Abstract
A novel extracellular enzyme with strong fibrinolytic activity, produced by Bacillus tequilensis, which was isolated from the soil of Zhuhai City (China) was purified and characterized. The enzyme was secreted by cultured B. tequilensis in solid state and purified at a high efficiency using the combination of salting out, ion exchange chromatography, and size exclusion chromatography. The enzyme was estimated to have a molecular weight of approximately 27 kDa, pI of 8.9 ± 0.1, to stable at pH 5.0–12.0 and up to 50 °C; the optimum pH and temperature are 10.5 and 45 °C (2373.59 ± 54.81 U/mg), respectively. The fibrinolytic activity was enhanced by K+, Na+, Mg2+, Mn2+, Ca2+, and Ba2+ and inhibited by Cu2+, Zn2+, and Fe3+. Moreover, the activity was slightly enhanced by PMSF and EDTA at low concentrations and inhibited by β-mercaptoethanol. The N-terminal amino acid sequence is AQSVPYGISQI. The enzyme has a higher enzymatic activity than most other fibrinolytic enzymes. The high thermal stability indicated that it is easy to preserve and could be activated under high-temperature conditions.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1115-4) contains supplementary material, which is available to authorized users.
Keywords: Bacillus tequilensis, Fibrinolytic enzyme, Purification, Identification, Characterization
Introduction
Intravascular thrombosis, resulting from the accumulation of fibrin in the blood vessel, is a main cause of cardiovascular disease which is seriously harmful to human health (Basson 2008). In 2011, according to the statics of America Heart Association, CVD accounted for 31.3% of mortality of Americans (Mozaffarian et al. 2015). Normally, fibrin is a key structural support protein and the main protein component of a thrombus, and is removed from the vessel wall by the blood’s fibrinolytic system (Kannel et al. 1987). Under certain specific physiological conditions, the fibrinolytic effect is inefficient, and fibrin clots will remain in the vessel and obstruct the flow of blood (Astrup 1956). Ancrod, a thrombin-like enzyme in viper venom, can efficiently degrade fibrin, which has strongly demonstrated that the fibrinolytic enzyme existing in nature can be used for CVD therapy (Liu et al. 2011). In addition to ancrod, urokinase, streptokinase, and anisoylated plasminogen streptokinase activator complex (APSAC) are common natural fibrinolytic enzymes used as thrombolytic agents clinically. In 1987, Bacillus subtilis, which is used to produce traditional fermented Japanese food natto, was found to have the ability to produce nattokinase, an enzyme with a strong fibrinolytic activity (Sumi et al. 1987). A number of bacteria species have since been identified that produce nattokinase-like fibrinolytic enzymes, such as Streptomyces sp. (Wang et al. 1999), Bacillus amyloliquefaciens (Peng et al. 2003), and Bacillus pumilus (Afifah et al. 2014). Thus, bacteria appear to be a very good source of fibrinolytic enzymes. The aim of this study was to purify and characterize a fibrinolytic enzyme produced by a strain of bacteria isolated from the soil of Zhuhai, Guangdong province in China.
Materials and methods
Chemicals, reagents, and standards
Q-HP 16/25 pre-packed column, Sepharcryl-100 16/60 pre-packed column and iso-electric focusing (IEF) calibration kit were purchased from GE Healthcare (Sweden); bovine serum albumin, sodium dodecyl sulfate, Precision Plus Protein Standard, phenylmethylsulfonyl fluoride, ethylene diamine tetra acetic acid, iodoacetic acid, and β-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). The PTH-amino acids mixture standard solution was purchased from Wako, Japan. All other chemicals-(NH4)2SO4, MgCl2, Na2CO3, NaHCO3, NaCl, NaOH, KCl, MgSO4, MnCl2, CaCl2, CuCl2, ZnCl2, Ba(SO4)2, and FeCl3 were obtained from local chemical suppliers.
Strain and fermentation for enzyme production
Bacillus tequilensis (No. 11462) was obtained from the Chinese General Microbiological Culture Collection Center (CGMCC), China. The 16S rRNA gene sequence accession number of GenBank is MG733132. It was grown in Luria–Bertani broth medium (Afifah et al. 2014) at our laboratory and used for the experiments. After 2 days of incubation at 37 °C, the strains were transferred to a solid production medium containing 40 g soybeans, 4 g MgCl2, and 20 mL distilled water. After 72 h incubation at 32 °C, the fermented soybeans were collected for fibrinolytic enzyme purification.
Enzyme purification
The fibrinolytic enzyme was purified by the method of Kim et al. (1997) with slight modification. Briefly, 5 g of fermented soybeans was washed with 50 mL DI water followed by centrifugation at 11,000×g for 20 min to remove the solid debris. The enzyme was precipitated with ammonium sulfate (75% saturation) and dissolved in buffer A (Na2CO3/NaHCO3, 30 mM, pH 9.5). After dialysing against buffer A for 36 h, the enzyme solution was loaded on a Q HP column (1.6 × 5 cm) pre-equilibrated with buffer A and then eluted with buffer A containing 1 mol NaCl with a linear gradient of 0.0–1.0 mol NaCl at a flow rate of 2.5 mL/min. Fractions with fibrinolytic activity were collected and concentrated. The concentrated samples were loaded on a Sephacryl-100 column (1.6 × 60 cm) pre-equilibrated buffer A and eluted with the same buffer. Fractions with a single peak of fibrinolytic activity were pooled, concentrated, and separated by electrophoresis (SDS-PAGE).
