Abstract
Ajuga multiflora Bunge cells contain β-ecdysterone (β-EC) that regulates the molting process of insect larvae. In this study, different conditions of culture have been studied to optimize the production of β-EC. A. multiflora Bunge growth fitted the curve of logistic equation with one growth cycle of 17 days. The electric conductivity of medium had a negative correlation with not only the weight of dry cell but also the β-EC accumulation, and thus, could be used for monitoring the peak of both cell growth and β-EC accumulation. The pH value of the culture medium varied from 4.67 to 5.84 and reached the maximum at the end of the culture (on the 17th day). The relation of cell growth and nutrient consumption in A. multiflora Bunge cell suspension culture was distinctly correlated. Continuous subculture caused a reduction in β-EC synthesis; passages 7–15, the β-EC content declined (p < 0.05). At passage 11, the β-EC content was only 42.72% of that at passage 5. Additives such as mevalonic acid (MVA), l-phenylalanine (l-Phe), α-pinene, terpineol, and nitric oxide (NO) in the suspension culture medium, could significantly promote the cell growth and stimulate β-EC accumulation. The optimal concentrations of l-Phe, MVA, terpineol, and α-pinene were 0.2 mmol/l, 10 mg/l, 1 mmol/l and 6 mmol/l, respectively, with the β-EC concentrations as 1.914 ± 0.1948 mg/g (p < 0.01), 6.012 ± 0.4252 mg/g (p < 0.01), 5.147 ± 0.4819 mg/g (p < 0.01), 2.801 ± 0.1253 mg/g (p < 0.01), respectively. The optimal concentration of sodium nitroprusside, the provider of NO, was 3 mmol/l with the β-EC concentration 2.87 ± 0.2493 mg/g (p < 0.01). The results offer a strategy for massive production of β-EC.
Keywords: Biological control, Cell culture, Hormone synthesis, Phytoecdysteroids
Introduction
Ecdysterone is the steroidal hormone in arthropods, including insects and probably invertebrates. It controls molting and metamorphosis in insects (Laurence 2001). Ecdysterone exhibits essential roles at every stage of the life cycle of the insect (Savchenko et al. 1998). Phytoecdysteroids produced by some plants have a structure, similar to that of insect-derived ecdysterone. Thus, at certain dosages, the larvae can not only develop into pupae, but also induce the insect into diapause or lead the pests to mortality (Lafont and Horn 1989; Laurence 2001; Chi et al. 2002). Phytoecdysteroids can be applied for the improvement of the output of silk and pest control (Chou and Lu 1980; Ninagi and Maruyama 1996). The pesticide characteristic of phytoecdysteroids on some pests, such as Clostera anastomosis (Linnaeus), Stilpnotia candida (Staudinger), Tuberolachnus salignus (Gmelin), Hyphantria cunea (Drury), Aporia crataegi Linnaeus, Malacosoma neustria testacea Motschulsky, Lymantria dispar L., Aphrophora intermedia Uhler, Parthenolecanium corni (Bouche), and Myzus persicae (Sulzer) have been studied (Shao et al. 1997; Chi et al. 1997a, b; Darvas et al. 1997). In 2002, eight types of phytoecdysteroids extracted from Ajuga multiflora Bunge were found to have killing effects on the larvae of Cryptorrhynchus lapathi L. (Chi et al. 2002).
Phytoecdysteroids has been utilized to make the larvae of Bombyx mori (L.) pupate synchronously in late autumn in China (Nie and Qiu 1987). Accumulated evidences showed that the acute toxicity of phytoecdysteroids to mammals or humans is extremely low. Reportedly, the phytoecdysteroids possess a great many helpful pharmacological effects, for example, it can control diabetes and heal wound (Yoshida et al. 1971; Ogawa et al. 1974; Kosar et al. 1997; Hou et al. 2007; Zhu et al. 2014). The phytoecdysteroids can be obtained from over 100 terrestrial plant families representing ferns, gymnosperms and angiosperms. More than 130 kinds of phytoecdysteroids have been found in both annuals and perennials plants (Laurence 2001). The most common compounds are 20-OH ecdysone, cyasterone, makisterone, ajugalactone, and makisterone (Darvas et al. 1997). The concentration of phytoecdysteroids in plants is higher than that in insects (Qian et al. 2015). β-Ecdysterone (β-EC) is a type of phytoecdysteroids commonly found in most of the plants (Mamadalieva et al. 2003; Shoeb et al. 2006; Coll et al. 2007; Ramazonov et al. 2017; Snogan et al. 2007).
As a perennial herb, Ajuga multiflora Bunge (Lamiaceae, Ajuga L.) is distributed in many Chinese regions (Heilongjiang, Inner Mongolia, Hebei, Liaoning, Jiangsu, and Anhui), Korea, and Siberia of Russia and is utilized to treat fever in the folk medicine in Korea (Liu et al. 2010; Sivanesan et al. 2016). β-EC is commonly extracted from A. multiflora Bunge. The wild resources of A. multiflora Bunge were limited, and a prolonged duration was required for cultivation. Moreover, the content of β-EC in the artificially cultivated A. multiflora Bunge was low. A previous study found the possibility of obtaining ecdysteroids, ecdysterone, and turkesterone from the culture of tissues and cells of the plant Ajuga turkestanica (Lev et al. 1990). Alternatively, the tissue of the plant and cell cultures could be selected to breed A. multiflora Bunge, which required more short-term growth and was not affected by both seasons and by environment. The β-EC is primarily contained in leaves of A. multiflora Bunge. Several studies have focused on producing β-EC by leaves and callus cultures (Sun et al. 2015). A simple, fast, and convenient cell engineering technique has been applied to culture the cells of the leaves of A. multiflora Bunge (Zhao et al. 2016). The cell culture of A. multiflora Bunge not only can provide sufficient raw materials for producing β-EC in large scale, but also decrease the costs.
A. multiflora Bunge was collected from Fuling Forest Park (41°50′N, 123°35′E) in Shenyang, Liaoning Province. The effective propagation and preliminary suspension culture of A. multiflora Bunge were built by Insect Laboratory of the Department of Forest (Zhao et al. 2016). Since then, the conditions of the system of suspension culture have been adapted to optimally produce the secondary metabolites β-EC and facilitate an efficient β-EC extraction. In this study, to design an optimal culture system for producing β-EC, the correlations between the consumption of nutrient, electric conductivity, growth of cells, and the accumulation of biomass have been analyzed.
β-EC is synthesized by the pathway of mevalonate acid (MVA) or the pathway of 5-phosphate-d-deoxyxylulose/2-C-methy-d-erythritol-4-phosphate (DOXP/MEP). The MVA pathway leads to the generation of terpenes and steroid ketones by reduction to mevalonate. On the other hand, the DOXP/MEP pathway produces terpenes by utilizing GA-3P and pyruvic acid as precursors. Although intermediate products of both pathways are isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMAPP), the mechanisms underlying the synthesis are varied based on the intracellular localization of metabolic end products monocyclic monoterpene can influence the pathways of both MEP and MVA. The α-pinene is capable of alternating the pathway of synthesis to increase yield of steroid ketones by inhibiting the synthesis of terpene. Terpineol, a monocyclic terpene alcohol produced by α-pinene metabolism, can also function as an inhibitor of terpene biosynthesis (Rohmer et al. 1993; Jomaa et al. 1999; Reuter et al. 2002; Liao et al. 2006; Qian et al. 2016).
The present study was aimed at investigating the molecular effect which intervenes in pathway-based generation of β-EC. MVA could be regarded as one precursor for the synthesis of sterone; moreover, the fundamental amino acid, l-phenylalanine (l-Phe) is treated as one frequently used precursor for different pathways of secondary metabolism. As nitric oxide (NO) is extensively utilized as one elicitor of the secondary metabolism of plants, these substrates have been supplemented in the A. multiflora Bunge suspension culture system as additives, and their influence on the production of the secondary metabolite, β-EC, has been evaluated (Luo et al. 2003; Cao et al. 2012; Qiao et al. 2015; Qian et al. 2016).
