Abstract
Soybean (Indian variety, JS 335) callus growth and its folate content was sustained on Murashige and Skoog (MS) medium supplemented with optimized levels of auxins and cytokinins. Callus cultures exhibited moderate production of total folate. Callus growth was stimulated with highest biomass content of 21.3 g/L DW (24 days culture) on medium supplemented with 1.5 mg/L 2,4-dichlorophenoxy acetic acid (2,4-D) and 0.1 mg/L Kinetin (Kn). Total folate production was enhanced by an increase in indole-3-acetic acid (IAA) from 0.5 to 1.0 mg/L and decreased thereafter up to 1.5 mg/L. 2,4-D at 1.5 mg/L repressed the callus growth and also folate production. A concentration of 1.0 mg/L IAA and 0.2 mg/L Kn elicited folate production to a maximum total folate content of 323.82 µg/100 g DW. The results indicate that optimized medium for callus suspension cultures conceivably are applied for scale-up studies in bioreactors.
Keyword: Soybean, Biomass, Microbiological assay, Lactobacillus rhamnosus, Total folate
Introduction
Glycine max contains potential therapeutic isoflavones and other bioactives. In general, the productivity of such essential metabolites in plants is considerably influenced by several stress factors as shown in various plants (Avinash et al. 2015; Sridevi and Giridhar 2015). Folate (vitamin B9) is a general term referring to various derivatives of folic acid. Its intake is reported to be vital to prevent megaloblastic anemia, neural tube defects (Lyer and Tomar 2009), decreasing the risk of cardiovascular disease, and cognitive disorders (Lucock 2000; Coppen and Bolander-Gouaille 2005). Scanty reports indicate the folate content of soybeans in the range of 2207–2671 µg/kg dry matter (Mo et al. 2013). However, folate content depends on various factors (Sanke et al. 1971), and its poor bio-accessibility is a concern (Mo et al. 2013). Hence, it is imperative to study dietary folate sources and ways to naturally enhance folate intake. In vitro production of essential bioactives and cell growth in cultures depends on interaction and concentration of nutrient status in the culture media (Cloutier et al. 2008). Consequently, of the various nutrient components, plant growth regulators (PGRs) concentration has a vital role for enhanced production of metabolites of interest in callus suspension cultures (Rajasekaran et al. 2007; Akitha Devi and Giridhar 2014).
Sporadic reports dealt with the significance of vitamin production in cell cultures in specific, tocopherol in Carthamus tinctorius L. (Chavan et al. 2011) and folate in Coriandrum sativum (Puthusseri et al. 2012), but none in G. max cultures. In general, the variation in bioactive content in in vitro cultures resulted from up-regulation of enzyme biosynthesis and gene expression and also its concentration varies from species to genotype (Federici et al. 2003). Such in vitro folate models are useful for modulation of folate biosynthetic pathway genes for obtaining enriched production of folates. In soybean, reports on folates production in vitro are not available. Under this context, optimization of the medium and PGR concentration for efficient multiplication of callus cultures and the establishment of callus suspension cultures of soybean are required for in vitro production of folic acid.
Diverse biotic and abiotic stress factors might amend the folate content under several stress factors (Nazki et al. 2014). However, of the several metabolites enhancement strategies adapted in plant cell cultures, elicitation found significance (Saini et al. 2014; Sridevi and Giridhar 2015). Such technologies have improved production or to persuade de novo synthesis of commercially significant bioactives (Luczkiewicz and Kokotkiewicz 2012; Złotek et al. 2014). Elicitors have been used to increase accumulation of various metabolites in several plant species in vitro and in vivo (Kim et al. 2005; Pérez-Balibrea et al. 2011).
Accordingly, the present communication aims to optimize a medium for in vitro production of folates through soybean callus cultures and its identification and confirmation by appropriate analytical methods.
Materials and methods
Chemicals
Folic acid, protease (source from Streptomyces griseus (Type XIV), α-amylase (source from Aspergillus oryzae), trichloroacetic acid (TCA), dithiothreitol, and mercaptoethanol were purchased from Sigma-Aldrich, Bangalore. All the plant growth regulators (PGRs) such as 1-naphthalene acetic acid (α-NAA), 2,4-dichloroacetic acid (2,4-D), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), 6-benzyladenine (BAP) and kinetin (Kn), and fluorescein diacetate (FDA) used in the present study were obtained from Sigma-Aldrich, Bangalore. Salicylic acid (SA), methyl jasmonate (MJ), chitosan (≥ 75% deacetylated; from shrimp), and sodium alginate were procured from Sigma-Aldrich, Bangalore. Solvents used for the analyses are HPLC grade (Sisco Research Laboratories-SRL, Mumbai, India). All other analytical-grade chemicals were purchased from Merck (Darmstadt, Germany).
Lactobacillus rhamnosus (ATCC 7469), Lactobacillus agar and broth, and folic acid assay medium were from Hi-Media (Mumbai, India) and microtiter plate obtained from Tarsons products Pvt. Ltd (Kolkata, India). As a conjugase source, rat serum was collected from CFTRI animal house. All other reagents used for the present study were of analytical grade, and all the glassware used for folate analysis was covered with aluminium foil to circumvent direct light exposure.