Determination of protein concentration and enzymatic activity
Protein concentration was determined according to the method of Smith et al. (1985). Enzymatic activity was determined using the fibrin plate method described by Astrup and Mullertz (1952) with slight modifications. Briefly, A 72 mg fibrinogen was dissolved in 10 mL 0.96% NaCl (w/v) solution, and mixed with 9 mL 2.35% agarose (w/v) solution along with 1 mL of thrombin solution (500 U/mL). The fibrin plate was left for 1 h at room temperature to form the fibrin clot. The plate was hole punched by an autoclaved puncher with the diameter of 5 mm. The sample of 30 μL was added into the hole and the plate was incubated for 6 h. The enzymatic activity was quantified by measuring the diameter of the lysis cycle on the plate surface.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was performed using a Bio-Rad system based on the method described by Laemmli (1970).
Determination of isoelectric point (pI)
The pI of the purified fibrinolytic enzyme was measured and compared with standards (pI 3–10) from the IEF calibration kit according to the instruction manual of manufacturer. The measurement was performed based on the method described by Vesterberg (1972).
N-terminal amino acid sequence analysis
The enzyme was isolated using SDS-PAGE and then electro-transferred onto a polyvinylidene difluoride membrane (PVDF). Proteins were stained with Coomassie blue R-250 after electrophoresis. N-terminal amino acid sequences were analyzed by the Edman degradation method (Edman 1950) followed by sequencing using a PPSQ-33A automated protein and peptide sequencer (Shimadzhu, Japan).
Effects of pH and temperature
The effect of pH and temperature on the enzyme was determined by the method of Hua et al. (2008). The optimal pH was determined by measuring the activity of the purified enzyme over the pH range of 3.0–11.0 (pH 3.0, 20 mM citrate buffer; pH 4.0–5.0, 50 mM acetate buffer; pH 6.0–7.0, 30 mM phosphate buffer; pH 8.0, 0.05 mM tris buffer; pH 9.0–11.0 0.03 mM sodium carbonate/sodium bicarbonate buffer at 45 °C). The optimal temperature of the fibrinolytic enzyme was determined by measuring activity over the temperature range of 20–70 °C at pH 10.5.
The residual fibrinolytic activity of the enzyme was measured by incubating the enzyme in various buffers over the pH range of 3.0–13.0 (100 mM glycine–NaOH was used as pH 12.0–13.0 buffer) at 45 °C for 0.5, 3 and 12 h to determine the pH stability. The residual fibrinolytic activity of the enzyme was measured by incubating the enzyme in pH 10.5 buffer over the temperature range of 20–70 °C for 0.5, 3, and 12 h to determine the temperature stability.
Effects of metal ions or inhibitors
The effect of metal ions and inhibitors on the purified enzyme was checked using the method of Hua et al. (2008) with slight modifications. The residual activity of the purified enzyme was determined by incubating it in buffer-A containing various concentrations (1, 5 and 10 mM) of metal ions (Na+, K+, Mg2+, Mn2+, Ca2+, Cu2+, Ba2+, Zn2+, and Fe3+) or various concentrations (1, 2.5, and 5 mM) of phenylmethanesulfonyl fluoride (PMSF), ethylene diamine tetra acetic acid (EDTA), iodoacetic acid (IAA), and β-mercaptoethanol.
Statistical analysis
Results were expressed as averages of mean of three independent samples with standard deviation. The data were analyzed by one-way analysis of variance (ANOVA) using Microsoft Excel XP (Microsoft Corp., Redmond, WA, USA), and the post hoc mean separations were performed by Duncan’s multiple-range test at P < 0.05.
