Abstract
Lipocalin-2 (LCN2), a secreted glycoprotein belonging to the lipocalin superfamily was reported to participate in various biological processes including cell migration, cell survival, inflammatory responses, and insulin sensitivity. LCN2 is expressed in the multiple tissues such as kidney, liver, uterus, and bone marrow. The receptors for LCN2 were additionally found in microglia, astrocytes, epithelial cells, and neurons, but the role of LCN2 in the central nervous system (CNS) has not been fully understood yet. Recently, in vitro, in vivo, and clinical studies reported the association between LCN2 and the risk of Alzheimer's disease (AD). Here, we reviewed the significant evidences showing that LCN2 contributes to the onset and progression of AD. It may suggest that the manipulation of LCN2 in the CNS would be a crucial target for regulation of the pathogenesis and risk of AD.
Keywords: Lipocalin-2, Brain, Alzheimer disease, Inflammation, Insulin resistance
INTRODUCTION
Lipocalin-2 (LCN2) is a 25 kDa secreted glycoprotein belonging to the lipocalin family which contributes to the transport of small hydrophobic molecules [1,2]. LCN2 participates in the control of various biological processes such as cell death [3], cell migration [4], cell differentiation [5], and innate immunity [6,7]. It is also known as neutrophil gelatinase-associated lipocalin (NGAL), because it was first identified from human neutrophils [8]. LCN2 is expressed in multiple tissues, i.e., kidney, liver, adipocytes, bone marrow, and uterus [9,10]. LCN2 was suggested as a sensitive biomarker for various forms of kidney injuries as LCN2 in the renal tubules is highly expressed about 1,000-fold, and released into plasma under infection, or ischemic condition [11,12,13,14]. Several studies also reported that circulating levels of LCN2 are associated with obesity, metabolic syndrome, diabetes, cardiovascular disease, and cancer [15,16]. Recently, the receptors of LCN2 were found in microglia [17], astrocytes [18], neurons [19], and choroid plexus [20]. LCN2 secreted by glial cells regulates neuronal morphological changes [17], contributes to neuronal cell death [21], is associated with reactive astrocytosis [18] and neuroinflammation [22], and act as a chemokine inducer in the central nervous system (CNS) [23]. Among the CNS disease, the risks of experimental autoimmune encephalomyelitis [24], intracerebral hemorrhage [25], and spinal cord injury [26] was reported to be positively associated with circulating LCN2 levels. However, the role of LCN2 in the CNS has not been fully elucidated yet.
In this paper, we particularly reviewed the significant evidences showing that LCN2 is implicated in Alzheimer's disease (AD) [27,28] which may suggest that LCN2 could control neuroinflammation [29], insulin signaling [30], neurite maturation [31] related to AD pathogenesis. This review may provide the possibility of LCN2 for the regulation of the AD pathogenesis.
THE ROLE OF LCN2 IN THE BRAIN
LCN2 known as NGAL [32], or growth factor-stimulated inducible protein 24 [33] is a 25 kDa glycoprotein purified from human neutrophils [34]. LCN2 is composed of extracellular ligand-binding proteins with a high specificity for hydrophobic molecules [35,36]. LCN2 binds to 2 specific receptors such as brain type organic cation transporter (24p3R) and megalin [14,37,38]. The 24p3R exists not only in the neutrophils [39] particularly with high levels, but also in the microglia [17], astrocytes [18], and neurons [19]. Megalin is mainly expressed in the brain capillaries, choroid plexus [20], the ependymal cells of the lateral ventricles [40], neural tube [41], astrocytes [42], and neurons [43,44]. Previous studies demonstrated that lipocalins acts as a critical role in multiple physiological processes including immune responses, cell migration, proliferation [45], and the regulation of cell homeostasis against exogenous compounds [2]. Particularly, LCN2 promotes neuronal death [46] and reactive gliosis [22,47,48], and triggers insulin resistance (IR) [30] in the CNS. Recently, several studies suggested positive relationship between LCN2 and pathophysiology of AD [28,49]. Increased LCN2 levels were observed in the postmortem of AD brains [28] as well as in people with mild cognitive impairment (MCI) [50]. Based on these evidences, alteration of LCN receptors and the increased level of LCN2 in the CNS may contribute to the progression of AD which may be related to neuronal cell death, glia's activation [51], and IR [52]. Even though the role of LCN2 in AD brain was not fully understood until now, recent studies suggest that LCN2 would be a potential marker for AD progression.