Plant material and surface sterilization
Uniform size soybean [Glycine max L. (Merr.)] JS 335 variety seeds were handpicked, surface-sterilized, and germinated using a standardized protocol (Nedev et al. 2007). Seeds were surface-sterilized with 70% (v/v) ethanol for 30 s, subsequently 0.2% (w/v) carbendazim for 5 min, and then 0.1% (w/v) mercuric chloride for 1 min. Surface-sterilized seeds were washed thrice with sterile distilled water and then cultured in petri dishes containing Murashige and Skoog (MS) basal medium (Murashige and Skoog 1962) for germination. The pH of the MS medium was set up to 5.8 and then autoclaved at 121 °C for 20 min. The inoculated cultures were maintained at 25 ± 2 °C at 16 h photoperiod.
Callus induction
In vitro germinated seedlings of 3 weeks old were used as an explant source for the initiation of callus cultures. Cotyledonary nodal leaves were incised into small pieces (9–10 mm) and then aseptically transferred to callus induction medium containing MS full strength solid medium. The medium includes 3% sucrose and 0.8% (w/v) agar, supplemented with varying concentrations of individual and combinations of cytokinins (BAP and Kn 0.1–1.0 mg/L) and varying concentrations of 2,4-D (0.5–2.0 mg/L). The media pH was adjusted to 5.7 before autoclaving (121 °C and 1.2 kg/cm2 pressure for 20 min). Cultures were routinely subcultured every 3 weeks, and further suspension cultures were initiated from friable callus production. This optimized media’ hormonal combinations were used to establish suspension cultures (Table 1).
Table 1.
Media with single and combination of auxins and cytokinin for folate production in G. max callus suspension cultures
| Media combination | Combination of cytokinin and auxins |
|---|---|
| MS + Kn (0.1, 0.2 and 0.5 mg/L) MS + 2,4-D (0.5, 1.0 and 1.5 mg/L) MS + α-NAA (0.5, 1.0 and 1.5 mg/L) MS + IAA (0.5, 1.0 and 1.5 mg/L) MS + IBA (0.5, 1.0 and 1.5 mg/L) |
MS + 2,4-D (0.5 mg/L) and Kn (0.2 mg/L)—FA1 |
| MS + 2,4-D (1.0 mg/L) and Kn (0.2 mg/L)—FA2 | |
| MS + α-NAA (1.0 mg/L) and Kn (0.2 mg/L)—FA3 | |
| MS + α-NAA (1.5 mg/L) and Kn (0.2 mg/L)—FA4 | |
| MS + IAA (1.0 mg/L) and Kn (0.2 mg/L)—FA5 | |
| MS + IAA (1.5 mg/L) and Kn (0.2 mg/L)—FA6 |
Optimization of growth regulators for friable callus biomass production
Furthermore, the callus cultures were propagated in 150 mL Erlenmeyer flasks containing 40 mL of liquid medium supplemented with varying concentrations of auxins (2,4-D, NAA, IAA, and IBA) and single concentration of Kn (0.2 mg/L), as shown in Table 1. These culture flasks were incubated on a rotary shaker at 100 rpm with a 16 h light/8 h dark photoperiod at 26 ± 1 °C. Callus suspension cultures grew well uniformly after 8–10 passages, and then, regular subcultures were performed by transferring a packed cell volume (PCV) of cells to fresh medium at 2 week intervals. On the basis of callus biomass and folate content, for initiation of callus suspension cultures, the callus derived from the FA5 medium (2, 4-D 1.0 mg/L and Kn 0.2 mg/L) was selected for total folate content analysis. All the experiments have at least three replicates, and each growth regulator combination was repeated three times.
Elicitor preparation
Biotic elicitor
The biotic elicitors were prepared (Giridhar and Parimalan 2010), using two fungal cultures Aspergillus niger and Rhizopus oligosporus that were obtained from Microbiology and Fermentation Technology Department (CSIR-CFTRI). Fresh cultures of R. oligosporus (RO) and A. niger (AN) were cultured grown on PDA (HiMedia, Mumbai) slants and then incubation at 37 °C for 7 days. Further respective fungal spores were selected for spore suspension preparation in 0.1% sodium lauryl sulfate (w/v) and then diluted with sterile distilled water under sterile conditions to obtain a spore density of ~ 2.5 × 106 spores/mL. Afterward, the spores were then inoculated in 40 mL of potato dextrose agar prepared in 150 mL Erlenmeyer conical flasks and then incubated for 10 days in dark. Subsequently incubated cultures were autoclaved and separated the mycelium from the culture broth by means of filtration and fresh weight was recorded. The aqueous extract was prepared by homogenization. The extract was then filtered (using Whatman No.1 filter paper) and set aside as the stock solution. From the stock, broad working concentration (fungal mycelium wet weight in 1 L of distilled water) range of individual fungal mycelial extracts was done in sterile water and used for conducting elicitor experiments (Table 2). Chitosan was prepared by dissolving 1% (w/v) in 0.5% acetic acid solution, and then adjusted the final volume to 100 mL using distilled water and then the pH adjusted to 5.8 with NaOH.
Table 2.