Results and discussion
Purification of the fibrinolytic enzyme
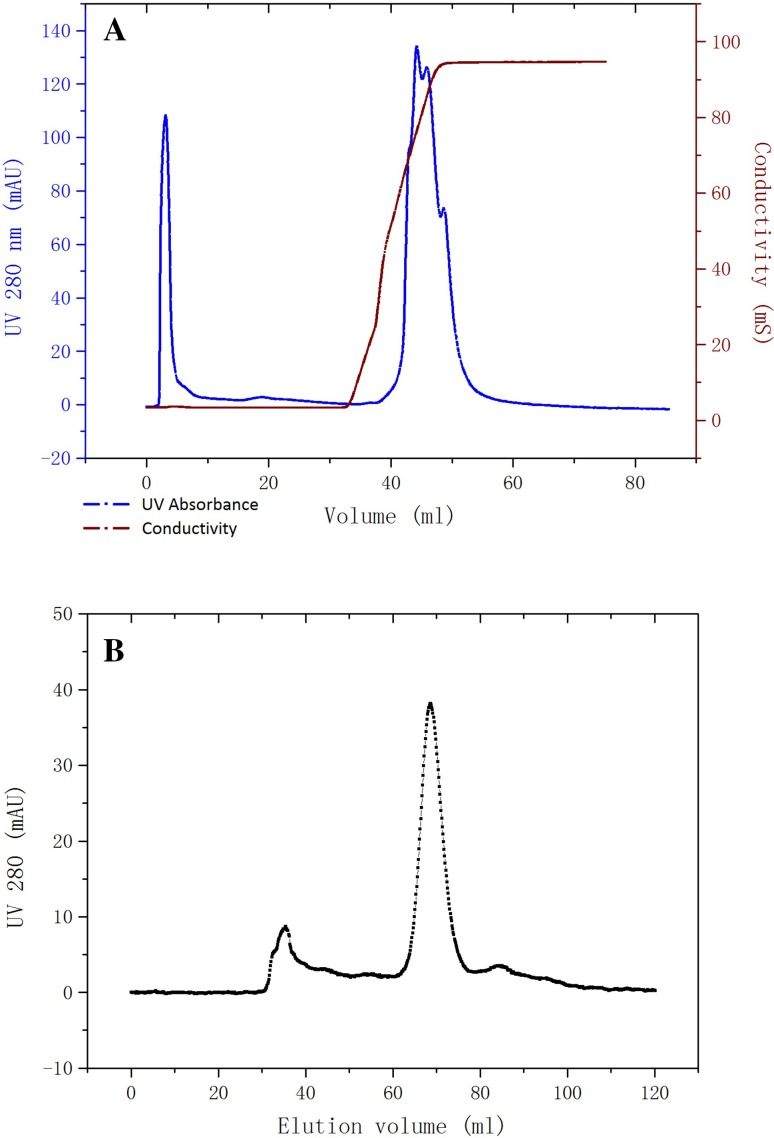
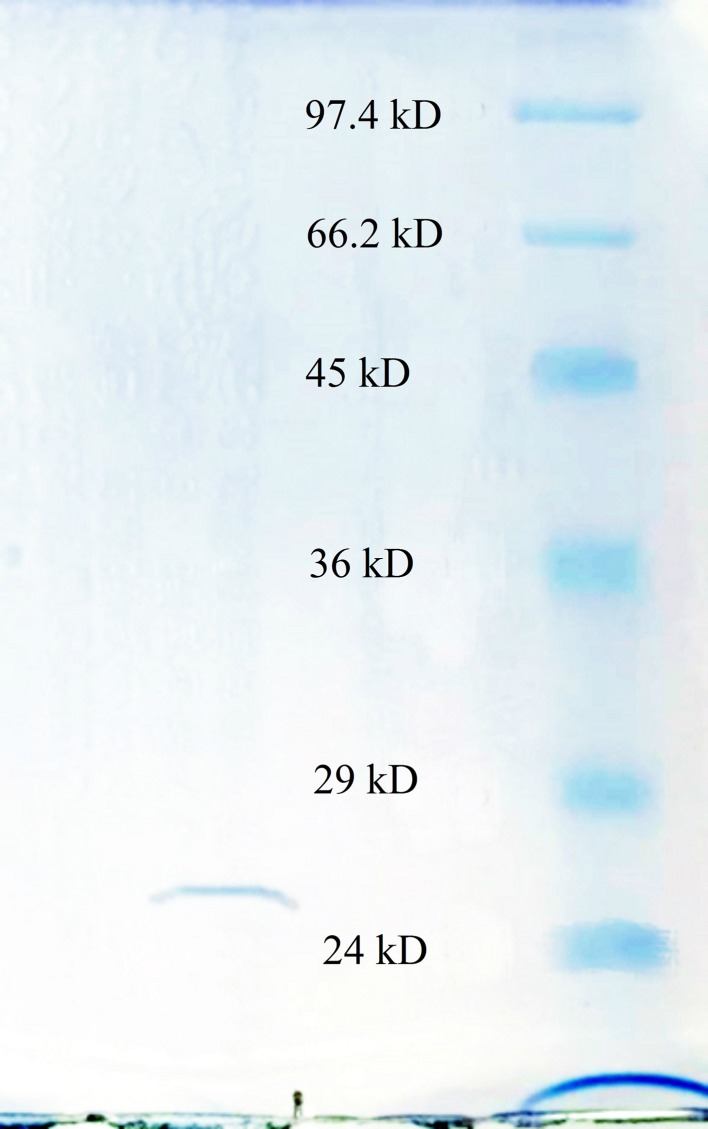
Fibrinolytic enzymes were recovered at rates of approximately 82.6, 50.7, and 42.7% after precipitation with ammonium sulfate, elution with Q HP, and Sephacryl-100 chromatography, respectively (Table 1). The majority of the contaminants were removed by Q anion exchange chromatography and the fraction with fibrinolytic activity was collected from 8 to 23 mL as unbound part (Fig. 1a). Sepharcryl-100 chromatography revealed three peaks and only the middle peak showed fibrinolytic activity (Fig. 1b). The concentrated final product showed single bank at SDS-PAGE (Fig. 2). The fibrinolytic activity of final product increased by 329.76-fold with a specific activity of 2373.59 (units/mg).
Table 1.