Materials and methods
Callus induction and suspension culture of cells
The leaves of A. multiflora Bunge served as the explants for the induction of callus [culture contents: Murashige and Skoog culture medium (MS), 6-benzylaminopurine (BA) 0.2 mg/l, kinetin (KT) 0.2 mg/l and 2,4-dichlorophenoxyacetic acid (2,4-D) 0.4 mg/l]. After incubation for 14 days, the tissues of callus were subjected to continuous cultivation (culture contents: MS and 2,4-D 0.4 mg/l) (Zhao et al. 2011). Subsequently, cells were harvested for the suspension culture. In this study, the basic liquid medium included MS media, 0.6 mg/l of 2,4-D. The pH value was set to be 5.8, the concentration of sucrose was 3%, and the ratio of inoculation was 10% (5 g cells in 50 ml of medium). Cell culture was made in one 16/8 h light/dark cycle with the light intensity of 2000 lx at 25 ± 1 °C and humidity of 70% with agitation at 120–130 rpm. After 24 h, the large and compact cell masses were removed from the callus tissues for continuous cultivation for 8–12 days under the same conditions as described above after passing through a 300-mesh sieve, which retrieved the seed cells (Zhao et al. 2016).
These seed cells were inoculated at 10% ratio into the basic liquid medium as described above and cultured for 1, 3, 5, 7, 9, 11, 13, 15, and 17 days for the test.
Measurement of weight of cells and construction of kinetic model
Suspension of cells was made through one 300-mesh sieve and dried by filter paper; fresh weight (FW) was recorded down. Subsequently, the cells were dried at the temperature of 60 °C, so that dry weight (DW) could be measured.
The growth kinetics of cells were calculated by the following formula:
where represented the highest cell concentration (g/l DW), dt indicated parameter acquired in data fitting, and K represented scaling factor.
Measurement of β-EC content in cell suspension
DW at 0.2 g of A. multiflora Bunge cells were soaked in 5 ml methanol for 24 h, handled by ultrasound (YH-200DH, Yuhao, China) for 1–2 h at 40 kHz, and digested by utilizing a microwave (WT-8000 microwave digestion system, digestion conditions: T = 50 °C, p = 2P0, T = 10 min, W = 300 × 2). The suspension of resultant was filtered with an organic membrane (Lu Teng Co., China), and the β-EC content of the filtrate was evaluated by HPLC. An MSC18 column (4.6 × 250 mm2, 5 μm particle size) (Agilent Co., USA) and one UV–vis detector (range of detection: 190–800 nm) were adopted to detect β-EC at a wavelength of 242 nm. The flow rate of the mobile phase (ratio of methanol to water = 50:50) was 0.6 ml/min, and the loading volume of sample was 10 μl (Mu et al. 2011).
Standards of β-EC with the concentration of 0, 0.1, 0.2, 0.4, and 0.8 mg/ml were prepared and loaded in 10 μl volume. Detections of all standards were made repeatedly for three times. The concentration was used as horizontal axis and the mean peak area as vertical axis; the equation of regression of β-EC was acquired (y = 18,498x + 226.24, R2 = 0.9996). The content of β-EC of all samples was calculated by standard curve. The final concentration of β-EC = (y − 226.24)/18,498, (y = peak area).
The kinetic model of β-EC production fitted in the reference logistic equation:
where represented maximum concentration of the product (mg/g), represented initial concentration of product (mg/g), P represented concentration of the product (mg/g), k indicated formation coefficient of product and t represents culture duration (d).
Measurement of culture medium pH
The pH of culture medium was measured by S-25 pH meter (Shanghai Leici Instrument Factory, China) (Chen et al. 2014).
Measurement of the electric conductivity of culture medium
The conductivity of culture medium was measured by one BEC microprocessor-based conductivity meter (BEC-11AW, Bell Analysis Instrument Co., Ltd.) (Ge et al. 2010).
Measurement of the consumption of nutrient and sugar
The method of molybdenum blue was employed to measure the content of phosphate of the culture medium. Salicylic acid spectrophotometry was adopted to determine the content of nitrate, whereas, ninhydrin assay was utilized for measuring the level of ammonium salt content. The total content of soluble sugar was estimated by the anthrone colorimetric method (Qian et al. 2016).
The kinetics of consumption of sugar was calculated by the following formula:
where represents yield in theory, m is maintenance factor of cells, is macro-yield, refers to initial concentration of substrate (mg/l DW), refers to concentration of substrate (mg/l), and t is time of culture (d).
Measuring the influence of passage on β-EC content
The β-EC content of the cells of A. multiflora Bunge was measured in the initial inoculation (passage 1), and then preceding every passage at once, which was conducted by the inoculation of 5 g of cells in 50 ml of liquid medium per 10 days for 15 passages.
Measuring the influence of exogenous substrates on the growth of cells and content of β-EC
In order to evaluate the influence of α-pinene, MVA, l-Phe or terpineol on the growth of cells and content of β-EC, cells of A. multiflora Bunge at passage 11 were inoculated (5 g) in 50 ml of liquid medium with the supplement of indicated additives separately or in combination and cultured for 7 days. Both the growth of cells and content of β-EC were measured during the measurement, every treatment was repeated three times.
The l-Phe (0.1652 g) was diluted with sterile ddH2O (10 ml) at the concentration of 0.1 mol/l, then it was added to the culture medium to yield a final concentration of 0, 0.1, 0.2, and 0.4 mmol/l. The MVA was diluted with KOH (0.1 N) at the concentration of 50 g/l, then it was added to the culture medium to yield a final concentration of 0, 5, 10, 20, and 60 mg/l. The α-pinene was diluted with 100% ethanol (ratio of α-pinene to ethanol = 8:2 in volume), then it was added to the culture medium to yield a final concentration of 0, 3, 6, and 12 mmol/l. The terpineol (8.4 ml) was diluted with 1.6 ml of 100% ethanol, then it was added to the culture medium to yield a final concentration of 0, 0.5, 1, 2, and 3 mmol/l. After 2 days growth of cells, the activity of cells and content of β-EC were measured.
In order to evaluate the influence of NO on the growth of cells and content of β-EC, cells of A. multiflora Bunge at passage 11 were inoculated (5 g) in 50 ml of liquid medium and cultured for 7 days. Sodium nitroprusside (SNP, 1 mol/l, 29.80 g SNP in 100 ml ddH2O), the donor of NO, was added to the culture medium to yield a final concentration of 0, 0.1, 0.5, 1, 3, 5, 10 mmol/l (0.5 mol/l of SNP that can release 2.0 μmol/l NO). After 2 days growth of cells, the activity of cell and content of β-EC were measured.
The assay of tetrazolium chloride (TTC) was adopted to evaluate the activity of cells. The fresh cells (0.4 g) were incubated with phosphate buffered solution (PBS) (2.5 ml, pH 7) and 0.4% TTC (2.5 ml) for 14 h in the dark. Then the suspension was centrifuged, the supernatant was abandoned, and the cells were rinsed with ddH2O for three times. 95% ethanol (5 ml) was added to washed pellet and the cells were decolorized in water bath at 60 °C for 30 min (the flask was agitated for 5 min at each step). The absorbance was measured at 485 nm by using one spectrophotometer (Qian et al. 2016).
Experimental design and statistical analysis
In this study, three samples (same culture time) were assessed in each group, to assess the cell DW and FW, β-EC content in the culture medium, the impact of passage on β-EC content, pH of the culture medium, electric conductivity, soluble sugar, phosphate, nitrate, and ammonium salt. The results are the mean value of three samples. The mean value (MV) and standard deviation (SD) was calculated by Microsoft Excel 2007 (Microsoft Co. Redmond, USA). Experimental data were sorted and recorded by Microsoft Excel 2007 (Microsoft Co. Redmond, USA). Kinetic fitting of β-EC production and sugar consumption was performed by OriginLab 9 (OriginLab Co. Northampton, USA). The significant variation between each group was analyzed by Duncan’s multiple range test (DMRT), significant differences were determined at p = 0.01 and p = 0.05 level with the aid of SPSS 22 (SPSS Inc. Chicago, USA).