Elicitors and their concentration used for folate production in G. max callus suspension cultures
| Elicitor | Unit | Concentration of elicitor |
|---|---|---|
| Salicylic acid | µM | 5, 10, 25, 50 |
| Methyl jasmonate | µM | 5, 10, 25, 50 |
| Aspergillus niger | % | 0.1, 0.5, 1, 2 |
| Rhizopus oligosporus | % | 0.1, 0.5, 1, 2 |
| Chitosan | µg | 2, 5, 10, 25 |
Abiotic elicitor
Stock solutions of salicylic acid (SA) and methyl jasmonate (MJ) were prepared by dissolving in sterile distilled water, then filter-sterilized, and diluted to the wide range of concentrations (Table 2).
Elicitor treatment in G. max callus suspension cultures
Each elicitor with desired concentration was added to the G. max callus suspension culture. The concentration of each elicitor administered for folate analysis was depicted in Table 2. The biotic elicitors were added to the culture medium before autoclaving, but abiotic elicitors were added to the medium after autoclaving. Culture with equal volumes of water without elicitors was maintained as a control. Cultures were harvested at 3, 6, and 9 days (for biomass and folate estimation) after the addition of elicitors. The experiment was repeated thrice for the analysis of growth parameters and metabolites.
Effect of elicitors on total folate content in G. max plants grown under greenhouse conditions
The effect of floral administration of several abiotic and biotic elicitors on total folate content was analyzed in JS 335 and MAUS-2 variety using microbiological assay. Elicitors at different concentrations had varying influence on folate levels in soybean seeds.
Total folate content analysis
Sample preparation and extraction from seeds
For folate analysis, sample preparations and extractions performed under subdued light with a slight modification of Ruggeri et al. (1999). About 10 g of powdered seeds were homogenized in 100 mL extraction buffer containing 0.1 M potassium phosphate buffer and 1% ascorbic acid with pH 6.1. Subsequently, the homogenate was autoclaved at 121 °C for 10 min cooled instantly and centrifuged at 1000g for 15 min. Then, the aliquots of the supernatant of the sample (10 mL) were stored at − 20 °C in sterile amber bottles and used within a week for further analysis. The samples were concentrated by keeping for lyophilization. After freeze-drying, the samples were hydrolyzed and deconjugated using tri-enzyme treatment.
Extraction from callus cultures
The combinations mentioned in Table 1 were selected on the basis of the response for folate content and biomass evaluated from the single concentration of PGRs. The growth of callus cells was determined as growth index (GI) as reported earlier (Akitha Devi and Giridhar 2014). The biomass in the callus suspension cultures established for folate analysis was calculated from the lyophilized culture (days 0, 3, 6, 9, and 12) as dry weight. Known quantity of this callus was extracted with extraction buffer (1:10 ratio) containing 0.1 M potassium phosphate buffer and 1% ascorbic acid with pH 6.1 as explained above for extraction from seeds.
Tri-enzyme treatment
Enzymes protease (EC 3.4.21.62) and α-amylase (EC 3.2.1.1) were prepared in triple distilled-sterilized water as per the procedure of Rader et al. (1998) and sterilized using microfilter (0.22 µm). The rat serum was used as folate conjugase (γ-glutamyl hydrolase) source. To remove the endogenous folate, the rat serum was initially mixed with charcoal of 1:10 w/v by stirring for 1 h on ice followed by filter (0.22 µm) sterilization (Aiso and Tamura 1998). Store the conjugase aliquots at − 70 °C until use.
Hydrolysis
Prior to tri-enzyme treatment, the pH of sample extracts was adjusted to 4.5 using 0.1 M HCl. For the hydrolysis, 1.6 mL of protease (2 mg/mL) added to 10 mL extract, followed by incubation for 16 h at 37 °C. Furthermore, the enzyme was inactivated by heating the reaction mixture for 5 min in boiling water and then cooled, digestion with α- amylase (20 mg/mL) of 1.6 mL and kept for incubation at 37 °C for 4 h. Consequently, the reaction was stopped by boiling for 5 min in a water bath.
Deconjugation
The hydrolyzed extract after cooling was adjusted to pH 7.2 for deconjugation using conjugase (from rat serum) and incubated at 37 °C for 3 h (Rader et al. 1998). After that, the enzyme deactivation was done by boiling in a water bath for 5 min followed by cooling on ice. The extract was finally centrifuged at 1000g for 10 min, and the supernatant was stored in amber tubes and kept at − 20 °C until purification (within a week). Similarly, an enzyme blank without sample addition was incorporated to assess the folate content in the used enzymes. The resultant blank value was deducted from the sample folate content to find the concentration of actual folate.
Microbiological assay
Assay for the total folate content was performed using L. rhamnosus (ATCC 7469) in a 96-well microtiter plate (Wilson and Horne 1982) with minor modifications. The reaction mixture for the assay contains: 8 µL of working buffer (1.42 g sodium phosphate dibasic and 1 g ascorbic acid in 100 mL distilled water, pH 6) sterilized using 0.22 µm filter and then pipetted to each well in the plate. The added wide range of concentration sample extract/standards (up to 122 µL) and then made up the final volume to 150 µL with sterile water. Then to each well, added 150 µL of folic acid casei medium and 20 µL of inoculums (L. casei) followed by covering the plate with polystyrene cover. Subsequently, the plates were kept for 18 h incubation at 37 °C and then read the absorbance at 600 nm using ELISA reader (Spectramax 340PC384, Molecular Devices, Sunnyvale, CA). The content of total folate in each sample extract was determined from the calibration curve of the standard value. All sample extracts were analyzed in five replicates with varying concentrations to circumvent the error caused by any external contaminants.