LCN2 CONTRIBUTES TO NEUROINFLAMMATION IN THE AD BRAIN
Neuroinflammation aggravates the pathogenesis of AD
Neuroinflammation is known to be mediated by microglia as the resident brain macrophage, astrocytes, neurons, neutrophils, and various inflammatory mediators released from these cells [51]. In the initiation state, neuroinflammation is beneficial for repairing the damaged tissue, but the excessive inflammatory responses are detrimental for neuronal regeneration [53]. Recent studies demonstrated that excessive neuroinflammatory response could impair the neuronal circuit in the CNS [54], and chronic neuroinflammation is critical for the onset and the progression of neurodegenerative diseases including AD [55,56]. Moreover, current reports indicate that high levels of inflammatory mediators have influence on diverse regions in the brain, and contribute to the cognitive dysfunction in the AD [57,58,59]. The study on neuroinflammatory mechanisms in AD brain is needed to ascertain the strategies to prevent the onset and the progression of AD.
LCN2 contributes to the inflammation in the brain
It has been reported that LCN2 contributes to the immune responses leading to the pathogenesis of neurodegenerative diseases [28,29,47,60]. Neuroinflammation by the brain injury [61] or infection [62] could promote the secretion of LCN2 from astrocyte, microglia, endothelial cell as well as neurons [25]. Released inflammatory mediators from the activated microglia and astrocytes play a critical role in cell migration and recruitment of these glial cells to the injury site [63,64]. LCN2 accelerates neuronal motility and its morphological alteration [21], and cell migration in glial cells and endothelial cells [23,65]. LCN2 promotes phenotypic changes of glial cells by a Rho-ROCK (Rho kinase) [31], and the migration of astrocyte, microglia and neurons by secreting chemokines such as CXCL10 [65]. Moreover, chronic and excessive immune response triggers the over-secretion of inflammatory cytokines including LCN2 in the CNS, subsequently aggravates the neural imbalance in hippocampus, and leads to the impaired long-term behavior [66,67,68]. In fact, increased LCN2 levels were found in the brain of the AD [28,61,69], and the MCI [50]. Considering the previous findings, LCN2 contributes to the neuroinflammation in the brain, and is especially linked to the neuroinflammatory responses in the AD brain. Given that the neuroinflammation contributes to the aggravation of AD pathogenesis [54,57], further studies are needed to elucidate the role of LCN2 on inflammatory mechanisms in AD brain.
LCN2 CONTRIBUTES TO IR IN THE AD BRAIN
IR aggravates the pathogenesis of the AD brain
Several epidemiologic studies have shown that type 2 diabetes [70], and demonstrated that IR as impaired insulin mechanism is related to the pathogenesis of cognitive declines in the AD [71,72,73]. Previous studies suggested that impaired insulin signaling was found in the postmortem of AD brains [52,74]. Normally, insulin signaling contributes to the inhibition of beta amyloid (Aβ) oligomerization and tau phosphorylation in the brain [52] as well as the transportation of Aβ out of the brain [75], while impaired insulin signaling leads to the pathogenesis of AD [76,77]. One in vitro study demonstrated that reduced activations of the insulin receptor/insulin receptor substrate 1 (IR/IRS1)/PI3K/AKT, and insulin growth factor-1 receptor (IGF-1R)/IRS2/PI3K signaling were found in the AD brains [74]. Moreover, disrupted insulin signaling is negatively associated with the volumes of hippocampus [71], medial prefrontal cortex, and medial temporal area [78]. Additionally, IR results in the disconnection in the cerebellum-frontal-temporal cortex [79]. Recent studies demonstrated that the site of IRS1 phosphorylation which is important to identify IR [80,81], is a key in the progression of AD [82]. Recent studies suggested that IR may be one of important causes which lead to diabetes induced dementia involving to cognitive decline [9,10]. Collectively, IR is a critical issue in the AD, and should be highlighted to find the mechanisms related to the onset and progression of AD.