Summary of the purification efficiency at each step
| Procedure | Total protein (mg)A | Total activity (U)B | Specific activity (U/mg) | Enzyme purification (fold) | Recovery (%) |
|---|---|---|---|---|---|
| Crude extract | 304.86 ± 10.54a | 2340.28 ± 60.23a | 7.76 ± 0.45d | 1.0 ± 0.16c | 100a |
| (NH4)2SO4 ppt | 297.42 ± 8.56b | 1932.85 ± 51.32b | 6.54 ± 0.28c | 0.84 ± 0.05d | 82.6 ± 6.29b |
| Q FF | 0.84 ± 0.12c | 1186.42 ± 57.98c | 1416.83 ± 25.69b | 184.24 ± 9.01b | 50.7 ± 3.25c |
| Sephacryal 100 | 0.42 ± 0.08d | 999.26 ± 32.76d | 2373.59 ± 54.81a | 329.76 ± 12.34a | 42.7 ± 2.17d |
Values were expressed as mean ± SD (n = 3). Values not sharing a similar superscript within the same column were significantly different (P < 0.05) as determined by ANOVA
AProtein concentration was measured by BCA method of using BSA as standard
BEnzymatic activity was measured in 30 mM sodium carbonate/sodium bicarbonate buffer (pH 10.5) at 45 °C for 6 h
Fig. 1.
The fraction with specific activity was collected from 8 to 23 mL from the Q HP chromatography (a) and the fraction with specific activity was collected from 65 to 75 mL from the Sepharcryl-100 chromatography (b)
Fig. 2.
SDS-PAGE results of the fibrinolytic enzyme. The molecular markers used were phosphorylase b (97.4 kDa), bovine serum albumin (66.2 kDa), chicken egg ovalbumin (45 KDa), glyceraldehyde-3-P dehydrogenase (36 kDa), bovine carbonic anhydrase (29 kDa), and bovine trypsinogen (24 kDa)
Determination of molecular weight and pI value of the enzyme
The molecular weight (MW) of this enzyme estimated by SDS-PAGE is approximately 27 kDa (Fig. 2). The retention volume of activated peak of Sephacryl-100 chromatography is 70 mL, which also corresponds to the MW of about 27 kDa according to the instruction of manufacturer. The pI of this enzyme determined by IEF was 8.9 ± 0.1.
N-terminal amino acid sequence determination
The N-terminal amino acid sequence of the purified enzyme was analyzed using the automated Edman method following SDS-PAGE. The sequence of the first ten residues was AQSVPYGISQ. After being calibrated with the PTH-amino acids standard, the sample was tested and the result was compared with standard. According to the comparison, the first amino acid of N-terminal was alanine (Ala), the next was glutamine (Gln), serine (Ser), valine (Val), proline (Pro), tyrosine (Tyr), glycine (Gly), isoleucine (Ile), serine (Ser), and glutamine (Gln). The first ten N-terminal amino acids sequence of enzyme T were NH2-Ala-Gln-Ser-Val-Pro-Tyr-Gly-Ile-Ser-Gln (authors provided supplementary Figures S1, S2 and S3), which is consistent with N-terminal sequence of nattokinase.
Effects of pH and temperature
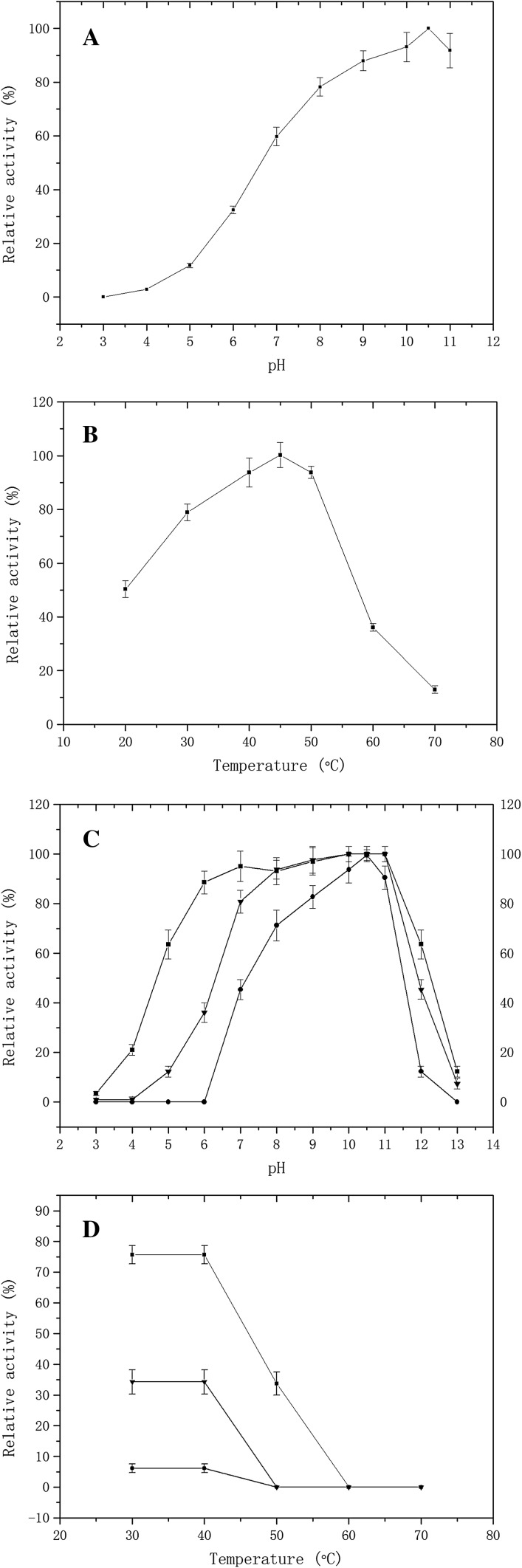
The optimal pH and temperature for this enzyme were determined to be 10.5 and 45 °C, respectively (Fig. 3a, b). The enzyme was stable between pH 5.0 and 12.0 and up to 50 °C. The enzymatic activity remained 63.58 and 12.33%, respectively, after 0.5 and 3 h of incubation at the buffer of pH 5.0, remained 63.58 and 45.38% after 0.5 and 3 h of incubation at the buffer of pH 12.0 (Fig. 3c). When the pH was adjusted to 4.0, the enzymatic activity was decreased to 1.11% after 3 h. After incubation the enzyme at the buffer of pH 10.5 under the temperature of 50 °C for 0.5 h, the enzymatic activity was decreased to 33.92% (Fig. 3d). When the enzyme was incubated at room temperature (20 °C), the residual activity remained 100% after 24 h.