Statistical analysis
Folate analysis of each variety by HPLC was performed in four replicates with two extracts. The data were analyzed statistically by SPSS 17.0 software using one-way analysis of variance (ANOVA), and the difference between the means of the sample was analyzed by the least significant difference (LSD) test at a probability level of 0.05.
Results and discussion
The initiated callus cultures were established on BAP, Kn, and 2,4-D. Later, the optimization of culture conditions for friable callus suspensions after 8–10 passages was used for further experiments to study the biomass and folate content in in vitro cultures.
Analysis of biomass and folate content in in vitro cultures
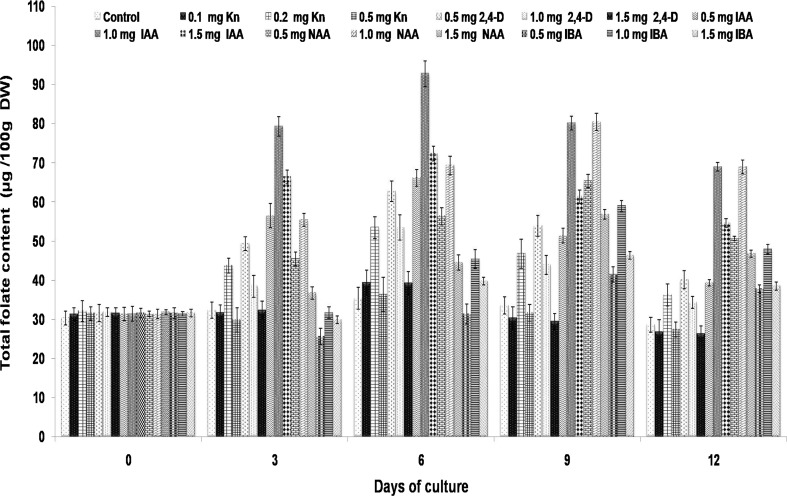
The results of best media concentration of PGRs for the total folate content and biomass were presented in Fig. 1. Since the folate content reached maximum by the 6th day of culturing, the biomass content was analyzed in the study, up to day 12. Among the individual PGRs’ treatments, maximum biomass has been recorded for 2,4-D at 1.5 mg/L (4.52 g/L). For other auxins and cytokinins, the biomass was marginally less to that of 2,4-D 1.5 mg/L (data not provided).
Fig. 1.
Effect of varying concentrations of auxins and cytokinins on total folate content in Glycine max callus suspension cultures. Values are mean ± SD with significant at p < 0.05
When 0.2 mg/L kinetin alone was used in the culture medium, a significant increase in total folate production was noticed till 6th day, and subsequently, it decreased to 36.24 µg/100 g DW by the 12th day of culturing. IBA at 1.0 mg/L presence in culture medium showed the poor response for total folate production by callus suspension cultures, wherein a maximum of 58.91 µg/100 g DW folate was produced. The best response for total folate content was evident in the presence of 1.0 mg/L IAA and found maximum content by 6th days of culturing (92.78 µg/100 g DW), followed by 80.42 mg/100 g DW on 9th day for 1.0 mg/L NAA, and 62.69 µg/100 g DW on 6th day for 0.5 mg/L 2,4-D, respectively.
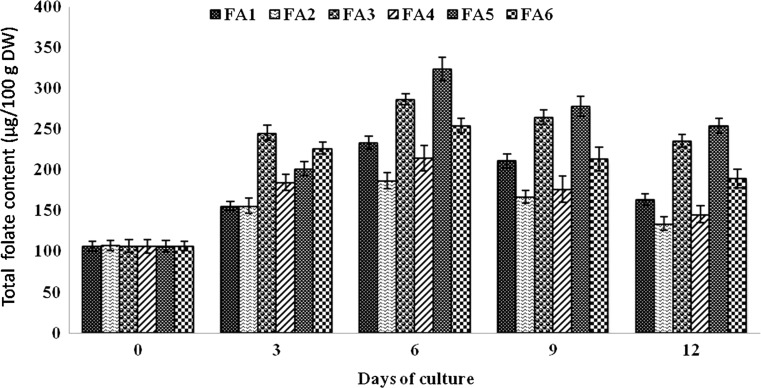
The combination of kinetin with different auxins (FA1–FA5) showed varied response for total folate production in callus suspension cultures of soybean. Among these five different media, the best response for folate content was on FA5, followed by FA3, FA6, FA1, FA4, and FA2 by 6 days of culturing, wherein a total folate content of 325, 290, 260, 240, 225, and 180 µg/100 g DW was recorded respectively. For all the six media, there was a progressive increase in folate content up to 6th day, and then, it was decreased.