Results
Growth curve of Ajuga multiflora Bunge cells under basic liquid culture medium
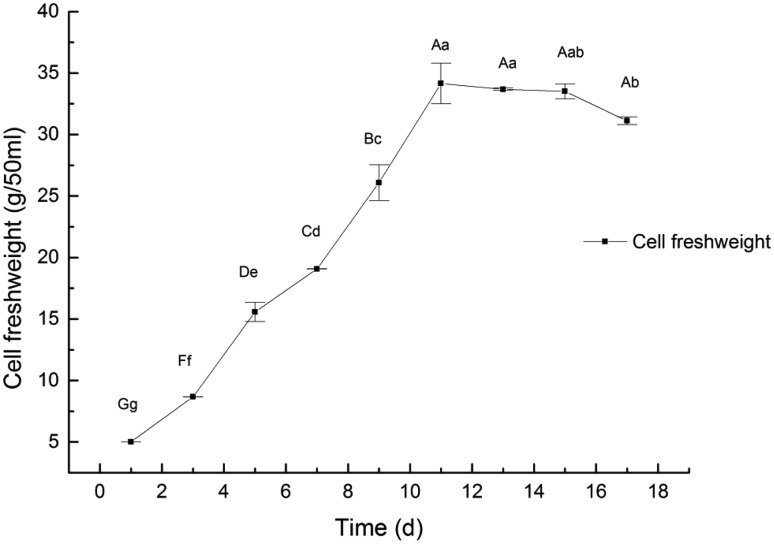
The growth cycle of the cells of A. multiflora Bunge lasted for 17 days, and the growth curve was in S-shape. In lag phase (1st to 3rd day), cells grew and biomass accumulated at a slow speed. Between 3rd and 11th days, the logarithmic growth phase occurred. From 11th to 15th days, the cell growth waned gradually, entering the stationary phase, when the biomass accumulation (FW) reached 33.67 ± 0.17 g · 50 m/l; this was 6.734-fold of the inoculum amount. The stationary phase lasted until the 15th day, after which, the cells entered into the declining phase, and the accumulation of biomass was reduced (Fig. 1).
Fig. 1.
Dynamic changes of cell fresh weight in A. multiflora Bunge culture. Note: basic liquid medium consisted of MS media, 0.6 mg/l of 2,4-D. a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
Electric conductivity of cell culture, cell DW, and β-EC content in the culture medium
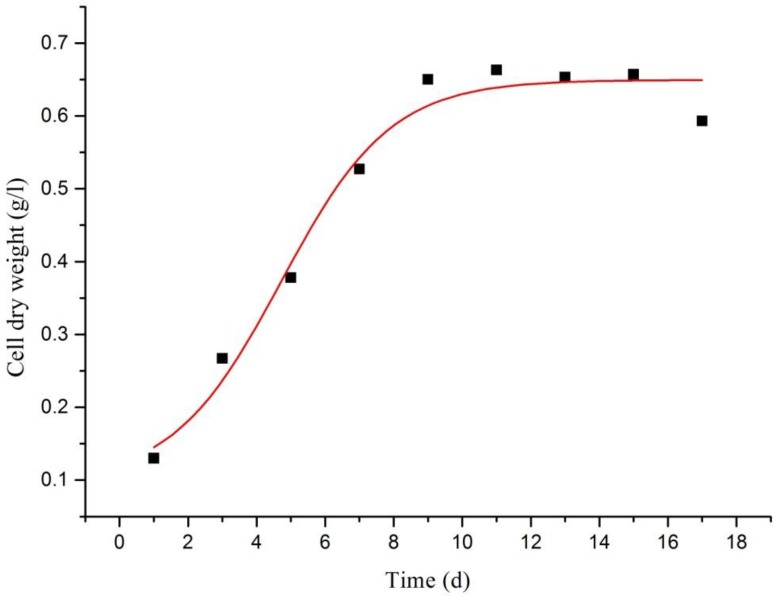
During the suspension culture of A. multiflora Bunge, the conductivity of culture medium was lowered, whereas the DW of cells were raised (Fig. 2). On day 11, DW reached its zenith, 0.663 ± 0.045 g, and relevant electric conductivity was 1.69 ± 0.036 ms/cm. After cells entered into stationary phase, the electric conductivity went down. The variations in DW and growth of cells were suitable for the following equation:
Fig. 2.
Cell dry weight in A. multiflora Bunge culture (spots indicate the cell dry weight at different culture times, curve is cell dry weight kinetics)
The parameters acquired in data fitting were as follows: Xmax = 0.67643, X0 = 0.1504, t0 = 4.97272d, dt = 2.94306, and the correlation coefficient R2 = 0.98086, which demonstrated that the model well reflected the kinetics of the cell growth of A. multiflora Bunge in suspension culture.
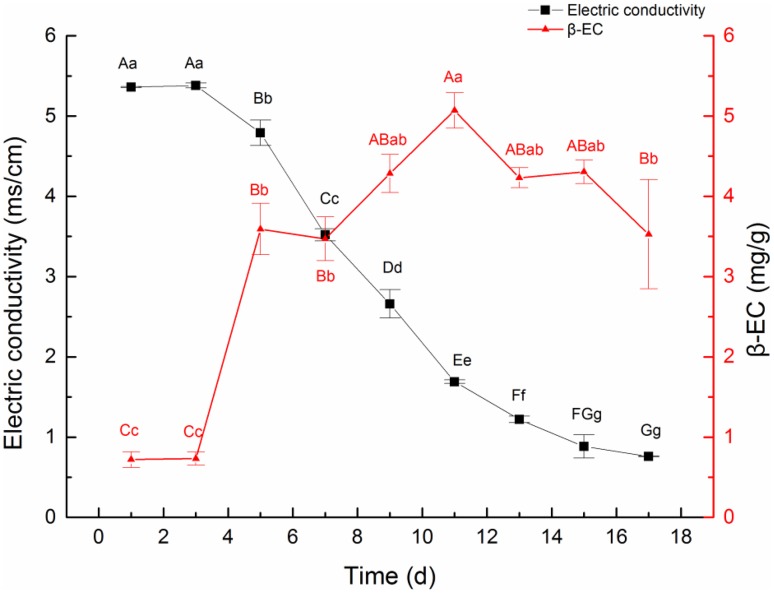
The content of β-EC had a negatively correlation with the electric conductivity of medium (Fig. 3). When electric conductivity rose to 1.69 ± 0.036 ms/cm, the β-EC content peaked at 5.068 ± 0.382 mg/g. In stationary phase, the electric conductivity was continually reduced until reach the declination phase, which was a potential result of the out flow of intracellular ions after the rupture of cell membrane.
Fig. 3.
Dynamic changes in cell suspension electric conductivity and β-EC content. Note: a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
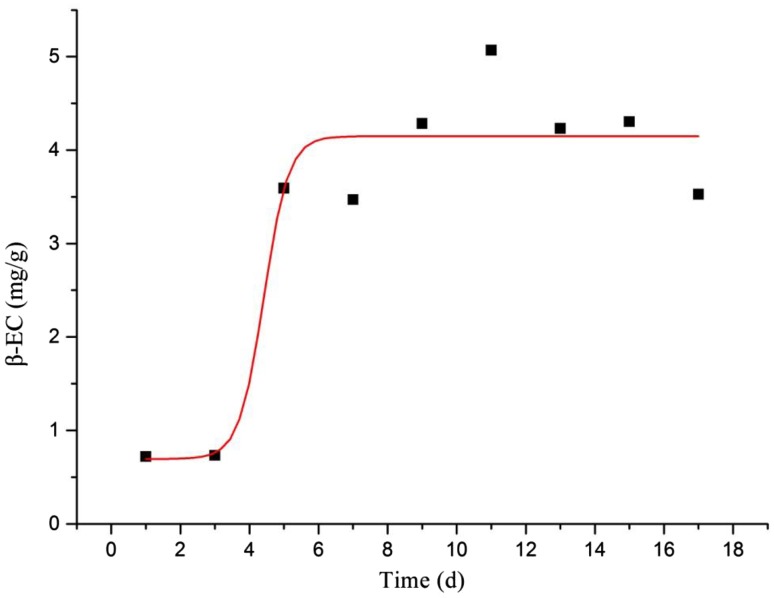
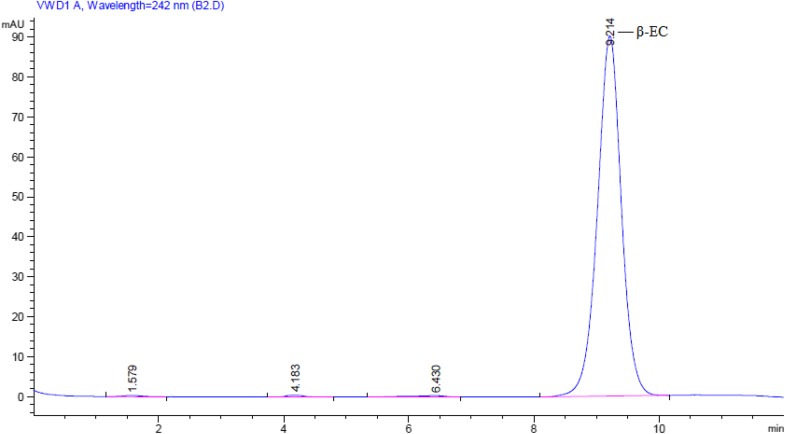
The correlation coefficient R2 = 0.8931, Pmax = 0.204 and k = 1.3505 was included in the parameters which described the accumulation of β-EC in suspension culture and were acquired in data fitting (Fig. 4). In HPLC experiment, the zenith of standard β-EC appeared at 9.214 min (Fig. 5).