LCN2 is associated with IR in the brain
Considering the previous evidences, circulating LCN2 levels were associated with hyperglycemia, IR, metabolic syndrome, diabetes, cardiovascular disease [16,30,83,84,85], in vitro [86,87], in vivo [83,88], and clinical researches [89] reported that LCN2 is involved in the negative regulation of insulin sensitivity, and could directly reduce the insulin sensitivity. Knockdown of LCN2 gene in the adipose tissue was reported to reduce IR closely related to obesity and diabetes [30]. Increased levels of LCN2 in both circulation and adipose tissue were observed in the obese and type 2 diabetic patients [16,83]. These evidences suggest that the reduction of LCN2 levels may contribute to the improvement of IR in the AD although the relationship between LCN2 and IR in the brain was not fully elucidated. Taken together, specific mechanisms on the association between LCN2 and IR in AD brain should be further investigated, which may suggest that LCN2 could aggravate IR which progress AD [30,86].
LCN2 CONTRIBUTES TO THE COGNITIVE DECLINE
Recent studies demonstrated that LCN2 is involved in some behaviors such as cognitive functions, emotional behaviors, depression, and anxiety [68,90,91]. Especially, LCN2 plays a role as a pivotal regulator in the cognitive behaviors [90]. As the LCN2 gene expression was modulated by glucocorticoids which is related to cognitive function [92], and high levels of LCN2 were found in patients with MCI dementia [47,50], high levels of LCN2 may lead to the cognitive decline. It was reported that high levels of LCN2 triggers inflammation, and subsequently reduces cognitive function [48,50]. Moreover, LCN2 could regulate the formation of dendritic spines and maturation of dendrites [93]. It could also control the neuronal morphology and plasticity by transporting iron which suggested that synaptic plasticity is related to iron metabolism [94]. Based on the significant evidences, LCN2 is an important factor to regulate cognitive function in the AD. Furthermore, the association between LCN2 and IR in the brain also supports the possibility of LCN2 to control the cognitive decline [6,9].
CONCLUSION
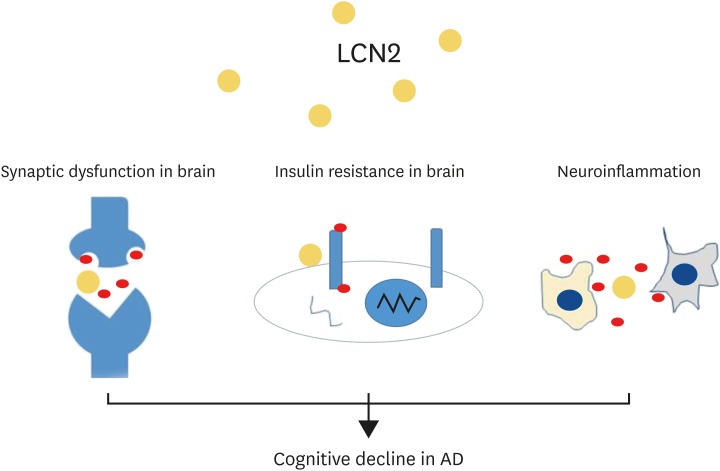
In this review, we highlight 3 points: first, LCN2 could aggravate inflammation in the AD brain; second, LCN2 is involved in IR in the AD brain; and third, LCN2 could contribute to cognitive decline in the AD by modulating inflammation and controlling synaptic plasticity (Figure 1). Taken together, we suggest that the modulation of LCN2 may be a key to attenuate the pathogenesis of AD. In addition, further studies such as in vivo study using transgenic animal model, and long-term clinical studies are needed to decipher the role of LCN2 in the pathogenesis of AD and cognitive impairment.
Figure 1.
The schematic image regarding the role of LCN2 in AD brain. LCN2 is involved in the neuroinflammatory responses in AD brain, and may aggravate insulin resistance in the AD brain, and cognitive decline in the AD by controlling inflammation and synaptic plasticity.
LCN2, lipocalin-2; AD, Alzheimer's disease.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea (grant number: 2016R1D1A1B03930394 [J.S.], 2016R1A2B4013627 [O.Y.K]).