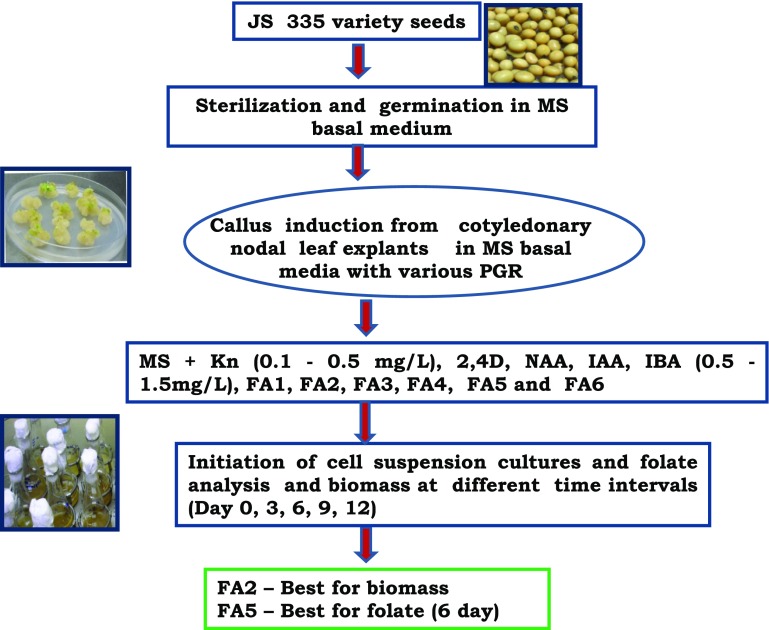
An increase in the total folate content with 92.78 µg/100 g DW compared to control was observed for 6-day-old culture medium supplemented with 1.0 mg/L IAA. The folate content of in vitro callus cultures in the present study was significantly less in comparison with seeds. This result was in agreement with Puthusseri et al. (2012) who analyzed the folate content in callus cultures induced in varying PGR concentrations for Coriander sativum. Therefore, media combinations with cytokinins and auxins (FA1–FA6) were selected with the intention to augment the production in callus suspension cultures (Fig. 2). The combinations of PGRs significantly improve the biomass as well as folate content. In the FA5 medium supplemented with IAA (1.0 mg/L) and Kn (0.2 mg/L), the total folate content was 1.4-fold (323.82 µg/100 g DW) high compared to seeds. However, the biomass content varied in the media combinations with the maximal production observed in FA2 medium. In addition, the results found in the present study were in agreement with Geipel et al. (2013) and Chavan et al. (2011) that combinations of PGRs improved the vitamin E production in cell cultures of Helianthus annuus and Carthamus tinctorius, respectively. In the present study, enhanced production of folate content observed in the cell cultures might be due to the up-regulation of genes involved in the biosynthesis pathway as shown for different bioactive compounds production in various plant cultures in vitro (Besher et al. 2014). The total folate content of 92.78 µg/100 g DW in established suspension cultures in the present study may be quite less to that of 188.47 ± 6.14 of raw seeds of the same JS335 variety. According to earlier reports (Arcot et al. 2002; Ginting et al. 2003; Ginting and Arcot 2004; Mo et al. 2013), the folate content is moderately high in fermented soy products such as tempe (298–416 µg/100 g DW) compared to raw soybeans (220–267 µg/100 g DW). Under this context, the data obtained in the present study is significant from in vitro cultures point of view, although folate content varies with the soybean variety. Figure 3 shows the overall representation of the schematic methodology for in vitro folate production in callus cultures of soybean JS335 variety.
Fig. 2.
Effect of combinations of cytokinins on total folate content in callus suspension cultures of G. max. Values are mean ± SD with significance at p < 0.05
Fig. 3.
Schematic representation of the methodology for in vitro folate production in callus cultures of soybean JS335 variety
Influence of elicitors on biomass and total folate content in G. max in in vitro cultures
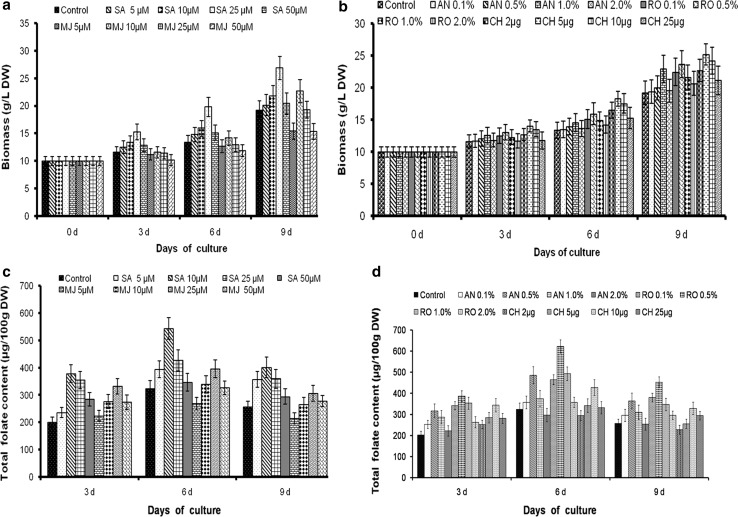
The effect of varying concentrations of abiotic elicitors on biomass and folate production in vitro G. max cultures was evident (Fig. 4a). Since the folate content reached maximum at the day 6 culture, the biomass content was analyzed in the study up to day 9. The highest biomass of 26.83 g/L DW was observed in SA (25 µM)-treated cultures. Abiotic elicitors significantly influenced the biomass content than biotic elicitors. Amongst the biotic elicitors, chitosan (5 µg)-treated cultures responded with the highest biomass 25.21 ± 2.24 g/L DW than control cultures (Fig. 4b).
Fig. 4.