Fig. 4.
β-EC content accumulation in A. multiflora Bunge culture at different times (spots indicate the β-EC production at different culture times, curve is β-EC accumulation kinetics)
Fig. 5.
The HPLC chromatogram of β-EC
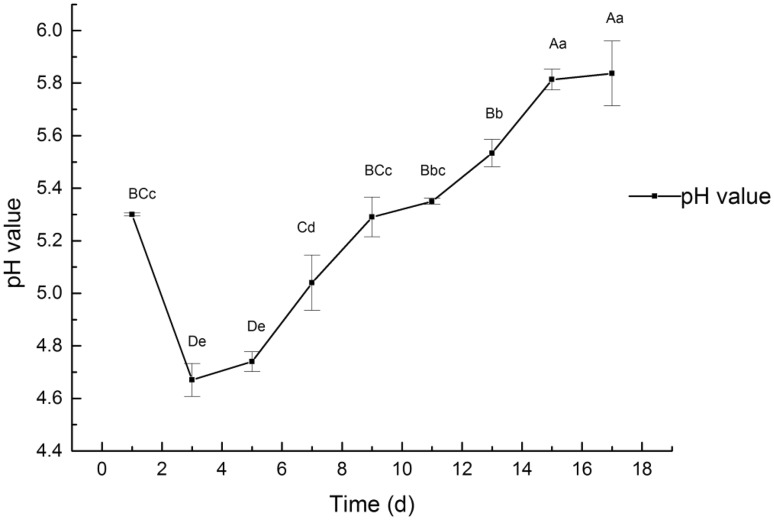
The pH variation of the cell culture medium during A. multiflora Bunge cell culture process
Throughout the whole growth cycle, the medium pH varied and immediately declined after the inoculation. On day 3 after inoculation, the pH value reached a minimum, which was 4.67 ± 0.24, following which, it increased gradually within the range 4.74–5.84. On day 17, the pH had raised to 5.84 ± 0.21 (Fig. 6).
Fig. 6.
The pH values in A. multiflora Bunge cells suspension culture at different times. Notes: pH initial change from 5.30 to 4.67 attributed to the cells absorb ammonium ion to adapt the suspension culture environment. a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
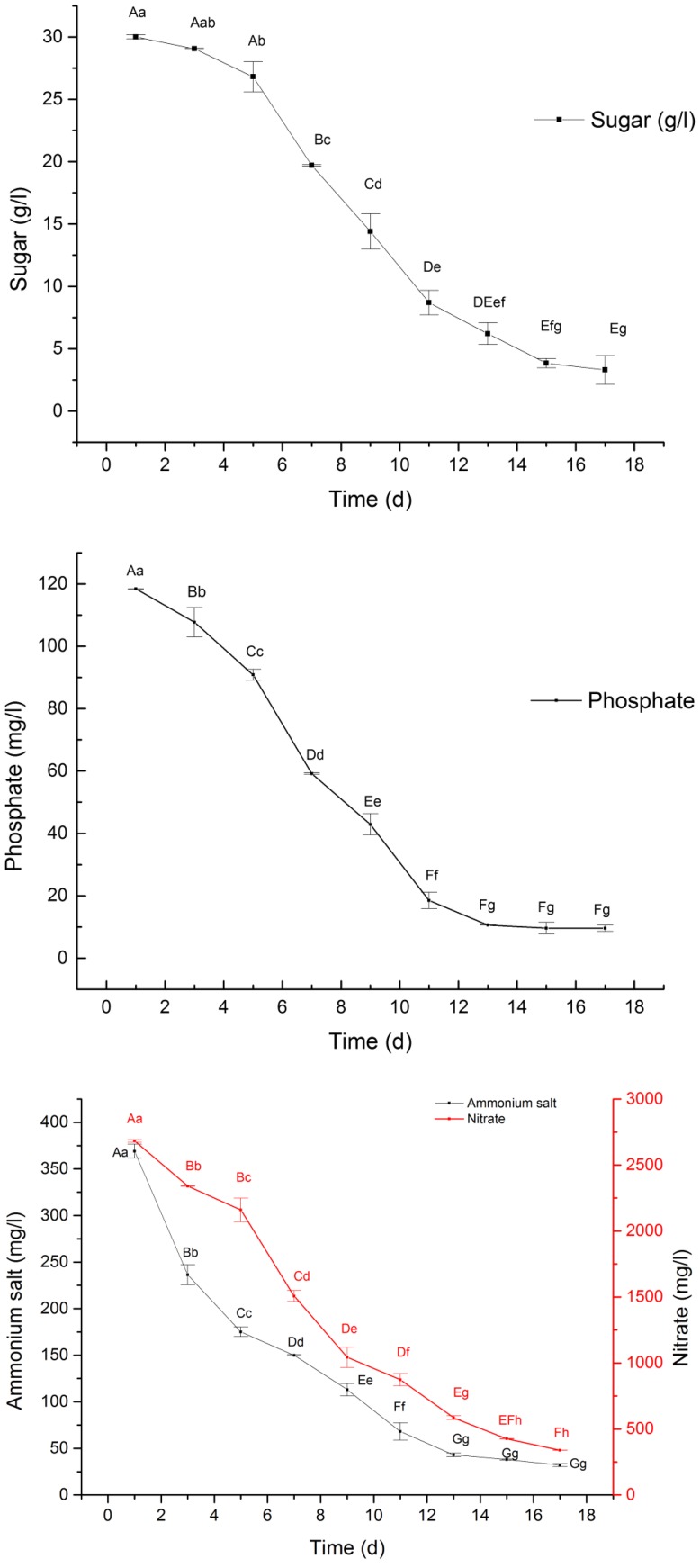
Substrate consumption during A. multiflora Bunge culture process
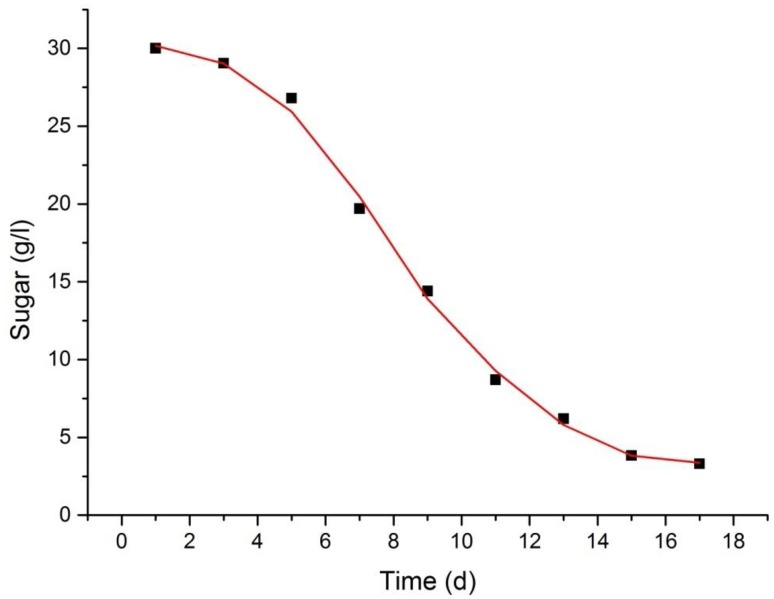
The curve comparison of the growth of cells and consumption of substrate suggested that these two indicators had a significant association. The contents of phosphate, sugar, ammonium salt and nitrate were found to slowly decline with the growth of cells in suspension culture (Fig. 7). However, on day 17, 12.64% of the medium nitrates remained unabsorbed, whereas 8.67% of the ammonium salts were unabsorbed. The consumption of total sugar conformed to the kinetic model of the consumption of sugar; m = 0.1033 (Fig. 8). The correlation coefficient, R2 = 0.9988, showed that the curve well reflected the consumption rate of sugar in the process of suspension culture.