Fig. 3.
Effect of pH and temperature (filled square 0.5 h, filled triangle 3 h, filled circle 12 h). a The optimum pH of the purified enzyme was determined by measuring the activity over the pH range of 3.0–11.0 (pH 3.0, 20 mM citrate buffer; pH 4.0–5.0, 50 mM acetate buffer; pH 6.0–7.0, 30 mM phosphate buffer; pH 8.0, 50 mM tris buffer; pH 9.0–11.0, 30 mM sodium carbonate/sodium bicarbonate buffer) at 45 °C; b The optimal temperature of the fibrinolytic enzyme was determined by measuring activity over the temperature range of 20–70 °C at pH 10.5; c The pH stability was determined by measuring the residual enzymatic activity after 0.5, 3, and 12 h incubation of the purified enzyme in various buffers over the pH range of 3.0–13.0 (pH 12.0–13.0 buffer, 100 mM glycine–NaOH) at 45 °C; d The temperature stability was determined by measuring the residual enzymatic activity after 0.5, 3, and 12 h incubation of the purified enzyme over the temperature range of 20–70 °C at the buffer of pH 10.5
Effects of metals and chemicals
As shown in Table 2, the fibrinolytic activity of this enzyme was inhibited by Cu2+ and Zn2+ at concentrations higher than 5 mM and inhibited by Fe3+ at concentration higher than 10 mM. The activity was enhanced by K+, Na+, Mg2+, Mn2+, Ca2+, and Ba2+, especially by K+, Mg2+, and Mn2+ at very low concentrations. The activity of this newly purified enzyme was slightly enhanced by PMSF and EDTA at a concentration of 2.5 mM but was inhibited at 5 mM; β-mercaptoethanol had the strongest inhibitory effect among the chemicals tested, while IAA did not inhibit the newly purified enzyme (Table 3).
Table 2.
Residual fibrinolytic activity of the enzyme when existing at the different concentrations of metal ions
| Metal ions concentration (mM) | Relative activity (%) | ||
|---|---|---|---|
| 1.0 | 5.0 | 10.0 | |
| NaCl | 186.13 ± 8.56c | 118.18 ± 5.23c | 89.46 ± 3.89b |
| KCl | 215.45 ± 9.12b | 136.23 ± 6.78b | 100.03 ± 5.05a |
| MgSO4 | 244.67 ± 6.33a | 118.98 ± 5.14c | 100.67 ± 6.32a |
| MnCl2 | 244.89 ± 5.68a | 155.08 ± 4.39a | 100.89 ± 5.51a |
| CaCl2 | 186.02 ± 8.21c | 118.24 ± 8.40c | 100.75 ± 4.64a |
| CuCl2 | 100.15 ± 5.69d | 86.06 ± 4.79e | 36.68 ± 1.85d |
| Ba(SO4)2 | 186.85 ± 7.53c | 100.23 ± 5.63d | 100.03 ± 6.37a |
| ZnCl2 | 100.05 ± 4.68d | 81.76 ± 3.81f | 78.98 ± 2.89c |
| FeCl3 | 100.32 ± 5.03d | 100.95 ± 4.05d | 75.99 ± 3.56c |
| Control | 100.68 ± 4.95d | 100.63 ± 3.26d | 100.02 ± 5.35a |
Residual enzymatic activity was measured in 30 mM Na2CO3/NaHCO3 buffer (pH 10.5) containing various metal ions at 45 °C for 6 h. Values were expressed as mean ± SD (n = 3). Values were not sharing a similar superscript within the same column were significantly different (P < 0.05) as determined by ANOVA
Table 3.