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 2.Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehrer JP. Lipocalin-2: pro- or anti-apoptotic? Cell Biol Toxicol. 2010;26:83–89. doi: 10.1007/s10565-009-9119-9. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA. 2009;106:3913–3918. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16:660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Q, Zhang J, Li CY, Wang Y, Zeng MJ, Cai ZX, Tian RB, Jia W, Li XH. Insulin resistance-induced hyperglycemia decreased the activation of Akt/CREB in hippocampus neurons: molecular evidence for mechanism of diabetes-induced cognitive dysfunction. Neuropeptides. 2015;54:9–15. doi: 10.1016/j.npep.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Pratchayasakul W, Sa-Nguanmoo P, Sivasinprasasn S, Pintana H, Tawinvisan R, Sripetchwandee J, Kumfu S, Chattipakorn N, Chattipakorn SC. Obesity accelerates cognitive decline by aggravating mitochondrial dysfunction, insulin resistance and synaptic dysfunction under estrogen-deprived conditions. Horm Behav. 2015;72:68–77. doi: 10.1016/j.yhbeh.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta. 2015;444:18–23. doi: 10.1016/j.cca.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Neergaard JS, Dragsbæk K, Christiansen C, Nielsen HB, Brix S, Karsdal MA, Henriksen K. Metabolic syndrome, insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes. 2017;66:1957–1963. doi: 10.2337/db16-1444. [DOI] [PubMed] [Google Scholar]
- 11.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 2010;120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraman A, Roberts KA, Yoon J, Yarmush DM, Duan X, Lee K, Yarmush ML. Identification of neutrophil gelatinase-associated lipocalin (NGAL) as a discriminatory marker of the hepatocyte-secreted protein response to IL-1beta: a proteomic analysis. Biotechnol Bioeng. 2005;91:502–515. doi: 10.1002/bit.20535. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970. doi: 10.1038/sj.ki.5002165. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 15.Wang MR, Zhu XJ, Yang JS, Dai ZM, Mahmood K, Yang F, Yang WJ. Prawn lipocalin: characteristics and expressional pattern in subepidermal adipose tissue during reproductive molting cycle. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:222–229. doi: 10.1016/j.cbpb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Yang Z, Ye Z, Li Q, Wen J, Tao X, Chen L, He M, Wang X, Lu B, Zhang Z, Zhang W, Qu S, Hu R. Lipocalin-2, glucose metabolism and chronic low-grade systemic inflammation in Chinese people. Cardiovasc Diabetol. 2012;11:11. doi: 10.1186/1475-2840-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007;179:3231–3241. doi: 10.4049/jimmunol.179.5.3231. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Park JY, Lee WH, Kim H, Park HC, Mori K, Suk K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon S, Jha MK, Ock J, Seo J, Jin M, Cho H, Lee WH, Suk K. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. J Biol Chem. 2013;288:24116–24127. doi: 10.1074/jbc.M113.454140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Lee WH, Lee MS, Mori K, Suk K. Regulation by lipocalin-2 of neuronal cell death, migration, and morphology. J Neurosci Res. 2012;90:540–550. doi: 10.1002/jnr.22779. [DOI] [PubMed] [Google Scholar]
- 22.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HH, Liao CJ, Lee YC, Hu KH, Meng HW, Chu ST. Lipocalin-2-induced cytokine production enhances endometrial carcinoma cell survival and migration. Int J Biol Sci. 2011;7:74–86. doi: 10.7150/ijbs.7.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berard JL, Zarruk JG, Arbour N, Prat A, Yong VW, Jacques FH, Akira S, David S. Lipocalin 2 is a novel immune mediator of experimental autoimmune encephalomyelitis pathogenesis and is modulated in multiple sclerosis. Glia. 2012;60:1145–1159. doi: 10.1002/glia.22342. [DOI] [PubMed] [Google Scholar]
