R. Is the inattentive subtype of ADHD different from the combined/hyperactive subtype? Journal of Attention Disorders. 2010;13(6):649–657. doi: 10.1177/1087054709347200. [DOI] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, … Lane FG. Choice impulsivity: Definitions, measurement issues, and clinical implications. Personality Disorders: Theory, Research, and Treatment. 2015;6(2):182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: A meta-analytic review. Journal of Attention Disorders. 2012;16(3):190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Peterson DJ, Rosch KS, Crocetti D, Mori S, Mostofsky S. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperac-tivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2015;54(11):938–946. doi: 10.1016/j.jaac.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL. Examining relationships between executive functioning and delay aversion in attention deficit hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology. 2011;40(6):837–847. doi: 10.1080/15374416.2011.614578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children – Lifetime Version. Pittsburgh, PA: Western Psychiatric Institute and Clinic and Yale University; 2013. Kiddie-SADS-PL 2013 Working Draft. [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4(1):35–45. doi: 10.20982/tqmp.04.1.p035. [DOI] [Google Scholar]
- Luciana M. Practitioner review: Computerized assessment of neuropsychological function in children: Clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB) Journal of Child Psychology and Psychiatry. 2003;44(5):649–663. doi: 10.1111/jcpp.2003.44.issue-5. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience & Biobehavioral Reviews. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O’Brien J, Kaufmann WE, Denckla MB, Mostofsky SH. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society. 2011;17(6):1047–1057. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- O’Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of Clinical Neuropsychology. 2010;25(7):656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review. 2016;43:162–174. doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Patros CH, Alderson RM, Lea SE, Tarle SJ, Kasper LJ, Hudec KL. Visuospatial working memory underlies choice-impulsivity in boys with attention-deficit/hyperactivity disorder. Research in Developmental Disabilities. 2015;38:134–144. doi: 10.1016/j.ridd.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: Understanding variability. Trends in Cognitive Sciences. 2011;15(5):227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: Implications for DSM-V and ICD-11. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):217–228. e211–213. [PMC free article] [PubMed] [Google Scholar]
- Reed DD, Kaplan BA, Brewer AT. A tutorial on the use of Excel 2010 and Excel for Mac 2011 for conducting delay-discounting analyses. Journal of Applied Behavior Analysis. 2012;45(2):375–386. doi: 10.1901/jaba.2012.45-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children And Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Retz W, Retz-Junginger P, Hengesch G, Schneider M, Thome J, Pajonk FG, … Rösler M. Psychometric and psychopathological characterization of young male prison inmates with and without attention deficit/hyperactivity disorder. European Archives of Psychiatry and Clinical Neurosciences. 2004;254(4):201–208. doi: 10.1007/s00406-004-0470-9. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40(2):305–315. doi: 10.1016/j.paid.2005.03.024. [DOI] [Google Scholar]
- Rosch KS, Fosco WD, Pelham WE, Jr, Waxmonsky JG, Bubnik MG, Hawk LW., Jr Reinforcement and stimulant medication ameliorate deficient response inhibition in children with attention-deficit/hyperactivity disorder. Journal of Abnorm Child Psychology. 2016;44(2):309–321. doi: 10.1007/s10802-015-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch KS, Mostofsky SH. Increased delay discounting on a novel real-time task among girls, but not boys, with ADHD. Journal of the International Neuropsychological Society. 2016;22(1):12–23. doi: 10.1017/S1355617715001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America. 2010;33(2):357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419–368. [DOI] [PubMed] [Google Scholar]
- Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: Task and symptom specific effects. Journal of Neural Transmission. 2008;115(2):221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Mostofsky SH, Rosch KS. Cognitive load differentially impacts response control in girls and boys with ADHD. Journal of Abnormal Child Psychology. 2016;44(1):141–154. doi: 10.1007/s10802-015-9976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour KE, Tang X, Crocetti D, Mostofsky SH, Miller MI, Rosch KS. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Research: Neuroimaging. 2017;261:20–28. doi: 10.1016/j.pscychresns.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, … Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36(6):903–913. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels Rosch K, Dirlikov B, Mostofsky SH. Increased intrasubject variability in boys with ADHD across tests of motor and cognitive control. Journal of Abnormal Child Psychology. 2013;41(3):485–495. doi: 10.1007/s10802-012-9690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, … Newcorn JH. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. Journal of Abnormal Child Psychology. 2007;35(5):729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD - A dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130(1–2):29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD–a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130(1–2):29–36. doi: 10.1016/S0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neuroscience & Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Bitsakou P, Thompson M. Beyond the dual pathway model: Evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Tarle SJ, Alderson RM, Patros CH, Lea SE, Arrington EF. Working memory and behavioral inhibition in attention-deficit/hyperactivity disorder: Central executive processes differentially mediate stop-signal and go/no-go performance Under Review. [Google Scholar]
- Tripp G, Wickens JR. Research review: Dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Wahlstedt C, Thorell LB, Bohlin G. Heterogeneity in ADHD: Neuropsychological pathways, comorbidity and symptom domains. Journal of Abnormal Child Psychology. 2009;37(4):551–564. doi: 10.1007/s10802-008-9286-9. [DOI] [PubMed] [Google Scholar]
- Wechsler DL. Wechsler Individual Achievement Test. 2. San Antonio, TX: The Psychological Corporation; 2002. (WIAT-II) [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children. 4. San Antonio, TX: The Psychological Corporation; 2003. (WISC-IV) [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children. 5. San Antonio, TX: The Psychological Corporation; 2014. (WISC-V) [Google Scholar]
- Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: A four-factor model of impulsivity. European Journal of Personality. 2005;19(7):559–574. doi: 10.1002/per.556. [DOI] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT. Delay discounting of reward in ADHD: Application in young children. Journal of Child Psychology and Psychiatry. 2011;52(3):256–264. doi: 10.1111/j.1469-7610.2010.02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]