Residual fibrinolytic activity of the enzyme when existing at the different concentrations of enzyme inhibitors
| Inhibitors concentration (mM) | Relative activity (%) | ||
|---|---|---|---|
| 1.0 | 2.5 | 5.0 | |
| PMSF | 100.45 ± 3.98a | 122.86 ± 5.67a | 77.03 ± 5.92c |
| EDTA | 100.87 ± 2.56a | 113.45 ± 8.25b | 75.68 ± 4.38c |
| β-Mercaptoethanol | 87.61 ± 3.45b | 77.62 ± 6.39d | 68.73 ± 5.25d |
| IAA | 100.95 ± 6.32a | 100.05 ± 5.63c | 93.25 ± 2.58b |
| Control | 100.82 ± 4.28a | 100.24 ± 4.97c | 100.98 ± 3.67a |
Residual enzymatic activity was measured in 30 mM sodium carbonate/sodium bicarbonate buffer (pH 10.5) containing various enzyme inhibitors at 45 °C for 6 h. Values were expressed as mean ± SD (n = 3). Values that were not sharing a similar superscript within the same column were significantly different (P < 0.05) as determined by ANOVA
As early as 1990, the fibrinolytic enzyme produced by Bacillus subtilis was reported to be used as the fibrinolytic therapy. After being orally administrated in human subjects, the fibrin was degraded dramatically and the level of tissue plasminogen activator (t-PA) in blood was also increased (Sumi et al. 1990). Then bacteria were found to be the most important source of fibrinolytic enzymes. B. tequilensis used for enzyme production in this report is one species of Bacillus genus bacteria. The morphological characteristic is different from B. subtilis which is the most common bacteria used for fibrinolytic enzyme production. The cell of this strain is spore-forming, motile, catalase-positive, oxidase-positive, aerobic, and gram-positive bacilli. The strain was previously reported to have the ability to produce a number of functional ingredients, such as polygalacturonase (Shah et al. 2013), lipase (Bonala and Mangamoori 2012), and laccase (Sondhi et al. 2014). The fibrinolytic enzyme producing ability described by this report was an important supplement for the application research of B. tequilensis. A strain of B. tequilensis (CWD-67) was reported to have the ability to secrete a fibrinolytic enzyme with MW of 22 kDa, different from the 27 kDa of enzyme obtained by this report. There was no statistical analysis on the characterization results of the biochemical properties of the enzyme produced by B. tequilensis (CWD-67). Based on this, we believe this report provided a more accurate characterization of the fibrinolytic enzyme secreted by B. tequilensis.
The enzyme was purified by a logical combination of salting out, ion exchange chromatography, and size exclusion chromatography. The purification efficiency was much higher than previously reported for the purification of other fibrinolytic enzymes, such as 9.2-fold enzymatic activity increase with 9.5% recovery for nattokinase from B. subtilis (YJ1) (Yin et al. 2010) and 4.97-fold with 6.28% recovery for the fibrinolytic enzyme BSF1 from B. subtilis (A26) (Agrebi et al. 2009). It was close to the most effective result to date for the purification of a fibrinolytic enzyme from Rhizopus chinensis at 893.1-fold and 42.6% recovery (Liu et al. 2005). The molecular weight of this enzyme is similar to many other fibrinolytic enzymes such as nattokinase (27.7 kDa) (Cai et al. 2017), subtilisin DFE (28 kDa) (Peng et al. 2003), and CK (28.2 kDa) (Kim et al. 1996), but lower than t-PA (72 kDa) (Rijken and Collen 1981) and urokinase (56 kDa) which is most commonly used as thrombolytic agents in clinical treatment (Husain et al. 1983). It is well known that lower MW always indicates lower possibility of resulting in antigenicity. Thus, the enzyme should be a safe thrombosis agent. The N terminal amino acid sequence of this enzyme is identical to nattokinase (Cai et al. 2017), subtilisin DFE (Peng et al. 2003), subtilisin QK-2 (Ko et al. 2004), and subtilisin DJ-4 (Kim and Choi 2009), but different from those of bacillokinase-II and KA38, which are ARAGEALRDI and VYPFPGPIPN, respectively (Jeong et al. 2004; Kim et al. 1997). The MW of first 12 amino acids of nattokinase is 1291.298 (corresponding to AQSVPYGISQIK) which is a typical peak of nattokinase after being digested with trypsin, while there is no matched MW identified when analyzing this purified enzyme by a peptide mass fingerprint (PMF) method, indicating at least one residue of 11th and 12th is different with nattokinase (data not shown).
The optimal temperature of this purified enzyme was similar to those of the fibrinolytic enzyme from R. chinensis 12 at 45 °C (Liu et al. 2005) and subtilisin DJ-4 at 40 °C (Kim and Choi 2009), but lower than subtilisin QK2 at 55 °C (Ko et al. 2004). The thermal stability of the purified enzyme is close to that of most thermal-stable fibrinolytic enzymes (up to 60 °C) produced by B. subtilis KCK-7 (Paik et al. 2004). The stable pH range of this enzyme is also similar to that of most of other fibrinolytic enzymes, such as subtilisin DFE (stable at pH 6–10, optimal pH of 9) (Peng et al. 2003), and nattokinase (stable at pH 6.0–12.0 optimal pH of 9) (Cai et al. 2017). The most stable fibrinolytic enzyme is subtilisin QK-2, which is stable at pH 3.0–12.0 and has an optimal pH of 8.5 (Ko et al. 2004). Thus, the optimal pH of the newly purified enzyme is higher than that of many other fibrinolytic enzymes. It could be used for preparing the agents activated in more alkaline conditions. Meanwhile, high optimal temperature and thermal stability allow this enzyme to be preserved at normal conditions, and can be used in specific circumstance which requires high temperature.