- 25.Dong M, Xi G, Keep RF, Hua Y. Role of iron in brain lipocalin 2 upregulation after intracerebral hemorrhage in rats. Brain Res. 2013;1505:86–92. doi: 10.1016/j.brainres.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathore KI, Berard JL, Redensek A, Chierzi S, Lopez-Vales R, Santos M, Akira S, David S. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J Neurosci. 2011;31:13412–13419. doi: 10.1523/JNEUROSCI.0116-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's disease. Neuroimage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naudé PJ, Nyakas C, Eiden LE, Ait-Ali D, van der Heide R, Engelborghs S, Luiten PG, De Deyn PP, den Boer JA, Eisel UL. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. FASEB J. 2012;26:2811–2823. doi: 10.1096/fj.11-202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JL, Reeves TM, Phillips LL. Osteopontin expression in acute immune response mediates hippocampal synaptogenesis and adaptive outcome following cortical brain injury. Exp Neurol. 2014;261:757–771. doi: 10.1016/j.expneurol.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolando C, Parolisi R, Boda E, Schwab ME, Rossi F, Buffo A. Distinct roles of Nogo-a and Nogo receptor 1 in the homeostatic regulation of adult neural stem cell function and neuroblast migration. J Neurosci. 2012;32:17788–17799. doi: 10.1523/JNEUROSCI.3142-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudd PM, Mattu TS, Masure S, Bratt T, Van den Steen PE, Wormald MR, Küster B, Harvey DJ, Borregaard N, Van Damme J, Dwek RA, Opdenakker G. Glycosylation of natural human neutrophil gelatinase B and neutrophil gelatinase B-associated lipocalin. Biochemistry. 1999;38:13937–13950. doi: 10.1021/bi991162e. [DOI] [PubMed] [Google Scholar]
- 33.Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 34.Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 35.Ganfornina MD, Gutiérrez G, Bastiani M, Sánchez D. A phylogenetic analysis of the lipocalin protein family. Mol Biol Evol. 2000;17:114–126. doi: 10.1093/oxfordjournals.molbev.a026224. [DOI] [PubMed] [Google Scholar]
- 36.Dittrich AM, Meyer HA, Hamelmann E. The role of lipocalins in airway disease. Clin Exp Allergy. 2013;43:503–511. doi: 10.1111/cea.12025. [DOI] [PubMed] [Google Scholar]
- 37.Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Richardson DR. 24p3 and its receptor: dawn of a new iron age? Cell. 2005;123:1175–1177. doi: 10.1016/j.cell.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Eller K, Schroll A, Banas M, Kirsch AH, Huber JM, Nairz M, Skvortsov S, Weiss G, Rosenkranz AR, Theurl I. Lipocalin-2 expressed in innate immune cells is an endogenous inhibitor of inflammation in murine nephrotoxic serum nephritis. PLoS One. 2013;8:e67693. doi: 10.1371/journal.pone.0067693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Götz M, Kempermann G, Peterson AS, Willnow TE, Hammes A. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- 41.Kur E, Mecklenburg N, Cabrera RM, Willnow TE, Hammes A. LRP2 mediates folate uptake in the developing neural tube. J Cell Sci. 2014;127:2261–2268. doi: 10.1242/jcs.140145. [DOI] [PubMed] [Google Scholar]
- 42.Bento-Abreu A, Velasco A, Polo-Hernández E, Pérez-Reyes PL, Tabernero A, Medina JM. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J Neurochem. 2008;106:1149–1159. doi: 10.1111/j.1471-4159.2008.05462.x. [DOI] [PubMed] [Google Scholar]
- 43.Chung RS, Penkowa M, Dittmann J, King CE, Bartlett C, Asmussen JW, Hidalgo J, Carrasco J, Leung YK, Walker AK, Fung SJ, Dunlop SA, Fitzgerald M, Beazley LD, Chuah MI, Vickers JC, West AK. Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J Biol Chem. 2008;283:15349–15358. doi: 10.1074/jbc.M708446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming CE, Mar FM, Franquinho F, Saraiva MJ, Sousa MM. Transthyretin internalization by sensory neurons is megalin mediated and necessary for its neuritogenic activity. J Neurosci. 2009;29:3220–3232. doi: 10.1523/JNEUROSCI.6012-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flower DR. The lipocalin protein family: a role in cell regulation. FEBS Lett. 1994;354:7–11. doi: 10.1016/0014-5793(94)01078-1. [DOI] [PubMed] [Google Scholar]