Fig. 7.
The nutrients consumption of A. multiflora Bunge culture. Note: a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
Fig. 8.
Sugar kinetics of A. multiflora Bunge culture
The impact of cell passaging on β-EC accumulation
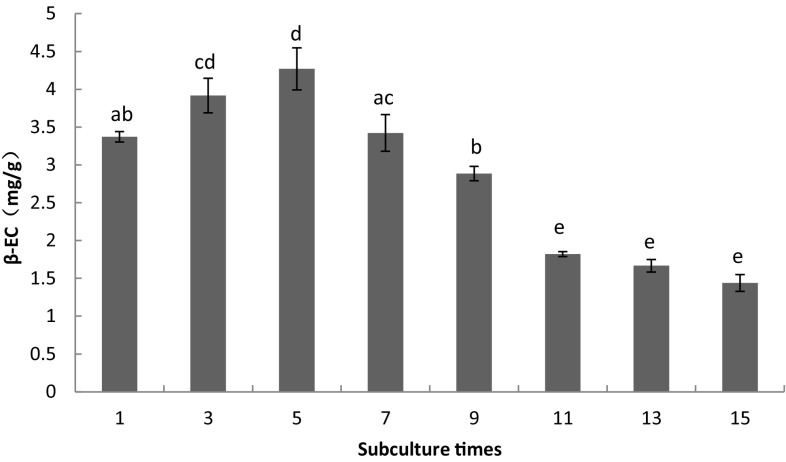
The β-EC content in A. multiflora Bunge suspension culture cells increased from the first to fifth subcultures and reached to 4.27 ± 0.48 mg/g at passage 5. Nevertheless, from passages 7–15, the content of β-EC decreased (p < 0.05) (Fig. 9), and the content of β-EC at passage 11 accounted for only 42.72% of that at passage 5.
Fig. 9.
The impact of subculture times on β-EC accumulation in suspension culture within 15 days. Notes: a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
The impact of exogenous substrates on the activity of A. multiflora Bunge and accumulation of β-EC
The exogenous culture additives were supplemented to passage 11 A. multiflora Bunge culture to estimate their influence. At this passage, we observed less β-EC production than in the younger cells, thereby, speculating that the exogenous additives increased the accumulation of β-EC.
The impact of l-Phe on A. multiflora Bunge cell activity and β-EC accumulation
Supplemented with 0–0.4 mmol/l. l-Phe, the activity of cells at passage 11 A. multiflora Bunge suspension culture was increased significantly (p < 0.01; Table 1). Nevertheless, the impact of l-Phe was not significant on the content of β-EC. Taking the influence of subculture into account, the activity of cells with 0.2 mmol/l l-Phe was 2.2-fold that of the control and could be used as an activator of the suspension cells at passage 11.
Table 1.
Effect of additives on A. multiflora Bunge cell suspension culture on the seventh day
| Additives | Concentration (mmol/l or mg/l) | Cell growth | Cell activity MV ± SD | β-EC (mg/l) MV ± SD |
|---|---|---|---|---|
| l-Phe | 0 | ++ | 0.31 ± 0.045Cd | 1.82 ± 0.0569Aa |
| 0.1 | ++ | 0.45 ± 0.015Bc | 1.808 ± 0.0515Aa | |
| 0.2 | ++ | 0.68 ± 0.01Aa | 1.914 ± 0.1948Aa | |
| 0.4 | ++ | 0.57 ± 0.08ABb | 1.865 ± 0.656Aa | |
| MVA | 0 | ++ | 0.31 ± 0.045Bb | 1.82 ± 0.0569Dd |
| 5 | ++ | 0.34 ± 0.026Bb | 4.59 ± 0.2094Bb | |
| 10 | + | 0.38 ± 0.036ABb | 6.012 ± 0.4252Aa | |
| 20 | – | 0.45 ± 0.03Aa | 3.235 ± 0.5723Cc | |
| 60 | – | – | – | |
| α-Pinene | 0 | ++ | 0.31 ± 0.045Bc | 1.82 ± 0.0569Cc |
| 3 | + | 0.38 ± 0.02Bb | 1.961 ± 0.0929Cc | |
| 6 | + | 0.59 ± 0.0Aa | 2.801 ± 0.1253Aa | |
| 12 | – | 0.36 ± 0.039Bbc | 2.413 ± 0.1812Bb | |
| Terpineol | 0 | ++ | 0.31 ± 0.045Aa | 1.82 ± 0.0569Cc |
| 0.5 | + | 0.3 ± 0.033Aa | 2.761 ± 0.1503Bb | |
| 1 | + | 0.28 ± 0.057Aa | 5.147 ± 0.4819Aa | |
| 2 | – | – | 1.422 ± 0.2696Cc | |
| 3 | – | – | 1.382 ± 0.2927Cc |
“++” cells are growing very well; “+” cells are growing
a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
The impact of MVA on A. multiflora Bunge cell activity and β-EC accumulation
The cell activity of A. multiflora Bunge cell suspension, supplemented with 0–60 mg/l MVA increased significantly (p < 0.01; Table 1). The addition of 5 or 10 mg/l MVA promoted the synthesis of β-EC, enhanced the content of β-EC by 1.527- and 2.297-fold that of the control, respectively (p < 0.05 and p < 0.01, respectively). However, the growth of cell was inhibited by 20 or 60 mg/l MVA dose-dependently, while the ordinary growth was enhanced by the cell suspension system supplemented with 20 mg/l MVA, and the color of medium was gray. On the other hand, the color cell suspension culture incubated with 60 mg/l MVA was brown, even death, and the accumulation of β-EC was dramatically decreased.
The impact of α-pinene on the cell activity A. multiflora Bunge and accumulation of β-EC
Adding 12 mmol/l α-pinene into A. multiflora Bunge cell culture medium reduced the growth of cells (Table 1); however, 3 and 6 mmol/l α-pinene enhanced the cell activity (p < 0.05). In presence of 6 mmol/l α-pinene (p < 0.01), the accumulation of β-EC was elevated to 2.801 ± 0.1253 mg/g, which was 1.9-fold than that of the control.
The impact of terpineol on A. multiflora Bunge cell activity and β-EC accumulation
Supplementing the A. multiflora Bunge cell culture with 1 mmol/l terpineol significantly stimulated the synthesis of β-EC synthesis, elevating the content 1.81-fold that of the control (p < 0.01; Table 1). However, when the concentration was > 2 mmol/l, terpineol reduced the cell growth and activity, as well as resulted in browning and death.