Normally, the activity of most nattokinase-like fibrinolyitc enzyme is enhanced by K+ and inhibited by Cu2+, Zn2+, and Fe3+. For example, the activity of enzyme subtilisin BSF1 was increased to 101.7% with 0.005 mol/L K+, but decreased to 77.9 and 87.3% with 0.005 mol/L Cu2+ and Zn2+, respectively (Agrebi et al. 2009). The fibrinolytic activity of the enzyme from B. subtilis YJ1 increased to 103, 114, and 117% by 1 mM, 5 mM, and 10 mM K+, and was decreased to 0% by 5 mM Zn2+ and Fe3+, and 10 mM Cu2+ (Yin et al. 2010). Other fibrinolytic enzymes such as the enzyme from Bacillus sp. strain DJ-4 (Kim and Choi 2009) are also inhibited by Fe3+, but the activity of the enzyme from B. subtilis KCK-7 is enhanced by Cu2+ (Paik et al. 2004) Similarly with these nattokinase-like fibrinolytic enzymes, the activity of this purified enzyme was also enhanced by K+ and inhibited by Cu2+, Zn2+ and Fe3+, while other metal ions could also enhance the activity of this purified fibrinolytic enzymes.
The chemicals effect of this enzyme is similar with that of fibrinolytic enzyme of serine protease, such as enzyme subtilisin BSF1, the activity is completely inhibited by PMSF, and the enzyme produced by B. subtilis YJ1, which is inhibited by EDTA (Yin et al. 2010; Agrebi et al. 2009). However, the chemical endurance ability of this purified enzyme is higher than most of serine protease. The fibrinolytic activity still remains when the concentration of PMSF and EDTA is up to 5 mM, so it could be activated at more broad conditions. The fibrinolytic enzyme produced by B. tequilensis has a higher fibrinolytic activity than most other fibrinolytic enzymes. The biochemical properties of this enzyme were determined accurately in this article, and the results indicated a great potential of this enzyme to be developed as the ingredient of functional food or clinical thrombolytic agent. The required activated conditions are simpler than many other fibrinolytic enzymes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank a research Grant no. 201319 by Beijing Normal University-Hong Kong Baptist University United International College for providing financial support for this project.
Abbreviations
- CGMCC
General Microbiological Culture Collection Center
- PMSF
Phenyl methyl sulfonyl fluoride
- EDTA
Ethylene diamine tetra acetic acid
- IAA
Iodoacetic acid
- CVD
Cardiovascular disease
- SDS
Sodium dodecyl sulfate
- APSAC
Anisoylated plasminogen streptokinase activator complex
- pI
Isoelectric point
- DI
Deionized water
- BCA
Bicinchoninic acid
- BSA
Bovine serum albumin
- QFF
Q sepharose fast flow ion exchange chromatography
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PVDF
Polyvinylidene difluoride membrane
- PTH
Phenylthiohydantoins
- MW
Molecular weight
- kDa
Kilodaltons
- IEF
Iso-electric focusing
- t-PA
Tissue plasminogen activator
- PMF
Peptide mass fingerprint
- (NH4)2SO4 ppt
Ammonium sulfate precipitation
- MgCl2
Magnesium chloride
- Na2CO3
Sodium carbonate
- NaHCO3
Sodium bicarbonate
- NaCl
Sodium chloride
- NaOH
Sodium hydroxide
- KCl
Potassium chloride
- MgSO4
Magnesium sulfate
- MnCl2
Manganese chloride
- CaCl2
Calcium chloride
- CuCl2
Copper chloride
- ZnCl2
Zinc chloride
- Ba(SO4)2
Barium sulfate
- FeCl3
Iron chloride
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1115-4) contains supplementary material, which is available to authorized users.