- 46.Tong J, Huang C, Bi F, Wu Q, Huang B, Liu X, Li F, Zhou H, Xia XG. Expression of ALS-linked TDP-43 mutant in astrocytes causes non-cell-autonomous motor neuron death in rats. EMBO J. 2013;32:1917–1926. doi: 10.1038/emboj.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci USA. 2013;110:4069–4074. doi: 10.1073/pnas.1218497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang E, Lee S, Kim JH, Kim JH, Seo JW, Lee WH, Mori K, Nakao K, Suk K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013;27:1176–1190. doi: 10.1096/fj.12-222257. [DOI] [PubMed] [Google Scholar]
- 49.Naudé PJ, Eisel UL, Comijs HC, Groenewold NA, De Deyn PP, Bosker FJ, Luiten PG, den Boer JA, Oude Voshaar RC. Neutrophil gelatinase-associated lipocalin: a novel inflammatory marker associated with late-life depression. J Psychosom Res. 2013;75:444–450. doi: 10.1016/j.jpsychores.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Choi J, Lee HW, Suk K. Increased plasma levels of lipocalin 2 in mild cognitive impairment. J Neurol Sci. 2011;305:28–33. doi: 10.1016/j.jns.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G. Neuroinflammation pathways: a general review. Int J Neurosci. 2017;127:624–633. doi: 10.1080/00207454.2016.1212854. [DOI] [PubMed] [Google Scholar]
- 52.Talbot K, Wang HY. The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer's disease. Alzheimers Dement. 2014;10:S12–25. doi: 10.1016/j.jalz.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo MV, McGavern DB. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353:783–785. doi: 10.1126/science.aaf6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busche MA, Konnerth A. Impairments of neural circuit function in Alzheimer's disease. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150429. doi: 10.1098/rstb.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen WW, Zhang X, Huang WJ. Role of neuroinflammation in neurodegenerative diseases. Mol Med Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Zheng Z, Ruan J, Li Z, Tzeng CM. Chronic inflammation links cancer and Parkinson's disease. Front Aging Neurosci. 2016;8:126. doi: 10.3389/fnagi.2016.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaik-Dasthagirisaheb YB, Conti P. The role of mast cells in Alzheimer's disease. Adv Clin Exp Med. 2016;25:781–787. doi: 10.17219/acem/61914. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Dong H, Li N, Zhang S, Sun J, Zhang S, Qian Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J Neuroinflammation. 2016;13:127. doi: 10.1186/s12974-016-0592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amor S, Woodroofe MN. Innate and adaptive immune responses in neurodegeneration and repair. Immunology. 2014;141:287–291. doi: 10.1111/imm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J Neuroinflammation. 2013;10:149. doi: 10.1186/1742-2094-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu ZL, Ciallella JR, Flood DG, O'Kane TM, Bozyczko-Coyne D, Savage MJ. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol Aging. 2006;27:377–386. doi: 10.1016/j.neurobiolaging.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Marques F, Rodrigues AJ, Sousa JC, Coppola G, Geschwind DH, Sousa N, Correia-Neves M, Palha JA. Lipocalin 2 is a choroid plexus acute-phase protein. J Cereb Blood Flow Metab. 2008;28:450–455. doi: 10.1038/sj.jcbfm.9600557. [DOI] [PubMed] [Google Scholar]
- 63.Edye ME, Lopez-Castejon G, Allan SM, Brough D. Acidosis drives damage-associated molecular pattern (DAMP)-induced interleukin-1 secretion via a caspase-1-independent pathway. J Biol Chem. 2013;288:30485–30494. doi: 10.1074/jbc.M113.478941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 65.Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011;286:43855–43870. doi: 10.1074/jbc.M111.299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm (Vienna) 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 67.Jin M, Kim JH, Jang E, Lee YM, Soo Han H, Woo DK, Park DH, Kook H, Suk K. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2014;34:1306–1314. doi: 10.1038/jcbfm.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Almeida-Suhett CP, Li Z, Marini AM, Braga MF, Eiden LE. Temporal course of changes in gene expression suggests a cytokine-related mechanism for long-term hippocampal alteration after controlled cortical impact. J Neurotrauma. 