The impact of NO on A. multiflora Bunge cell activity and β-EC accumulation
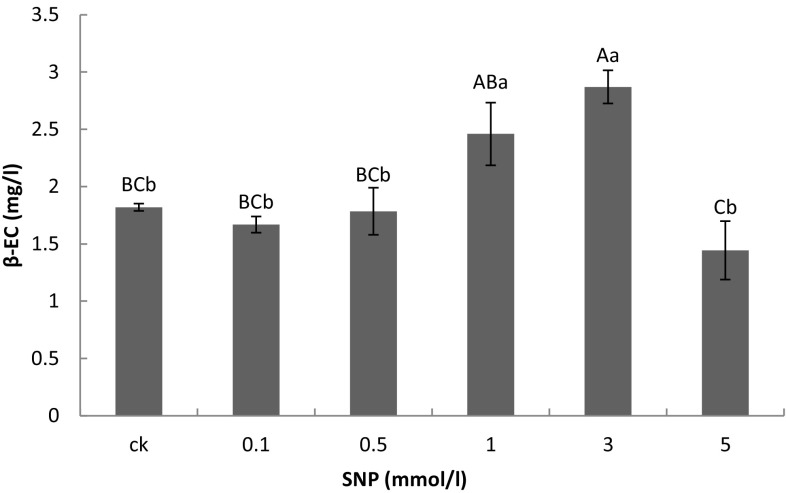
Distinct differences were not observed in the β-EC accumulation at 0.1 and 0.5 mmol/l in the presence of SNP. The β-EC accumulation reached 2.46 ± 0.4753 mg/g in presence of 1 mmol/l SNP (p < 0.05). However, β-EC accumulation reached 2.87 ± 0.2493 mg/g in presence of 3 mmol/l SNP (p < 0.01). In addition, β-EC accumulation was decreased slightly in presence of 5 mmol/l SNP, but did not achieve statistical significance (Fig. 10).
Fig. 10.
The impact of sodium nitroprusside (SNP) at different concentrations on β-EC accumulation in suspension culture. Notes: a, b, c, d, e: different letter means differences are significant; the same letter means differences are not significant; capital letters indicate a very significant difference (p < 0.01), lowercase letters indicate a significant difference (p < 0.05)
Discussion
The pH of the culture media is critical for the regulation and maintenance of cellular activities (Teo et al. 2014). The cells and tissues of plants need an optimal pH to grow and develop in cultures. The pH can affect the nutrients absorption and hormonal and enzymatic activities in plant cells (Bhatia and Ashwath 2005). In this study, pH varied throughout the entire growth cycle. During the lag phase, the decreased pH from 5.30 to 4.67 in the culture medium might be attributed to the adaptation of cells to the environment of suspension culture. After a short lag period, during which the suspension cells adapt to the culture condition, the pH begins to rise resulting from the absorption of ammonium ions by the cells. When exposed to an environment with unstable ambient pH, for growth and metabolism, the cells adjusted to maintain a relatively stable environment (OH et al. 2008).
The electric conductivity has been used successfully to monitor the cell growth as the increase in biomass can decrease the medium electric conductivity (Wen and Zhong 1996). In the culture medium, the electric conductivity can reflect the concentration of ions in culture medium in a direct manner and the nutrient contents occurring as ions in indirect manner. Along with cell growth and secondary metabolism, the nutrition in the cell culture was consumed gradually, thereby depleting the quantity in the suspension culture. The present study found that with an increased β-EC accumulation, the electric conductivity decreased, which was similar to the result from the study of electric conductivity in the culture medium of suspension culture of Ajuga lobata D. Don for 20-hydroxyecdysone accumulation (Qian et al. 2016). This phenomenon might be a characteristic of both Ajuga lobata D. Don and A. multiflora Bunge belonging to the Ajuga genus. Therefore, the culture medium electric conductivity can reflect cell growth and β-EC accumulation in A. multiflora Bunge cell suspension culture.
In this study, the relationship between consumption of nutrient, growth of cells, and accumulation of biomass was analyzed, and it was found that the growth curve of cells corresponded to nutrient consumption curve. Nevertheless, the consumption of nitrate and ammonium salt was not synchronous; the consumption rate of ammonium salt was faster than that of the nitrate during the initial 3 days of the lag phase, but slower in both logarithmic and stationary growth phases. The consumption of these substrates came to stop after cells entered into declining phase. At the end of the culture, the content of the residual nitrates and ammonium salts differed significantly in the culture medium, which might be attributed to the mechanisms underlying the growth of A. multiflora Bunge cells requiring ammonium salt. On the other hand, the β-EC accumulation was significantly associated with nitrate in the logarithmic growth period. A study also found that NH4+ and NO3+ absorbed by cells were assimilated immediately because the high concentration of NH4+ renders toxicity that will inhibit the synthesis of ATP and hydrolysis during photoreaction (Oh et al. 2008). The study of growth kinetics of Sorbus aucuparia L. culture suspension suggested that kinetics can optimize the cell culture conditions (Xiao et al. 2013). For example, Subhashini et al. (2014) utilized growth kinetics in order to establish a cell suspension culture for sea grass Halodule pinifolia. The growth kinetics also laid the foundation for the stimulation of cell growth of A. lobata D. Don. The curve of growth indicated that the cells of A. lobata D. Don fit the logistic function that represented the kinetics of cell growth (Qian et al. 2016).
Owing to the complexity of the biochemical reactions, none of the models could incorporate all the influencing factors during cell growth. Thus, in this study, only sucrose was utilized as the limiting factor, supposing that the accumulation of biomass was synchronous with β-EC synthesis. Therefore, in order to design suitable cell culture medium, a kinetic model of sucrose consumption was established.
Furthermore, not only environmental factors and hormones but also cell groups, cell tissues, and cell culture can influence the in vitro-cultured plant cells, such as loss of metabolites or cell death during passage (Hu et al. 2003; Fang et al. 2005). In this study, β-EC production was weakened in A. multiflora Bunge cells after subculture. Thus, in order to stimulate the secondary metabolism, precursor concentration, temperature, nutrient and hormone additives were altered in the suspension culture conditions; also, new suspension culture cycle could be established by new callus tissue. Herein, exogenous culture additives were supplemented to the culture of A. multiflora Bunge cells, so that the influencing capacity of these exogenous additives of culture could be measured. At passage 11 of A. multiflora Bunge cells, β-EC production was less than that in younger cells. The additives indicated above were added to passage 11 aspiring to measure the influence of those exogenous additives on β-EC accumulation.
Precursors are defined as those primary metabolisms that participate in the synthesis of target secondary metabolisms directly (Qian et al. 2016). Zhou et al. (2002) found that the precursors of serine (2-amino-3-hydroxypropanoic acid) and sodium benzoate (E211, benzoate of soda) enhanced the accumulation of taxol in Taxus chinensis (Pilger) Rehd cell suspension. Another study also found that the precursors such as l-Phe, tyrosine, and cinnamic acid enhanced the accumulation of glycyrrhizic flavone in Glycyrrhiza inflata Bat cell suspension culture (Yang et al. 2007). MVA directly enhanced the accumulation of plant secondary metabolites (Sun et al. 2000). In this study, the growth of A. multiflora cells in suspension was inhibited; however, the activity of the cells was increased, and the accumulation of β-EC was significantly enhanced after MVA was added to the culture. Nevertheless, the cells might display browning and even result in mortality as a result of high concentrations of MVA added to the culture system. This result was similar to that obtained from the study, wherein MVA was applied for the accumulation of 20-hydroxyecdysone in A. lobata D. Don suspension culture (Qian et al. 2016). This phenomenon might be attributed to the toxicity induced by the high concentration of MVA in suspension cells, which in turn, might inhibit the absorption of nutrient substances or influence the synthesis of metabolites.
Some substances can be used as inhibitors of the activity of specific enzymes involved in metabolism. As a result, the cells can be promoted to synthesize a specific compound. Many studies postulated that the addition of inhibitors alternated the pathways by stimulating the secondary metabolism. A study found that the inhibitors added to the Artemisia annua L. cell suspension culture could enhance the accumulation of artemisinin (Li et al. 1999). Another study also found that inhibitors, gibberellic acid and ancymidol, added to the cell culture of Taxus brevifolia could enhance the accumulation of taxol (Collins-Pavao et al. 1996). In present study, the MAV and DOXP/MEP-based pathways were redirected to enhance the accumulation of β-EC by the addition of inhibitors, such as derivative terpineol and α-pinene. The two inhibitors could enhance the accumulation of β-EC; however, the high concentration of these two inhibitors inhibited the cell activity as well as the accumulation of β-EC. This result was similar to that obtained from the study of accumulation of 20-hydroxyecdysone in the suspension culture of A. lobata D. Don using these two inhibitors (Qian et al. 2016).