References
- Afifah DN, Sulchan M, Syah D. Purification and characterization of a fibrinolytic enzyme from Bacillus pumilus 2 g isolated from gembus, an Indonesian fermented food. Prev Nutr Food Sci. 2014;19:213–219. doi: 10.3746/pnf.2014.19.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrebi R, Haddar A, Hmidet N, Jellouli K, Manni L, Nasri M. BSF1 fibrinolytic enzyme from a marine bacterium Bacillus subtilis A26: purification, biochemical and molecular characterization. Process Biochem. 2009;44:1252–1259. doi: 10.1016/j.procbio.2009.06.024. [DOI] [Google Scholar]
- Astrup T. The biological significance of fibrinolysis. Lancet. 1956;271:565–568. doi: 10.1016/S0140-6736(56)92048-7. [DOI] [PubMed] [Google Scholar]
- Astrup T, Mullertz S. The fibrin plate method for the estimating fibrinolytic activity. Arch Biochem Biophys. 1952;40:346–356. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Basson M. Cardiovascular disease. Nature. 2008;451:903. doi: 10.1038/451903a. [DOI] [Google Scholar]
- Bonala KC, Mangamoori LN. Production and optimization of lipase from Bacillus tequilensis NRRL B-41771. Int J Biotechnol Appl. 2012;4:134–136. doi: 10.9735/0975-2943.4.1.134-136. [DOI] [Google Scholar]
- Cai D, Zhu C, Chen S. Microbial production of nattokinase: current progress, challenge and prospect. World J Microbiol Biotechnol. 2017;33:84. doi: 10.1007/s11274-017-2253-2. [DOI] [PubMed] [Google Scholar]
- Edman P. Method for determination of the amino acid sequence in peptides. Acta Chem Scand. 1950;4:283–293. doi: 10.3891/acta.chem.scand.04-0283. [DOI] [Google Scholar]
- Hua Y, Jiang B, Mine Y, Mu W. Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. nov. SK006 isolated from an Asian traditional fermented shrimp paste. J Agric Food Chem. 2008;56:1451–1457. doi: 10.1021/jf0713410. [DOI] [PubMed] [Google Scholar]
- Husain SS, Gurewich V, Lipinski B. Purification and partial characterization of a single-chain high molecular-weight form of urokinase from human urine. Arch Biochem Biophys. 1983;220:31–38. doi: 10.1016/0003-9861(83)90383-1. [DOI] [PubMed] [Google Scholar]
- Jeong YK, Kim JH, Gal SW, Kim JE, Park SS, Chung KT, Kim YH, Kim BW, Joo WH. Molecular cloning and characterization of the gene encoding a fibrinolytic enzyme from Bacillus subtilis Strain A1. World J Microbiol Biotechnol. 2004;20:711–717. doi: 10.1007/s11274-003-4514-5. [DOI] [Google Scholar]
- Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease-the Framingham study. JAMA. 1987;258:1183–1186. doi: 10.1001/jama.1987.03400090067035. [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi NS. Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp strain DJ-4 screened from Doen-Jang. Biosci Biotechnol Biochem. 2009;64:1722–1725. doi: 10.1271/bbb.64.1722. [DOI] [PubMed] [Google Scholar]
- Kim W, Choi K, Kim Y, Park H, Choi J, Lee Y, Oh H, Kwon I, Lee S. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol. 1996;62:1488–2482. doi: 10.1128/aem.62.7.2482-2488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Kim GT, Kim DK, Choi WA, Park SH, Jeong YK, Kong IS. Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. KA38 originated from fermented fish. J Ferment Bioeng. 1997;84:307–312. doi: 10.1016/S0922-338X(97)89249-5. [DOI] [Google Scholar]
- Ko JH, Yan JP, Zhu L, Qi YP. Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:65–74. doi: 10.1016/j.cca.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu XL, Du LX, Lu FP, Zheng XQ, Xiao J. Purification and characterization of a novel fibrinolytic enzyme from Rhizopus chinensis 12. Appl Microbiol Biotechnol. 2005;67:209–214. doi: 10.1007/s00253-004-1846-5. [DOI] [PubMed] [Google Scholar]
- Liu S, Marder VJ, Levy DE, Wang SJ, Yang F, Paganini-Hill A. Ancrod and fibrin formation: perspectives on mechanisms of action. Stroke. 2011;42:3277–3280. doi: 10.1161/STROKEAHA.111.622753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Huffman MD. Executive summary: heart disease and stroke statistics-2015 update a report from the American heart association. Circulation. 2015;131:434–441. doi: 10.1161/CIR.0000000000000157. [DOI] [PubMed] [Google Scholar]
- Paik HD, Lee SK, Heo S, Kim SY, Lee H, Kwon TJ. Purification and characterization of the fibrinolytic enzyme produced by Bacillus subtilis KCK-7 from Chungkookjang. J Microbiol Biotechnol. 2004;14:829–835. [Google Scholar]
- Peng Y, Huang Q, Zhang RH, Zhang YZ. Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Comp Biochem Physiol B Biochem Mol Biol. 2003;134:45–52. doi: 10.1016/S1096-4959(02)00183-5. [DOI] [PubMed] [Google Scholar]
- Rijken DC, Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981;256:7035–7041. [PubMed] [Google Scholar]
- Shah KP, Chandok KH, Rathore P, Sharma MV, Yadav M, Nayarisseri SA. Screening, isolation and identification of polygalacturonase producing Bacillus tequilensis strain EMBS083 using 16S rRNA gene sequencing. Eur J Biol Sci. 2013;5:09–13. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sondhi S, Sharma P, Saini S, Puri N, Gupta N. Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One. 2014;9:e96951. doi: 10.1371/journal.pone.0096951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43:1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinases. Acta Haematol. 1990;84:139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Aca. 1972;257:11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang M, Wang Y. Purification and characterization of a novel fibrinolytic enzyme from Streptomyces spp. Chin J Biotechnol. 1999;15:83–89. [PubMed] [Google Scholar]
- Yin LJ, Lin HH, Xiao ZR. Purification and characterization of a cellulase from Bacillus subtilis YJ1. J Mar Sci Technol. 2010;18:466–471. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.