Effect of abiotic (a) and biotic (b) elicitors on biomass content and in total folate content abiotic (c) and biotic (d) in G. max cultures. Values are mean ± SD with significant at p < 0.05
Similar to control cultures, day 6 elicitor-treated cultures showed increase in the total folate content. Both biotic and abiotic elicitors significantly contributed to the total folate content as analyzed using microbiological assay (Fig. 4c, d). Supplementation of abiotic elicitor, SA (10 µM) prominently augments the total folate content with a maximum of 68% increase than control. The results were in contrast with the earlier report that the higher concentration of SA significantly enhances the folate content in coriander cultures (Puthusseri et al. 2012). Similarly, biotic elicitor R. oligosporus treatment at 0.1, 1.0, and 2.0% level to callus cultures showed 43, 52, and 10% improvement in folate content, respectively. Overall, a substantial increase in folate content (621.73 µg/100 g DW) with an increase of 92% than control was evident at R. oligosporus (0.5%) treatment. Chitosan and methyl jasmonate exhibited moderate response for folate production in callus cultures.
These observations were in concomitant with that of Chavan et al. (2011) and Geipel et al. (2013) for vitamin E production improvement in cell cultures of Helianthus annuus and Carthamus tinctorius, respectively, under elicitors’ treatment and this enhanced production of folate content observed in the cell cultures might be due to the up-regulation of genes involved in the biosynthesis pathway.
Effect of elicitors on total folate content in G. max plants grown under greenhouse conditions
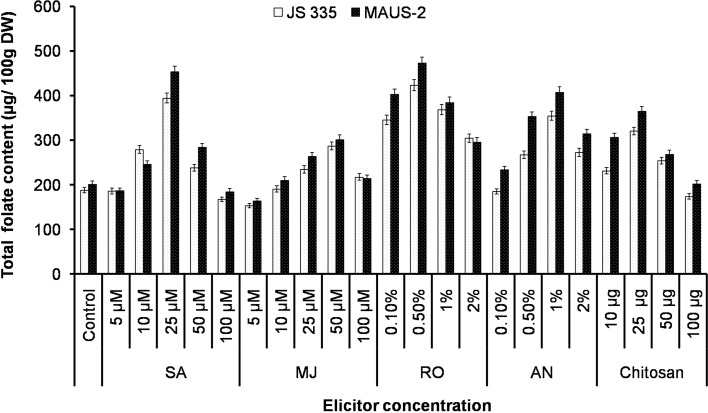
In JS 335 variety, application of R. oligosporus (0.5%) resulted in 2.57-fold increase with 423.36 ± 12.48 μg/100 g DW of folate content compared to control (164.37 ± 4.21 μg/100 g DW). In case of abiotic elicitors, SA (25 μM) was found to be effective for enhancement of folate content with 394.48 ± 11.34 μg/100 g DW, while MJ (50 μM) moderately enhanced the folate content (287.21 ± 9.11 μg/100 g DW). In addition, biotic elicitors A. niger (1.0%), R. oligosporus (0.5%), and chitosan (25 µg) enhance the total folate content by 2.15, 2.57, and 1.95-fold respectively (Fig. 5).
Fig. 5.
Influence of elicitors on total folate content in in vivo G. max plants. Values are mean ± SD with significant at p < 0.05
In MAUS-2 variety, the level of folate in treated seeds was high compared to JS 335 variety seeds. However, the fold change was more in elicitor-treated seeds of JS 335 than MAUS-2. Among the elicitors, R. oligosporus (0.5%) enhanced the folate content with an increase of 2.38-fold (472.61 ± 13.38 μg/100 g DW) compared to control (198.32 ± 6.11 μg/100 g DW). Abiotic elicitor SA (25 μM) was found to be efficient for improvement of folate content with 453.38 ± 12.36 μg/100 g DW. Similarly, MJ (50 μM) influences 1.51-fold increase in the folate content (301.27 ± 10.34 μg/100 g DW). Biotic elicitors with a concentration of 1.0% A. niger, 0.5% R. oligosporus and 25 µg chitosan augment the total folate content by 2.05, 2.38, and 1.83-fold, respectively (Table 3). Similar enhancement in the folate content in Coriander sativum by foliar application of salicylic acid and MJ was reported (Puthusseri et al. 2012). The data demonstrated the augmentation of folate content in treated seeds of JS 335 and MAUS-2. In this study, R. oligosporus was found to be most efficient for augmentation of folate content in soybean seeds. The comparison of total folate content between elicitor-treated in vitro cultures and potted plants was presented (Table 3).
Table 3.
Comparison of total folate content between elicitor-treated in in vitro cultures/potted plants
| Treatment | In vitro cultures | In vivo (green house) | ||
|---|---|---|---|---|
| Best concentration | Increased folate content (mg/g/L DW)* | Best concentration | Increased folate content (mg/g DW)* | |
| Salicylic acid (µM) | 10 | 544.01 ± 6.72b | 25 | 394.48 ± 4.01ab |
| Methyl jasmonate (µM) | 25 | 396.67 ± 4.12d | 50 | 287.21 ± 3.01c |
| Rhizopus oligosporus (%) | 0.5 | 621.73 ± 6.33a | 0.5 | 423.36 ± 5.21a |
| Aspergillus niger (%) | 0.5 | 485.41 ± 4.33c | 1.0 | 354.64 ± 3.12b |
| Chitosan (µg) | 10 | 426.32 ± 4.78cd | 25 | 320.42 ± 3.78bc |
| Control | – | 323.82 ± 3.63e | – | 188.47 ± 2.01d |
* Each value represents mean ± SD with significant at p < 0.05
Values with different superscript were found to be significant difference from each other
It is well documented that being signal molecules both salicylic acid and methyl jasmonate stimulate various secondary metabolites production in vitro and in vivo (Giridhar and Parimalan 2010). The reasons for their response are mainly due to activation of respective biosynthetic pathway gene transcripts which leads to the augmented production of metabolites such as carotenoids (Parimalan et al. 2011), isoflavones (Saini et al. 2013), and caffeine alkaloids (Avinash and Giridhar 2015). Therefore, the major influence is supposed to be on biosynthetic pathways only as demonstrated in marigold, wherein SA increased biomass and metabolites production without altering photosynthesis activity (Pacheco et al. 2013).