The elicitors constitute the series of substances that can alter the metabolic pathways or induce the defense responses of plants. The NO is generally accepted as one elicitor for the secondary metabolism during the suspension culture of plant cells. The primary role of NO is to regulate the metabolic process of specific enzyme activity and the transcription level of some key enzymes; thus, NO served as a molecular switch (Zang et al. 2006; Wang et al. 2015; Qian et al. 2016). A study found that lipoxygenase played a major role in stimulating the elicitors. The activity of lipoxygenase had a positive correlation with the yield of paclitaxel, while methyl jasmonate was a principal product in the lipoxygenase pathway. The addition of methyl jasmonate induces the synthesis of taxol by lipoxygenase pathway, which in turn, synthesizes several molecules with activity for signal transmission (Huang et al. 2005).
The NO added to the A. lobata cell suspension culture promotes the cell growth and influences the β-EC accumulation (Qian et al. 2015). In this study, the high concentration of NO did not enhance the accumulation of β-EC efficiently. A. multiflora Bunge suspension cells treated with 5 mmol/l SNP exhibited the low level of secondary metabolites and β-EC content than the cells treated with 3 mmol/l SNP. The level of β-EC in A. multiflora Bunge suspension cells treated by 1 or 3 mmol/l SNP was remarkably higher than that in control group.
This study provided an experimental insight into the massive production of β-EC, which can be used as a safe and pollution-free biological pesticide; however, whether the combination of the different precursors, inhibitor, and elicitors, could enhance the accumulation of β-EC in A. multiflora Bunge cell suspension necessitates further exploration.
Conclusions
Since there is a high demand for pest control using environmentally friendly methods, β-EC has a great potential as an effective substance to control pests. But mass production of β-EC is difficult and costly. In our present study, different culture conditions have been studied to optimize the β-EC production. Furthermore, we found that including many additives (MVA, l-Phe, α-pinene, terpineol, and NO) at specific concentration to the suspension culture medium not only could significantly promote the cell growth and stimulate β-EC accumulation, but also these additives can be easily obtained. The implication of this study is that the production cost of this environmentally friendly substance can be brought down. Also the study makes massive production of β-EC become possible.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant number 31370649).
Abbreviations
- β-EC
β-Ecdysterone
- 2,4-D
2,4-Dichlorophenoxyacetic acid
- BA
6-Benzylaminopurine
- DMAPP
Dimethylallyl pyrophosphate
- DMRT
Duncan’s multiple range test
- DOXP/MEP
5-Phosphate-d-deoxyxylulose/2-C-methy-d-erythritol-4-phosphate
- DW
Dry weight
- FW
Fresh weight
- IPP
Isopentenyl pyrophosphate
- KT
Kinetin
- l-Phe
l-Phenylalanine
- MEP
2-C-methy-d-erythritol-4-phosphate
- MS
Murashige and Skoog culture medium
- MV
Mean value
- MVA
Mevalonic acid
- PBS
Phosphate buffered solution
- SD
Standard deviation
- SNP
Sodium nitroprusside
- TTC
Tetrazolium chloride
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- Bhatia P, Ashwath N. Effect of medium pH on shoot regeneration from the cotyledonary explants of tomato. Biotechnology. 2005;4:7–10. doi: 10.3923/biotech.2005.7.10. [DOI] [Google Scholar]
- Cao XB, Wang Z, Peng S, Wang XM, Zhang R, Ye BX. Effects of lanthanum nitrate and l-phenylalanine on callus growth and total alkaloid accumulation of Pinellia ternata. Shandong Agric Sci. 2012;44(7):26–28. [Google Scholar]
- Chen JG, Shang Guan XC, Yin ZP, Ren MH, Fu X. Establishment of the cell suspension culture system of Cyclocarya paliurus and matrix consumption laws. Mod Food Sci Technol. 2014;30(1):44–50. [Google Scholar]
- Chi DF, Darvas B, Rafael OR. Effects of some materials extracted from Ajuga species on the larvae of Hyphantria cunea and its natural enemies. J For Res. 1997;8(2):99–103. doi: 10.1007/BF02864977. [DOI] [Google Scholar]
- Chi DF, Shao JW, Sun F, Wang C, Mu Y, Zhou Z. Effects of phytoecdysones from Ajuga plants on the nymph of piercing–sucking mouthparts pests and their natural enemies. J Northeast Fore Univ. 1997;25(5):91–96. [Google Scholar]
- Chi DF, Sun MX, Xia WF. Pesticidal character of phytoecdysteroids from Ajuga multiflora Bunge (Labiatae) on larvae of Cryptorrhynchus lapathi L. (Coleoptera: Curculionidae) J For Res. 2002;13(3):177–182. doi: 10.1007/BF02871693. [DOI] [Google Scholar]
- Chou WS, Lu MS. Growth regulation and silk production in Bombyx mori L. from phytogenous ecdysteroids. In: Hoffmann JA, editor. Developments in endocrinology. Amsterdam: Elsevier/North-Holland Biomedical Press; 1980. pp. 281–297. [Google Scholar]
- Coll J, Tandron YA, Zeng XN. New phytoecdysteroids from cultured plants of Ajuga nipponensis Makino. Steroids. 2007;72(3):270–277. doi: 10.1016/j.steroids.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Collins-Pavao M, Chin CK, Pedersen H. Taxol partitioning in two-phase plant cell cultures of Taxus brevifolia. J Biotechnol. 1996;49(1–3):95–100. doi: 10.1016/0168-1656(96)01525-8. [DOI] [Google Scholar]
- Darvas B, Coll J, Chi DF. Control ability of extracts from Ajuga plants on some pests. J For Res. 1997;8(3):160–163. doi: 10.1007/BF02855410. [DOI] [Google Scholar]
- Fang WJ, Han LB, Zeng HM. Research advances in factors affecting establishment of plant cell suspension culture. Biotech Bull. 2005;4(5):11–15. [Google Scholar]
- Ge F, Wang JP, Wang XD, Zhao B, Wang YC. Process study of Amoeba euchroma cells in suspension culture. Nat Prod Res Dev. 2010;22(3):460–465. [Google Scholar]
- Hou L, Wu X, Wang WJ. Experimental study on the effect of ecdysterone, a herbal active ingredient, on wound healing. J New Chin Med. 2007;39(4):106–108. [Google Scholar]
- Hu ZH, Chen HQ, Wu GT, Zhang XL, Jin W, Xia ZH, Chen JQ. Establishment of suspension cell lines and high frequency regeneration of green plants in tall fescue. Acta Pratacult Sin. 2003;12(3):95–100. [Google Scholar]
- Huang YF, Lan WZ, Chen C, Yu LJ. The role of lipoxygenase in elicitor-induced taxol production in Taxus chinensis cell cultures. Process Biochem. 2005;40(8):2793–2797. doi: 10.1016/j.procbio.2004.12.018. [DOI] [Google Scholar]
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285(5433):1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- Kosar K, Opletal L, Vokác K, Harmatha J, Sovová M, Cerovský J, Krátký F, Dvorák J. Embryotoxicity of 20-hydroxyecdysone and polypodine B from Leuzea carthamoides DC. Pharmazie. 1997;52(5):406–407. [PubMed] [Google Scholar]
- Lafont R, Horn DHS. Phytoecdysteroids: structures and occurrence. In: Koolman J, editor. Ecdysone from chemistry to mode of action. Stuttgart: Thieme Verlag; 1989. pp. 39–64. [Google Scholar]
- Laurence D. Phytoecdysteroids: biological aspects. Phytochemistry. 2001;57:325–339. doi: 10.1016/S0031-9422(01)00078-4. [DOI] [PubMed] [Google Scholar]