2014;31:683–690. doi: 10.1089/neu.2013.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mesquita SD, Ferreira AC, Falcao AM, Sousa JC, Oliveira TG, Correia-Neves M, Sousa N, Marques F, Palha JA. Lipocalin 2 modulates the cellular response to amyloid beta. Cell Death Differ. 2014;21:1588–1599. doi: 10.1038/cdd.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Rasgon NL, Kenna HA, Wroolie TE, Kelley R, Silverman D, Brooks J, Williams KE, Powers BN, Hallmayer J, Reiss A. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging. 2011;32:1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:1863–1864. doi: 10.1056/NEJMc1311765. [DOI] [PubMed] [Google Scholar]
- 74.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chua LM, Lim ML, Chong PR, Hu ZP, Cheung NS, Wong BS. Impaired neuronal insulin signaling precedes Aβ42 accumulation in female AβPPsw/PS1ΔE9 mice. J Alzheimers Dis. 2012;29:783–791. doi: 10.3233/JAD-2012-111880. [DOI] [PubMed] [Google Scholar]
- 77.Keeney JT, Ibrahimi S, Zhao L. Human ApoE isoforms differentially modulate glucose and amyloid metabolic pathways in female brain: evidence of the mechanism of neuroprotection by ApoE2 and implications for Alzheimer's disease prevention and early intervention. J Alzheimers Dis. 2015;48:411–424. doi: 10.3233/JAD-150348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morris JK, Vidoni ED, Perea RD, Rada R, Johnson DK, Lyons K, Pahwa R, Burns JM, Honea RA. Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience. 2014;270:139–147. doi: 10.1016/j.neuroscience.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 80.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 81.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. FASEB J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Lam KS, Kraegen EW, Sweeney G, Zhang J, Tso AW, Chow WS, Wat NM, Xu JY, Hoo RL, Xu A. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 84.Na GY, Yoon SR, An J, Yeo R, Song J, Jo MN, Han S, Kim OY. The relationship between circulating neutrophil gelatinase-associated lipocalin and early alteration of metabolic parameters is associated with dietary saturated fat intake in non-diabetic Korean women. Endocr J. 2017;64:303–314. doi: 10.1507/endocrj.EJ16-0233. [DOI] [PubMed] [Google Scholar]
- 85.Cruz DN, Gaiao S, Maisel A, Ronco C, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med. 2012;50:1533–1545. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, Houstis NE, Kahn BB, Rosen ED. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 87.Chan YK, Sung HK, Jahng JW, Kim GH, Han M, Sweeney G. Lipocalin-2 inhibits autophagy and induces insulin resistance in H9c2 cells. Mol Cell Endocrinol. 2016;430:68–76. doi: 10.1016/j.mce.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Guo H, Jin D, Zhang Y, Wright W, Bazuine M, Brockman DA, Bernlohr DA, Chen X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes. 2010;59:1376–1385. doi: 10.2337/db09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cakal E, Ozkaya M, Engin-Ustun Y, Ustun Y. Serum lipocalin-2 as an insulin resistance marker in patients with polycystic ovary syndrome. J Endocrinol Invest. 2011;34:97–100. doi: 10.1007/BF03347037. [DOI] [PubMed] [Google Scholar]
- 90.Ferreira AC, Pinto V, Dá Mesquita S, Novais A, Sousa JC, Correia-Neves M, Sousa N, Palha JA, Marques F. Lipocalin-2 is involved in emotional behaviors and cognitive function. Front Cell Neurosci. 2013;7:122. doi: 10.3389/fncel.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naudé PJ, Mommersteeg PM, Zijlstra WP, Gouweleeuw L, Kupper N, Eisel UL, Kop WJ, Schoemaker RG. Neutrophil gelatinase-associated lipocalin and depression in patients with chronic heart failure. Brain Behav Immun. 2014;38:59–65. doi: 10.1016/j.bbi.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 92.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 93.Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc Natl Acad Sci USA. 2011;108:18436–18441. doi: 10.1073/pnas.1107936108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]