Conclusion
From the data obtained in the present study, we conclude that callus growth of soybean (Indian variety, JS 335) was stimulated with highest biomass content of 21.3 g/L DW (24 days culture) on medium supplemented with 1.5 mg/L 2,4-D and 0.1 mg/L Kn. An increase in total folate content could be achieved by adjusting IAA from 0.5 mg/L to 1.0 mg/L. The presence of 1.0 mg/L IAA and 0.2 mg/L Kn supported maximum folate content of 323.82 µg/100 g DW in cultures. It is evident from the study that the established suspension cultures are a reliable experimental tool, for achieving optimized media with pronounced biomass and folate content.
Acknowledgements
The authors are thankful to CSIR, New Delhi for the financial assistance (CSIR-CFTRI-MLP-0114) and ADMK is grateful to CSIR for fellowship.
Compliance with ethical standards
Conflict of interest
Authors declared that there is no conflict of interest.
References
- Aiso K, Tamura T. Trienzyme treatment for food folate analysis: optimal pH and incubation time for α-amylase and protease treatments. J Nutr Sci Vitaminol. 1998;44:361–370. doi: 10.3177/jnsv.44.361. [DOI] [PubMed] [Google Scholar]
- Akitha Devi MK, Giridhar P. Isoflavone augmentation in soybean cell cultures is optimized using response surface methodology. J Agric Food Chem. 2014;62:3143–3149. doi: 10.1021/jf500207x. [DOI] [PubMed] [Google Scholar]
- Arcot J, Wong S, Shrestha AK. Comparison of folate losses in soybean during the preparation of tempeh and soymilk. J Sci Food Agric. 2002;82:1365–1368. doi: 10.1002/jsfa.1197. [DOI] [Google Scholar]
- Avinash K, Giridhar P. Salicylic acid and methyl jasmonate restore the transcription of caffeine biosynthetic N-methyltransferases from a transcription inhibition noticed during late endosperm maturation in coffee. Plant Gene. 2015;4:38–44. doi: 10.1016/j.plgene.2015.09.002. [DOI] [Google Scholar]
- Avinash K, Naik G, Simmi PS, Giridhar P. Salinity and drought response alleviate caffeine content of young leaves of Coffea canephora var. Robusta S274. J Appl Biol Biotechnol. 2015;3:50–60. [Google Scholar]
- Besher S, Al-Ammouri A, Murshed R. Production of tropane alkaloids in the in vitro and callus cultures of Hyoscyamus aureus and their genetic stability assessment using ISSR markers. Physiol Mol Boil Plants. 2014;20:343–349. doi: 10.1007/s12298-014-0242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SP, Lokhande VH, Nitnaware KM, Nikam TD. Influence of growth regulators and elicitors on cell growth and α-tocopherol and pigment productions in cell cultures of Carthamus tinctorius L. Appl Microbiol Biotechnol. 2011;89:1701–1707. doi: 10.1007/s00253-010-3014-4. [DOI] [PubMed] [Google Scholar]
- Cloutier M, Bouchard-Marchand É, Perrier M, Jolicoeur M. A predictive nutritional model for plant cells and hairy roots. Biotechnol Bioeng. 2008;99:189–200. doi: 10.1002/bit.21543. [DOI] [PubMed] [Google Scholar]
- Coppen A, Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19:59. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- Federici E, Touché A, Choquart S, Avanti O, Fay L, Offord E, Courtois D. High isoflavone content and estrogenic activity of 25 year-old Glycine max tissue cultures. Phytochem. 2003;64:717–724. doi: 10.1016/S0031-9422(03)00379-0. [DOI] [PubMed] [Google Scholar]
- Geipel K, Socher ML, Haas C, Bley T, Steingroewer J. Growth kinetics of a Helianthus annuus and a Salvia fruticosa suspension cell line: shake flask cultivations with online monitoring system. Eng Life Sci. 2013;13:593–602. doi: 10.1002/elsc.201200148. [DOI] [Google Scholar]
- Ginting E, Arcot J. High-performance liquid chromatographic determination of naturally occurring folates during tempeh preparation. J Agric Food Chem. 2004;52:7752–7758. doi: 10.1021/jf040198x. [DOI] [PubMed] [Google Scholar]
- Ginting E, Arcot J, Chox JM. Determination of folate retention during tofu preparation using trienzyme treatment and microbiological assay. Ind J Agric Sci. 2003;4:12–17. doi: 10.21082/ijas.v4n1.2003.p12-17. [DOI] [Google Scholar]
- Giridhar P, Parimalan R. A biotechnological perspective towards improvement of annatto color production for value addition–the influence of biotic elicitors. Asian Pac J Mol Biol Biotechnol. 2010;18:77–79. [Google Scholar]