- Lev SV, Zakirova RP, Saatov Z, Gorovits MB, Abubakirov NK. Ecdysteroids from tissue and cell cultures of Ajuga turkestanica. Khim Prir Soedin. 1990;1:51–52. [Google Scholar]
- Li HJ, Zhang Y, Guo Y, Yao RH. Artificial regulation of artemisinin biosynthesis metabolite in cultured cells of Artemisia annua L. Chin J Biochem Mol Biol. 1999;15(3):479–483. [Google Scholar]
- Liao ZH, Chen M, Gong YF, Miao ZQ, Sun XF, Tang KX. Isoprenoid biosynthesis in plants: pathways, genes, regulation and metabolic engineering. J Biol Sci. 2006;6(1):209–219. doi: 10.3923/jbs.2006.209.219. [DOI] [Google Scholar]
- Liu KQ, Deng HP, Ma XP. The research situation of Ajuga species in China. J Qujing Norm Univ. 2010;29(6):32–35. [Google Scholar]
- Luo YM, Liu AH, Li Q, Huang LQ. Study development on the biosynthesis path of plant terpenoids and its key enzyme. J Jiangxi Univ Tradit Chin Med. 2003;15(1):45–51. [Google Scholar]
- Mamadalieva NZ, Zibareva LN, Saatov Z. Phytoecdysteroids of Silene viridiflora. Chem Nat Compd. 2003;39(2):199–203. doi: 10.1023/A:1024822116031. [DOI] [Google Scholar]
- Mu L, Yang SC, Guan DJ, Yang T, Wen GS, Zhang WS. A study on accumulation regularity of content of β-ecdysone and optimal harvest time in Cyanotis arachnoidea C. B. Clarke. J Yunnan Agric Univ. 2011;26(2):194–198. [Google Scholar]
- Nie RL, Qiu MH. The phytoecdysones in residue of mother liquid of the molting hormone produced in factory from Cyanotis arachnoidea. Acta Bot Yunnanica. 1987;9(2):252–256. [Google Scholar]
- Ninagi O, Maruyama M. Utilization of 20-hydroxyecdysone extracted from a plant in sericulture. Jarq Jpn Agric Res Q. 1996;30:123–128. [Google Scholar]
- Ogawa S, Nishimoto N, Matsuda H. Pharmacology of ecdysones in vertebrates. In: Burdette WJ, editor. Invertebrate endocrinology and hormonal heterophylly. Berlin, Heidelberg: Springer; 1974. pp. 341–344. [Google Scholar]
- Oh K, Kato T, Xu HL. Transport of nitrogen assimilation in xylem vessels of green tea plants fed with NH4–N and NO3–N. Pedosphere. 2008;18(2):222–226. doi: 10.1016/S1002-0160(08)60010-7. [DOI] [Google Scholar]
- Qian JJ, Li X, Yang YY, Lin J, Chi DF. β-Ecdysterone accumulation and regulation in Ajuga lobata suspension culture. J Beijing Fore Univ. 2015;37(9):91–100. [Google Scholar]
- Qian JJ, Yang YY, Li X, Chi DF. 20-Hydroxyecdysone accumulation and regulation in Ajuga lobata D. Don suspension culture. Biosci Biotechnol Biochem. 2016;80(3):591–599. doi: 10.1080/09168451.2015.1116921. [DOI] [PubMed] [Google Scholar]
- Qiao M, Sun JW, Chen Y, Han SF, Hou CY, Liu G, Wang DM. Wheat suspension cell replication elicitor stimulation of Ca2+ and NO allergic reaction dynamics and its interactions. Bull Bot. 2015;50(1):1–11. doi: 10.3724/SP.J.1259.2015.00001. [DOI] [Google Scholar]
- Ramazonov NS, Mamadalieva NZ, Bobaev ID. Phytoecdysteroids from five species of the genus Silene. Chem Nat Compd. 2017;43(1):117–118. doi: 10.1007/s10600-007-0049-6. [DOI] [Google Scholar]
- Reuter K, Sanderbrand S, Jomaa H, Wiesner J, Steinbrecher I, Beck E, Hintz W, Klebe G, Stubbs MT. Crystal structure of 1-deoxy-d-xylulose-5-phosphate reductoisomerase, a crucial enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. J Biol Chem. 2002;277(7):5378–5384. doi: 10.1074/jbc.M109500200. [DOI] [PubMed] [Google Scholar]
- Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko T, Whiting P, Sarker SD, Dinan L. Distribution and identity of phytoecdysteroids in Gomphrena spp. (Amaranthaceae) Biochem Syst Ecol. 1998;26(3):337–346. doi: 10.1016/S0305-1978(97)00106-3. [DOI] [Google Scholar]
- Shao JW, Chi DF, Sun F, Zhao YJ, Mu YJ, Wang CY. Effects of phytoecdysones from Ajuga plants on the larvae of Lepidoptera species. J Northeast Fore Univ. 1997;25(5):87–90. [Google Scholar]
- Shoeb M, MacManus SM, Yashodharan K. American in a bioactive dibenzyl butyrolactone lignan from the seeds of Centaurea americana. Phytochem Anal. 2006;67(21):2370–2375. doi: 10.1016/j.phytochem.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Sivanesan I, Saini RK, Noorzai R, Zamany AJ, Kim DH. In vitro propagation, carotenoid, fatty acid and tocopherol content of Ajuga multiflora Bunge. 3 Biotech. 2016;6(1):1–10. doi: 10.1007/s13205-016-0376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snogan E, Vahirua-Lechat I, Ho R. Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm.) Phytochem Anal. 2007;18(5):441–450. doi: 10.1002/pca.1000. [DOI] [PubMed] [Google Scholar]
- Subhashini P, Raja S, Thangaradjou Establishment of cell suspension culture protocol for a seagrass (Halodule pinifolia): growth kinetics and histomorphological characterization. Aquat Bot. 2014;117(2014):33–40. doi: 10.1016/j.aquabot.2014.04.005. [DOI] [Google Scholar]
- Sun BX, Weng NQ, Liu D, Hu ZB. Influence of metabolic intermediate products on culture cell growth and taxol content of Taxus chinensis var. mairei. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai. 2000;14(3):54–56. [Google Scholar]
- Sun FF, Li CX, Fan GZ. Effects of exogenous CO on the accumulation of β-ecdysterone in Ajuga sp. Pratacult Sci. 2015;32(9):1438–1443. [Google Scholar]
- Teo A, Mantalaris A, Lim M. Influence of culture pH on proliferation and cardiac differentiation of murine embryonic stem cells. Biochem Eng J. 2014;90:8–15. doi: 10.1016/j.bej.2014.05.005. [DOI] [Google Scholar]
- Wang XJ, Li Z, Peng Y. The antioxidant enzyme activities and gene expression induced by spermidine in leaves of white clover. Acta Pratacult Sin. 2015;24(4):140–147. [Google Scholar]
- Wen ZY, Zhong JJ. Correlation between biomass and medium conductivity in suspension cultures of rice cells. Biotechnol Tech. 1996;10(5):309–312. doi: 10.1007/BF00173244. [DOI] [Google Scholar]
- Xiao WJ, Yang G, Guo LP, Hao QX, Lin SF. Study on the growth kinetics of Sorbus sucuparia L. in suspension cultured cells. Mod Chin Med. 2013;15(7):569–573. [Google Scholar]
- Yang Y, He F, Ji JX, Lei J, Chen XH, Yu LJ. The effect of precursor feeding on flavonoids biosynthesis in cell suspension cultures of Glycyrrhiza inflata Bat. J Wuhan Bot Res. 2007;25(5):484–489. [Google Scholar]
- Yoshida T, Otaka T, Uchiyama M, Ogawa S. Effect of ecdysterone on hyperglycemia in experimental animals. Biochem Pharmacol. 1971;20(12):3263–3268. doi: 10.1016/0006-2952(71)90431-X. [DOI] [PubMed] [Google Scholar]
- Zang X, Lv XH, Yang DZ, Zhang J. The study on enhancing taxol yield in cell culture of Taxus chinensis. J Fujian For Sci Technol. 2006;33(2):154–158. [Google Scholar]
- Zhao XJ, Li XC, Yu J, Chi DF. Effects of growth regulator and culture methods on rooting of Ajuga lobata and content of β-ecdysone. Chin Tradit Herb Drugs. 2011;42(9):1828–1832. [Google Scholar]
- Zhao JJ, Yu J, Chi DF. Establishment of a complete plant regeneration system on Ajuga multiflora. J Nat Sci Hum Norm Univ. 2016;39(1):30–34. [Google Scholar]
- Zhou ZQ, Mei XG, Wu QJ. Regulation of taxol production in cell suspension cultures of Taxus chinensis by elicitors, precursors and inhibitors. Nat Prod Res Dev. 2002;14(2):19–21. [Google Scholar]
- Zhu YX, Sun LS, Yang J, Chen Q. Effect of ecdysterone on expression of insulin receptor substrate 2 in liver cell of type 2 diabetic rats. Shandong Med J. 2014;54(33):10–12. [Google Scholar]