- Kim HJ, Chen F, Wang X, Rajapakse NC. Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.) J Agric Food Chem. 2005;53:3696–3701. doi: 10.1021/jf0480804. [DOI] [PubMed] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Gen Meta. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Łuczkiewicz M, Kokotkiewicz A. Elicitation and permeabilisation affect the accumulation and storage profile of phytoestrogens in high productive suspension cultures of Genista tinctoria. Acta Physiol Plant. 2012;34:1–16. doi: 10.1007/s11738-011-0799-4. [DOI] [Google Scholar]
- Lyer R, Tomar SK. Folate: a functional food constituent. J Food Sci. 2009;74:R114–122. doi: 10.1111/j.1750-3841.2009.01359.x. [DOI] [PubMed] [Google Scholar]
- Mo H, Kariluoto S, Piironen V, Zhu Y, Sanders MG, Vincken JP, Wolkers-Rooijackers J, Nout MR. Effect of soybean processing on content and bioaccessibility of folate, vitamin B12 and isoflavones in tofu and tempe. Food Chem. 2013;141:2418–2425. doi: 10.1016/j.foodchem.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nazki FH, Sameer AS, Ganaie BA. Folate: metabolism, genes, polymorphisms and the associated diseases. Gene. 2014;533:11–20. doi: 10.1016/j.gene.2013.09.063. [DOI] [PubMed] [Google Scholar]
- Nedev T, Todorova R, Kosturkova G, Akitha Devi MK, Sakthivelu G, Giridhar P, Rajasekaran T, Ravishankar G. Variation in in vitro morphogenic response to growth regulators in soybean genotypes from India and Bulgaria. Int Elect J Bioaut. 2007;8:193–200. [Google Scholar]
- Pacheco AC, Cabral C, Fermino ES, Aleman CC. Salicylic acid induced changes to growth, flowering and flavonoids production in marigold plants. J Med Plants Res. 2013;7:3158–3163. [Google Scholar]
- Parimalan R, Mahendranath G, Giridhar P, Ravishankar GA. Abiotic elicitor mediated augmentation of annatto pigment production in standing crop of Bixa orellana L. Ind J Fund Appl Life Sci. 2011;1:229–236. [Google Scholar]
- Pérez-Balibrea S, Moreno DA, García-Viguera C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011;129:35–44. doi: 10.1016/j.foodchem.2011.03.049. [DOI] [Google Scholar]
- Puthusseri B, Divya P, Lokesh V, Neelwarne B. Enhancement of folate content and its stability using food grade elicitors in coriander (Coriandrum sativum L.) Plant Foods Hum Nutr. 2012;67:162–170. doi: 10.1007/s11130-012-0285-1. [DOI] [PubMed] [Google Scholar]
- Rader JI, Weaver CM, Angyal G. Use of a microbiological assay with tri-enzyme extraction for measurement of pre-fortification levels of folates in enriched cereal-grain products. Food Chem. 1998;62:451–465. doi: 10.1016/S0308-8146(98)00089-2. [DOI] [Google Scholar]
- Rajasekaran T, Giridhar P, Ravishankar GA. Production of steviosides in ex vitro and in vitro grown Stevia rebaudiana Bertoni. J Sci Food Agric. 2007;87:420–424. doi: 10.1002/jsfa.2713. [DOI] [Google Scholar]
- Ruggeri S, Vahteristo LT, Aguzzi A, Finglas P, Carnovale E. Determination of folate vitamers in food and in Italian reference diet by high-performance liquid chromatography. J Chromatogr A. 1999;855:237–245. doi: 10.1016/S0021-9673(99)00674-3. [DOI] [PubMed] [Google Scholar]
- Saini RK, Akithadevi MK, Giridhar P, Ravishankar GA. Augmentation of major isoflavones in Glycine max L. through the elicitor-mediated approach. Acta Bot Croat. 2013;72:311–322. [Google Scholar]
- Saini RK, Harish Prashanth KV, Shetty NP, Giridhar P. Elicitors, SA and MJ enhance carotenoids and tocopherol biosynthesis and expression of antioxidant related genes in Moringa oleifera Lam. Leaves. Acta Physiol Plant. 2014;36:2695–2704. doi: 10.1007/s11738-014-1640-7. [DOI] [Google Scholar]
- Sanke Y, Miyamoto T, Murata K. Studies on the nutritional value of tempeh. IV. Biosynthesis of folate compounds with Rhizopus oligosporus. J Vitaminol. 1971;17:96–100. doi: 10.5925/jnsv1954.17.96. [DOI] [PubMed] [Google Scholar]
- Sridevi V, Giridhar P. Impacts of biotic and abiotic stress on major quality attributing metabolites of coffee beans. J Environ Biol. 2015;36:377–382. [PubMed] [Google Scholar]
- Wilson S, Horne D. Use of glycerol-cryoprotected Lactobacillus casei for microbiological assay of folic acid. Clin Chem. 1982;28:1198–1200. [PubMed] [Google Scholar]
- Złotek U, Świeca M, Jakubczyk A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.) Food Chem. 2014;148:253–260. doi: 10.1016/j.foodchem.2013.10.031. [DOI] [PubMed] [Google Scholar]